Abstract
Observations about the natural history of aging in Cornelia de Lange syndrome (CdLS) are made, based on 49 patients from a multidisciplinary clinic for adolescents and adults. The mean age was 17 years. Although most patients remain small, obesity may develop. Gastroesophageal reflux persists or worsens, and there are early long-term sequelae, including Barrett esophagus in 10%; other gastrointestinal findings include risk for volvulus, rumination, and chronic constipation. Submucous cleft palate was found in 14%, most undetected before our evaluation. Chronic sinusitis was noted in 39%, often with nasal polyps. Blepharitis improves with age; cataracts and detached retina may occur. Decreased bone density is observed, with occasional fractures. One quarter have leg length discrepancy and 39% scoliosis. Most females have delayed or irregular menses but normal gynecologic exams and pap smears. Benign prostatic hypertrophy occurred in one male prior to 40 years. The phenotype is variable, but there is a distinct pattern of facial changes with aging. Premature gray hair is frequent; two patients had cutis verticis gyrata. Behavioral issues and specific psychiatric diagnoses, including self-injury, anxiety, attention-deficit disorder, autistic features, depression, and obsessive-compulsive behavior, often worsen with age. This work presents some evidence for accelerated aging in CdLS. Of 53% with mutation analysis, 55% demonstrate a detectable mutation in NIPBL or SMC1A. Although no specific genotype–phenotype correlations have been firmly established, individuals with missense mutations in NIPBL and SMC1A appear milder than those with other mutations. Based on these observations, recommendations for clinical management of adults with CdLS are made.
Keywords: Cornelia de Lange syndrome, aging, Barrett esophagus, mutation, phenotype
INTRODUCTION
Although many individuals with well-characterized single gene disorders have reached adulthood, there has been little published on the natural history and the potential related risks and complications. Some specific syndromes have well-known risks related to aging. An increased risk for Alzheimer disease in Down syndrome is due to the deposition of neurofibrillary tangles in the brain. A high risk for developing various types of cancers is associated with many syndromes, such as Cowden syndrome, Gorlin syndrome, and the chromosomal breakage syndromes. In many connective tissue disorders, aneurysms may develop with age. A recent publication on adults with the 22q11 deletion syndrome noted that there is an increased incidence of schizophrenia, infections, scoliosis, and hernias [Bassett et al., 2005]. Few multiple malformation syndromes have had comprehensive evaluations of aging.
Cornelia de Lange syndrome (CdLS) is a well-known multiple malformation disorder with a distinctive physical appearance, small stature, medical complications, and developmental and behavioral issues. There is a broad range of severity, and many milder cases have been noted during the past 12 years. The cardiac, gastrointestinal (GI), craniofacial, genitourinary, musculoskeletal, and central nervous systems may all be involved. Developmental capabilities range from normal intelligence with learning disabilities to profound mental retardation (MR) [Jackson et al., 1993]. Several causal genes have been recently identified, including NIPBL on chromosome 5 and SMC1A on the X chromosome, [Krantz et al., 2004; Tonkin et al., 2004; Musio et al., 2006], however the diagnosis is made clinically.
Few publications related to CdLS have described adults. Several reports of affected adults having affected children have included a mildly affected father of a more severely involved daughter [Borck et al., 2006] and a severely affected father and daughter [Russell et al., 2001], as well as several maternally transmitted cases [de Die-Smulders et al., 1992; McConnell et al., 2003]. Single case reports have noted hematometra in an 18-year-old female [Doyle et al., 2005], keratitis with entropion in an 18-year-old female [Kim et al., 2005], and degenerative joint disease of the hip in a 32-year-old female [Grant et al., 1997]. Masumoto et al. [2001] reported a 15-year-old male with cecal volvulus. A 15-year-old male with CdLS was found to have cerebellar vermis hypoplasia [Ozkinay et al., 1998], and a 35-year-old male with self-injurious behavior was reported as having congenital dysgenesis of the brain with vascular and degenerative lesions [Vuilleumier et al., 2002].
Little is known about the natural history of CdLS. There has been a perceived need to assess this among members of the CdLS Foundation, the national support organization for families of individuals with CdLS. Because of this gap in research, we set up formal evaluations of older individuals with CdLS in a multidisciplinary clinic. From this, many observations and recommendations have been made, as described below, with respect to body system involvement, physical changes, and potential risks with aging. We precede a description of the findings with a typical case report.
CASE STUDY
This male patient was seen at the age of 28 years. He had been diagnosed in the newborn period with CdLS. He was born with a cleft palate, cryptorchidism, and absent forearms. His parents were told during infancy that it was likely that he would not survive, so he never underwent palatoplasty or orchiopexy. Feeding was always difficult and lengthy, but he never had a feeding tube placed. He had bilateral inguinal herniorraphy and several nasolacrimal duct probings for dacryostenosis. He had a history of sinusitis, and chronic eye discharge. He was thought to have had grand mal seizures at the age of 22 years and was started on medication, however several years later this was tapered off. Although he never had a known history of gastroesophageal reflux, at the age of 27 years he was noted to have some shaking episodes. He underwent a swallowing study, which revealed marked gastroesophageal reflux with aspiration into the lungs, an anterior cervical web and a hiatal hernia. He also had a history of constipation. He underwent esophagogastroduodenoscopy, which noted an abnormally formed pylorus and Barrett mucosa with goblet cells in the cervical esophagus with no evidence for dysplasia on biopsy. He was started on Prevacid and Reglan. He was never ambulatory, is said to have severe MR, and he continues to live at home. On physical examination, his length and weight were far below the second centile for males with CdLS, but his head circumference was around the 5th centile for males with CdLS, and he appeared extremely thin. His facies was typical for CdLS with arched synophrys, bilateral ptosis, long philtrum, and short nose (Fig. 1I). He had an open cleft of the soft palate. He had absent forearms with no digits and marked kyphoscoliosis. His legs demonstrated muscle wasting and he exhibited mild bunion deformities and severe flexion contractures of the legs. He had a cyst on the glans penis and the right testicle was not palpable in the scrotum. He had cutis marmarata. We recommended cleft palate repair and consideration of a Nissen fundoplication and a gastrostomy tube. The patient was lost to follow-up.
Figure 1.
A–J: Composite of more typical facies from younger to older; (K–R) composite of less typical facies from younger to older. Patients shown in subparts A,D,E,H,I,M,N,P,Q,T are females.
METHODS
Patients were recruited for a twice-yearly multidisciplinary clinic through contact with the CdLS Foundation. The families were notified via newsletter, website, cyber news bulletins, telephone contact, or during national and local meetings. Longitudinal study was approved by the hospital IRB, and informed consent was obtained. Medical records were reviewed, standard medical histories and family histories were taken, and physical examinations and photography were performed. Growth parameters were plotted on CdLS-specific growth charts previously developed [Kline et al., 1993a]. Molecular testing was offered to each patient. Laboratory and radiology studies were obtained in a standard hospital laboratory according to usual procedures. The biannual clinic has occurred over a 6-year period, from 2000 to 2006. Subspecialties represented at all or most clinics include: pediatric genetics, obstetrical genetics, internal medicine genetics, genetic counseling, pediatric ophthalmology, pediatric gastroenterology, child psychiatry, nutrition, pediatric dentistry, and pediatric orthopedics. During the clinic, each patient was evaluated by each subspecialist present, who made recommendations based on the clinical findings. Management and treatment recommendations were conveyed to the patient’s primary care physician and the family.
Diagnosis was confirmed clinically, based on diagnostic criteria described elsewhere [Kline et al., 2007]. In brief, facial features and two to three of six categories need to be involved, with at least one from the three major areas of growth, development and behavior. The other categories include musculoskeletal findings, neurosensory/skin involvement, and issues in other systems (e.g. cardiac, GI, craniofacial). Behavioral reports were based on parent information elicited by the clinicians.
Molecular testing was performed according to previously described protocols for the NIPBL gene on the short arm of chromosome 5 [Krantz et al., 2004] and the SMC1A gene on the X chromosome [Deardorff et al., 2007].
RESULTS
Patient Population
Forty-nine patients with age range 11–50 years were evaluated in the clinic. The mean age was 17 years 9 months, with a male to female ratio of 1.4:1. Three patients were of African-American origin, two were Hispanic, and the rest were Caucasian. The majority of the patients were diagnosed with CdLS prior to adulthood, with 57% percent at birth, 36% during early-mid childhood, and only 7% after age 18 years. Sixteen percent of the patients were living in a group home or residence, and the remainder with their parents. One patient had been adopted at birth. One patient was known to have an apparently balanced reciprocal translocation; all of the other patients had had normal chromosomes when younger, although these records were not always available. One patient has subsequently died. Thirty-three percent of the patients have been seen more than once by at least two of the authors.
Growth
Growth in CdLS has been previously described [Kline et al., 1993a]. Average adult growth parameters were reported for weight as 30.5 kg in females and 47.6 kg in males, for height as 131 cm in females and 156 cm in males, and for head circumference as 49 cm in both males and females. In the current study population, 31 (63%) were below the 5th centile for growth in all parameters, 43 (98%) were below the 5th centile for height, and 36 (73%) had microcephaly, when compared to standard growth curves.
In the current study population, 31 (63%) were below the 5th centile for growth in all parameters, 43 (98%) were below the 5th centile for height, and 36 (73%) had microcephaly, when compared to standard growth curves.
Although most patients remain thin, nine (18%) developed obesity with age, most commonly in the truncal region, although two patients did lose weight with dietary changes. Eleven percent had very low weight for height. No patients were tested for growth hormone.
Facial Features
The facial features in CdLS appear to evolve with time. Our older population could be divided into those with more typical facies and those with less typical facies (Fig. 1). The facies coarsens and lengthens slightly; one patient in particular appeared extremely coarse (Fig. 1Q).
Our older population could be divided into those with more typical facies and those with less typical facies. The facies coarsens and lengthens slightly; one patient in particular appeared extremely coarse.
Synophrys, present in all of the patients, may become bushier with age. The palpebral fissures are narrow and usually slightly down-slanting. The typical nasal configuration and prominent philtrum persist; 78% had a prominent philtrum and 78% had a short nose with anteverted nares. The jaw becomes more square and boney in appearance. In our population, 22 of the 49 patients (45%) had a square jaw and 47% had micrognathia. Three male patients (6%) demonstrated prognathism. Sixty-five percent had down-turned corners of the mouth. The more severely involved patients had a more typical facies than the milder patients. (Note in Fig. 1A–J are more severely involved than K–R in terms of major malformations and developmental delays.) In comparing more recent photographs to those when younger (data not shown), several patients appear coarser and less typical. In addition, as some individuals get older, a more aged appearance to the face seems to occur, with wrinkling and sagging, than would be expected when compared to chronologic age. For example, the patients shown in Figure 1J and R appear older than their ages of 36 and 50 years, respectively. There were several other patients similarly noted.
Gastrointestinal Manifestations
Gastroesophageal reflux (reflux) was the most common GI involvement, with 82% reporting reflux and three-quarters of these confirmed by GI study. The remainder, with no known prior history of reflux, has been recommended to undergo a work-up to rule out “silent” reflux. Forty-one percent were medically treated for gastroesophageal reflux disease (GERD). Fifty-five percent of the patients have had a Nissen fundoplication. Twenty-two percent received a gastrostomy tube, and one patient had a jejunostomy tube, most commonly for feeding issues. Thirteen percent had poor esophageal motility. Sixteen percent reported rumination. Endoscopy findings include: esophagitis (35%), gastritis (22%), duodenitis (13%), esophageal stricture (12%), and hiatal hernia (9%). Biopsy of proximal GI tract demonstrated eosinophilia (16%), H. pylori (8%), esophageal metaplasia (9%), and Barrett esophagus in 10%. The patients who had Barrett esophagus ranged in age from 19 to 36 years; esophageal metaplasia was seen as young as 15 years. Four patients (9%) had a history of bowel obstruction. GI malformations included cervical esophageal web (7%), pyloric stenosis (7%), malrotation (10%), and a single patient with Meckel’s diverticulum. One patient had pancreatitis secondary to a congenital anomaly of pancreatic ducts. In addition, 23 patients (46%) had moderate to severe constipation, nine patients (18%) had chronic diarrhea, 48% reported increased gas production, 36% reported dysphagia, and 4 patients (8%) had inguinal hernia repairs. Nine patients (18%) had lactose intolerance, and several patients had milk-protein allergy and/or gluten-sensitive enteropathy. One of our patients died from small intestinal obstruction and perforation following a volvulus; she had a known malrotation, which had been repaired and was found to have multiple adhesions.
Genitourinary Manifestations
Eighty-two percent of males had a history of cryptorchidism, and all but one patient had orchiopexy or orchiectomy. Malformations included hypospadias in 9% of the males and micropenis in 37%. The oldest male patient was found to have benign prostatic hypertrophy at age 39 years and underwent a transurethral resection of the prostate at age 40 years, and with residual incomplete voiding and frequency. Of the seven females who underwent pelvic examinations, all had normal anatomy and Pap smears. One patient had a history of hydronephrosis, two patients (4%) had vesiculoureteral reflux, and one patient had nephrotic syndrome.
Cardiac Manifestations
Congenital heart disease occurred in 22% of the patients. This included ventricular septal defect in four patients, one of whom also had an atrial septal defect, atrial septal defect in another patient, pulmonary artery stenosis in four patients, and mitral stenosis and tetralogy of Fallot in one patient each. One patient with pulmonary stenosis also had total anomalous pulmonary venous return. An additional patient had dextrocardia and another additional patient had mitral valve prolapse. Two patients developed mitral regurgitation. Four patients (8%) had hypertension, one of whom had nephrotic syndrome and pericardial effusion of unclear etiology, one of whom had pericardial effusion and congestive heart failure, with mitral and aortic insufficiency, and two with idiopathic hypertension, one of whom had a trivial to small pericardial effusion. There was no history of arrhythmias. Six patients were said to have had a heart murmur in infancy, which then resolved. Echocardiograms were obtained at our institution on four patients with a history of heart murmur. Two of these were found to have trivial to small pericardial effusion, felt to be incidental, and no lesions.
Otolaryngologic and Dental Manifestations
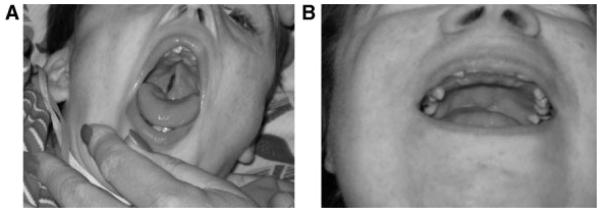
Eighteen (37%) of the patients had a cleft palate, including 11 (23%) with an overt cleft palate and 7 (14%) with a submucous cleft palate (Fig. 2B). Three of the overt cleft palates were never repaired (Fig. 2A). Five of the seven submucous cleft palates were not recognized prior to participation in the clinic. Thirty-seven percent of the patients had a history of chronic or serous otitis media in the past, but few had ongoing issues with this. Sixteen percent of this group had a cleft palate, of which 12% were overt and 5% submucous. Nineteen patients (39%) had a history of chronic sinusitis and six (12%) had nasal polyps. Thirty-two patients (65%) had a history of sensorineural hearing loss, of which half was mild and half moderate-severe. Audiology testing, when available, demonstrated high frequency loss. Recommendation for amplification had been made on three patients, however only one tolerated hearing aids. Conductive hearing loss was not a major issue. Most of the patients had dental crowding, with delayed secondary tooth eruption. A number had absent teeth, although panorex X-rays were not available on most patients. Dental caries were present particularly in the maxillary lingual surfaces of the teeth. Bruxism was noted in many patients.
Figure 2.
Otolaryngologic findings. Note unrepaired cleft palate in (A) and submucous cleft palate in (B).
Ophthalmologic Manifestations
Forty-one percent of the patients had ptosis, most of which was unilateral and none of which required surgery. Blepharitis was common, seen in 71%, and tear duct malformation had been found in 16%. Myopia was noted in half of the patients, and five (10%) had high myopia. One patient developed a detached retina secondary to the high myopia.
Myopia was noted in half of the patients, and five (10%) had high myopia. One patient developed a detached retina secondary to the high myopia.
Another patient had a detached retina from self-injury. Two patients had cataracts, with one subcapsular cataract diagnosed in early childhood, and one visually insignificant cataract diagnosed at age 14 years. A history of nystagmus was reported in three patients.
Musculoskeletal Manifestations
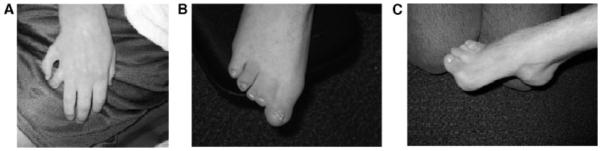
Musculoskeletal involvement was universal, although to varying degrees. The most severe finding of absent forearm(s) was seen in eight patients (16%), typically bilateral, often with a single digit (e.g. Fig. 1B,C,H,I). Nine percent had absent digit(s). No patients used prostheses. Three patients (6%) had polydactyly, two post-axial (e.g. Fig. 3A) and one insertional. Sixty-five percent had small hands and feet, with all measurements below third centile. Radial dislocation was noted in 53%, although limitation of elbow extension was present in 10% more. Proximally placed thumb, likely due to shortening of the first metacarpal, was noted in 20%. Other upper extremity findings included single palmar creases, fifth finger clinodactyly, brachydactyly of all digits, shoulder dislocation and contractures of the wrist. Pectus excavatum was seen in 13% of the patients, with none requiring surgery. Scoliosis was documented in 39% of the patients; one patient required Harrington rod placement. Seven percent had thoracic kyphosis, with the most severe being 118 degrees.
Figure 3.
Extremity findings. Note unusual presentation of postaxial polydactyly in (A) and of complete syndactyly of toes 1– 3 in (B). Unrepaired club foot, as in (C), was seen in several patients.
Scoliosis was documented in 39% of the patients; one patient required Harrington rod placement. Seven percent had thoracic kyphosis, with the most severe being 118 degrees.
Lower extremity findings were less severe than those involving the upper. Hip dislocation or dysplasia occurred in 15%, requiring surgery in childhood. Legg–Calve–Perthes disease had been reported in two patients. Other abnormalities included: leg length discrepancy (greater than 1 cm difference) in 26%, hip and knee contractures, club foot or feet in 10%, some of which were never repaired (Fig. 3C), overgrowth of toe, and 48% with partial syndactyly of toes 2–3. One patient had complete syndactyly of toes 1–3 (Fig. 3B). Bunion(s) were noted in 74% and the most severe occurred in those of older age. No patient underwent bunion repair. Twenty-two percent of the patients used orthotics.
Most patients (89%) were ambulatory; the remainder were able to walk with assistance and/or able to roll. Delayed skeletal maturity was noted in 10% of the patients. Orthopedic procedures were performed on 36% of the patients. Twenty percent of the patients reported fractures (clavicle, upper extremity, lower extremity). Low-bone density (osteopenia or osteoporosis) was confirmed in 86% of 14 patients over the age of 18 years who underwent DEXA scans, only one of whom had a fracture, and on two additional patients under age 18 years via radiographs and/or DEXA, both of whom had fractures.
Skin Manifestations
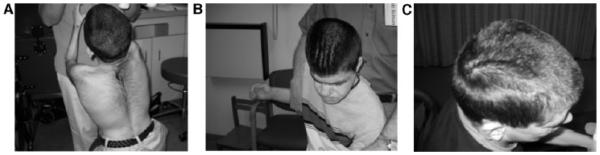
Hypertrichosis was present in 80% of the patients, typically on the face, back, and extremities. The amount varied, but could be extensive, particularly on the back (Fig. 4A). Abundant scalp hair was common, although two female patients had scalp hair loss of unknown etiology. Prematurely gray hair prior to age 20 years was noted in 18% unrelated to familial early graying. Sixty-one percent of patients had documented cutis marmarata. Two male patients were found to have cutis verticis gyrata (Fig. 4B,C).
Figure 4.
A: Marked hypertrichosis on the back of a 20-year-old patient. Cutis verticis gyrata in two unrelated male patients in (B,C).
Endocrine Involvement
There was some delay in developing physical changes of puberty by report, with average age of onset being 15 years for males, and 13 years for females, typically with the development of pubic hair and thelarche in females. Most females developed breast tissue (80%) and menses (87%), although 13% of females never achieved menarche. Menstrual periods were irregular in 53%, and premenstrual syndrome was prevalent. Only 33% of both males and females have axillary hair, while 90% demonstrate pubic hair. Scant chest hair was present in males. No lowering of the voice in males at puberty was appreciated.
Two patients developed type II diabetes, one of whom had truncal obesity with weight loss following dietary changes. One patient had a history of hypothyroidism when younger but was off medication when seen; no patients had hyperthyroidism.
Neurodevelopmental Manifestations
Neurologic exams were largely normal, with the frequent exception of increased ankle reflexes. Most patients had a history of high pain tolerance. Only two males had histamine skin testing as previously reported [Kline et al., 2001], one of which was positive. The patient with negative histamine testing had been found to have peripheral neuropathy on EMG and nerve conduction velocities and abnormal sympathetic skin response on sensory evoked potentials, thus demonstrating a possible autonomic dysfunction. Seizures occurred in 26% of the individuals, with typical age of onset prior to 18 years. Severe MR was present in 43% of the patients, moderate-severe MR in 8%, moderate MR in 16%, mild-moderate MR in 8%, mild MR in 16%, borderline IQ score in 6%, and normal intelligence with learning disabilities in 4%. Expressive language was the area of greatest deficit and even the patients with borderline or normal intelligence tended to be quiet and soft-spoken. No abnormalities were noted on the few cerebral CT or MRI scans that were available. Sleep disturbance was reported in 70% of the individuals. This included frequent awakening at night, minimal sleep requirement (as little as 2–4 hr per night), and ability to go for long periods of time without sleep (maximum 3 days). Medications that have been used for sleep include Melatonin, Clonidinem, and Risperdal, with minimal to variably positive results.
Behavioral Manifestations
Self-injury was present in 59% of the patients, with aggression in 37%. Attention deficit disorder with or without hyperactivity was noted in 20%. There was a roaming type of behavior in 35%, in which the individuals would not sit still, and wanted to roam constantly around the room. All of these individuals had moderate-severe to severe MR with absent speech. Anxiety was diagnosed in 33% and depression in 11% of the patients. Fifteen percent demonstrated obsessive-compulsive tendencies, and two patients had obsessive-compulsive disorder. Seven percent were found to have oppositional defiant disorder. Forty percent had autistic-like behaviors and 40% had quiet, shy, and retiring behavior. Of the several with normal or borderline intelligence, who also had learning disabilities, two have had substance abuse issues (alcohol in both, one with marijuana). A number of medications were used for behavioral issues including: Risperdal, Wellbutrin, Clonidine, selective serotonin reuptake inhibitors (e.g. Prozac), neuroleptics (e.g. Zyprexa), and anti-seizure medications at lower doses (e.g. Tegretol). Often, a patient was on two or three of these.
Molecular Testing
Molecular testing of the NIPBL and SMC1A genes was obtained on 53% of the patients. Mutations were detected in 55% of these samples; the rest of the samples will undergo testing as new genes or findings arise. See Table I for the specific changes. Ninety-three percent of detectable mutations occurred in the NIPBL gene and 7% in the SMC1A gene. Only two individuals, both fairly mildly affected, were found to have the same mutation, a missense mutation in exon 40, which is a relative “hotspot” for mutations. One individual had a familial missense mutation with a severely involved phenotype; his brother with CdLS had died as an infant. A fourth patient also had a missense mutation and was more mildly affected. No patient with missense mutations had absent forearms or cleft palate. Two patients had nonsense mutations and both of these were of very small stature and had major malformations. Three patients had splice site mutations, two of whom were severely involved with more proximal mutations. Four patients had frameshift mutations, two of whom were more severely affected and had more proximal mutations. The single patient with a mutation in the SMC1A gene had a missense mutation and was mildly involved. Of the patients without detectable mutations, their phenotype varied from mildly to severely involved.
TABLE I.
Mutation Analysis of NIPBL and SMC1A Genes in Older Individuals With CdLS
| Mutations | #/Sex | Growth | Developmental level of mental retardation |
Major malformations |
|---|---|---|---|---|
| NIPBL | 13 | |||
| Nonsense | 2 | |||
| Q1413X | F | Tiny | Severe | Absent forearm, CP, VSD |
| S1459X | F | Tiny | Severe | Absent forearm, CP, Hip disl., bowel obstr. |
| Missense | 4 | |||
| R1856Ta | M | Normal | Severe | RD |
| R2298C | F | Small | Mild | RD, VSD, club foot |
| R2298H | M | Normal | Mild | RD, Pulm. stenosis |
| G2381A | M | Small | Mild | RD, Eso. web, Crypt. |
| Splice site | 3 | |||
| 5574 + 1G>T | F | Tiny | Severe | RD, CP, ASD |
| 611-2A>G | F | Tiny | Severe | Absent forearm, hip disl., CP |
| R1890R | M | Normal | Severe | RD, Pulm. stenosis, MP |
| Frameshift | 4 | |||
| 3023del5 | F | Small | Severe | Absent forearm, CP |
| 2969delG | M | Small | Severe | Absent forearm, CP, Crypt., MP |
| 7210delC | M | Small | Mild | RD, Hip disl., Cataract, Crypt. |
| 14_15insAT | F | Small | Mild | Sz., mitral stenosis, CP |
| SMC1A | 1 | |||
| F1122L | F | Normal | Mild | Scoliosis |
Small, below 5th centiles for wt, ht on standard growth charts; tiny, below 50th centiles on CdLS growth charts.
CP, cleft palate; MP, micropenis; ASD/VSD, cardiac defects; Crypt., cryptorchidism; Pulm, pulmonary; Disl., dislocation; RD, radial dislocation; Sz, seizures.
Familial.
DISCUSSION
We present the largest group of older patients with CdLS followed to date. Formerly, it was thought that most individuals with CdLS, similar to other multiple malformation syndromes, would not live to adulthood. We are now aware that it is certainly possible to survive into at least the 50s with CdLS without major morbidity. Many of these patients, however, have had inconsistent medical care. It is through similar multidisciplinary clinics that there can be formal collection of data, observations collated, and specific recommendations made. A summary of findings by system is shown in Table II, also with comparison made between issues that arise in childhood, only in adulthood, or in both, as well as adult management recommendations.
TABLE II.
Clinical Findings of CdLS by System and Recommended Interventions in Adulthood
| Childhood involvement | Adult involvement | Adult management |
|---|---|---|
| Congenital heart disease | Typically stable | Echocardiogram if never had |
| Primary teeth tend to be retained | Panorex X-ray can be taken | |
| Secondary teeth eruption delayed Sinusitis and/or nasal polyps may produce symptoms |
Lingual caries may develop with GERD Sinusitis and/or nasal polyps may produce symptoms |
(Pediatric) dental visits every 4–6 months. ENT visits as needed; nasal polypectomy may be helpful |
| Cleft palate—should be repaired Pyloric stenosis requiring surgery Gastroesophageal reflux disease Complications of GERD Malrotation may be present |
Cleft palate stable—may need repair Constipation may persist GERD may worsen Barrett esophagus may occur Risk for volvulus, intestinal perforation |
Craniofacial team visits as needed Diet, medications as needed Regular gastrointestinal follow-up Biopsies every 1–3 years after diagnosis All patients need upper GI series and warning about presenting signs of volvulus (e.g. bilious vomiting) |
| Renal malformation, vesiculo-ureteral reflux (VUR) |
Renal malformation, VUR | Renal ultrasound on all patients, as indicated clinically |
| Cryptorchidism in males | Cryptorchidism in males | Orchiopexy and/or orchiectomy in childhood, monitor hormones later |
| Slightly delayed puberty females | Irregular or no menses | Hormonal treatment as needed; routine gynecology care with Pap smears every 3 years |
| Lacrimal duct malformations | Retinal detachment can occur with severe myopia |
Regular ophthalmology visits, surgery as needed |
| Blepharitis Hip dislocations |
Blepharitis tends to improve Leg length discrepancy, scoliosis, bunions may occur |
Baby shampoo rinses Orthopaedic visits as needed |
| Seizures may occur or worsen Peripheral neuropathy may produce symptoms |
Seizures may occur or worsen Peripheral neuropathy may produce symptoms |
Pediatric neurology, medications Medications may be helpful |
| Behavioral issues (SIB, anxiety, aggression) may worsen |
Behavioral issues (SIB, anxiety, aggression) may worsen |
Psychiatrist or psychologist intervention may be helpful |
There are some physical changes seen with aging. Facial features as expected show the most aging. It has previously been reported that with age, the face loses some of the typical appearance, the nasal height increases, the philtrum appears shorter and the upper vermilion border appears full and everted, although the crescent-shaped mouth with down-turned corners remains [Allanson et al., 1997]. We did not observe most of these findings, although down-turned mouth was common. For the less typical, milder patients (Fig. 1K–R), it was often helpful to review photographs from early childhood, which demonstrated more classical features. Regarding size, adult height was typically of short stature, but was partially dependent on familial factors, and adult weight was variable, with nearly one-fifth having truncal obesity. In several patients this was managed by healthier diet choices, which, along with increasing exercise, would be recommended for all older patients. In addition, 11% had extremely low weight for height and required nutritional supplements and/or cleft palate repair for improvement.
Patients with milder involvement could be difficult to diagnose with CdLS if evaluation occurred in adulthood.
Patients with milder involvement could be difficult to diagnose with CdLS if evaluation occurred in adulthood.
The classical findings of small stature, severe limb malformations, and typical facies should be recognizable during childhood, particularly if MR is present. The major conditions in the differential diagnosis would include fetal alcohol spectrum disorder (FASD), Rubinstein–Taybi syndrome and autistic spectrum disorder. The recognizable adult facies in CdLS includes synophrys in an arched pattern, narrow down-slanting palpebral fissures, short nose with anteverted nares and thick columella, prominent philtrum, thin down-turned lips and a square chin. The most striking findings in these older individuals are the square chin and slight elongation of the face, with slight coarsening in some patients. Although adults with Rubinstein–Taybi syndrome may exhibit slightly coarse features, the nose tends to be very different and extremity findings are important as well. Behavior can often be helpful in determining the correct CdLS diagnosis. Individuals with FASD tend to be talkative and conversational, whereas adults with CdLS, even with minimal developmental issues, tend to be quiet and reserved. Finally, patients with autistic spectrum disorder tend not to have dysmorphic features, small stature or extremity changes.
The foremost medical complication seen in CdLS is GERD, which is the most common GI manifestation. In some patients, evidence of significant GERD and its complications was found in the absence of obvious signs or symptoms.
The foremost medical complication seen in CdLS is GERD, which is the most common GI manifestation. In some patients, evidence of significant GERD and its complications was found in the absence of obvious signs or symptoms.
Barrett esophagus is typically reported in the general population beginning in the sixth decade of life. The incidence of 10% in our population is higher than expected, and onset at ages 19–36 years is also young for this finding [Bonino and Sharma, 2006]. There have been five previous reports of Barrett esophagus in CdLS patients [Sommer, 1993; Duvall and Walden, 1996; Pei et al., 2000; Luzzani et al., 2003], one of whom developed esophageal adenocarcinoma at age 25 years [Duvall and Walden, 1996]. This suggests three possibilities: either chronically unrecognized “silent” reflux or GERD under-treated for many years, the possibility of accelerated aging, or genetic predisposition to early development of Barrett esophagus in CdLS. Support for the presence of silent reflux, and for the morbidity of GERD in general, includes a high incidence of sinusitis and of maxillary lingual dental caries in our patients, both of which are recognized consequences of GERD [Linnett et al., 2002; DelGaudio, 2005; Poelmans and Tack, 2005]. In the general population, a trial of medical management is commonly used instead of or prior to work-up. In patients with CdLS a GI evaluation should always be obtained, since silent reflux may occur and esophageal metaplasia and Barrett esophagus are relatively common and relatively early complications. Intestinal obstructions, webs, stenoses and other anatomic abnormalities also should be sought and treated as necessary.
In the ophthalmologic system, myopia was common and two patients were found to have detached retina—one due to self-injury and the other to high myopia. This is a definite potential cause of morbidity with aging and should be monitored. Ptosis was also common but not clinically significant. Blepharitis, which is an important issue in younger patients with CdLS, appears to resolve with aging. There were two cases of cataracts, and none of the patients were found to have other eye changes associated with aging, such as glaucoma, severe vision deterioration, or macular degeneration. Several of the milder patients had gaze avoidance.
Musculoskeletal anomalies are known to range in severity in CdLS. Findings in our older patients included all of the typical upper extremity anomalies known in CdLS [Halal and Preus, 1979], as well as less commonly reported findings of leg length discrepancy with or without scoliosis, kyphosis, club feet, and hip dysplasia. More severe skeletal findings in our patients correlated with a lower range of intelligence, as previously reported [Roposch et al., 2004]. Orthopaedic procedures were performed more in the lower than the upper extremity, more often in patients with lower birth weights and more in patients with a higher intelligence level. Other orthopedic findings of note included Legg–Calve–Perthes disease, which had been reported previously [Roposch et al., 2004] and bunions. Bunion formation is a common manifestation of the aging process, but appears to be occurring at a younger age among patients with CdLS than in the general population. The development of bunions is also potentially significant as an occult source of pain, especially in the setting of unexplained behavioral changes. No patient, however, underwent bunion repair. Low bone density was a common finding, and could be related to hypogonadism, nutritional deficiency (especially calcium or vitamin D), insufficient weight-bearing exercise, and/or premature aging. Interestingly, most of the fractures were noted in patients without documented osteoporosis or osteopenia; this is an area of further investigation.
Neurodevelopmental issues were present in all patients. Those patients with seizures were well controlled, typically on monotherapy. Sleep disturbances were reported in over two thirds of the families when queried. These included frequent awakening at night, minimal sleep requirement (as little as 2–4 hr per night), and ability to go for long periods of time without sleep (maximum 3 days). Sleep disruption has been previously reported in CdLS [Berney et al., 1999], but not with specific details. There has been recent evidence that sleep disturbance in general can be due to silent reflux [Fass and Dickman, 2006]. Medications that have been used for sleep include Melatonin, Clonidine, and Risperdal, with minimal to variably positive results. Certainly this is an area for further investigation. Sensorineural hearing loss had been noted in 65% of our patients. The patients with milder loss tended to be more verbal, as expected. A few of the nonverbal patients communicated with sign language, which was also used by some of the verbal patients. Only two patients used hearing aids, although this has been reported successfully in conjunction with auditory training in a minority of younger patients with CdLS in Japan [Sakai et al., 2002]. Developmental levels of our patients were consistent with previous reports in the literature, and supported expressive language being the greatest area of deficit [Kline et al., 1993b].
Behavioral changes were frequent, and often were the primary issue of concern for the family. Although there is no specific behavioral phenotype in CdLS, self-injury, including head banging or hitting and/or skin picking, may increase in frequency during adolescence and then may improve in the 20s. Acutely increasing self-injurious behavior often has been traced to a source of hidden pain, such as dental abscess or cellulitis, sinusitis, otitis media, or worsening or developing gastroesophageal reflux, but sometimes remains inexplicable. Identifying and treating successfully an underlying organic cause has been helpful in many cases of new behaviors, and is of primary importance. In addition, many patients with CdLS have a very high pain tolerance. Several patients were prescribed neuropathic pain medications for unexplained behavioral issues, including the individual who had been shown to have mild peripheral neuropathy. Caretakers found some shortterm improvement in behavior but no long-term improvement. It is unclear if the failure to achieve long-term improvement with neuropathic pain medications has been due to inadequate control of pain or if the behavior is due to other causes. This is an area of further investigation. Finally, behavioral or psychiatric assessment is critical if issues such as anxiety, depression, attention-deficit with or without hyperactivity, or obsessive-compulsive symptoms arise or become overwhelming. The use of medication, or changes in medication regimens, was helpful in many cases. Some patients may need inpatient evaluation and management, as was noted in two of our patients.
Findings in our patients that have not been stressed in the literature include development of volvulus, leg length discrepancy, submucous cleft palate, and some of the behavioral issues, including anxiety, depression, and obsessive-compulsive tendencies. All of these could lead to the development of some morbidity or mortality, and providers and families should be made aware of them. Cecal volvulus has been reported in association with nonfixation of the cecum and ascending colon [Husain et al., 1994; Holthusen and Rottingen, 1998; Masumoto et al., 2001], and all patients should have an upper GI series to rule out malrotation. The relative high frequency of submucous cleft palate in our patients (14%), with two-thirds of these undetected prior to our clinic, should encourage caretakers to assess for this. Most had no morbidity associated, however, and this is most likely the reason for no prior detection.
Findings not previously reported in CdLS include cutis verticis gyrata, prematurely gray hair, rumination, and osteoporosis. Cutis verticis gyrata are multiple furrows found on the scalp with normal hair. This has been reported in individuals with microcephaly and found more frequently in males with MR [Schepis and Siragusa, 1995]. It does not appear to be associated with any morbidity. Rumination is found in individuals with moderate-severe MR, and is known to be associated with reflux [Schwarz et al., 2001]; it is likely also not specific to CdLS. It may lead to complications, so family and medical care providers should be made aware of the possibility. Osteoporosis was present at a younger age in our cohort than expected (e.g. some in the mid-teens), and this has prompted an additional ongoing study investigating potential hormonal or nutritional causes. Early findings include low vitamin D levels, low testosterone in many males, and occasionally low estrogen in some females (data not shown).
Management issues arose for many of the patients seen in the biannual clinic. At least a quarter of the patients had not had a screening echocardiogram or renal ultrasound. The latter, when obtained as a screening study, did not reveal any pathology, although 41% of patients with CdLS have been found to have structural urinary tract involvement [Selicorni et al., 2005]. GI and nutritional recommendations were made on most patients. For those patients without symptoms of reflux and no previous work-up, upper GI series was recommended and often endoscopy. On occasion, we suggested repair of previously unrepaired cleft palate but not of previously unrepaired club foot. Most of the time, these were never repaired because of the initial expectation of high risk of mortality in CdLS. For future similar patients in early childhood, these malformations should be repaired in a timely way so as not to increase morbidity later. The patient with the previously positive histamine test was encouraged to complete the work-up for peripheral neuropathy.
Some genotype–phenotype correlations between mutations in NIPBL and SMC1A have been made in the past [Gillis et al., 2004; Bhuiyan et al., 2006; Musio et al., 2006] and can be noted here, despite the fact that only 53% of our cases agreed to testing. In our patients, musculoskeletal findings appear to have some correlation with gene changes. Twelve of 14 patients (85%) with detectable mutations have absent forearms or radial head dislocation, in contrast to 50% of patients without detectable mutations. Proximal mutations appear more severe than distal, and missense mutations may present with milder musculoskeletal anomalies. In addition, cleft palate was present in half of the patients who were mutation positive, although found only in patients with nonsense, splice site and frameshift mutations and in no patient with missense mutations. Finally, only a single patient with seizures had a detectable mutation, and this was an insertion causing a frameshift mutation. Thus, as in previous reports, missense mutations appear milder, and more proximal mutations appear more severe than distal, as expected. Also, as in previous reports, the more severe patients exhibit mutations in NIPBL and milder may have SMC1A mutations [Bhuiyan et al., 2006; Musio et al., 2006]. It is likely that individuals with SMC1A mutations may remain undiagnosed. Forty-eight percent of our patients have not had mutation analysis to date, although plans exist to have this done in the future. All patients, however, had a confirmed clinical diagnosis, which is the recommended way to make the diagnosis currently. Furthermore, individuals with no other diagnosis who are similar to those with Cornelia de Lange syndrome could benefit from these recommendations in any case, as they proceed through adulthood.
Evidence for some premature aging include the early development of Barrett esophagus, osteoporosis present in the teenage years, prematurely graying of hair, and some changes to the skin of the face causing a more aged appearance compared to chronologic age. The genes NIPBL and SMC1A have broad roles in sister chromatid cohesion, chromosome condensation, DNA repair, and developmental gene expression regulation [Strachan, 2005]. The proteins interact with cohesin to carry out much of these functions and, if abnormally formed or truncated, could produce many of the developmental deficits seen in CdLS [Dorsett et al., 2005]. Affecting DNA repair could theoretically lead to premature physiologic aging, and there is precedent for this in some of the segmental aging syndromes [Navarro et al., 2006]. If premature aging is present in CdLS, it appears segmental. Prematurely gray hair in some patients may be an indication of premature aging in CdLS, although this is difficult to quantitate. Some of the progeroid syndromes (e.g. Werner syndrome) may have prematurely gray hair, although also present with a multitude of other findings not seen in CdLS.
Other body systems appear not to exhibit major changes associated with aging. From the cardiac point of view, there seems to be no premature aging, although it is of interest that 4% of the patients had a significant pericardial effusion. Six percent of the patients were found to have a trivial pericardial effusion of no apparent clinical significance on screening echocardiogram. Interestingly, an increased incidence of isolated pericardial effusion has been reported in Down syndrome [Concolino et al., 2005] and has been associated with acquired hypothyroidism in children with Down syndrome [Werder et al., 1993]. In addition, there does not seem to be an increased incidence of cancers or other diseases of older age (e.g. Parkinson, Alzheimer disease) at least so far in our patients, unlike a previous case report describing parkinsonism and dystonia [Fernandez et al., 2000]. Finally, early neurodegenerative changes in the brain were not noted in an autopsy report of a 35-year-old man with CdLS [Vuilleumier et al., 2002].
Specific recommendations for care in older individuals with CdLS can be made based on this study (Table II). Routine regular medical care and recommendations with aging, such as good nutrition and exercise, are important, and patients should be transitioned from pediatricians to internal medicine or family practice physicians comfortable with individuals with multiple needs in their late teens or early 20s.
Routine regular medical care and recommendations with aging, such as good nutrition and exercise, are important, and patients should be transitioned from pediatricians to internal medicine or family practice physicians comfortable with individuals with multiple needs in their late teens or early 20s.
Ophthalmologic evaluation should be made every 6–12 months for close monitoring, particularly if high myopia is present. A dental evaluation with a practitioner comfortable with caring for developmentally disabled adults should occur every 4–6 months, depending on the level of mental involvement, to minimize long-term complications for the teeth, particularly if reflux is present. Appropriate follow-up with other subspecialists should be made if clinically indicated (e.g. cardiology, orthopaedics, neurology, nephrology, audiology). All patients with CdLS, even without symptoms, should undergo thorough GI evaluation. With any clinical suspicion of worsening of gastroesophageal reflux, repeat evaluation (e.g. endoscopy, pH probe, swallowing study) is indicated. If GERD is found, aggressive treatment and close GI follow-up are important. All patients with CdLS should have had an upper GI series to rule out malrotation. Any sign of potential bowel obstruction or volvulus (e.g. bilious emesis) merits an immediate visit to an emergency department for evaluation and possible surgery. For females, Pap smears should be performed regularly (every 3 years if not sexually active). Hormonal therapy for protection against pregnancy and to help control menses should be considered. For any elective surgery, consider doing other recommended studies in sequence to minimize anesthesia exposure. This could include a pelvic exam, ophthalmologic evaluation, dental cleaning, and radiologic studies. Behavioral issues may worsen with age, starting at puberty, and should be addressed by the appropriate professional.
For older patients, molecular testing is likely not to be helpful. It would be useful only for recurrence testing in the family, or for genetic counseling for the autosomal dominant or X-linked recurrence risk in the affected individual’s offspring, if appropriate. Genetic counseling is encouraged. A medical care card is available from, among other places, the CdLS Foundation at the website www.cdlsusa.org that can be used among all caretakers and can accompany the patient at all times, describing CdLS and some potential complications. Additional support through the CdLS Foundation can be invaluable for families.
ACKNOWLEDGMENTS
The work of Ian Krantz is supported by the NOIH, grant number RO1 HD039323, and NICHD, grant number PO1 HD052860. We wish to acknowledge the help in grant awards and in general support from the Cornelia de Lange Syndrome Foundation, www. cdlsusa.org, and the participation of the member families. We appreciate the help of Georgette Saba in the preparation of this manuscript.
Contributor Information
Dr ANTONIE D. KLINE, Director of Pediatric Genetics in the Harvey Institute for Human Genetics at Greater Baltimore Medical Center. She is an Instructor in Pediatrics at the Johns Hopkins University School of Medicine and Clinical Assistant Professor at the University of Maryland School of Medicine. She is the Medical Director of the Cornelia de Lange Syndrome Foundation.
Dr MARCO GRADOS, Child Psychiatrist and an Assistant Professor of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine. He is also affiliated with the Kennedy Krieger Institute in Baltimore and a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr PAUL SPONSELLER, Professor in Orthopedic Surgery at Johns Hopkins University School of Medicine and the Chief of Pediatric Orthopedics at Johns Hopkins Hospital. He is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr HOWARD P. LEVY, Assistant Professor of Medicine and Genetics at Johns Hopkins University School of Medicine. He is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr NATALIE BLAGOWIDOW, Medical Director of the Genetics Prenatal Diagnostic Center in the Harvey Institute for Human Genetics, and the Director of the Mangione High Risk Obstetrical Center in the Department of Obstetrics at Greater Baltimore Medical Center. She is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr CHRISTIANNE SCHOEDEL, practicing Pediatric Ophthalmologist in York, Pennsylvania and the Medical Director of Neonatal and Pediatric Ophthalmological Services at York Hospital. She is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Ms. JONI RAMPOLLA, registered licensed Dietitian with a diverse background in corporate wellness and hospital-based health education in Maryland. She is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr DOUGLAS K. CLEMENS, practicing Pediatric Dentist in Baltimore, MD. He is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr IAN KRANTZ, Associate Professor at the University of Pennsylvania School of Medicine and is a Pediatric Geneticist and research scientist at The Children’s Hospital of Philadelphia. He is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Ms AMY KIMBALL, certified Genetic Counselor at the Harvey Institute for Human Genetics at Greater Baltimore Medical Center. She is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation
Dr CARMEN PICHARD, Orthopedic Resident at the Johns Hopkins Hospital. She has done research and clinical work with Cornelia de Lange Syndrome for 3 years
Dr DAVID TUCHMAN, Assistant Professor of Pediatrics at Johns Hopkins University School of Medicine and is the Director of the Division of Pediatric Gastroenterology and Nutrition at the Children’s Hospital at Sinai in Sinai Hospital of Baltimore. He is a member of the Clinical Advisory Board of the Cornelia de Lange Syndrome Foundation.
REFERENCES
- Allanson JE, Hennekam RC, Ireland M. De Lange syndrome: Subjective and objective comparison of the classical and mild phenotypes. J Med Genet. 1997;34:645–650. doi: 10.1136/jmg.34.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical Features of 78 adults with 22q11 deletion syndrome. Am J Med Genet Part A. 2005;138A:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney TP, Ireland M, Burn J. Behavioural phenotype of Cornelia de Lange syndrome. Arch Dis Child. 1999;81:333–336. doi: 10.1136/adc.81.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan ZA, Klein M, Hammond P, van Haeringen A, Mannens MM, Van Berckelaer-Onnes I, Hennekam RC. Genotype–phenotype correlations of 39 patients with Cornelia de Lange syndrome: The Dutch experience. J Med Genet. 2006;40:568–575. doi: 10.1136/jmg.2005.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonino JA, Sharma P. Barrett’s esophagus. Curr Opin Gastroenterol. 2006;22:406–411. doi: 10.1097/01.mog.0000231816.18396.26. [DOI] [PubMed] [Google Scholar]
- Borck G, Zarhrate M, Cluzeau C, Bal E, Bonnefont JP, Munnich A, Cormier-Daire V, Colleaux L. Father-to-daughter transmission of Cornelia de Lange syndrome caused by a mutation in the 5′ untranslated region of the NIPBL Gene. Hum Mutat. 2006;27:731–735. doi: 10.1002/humu.20380. [DOI] [PubMed] [Google Scholar]
- Concolino D, Pascuzzi A, Pietragalla E, Lia R, Canepa S, Strisciuglio P. High prevalence of isolated pericardial effusion in Down syndrome. Am J Med Genet Part A. 2005;132A:331–332. doi: 10.1002/ajmg.a.30399. [DOI] [PubMed] [Google Scholar]
- De Die-Smulders C, Theunissen P, Schrander-Stumpel C, Frijns JP. On the variable expression of the Brachmann-de Lange syndrome. Clin Genet. 1992;41:42–45. doi: 10.1111/j.1399-0004.1992.tb03628.x. [DOI] [PubMed] [Google Scholar]
- Deardorff M, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodriguez C, Arnedo M, Loeys B, Kine AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LG, Dorsett D, Krantz ID. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of Cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–494. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelGaudio JM. Direct nasopharyngeal reflux of gastric acid is a contributing factor in refractory chronic rhinosinusitis. Laryngoscopie. 2005;115:946–957. doi: 10.1097/01.MLG.0000163751.00885.63. [DOI] [PubMed] [Google Scholar]
- Dorsett D, Eissenberg JC, Misulovin Z, Martens A, Redding B, McKim K. Effects of sister chromatid cohesion proteins on cut gene expression during wing development in Drosophila. Development. 2005;132:4743–4753. doi: 10.1242/dev.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JO, Williams CD, Raymond CA. Hematometra in a patient with Cornelia de Lange syndrome. Obstet Gynecol. 2005;106:1202–1204. doi: 10.1097/01.AOG.0000160512.24767.08. [DOI] [PubMed] [Google Scholar]
- Duvall GA, Walden DT. Adenocarcinoma of the esophagus complicating Cornelia de Lange syndrome. J Clin Gastroenterol. 1996;22:131–133. doi: 10.1097/00004836-199603000-00012. [DOI] [PubMed] [Google Scholar]
- Fass R, Dickman R. Clinical consequences of silent gastroesophageal reflux disease. Curr Gastroenterol Rep. 2006;8:194–200. doi: 10.1007/s11894-006-0075-8. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Friedman JH, Famiglietti EV. Probable Cornelia de Lange syndrome with progressive parkinsonism and dystonia. Mov Disord. 2000;15:749–751. doi: 10.1002/1531-8257(200007)15:4<749::aid-mds1028>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Gillis LA, McCallum J, Kaur M, DeScipio C, Yaeger D, Mariani A, Kline AD, Li H, Devoto M, Jackson LG, Krantz ID. NIPBL mutational analysis in 120 individuals with Cornelia de Lange syndrome and evaluation of genotype–phenotype correlations. Am J Hum Genet. 2004;75:610–623. doi: 10.1086/424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RE, Schneider JA, Ferguson EJ, Cummings PB. Total hip reconstruction in a woman with Cornelia de Lange syndrome: A case report. J Natl Med Assoc. 1997;89:530–532. [PMC free article] [PubMed] [Google Scholar]
- Halal F, Preus M. The hand profile on de Lange syndrome: Diagnostic criteria. Am J Med Genet. 1979;3:317–323. doi: 10.1002/ajmg.1320030402. [DOI] [PubMed] [Google Scholar]
- Holthusen J, Rottingen JA. Cecal volvulus as a complication in Cornelia de Lange syndrome. A case report and literature review. Tidsskr Nor Laegeforen. 1998;118:1559–1560. [PubMed] [Google Scholar]
- Husain K, Fitzgerald P, Lau G. Cecal volvulus in the Cornelia de Lange syndrome. J Pediatr Surg. 1994;29:1245–1247. doi: 10.1016/0022-3468(94)90814-1. [DOI] [PubMed] [Google Scholar]
- Jackson L, Kline AD, Barr M, Koch S. de Lange syndrome: A clinical review of 310 individuals. Am J Med Genet. 1993;47:940–946. doi: 10.1002/ajmg.1320470703. [DOI] [PubMed] [Google Scholar]
- Kim IT, Park JW, Choi WC. A Korean case of Cornelia de Lange syndrome. Korean J Ophthalmol. 2005;19:153–155. doi: 10.3341/kjo.2005.19.2.153. [DOI] [PubMed] [Google Scholar]
- Kline AD, Barr M, Jackson LG. Growth manifestations in Brachmann-de Lange syndrome. Am J Med Genet. 1993a;47:1042–1049. doi: 10.1002/ajmg.1320470722. [DOI] [PubMed] [Google Scholar]
- Kline AD, Stanley C, Belevich J, Brodsky K, Barr M, Jackson LG. Developmental data on individuals with Brachmann-de Lange syndrome. Am J Med Genet. 1993b;47:1053–1058. doi: 10.1002/ajmg.1320470724. [DOI] [PubMed] [Google Scholar]
- Kline AD, Krantz I, Goldstein A, Koo B, Jackson LG. Cornelia de Lange syndrome: Evidence for a sensory neuropathy. Am J Hum Genet. 2001;69:280. [Google Scholar]
- Kline AD, Krantz ID, Sommer A, Kliewer M, Jackson L, FitzPatrick DR, Levin A, Selicorni A. Cornelia de Lange syndrome: Clinical review, diagnostic and scoring systems and anticipatory guidance. Am J Med Genet (in press) 2007 doi: 10.1002/ajmg.a.31757. [DOI] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScripio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophilia melanogaster Nipped-B. Nat Genet. 2004;36:631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett V, Seow WK, Connor F, Shepherd R. Oral health of children with gastroesophageal reflux disease: A controlled study. Aust Dent J. 2002;47:156–162. doi: 10.1111/j.1834-7819.2002.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Luzzani S, Macchini F, Valade A, Milani D, Selicorni A. Gastroesophageal reflux and Cornelia de Lange syndrome: Typical and atypical symptoms. Am J Med Genet Part A. 2003;119A:283–287. doi: 10.1002/ajmg.a.20191. [DOI] [PubMed] [Google Scholar]
- Masumoto K, Izaki T, Arima T. Cornelia de Lange syndrome associated with cecal volvulus: Report of a case. Acta Paediatr. 2001;90:701–703. [PubMed] [Google Scholar]
- McConnell V, Brown T, Morrison PJ. An Irish three-generation family of Cornelia de Lange syndrome displaying autosomal dominant inheritance. Clin Dysmorphol. 2003;12:241–244. doi: 10.1097/00019605-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Mehta AV, Ambalavanan SK. Occurrence of congenital heart disease in children with Brachmann-de Lange syndrome. Am J Med Genet. 1997;71:434–435. doi: 10.1002/(sici)1096-8628(19970905)71:4<434::aid-ajmg12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Musio A, Selicorni A, Focarelli ML, Gervsini C, Milani D, Russo S, Vezzoni P, Larizza L. X-linked Cornelia de Lange syndrome owing to SMC1A1 mutations. Nat Genet. 2006;38:528–530. doi: 10.1038/ng1779. [DOI] [PubMed] [Google Scholar]
- Navarro CL, Cau P, Levy N. Molecular bases of progeroid syndromes. Hum Mol Genet. 2006;15:R151–R161. doi: 10.1093/hmg/ddl214. [DOI] [PubMed] [Google Scholar]
- Ozkinay F, Cogulu O, Gunduz C, Levent E, Ozkinay C. A case of Brachmann-de Lange syndrome with cerebellar vermis hypoplasia. Clin Dysmorphol. 1998;7:303–305. doi: 10.1097/00019605-199810000-00013. [DOI] [PubMed] [Google Scholar]
- Pei RS, Lin CC, Mak SC, Chi CS, Chou G. Barrett esophagus in a child with de Lange syndrome: Report of one case. Acta Paediatr Taiwan. 2000;41:155–157. [PubMed] [Google Scholar]
- Poelmans J, Tack J. Extraesophageal manifestations of gastro-oesophageal reflux. Gut. 2005;54:1492–1499. doi: 10.1136/gut.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roposch A, Bhaskar AR, Lee F, Adedapo S, Mousny M, Alman BA. Orthopaedic manifestations of Brachmann-de Lange syndrome: A report of 34 patients. J Pediatr Orthop B. 2004;13:118–122. doi: 10.1097/00009957-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Russell KL, Ming JE, Patel K, Jukofsky L, Magnusson M, Krantz ID. Dominant paternal transmission of Cornelia de Lange syndrome: A new case and review of 25 previously reported familial recurrences. Am J Med Genet. 2001;104:267–276. doi: 10.1002/ajmg.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Watanabe T, Kaga K. Auditory brainstem responses and usefulness of hearing aids in hearing impaired children with Cornelia de Lange syndrome. Int J Pediatr Otorhinolaryngol. 2002;66:63–69. doi: 10.1016/s0165-5876(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Schepis C, Siragusa M. Primary cutis verticis gyrata or pachydermia verticis gyrata: A peculiar scalp disorder of mentally retarded adult males. Dermatology. 1995;191:292–294. doi: 10.1159/000246572. [DOI] [PubMed] [Google Scholar]
- Schwarz SM, Corredor J, Fisher-Medina J, Cohen J, Rabinowitz S. Diagnosis and treatment of feeding disorders in children with developmental disabilities. Pediatr. 2001;108:671–676. doi: 10.1542/peds.108.3.671. [DOI] [PubMed] [Google Scholar]
- Selicorni A, Sforzini C, Milani D, Cagnoli G, Fossali E, Bianchetti MG. Anomalies of the kidney and urinary tract are common in de Lange syndrome. Am J Med Genet. 2005;32:395–397. doi: 10.1002/ajmg.a.30445. [DOI] [PubMed] [Google Scholar]
- Sommer A. Occurrence of the Sandifer complex in the Brachmann-de Lange syndrome. Am J Med Genet. 1993;47:1026–1028. doi: 10.1002/ajmg.1320470719. [DOI] [PubMed] [Google Scholar]
- Strachan T. Cornelia de Lange syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev. 2005;15:258–264. doi: 10.1016/j.gde.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004;36:636–641. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]
- Vuilleumier N, Kovari E, Michon A, Hof PR, Mentenopoulos G, Giannakopoulos P, Bouras C. Neuropathological analysis of an adult case of the Cornelia de Lange syndrome. Acta Neuropathol (Berl) 2002;104:327–332. doi: 10.1007/s00401-002-0562-4. [DOI] [PubMed] [Google Scholar]
- Werder EA, Torresani T, Navratil F, Arbenz U, Eiholzer U, Pelet B, Burri M, Schwarzenbach P, Hunziker U. Pericardial effusion as a sign of acquired hypothyroidism in children with Down syndrome. Eur J Pediatr. 1993;152:397–398. doi: 10.1007/BF01955895. [DOI] [PubMed] [Google Scholar]