Abstract
Mammalian glutaredoxin 3 (Grx3) has been shown to be critical in maintaining redox homeostasis and regulating cell survival pathways in cancer cells. However, the regulation of Grx3 is not fully understood. In the present study, we investigate the subcellular localization of Grx3 under normal growth and oxidative stress conditions. Both fluorescence imaging of Grx3–RFP fusion and Western blot analysis of cellular fractionation indicate that Grx3 is predominantly localized in the cytoplasm under normal growth conditions, whereas under oxidizing conditions, Grx3 is translocated into and accumulated in the nucleus. Grx3 nuclear accumulation was reversible in a redox-dependent fashion. Further analysis indicates that neither the N-terminal Trx-like domain nor the two catalytic cysteine residues in the active CGFS motif of Grx3 are involved in its nuclear translocation. Decreased levels of Grx3 render cells susceptible to cellular oxidative stress, whereas overexpression of nuclear-targeted Grx3 is sufficient to suppress cells’ sensitivity to oxidant treatments and reduce reactive oxygen species production. These findings provide novel insights into the regulation of Grx3, which is crucial for cell survival against environmental insults.
Keywords: Oxidative stress, Cell death, Redox homeostasis, Glutaredoxin, Free radicals
Reactive oxygen species (ROS) are considered the by-products of aerobic metabolism in all oxygenic organisms [1,2]. Cells also actively generate ROS as signals through the activation of various oxidases and peroxidases in response to internal developmental cues and external stresses [3]. However, when in excess, ROS induce oxidative stress, causing a wide range of damage to macromolecules, and eventually lead to apoptotic or necrotic cell death [4,5], which are often associated with human diseases [6–9].
Glutaredoxins (Grxs) are ubiquitous, small heat-stable disulfide oxidoreductases that are conserved in both prokaryotes and eukaryotes [10,11]. Grxs seem to be involved in many cellular processes and play an important role in protecting cells against oxidative stress [12]. Grxs can be categorized into two major classes, dithiol Grxs that contain two cysteine residues in their active motif and monothiol Grxs that contain a single cysteine residue in their putative motif [12]. Mammalian cytosolic Grx1 is shown to be critical for protecting cardiomyocytes against oxidative stress-induced apoptosis by regulating the NF-κB-dependent cell survival pathway [13]. Furthermore, several recent studies indicate that Grx1 plays a crucial role in lung epithelial cells against bacterial infection by attenuating S-glutathionylation of NF-κB signaling pathways [14–16]. On the other hand, genetic analysis indicates that Grx1-knockout mice show attenuated lipopolysaccharide-induced lung inflammation and alveolar macrophage activation [17]. Grx2 is a mitochondrial oxidoreductase thought to have a vital function in protection against apoptotic stimuli [18]. Silencing of Grx2 increases the sensitivity of HeLa cells to external oxidative stress [19]. Furthermore, in primary lens epithelial cells, Grx2 plays a critical role in protecting cells against H2O2-induced injury by modulating peroxidase and dethiolase activities and mitochondrial function [20]. Interestingly, various expression patterns, isoforms, and subcellular localizations of Grx2 are correlated with various cellular functions [18].
Monothiol Grxs were initially identified in yeast (ScGrx3, 4, and 5) and subsequently found in all types of living organisms [21]. There is a growing body of evidence suggesting that monothiol Grxs may have multiple functions in biogenesis of iron–sulfur clusters, iron trafficking and homeostasis, protection of protein oxidation, cell growth, and proliferation [22–30]. In mammalian cells there are two monothiol Grxs, Grx3 and Grx5 [31,32]. Mammalian Grx5, a mitochondrial Grx, plays a critical role in iron–sulfur cluster biogenesis and heme synthesis in red blood cells [33,34] and seems to be crucial for protecting osteoblasts from oxidative stress-induced apoptosis [35]. Grx3, also termed thioredoxin-like 2 or PICOT (protein kinase C-interacting cousin of thioredoxin), was originally identified through a yeast two-hybrid screen, in which Grx3 physically interacted with the protein kinase C θ isoform [32]. Grx3 is an iron–sulfur binding protein and seems to be critical for Fe–S cluster biogenesis in vivo [36–38]. Grx3 has been also shown to regulate cellular stress responses, attenuate cardiac hypertrophy, and improve cardiac function when expressed in the heart [39–42]. Genetic studies also demonstrate that Grx3 is essential for early embryonic growth and development, as deletion of Grx3 causes embryonic lethality [43,44]. Our previous work indicates that Grx3 plays a critical role in regulating human breast cancer cell growth and metastasis via redox homeostasis and NF-κB signaling [45]. Furthermore, Grx3 seems to be involved in caspase 3-mediated apoptosis [46]. However, the precise function of Grx3 and its regulation under oxidative stress remain to be fully elucidated.
In this study, we investigated the subcellular localization of mammalian Grx3 and its dynamic changes under oxidative stress. We discovered that under reducing conditions, Grx3 was located in the cytoplasm. When cells were exposed to various oxidizing conditions, Grx3 was translocated from the cytoplasm into the nucleus, where it accumulated. We directly measured the cellular redox potential using redox-sensitive fluorescent proteins and tested the sensitivity of Grx3-knockdown (KD) HeLa cells under oxidative stress. Furthermore, we generated nuclear-targeted Grx3 and tested its ability to protect cells against environmental insults. Taken together, these findings suggest that the presence of mammalian Grx3 in the nucleus has important roles in controlling cell growth under oxidative stress.
Materials and methods
Reagents
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless stated otherwise. Trypan blue solution (0.4% in saline and potassium phosphate dibasic) was ordered from EMD (Gibbstown, NJ, USA). Catalase polyethylene glycol was ordered from Sigma–Aldrich. Dulbecco’s modified Eagle medium (DMEM) and HyClone newborn bovine calf serum (CS) were obtained from Thermo Scientific (Waltham, MA, USA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrence, GA, USA). EDTA with 0.25% trypsin was from Mediatech (Manassas, VA, USA). Penicillin–streptomycin solution (Penstrep) was from Global Cell Solutions (Charlottesville, VA, USA). Anti-Flag (M2) and anti-β-actin antibodies were bought from Sigma–Aldrich. Anti-PCNA, anti-lamin A/C, and secondary antibodies were from Cell Signaling Technology (Beverly, MA, USA). Anti-histone H3 and anti-Gapdh antibodies were purchased from Abcam (Cambridge, MA, USA). Monoclonal antibody against Grx3 was made in-house [44].
Cell culture, transfection, and cell viability assay
HeLa cells, MCF7 cells, MDA-MB-231 cells, and 3T3L1 fibroblasts were cultured in DMEM supplemented with 10% CS or FBS. Mouse embryonic fibroblasts (MEFs) were made from embryos at 12 days postgestation as previously described [44]. MEFs were cultured in DMEM with 10% FBS. All growth media contained 2 mM glutamine and 1% Penstrep. The cells were grown at 37 °C in 5% CO2.
Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) following the manufacturer’s instructions.
Cell viability was determined using the trypan blue exclusion and the neutral red uptake assays following the published procedure [47,48]. Because the trypan blue dye does not interact with the cell unless the membrane is damaged, unstained cells, which exclude the dye, are viable, whereas blue-stained cells are dead. For Fig. 5A and B, 1×105 HeLa cells were seeded in 24-well plates and in quadruplicate for each concentration of diamide or each time point for a single concentration of diamide. Cells were grown overnight followed by diamide treatment as indicated. For Fig. 5C, 1×105 HeLa and Grx3 ShRNA Nos. 1 and 2 KD cells were seeded in 24-well plates and in quadruplicate for each concentration of diamide. Cells were grown overnight and then treated with diamide for 14 h. After the treatment, the growth medium (containing dead cells) was collected from each well (1 ml for each well) and the cells were released by trypsin and then combined with the growth medium. All the cells were pelleted and suspended in 0.5 ml of fresh growth medium without serum. One-tenth milliliter of a 0.4% trypan blue solution was added in the tube, mixed well, and then incubated for 5 min at room temperature. Both blue-stained dead cells and unstained viable cells, which excluded the stain, were counted using a hemocytometer. For the neutral red uptake assay, in brief, after diamide treatment, HeLa and Grx3-KD cells, cultured in 24-well plates, were incubated with 0.033% neutral red solution in serum-free DMEM at 37 °C for 2 h, then the culture supernatant was removed, the cells were fixed in 0.1% CaCl2, 0.5% formaldehyde, and the incorporated dye was solubilized in a 1% acetic acid, 50% ethanol solution. The absorbance was measured using a microplate reader with a 540-nm test wavelength and a 690-nm reference wavelength. For the NuGrx3 complementation growth assay, after transfection of Grx3-KD cells with enhanced green fluorescent protein (eGFP; as vehicle control) and eGFP–NuGrx3, 5×104 cells were seeded in each well. After grown in normal growth medium for 24 h, the cells were treated with various concentrations of diamide for 14 h before neutral red uptake assay. All experiments were performed twice independently.
Fig. 5.
Grx3-knockdown cells are sensitive to oxidative stress. HeLa cells were (A) treated with various concentrations of diamide for 30 min or (B) treated with 1 mM diamide for various times. (C) HeLa cells (scrambled shRNA) and two Grx3 shRNA KD cell lines were treated with various concentrations of diamide as indicated for 14 h. Cell viability was determined with trypan blue exclusion assay. Shown is one representative of two independent experiments with similar results. In (A) and (B), each bar represents the mean±SD for diamide-treated viable or dead cells vs controls. Student’s t test, n=4; *p<0.05, **p<0.01, ***p<0.001. In (C), each bar represents the mean±SD for diamide-treated viable or dead cells for HeLa vs Grx3-KD (*) cells and shRNA 1 vs shRNA 2 ($). Statistical analysis using a two-way ANOVA, n=4; *p<0.05; $p<0.05. Consistent results were obtained by neutral red uptake assay (data not shown).
Irradiation treatment
Cells (human breast cancer cell lines MCF-7 and MDA-MB-231) were irradiated using a Gammacell 40 Exactor (Best Theratronics) cesium-137 irradiator. Plates containing cells were placed in a shielded chamber at room temperature. Radiation was then delivered that consisted of a single dose of 662 keV energy with a 1.06 Gy/min dose rate. Cells were exposed for the appropriate time to deliver the specified dose.
Construction of Grx3 variants and fusion genes
Full-length cDNA of mouse Grx3 was amplified by PCR using gene-specific primers (Supplementary Table S1). The N-terminal Trx-like domain (1–130 aa), the Grx domain I (G1, 131–238 aa), G2 (239–337 aa), and the C-terminal Grx domains (G1G2, 131–337 aa) were also amplified by PCR using domain-specific primers (Supplementary Table S1). Grx3 cysteine residues (Cys48, Cys148, Cys161, Cys231, and Cys263) were mutated to serine residues to generate each single Grx3 Cys mutant by site-directed mutagenesis [24] (Supplementary Table S1). Grx3 Tyr82Ala, Grx3 Leu139Ala, and Grx3 Leu139142Ala were also made by site-directed mutagenesis (Supplementary Table S1). We also generated eGFP–NuGrx3 by adding an SV40 nuclear-localized sequence (TPPKKKRKV) at the C-terminus of eGFP–NuGrx3 (Supplementary Table S1). All PCR products were cloned into pGEM-T Easy (Promega, Madison, WI, USA). The fidelity of all clones was confirmed by sequencing. Those genes were cloned into pENTR 4 (Invitrogen) and further subcloned into the mammalian expression vector pHAGE-N-Flag.
Subcellular localization
To visualize the subcellular localization of Grx3 in mammalian cells, three fusion gene constructs were made. Full-length mouse Grx3 was fused to the N-terminus of eGFP or red fluorescent protein (Clontech Laboratories, Inc., Mountain View, CA, USA). The fluorescent fusion protein constructs were subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen) as described previously [49]. The subcellular localization of the fused proteins was imaged by confocal microscopy [49]. The fluorescence signals were detected at 510 nm (excitation at 488 nm) for GFP and at 582 nm (excitation at 543 nm) for red fluorescent protein as previously described [49].
Nuclear and cytosolic fractionation and Western blot analysis
HeLa cells, MCF7 cells, MDA-MB-231 cells, 3T3 L1 cells, or MEFs were cultured in 10-cm dishes with 5×105 cells initially and grown to 70–80% confluence; then they were supplied with fresh serum-free DMEM and then starved overnight (16 h) before treatment. N-acetylcysteine (NAC; 200 mM) was added to the medium at a final concentration of 1, 5, or 10 mM for 3 h before diamide treatment. After treatment, the cells were released with 2 ml of trypsin (completely digested) and spun down at 4000 rpm for 5 min and washed with ice-cold phosphate-buffered saline (PBS) once and spun down again. Cell pellets were suspended in 200–400 μl ice-cold lysis buffer (10 mM Hepes, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.4% Nonidet P-40) with inhibitors (protease inhibitor cocktail tablet, Roche, Cat. No. 11697498001, plus 1 mM DTT, 0.5 mM PMSF, 1 mM Na vanadate) and incubated on ice for 10–15 min. The lysate was spun for 1 min in a microfuge at full speed at 4 °C and the supernatant (cytosolic fraction) was collected. The pellet contained nuclei and was washed with 500 μl lysis buffer without inhibitors. To the pellet was added 100 μl of extraction buffer (20 mM Hepes, pH 7.9, 0.4 M NaCl, 1 mM EDTA with inhibitors), and it was vortexed in a cold room for 5 min and spin for 10 min at full speed in a cold microfuge. The nuclear proteins were collected. All fractionations were stored at −70 °C before use. Western blot analysis was conducted following the established procedure [45]. For quantification of Western blotting, densitometry of the Western blot for the Grx3 antibody was calculated and quantified (triplicates for control and each treatment on the same blot), then the same blot was reprobed with an antibody against lamin A/C (for the cytosolic fraction a β-actin antibody was used) and the densitometry intensity of the Western blot was also calculated and quantified. The obtained signal intensity numbers for Grx3 antibody were normalized by dividing by the corresponding numbers for lamin A/C antibody (β-actin antibody for cytosolic fraction). The relative expression of Grx3 was calculated as the fold change (the ratio) between treated samples and controls. Significance was calculated by Student’s t statistics.
Detection of ROS
Ro-GFP2 plasmid DNA was a gift from Dr. James Remington (Departments of Biology, Chemistry, and Physics, Institute of Molecular Biology, University of Oregon, Eugene, OR, USA). Grx1-Ro-GFP2 plasmid DNA was a gift from Drs. Andreas Meyer (Heidelberg Institute of Plant Sciences, University of Heidelberg, Heidelberg, Germany) and Tobias Dick (Redox Regulation Research Group, German Cancer Research Center, Heidelberg, Germany). Both Ro-GFP2 and Grx1-Ro-GFP2 were subcloned into a lentiviral vector, pHAGE-CMV-N-Flag. Cells were seeded (5000/well) on 96-well plates for assessment of redox potential. Cells were transiently transfected with either Grx1-Ro GFP2 or Ro GFP2 using Lipofectamine 2000 and treated with diamide (0.7 mM) for 15 min before monitoring [50,51]. For catalase treatment, 400 or 800 units of PEGylated catalases was added into the cultured cells for 30 min before starting diamide treatment, then imaging was done as described below.
The redox-sensitive biosensors were excited at 403/12 nm and 470/20 nm using a Sutter Lamda DG-5 ultra-high-speed wavelength switcher, and the emission intensity was collected at 535/48 nm on a charge-coupled device camera (CoolSNAP MYO, Photometrics, Tucson, AZ, USA) attached to an Axio Observer (Zeiss) inverted microscope (40×H2O objective, 1.2 NA). After the basal level ratios were recorded, the cells were exposed to 0.7 mM diamide for 15 min to obtain a maximal oxidation, followed by DTT (10 mM) for 2 min to obtain maximal reduction of the biosensors. Regions of interest (ROI) were drawn on the cell and in the background. Ratio images (403/470) were created by subtracting the mean background ROI from the mean cell ROI. For dihydroethidium (DHE) measurement, HeLa Grx3-KD cells (shRNA 2) were transfected with eGFP–NuGrx3 for 6 h and then split into 24 wells with 5×104 initial cells for each well and grown for 24 h in growth medium. After treatment with or without diamide (0.7 mM diamide for 15 min), the cells were washed with PBS twice, then stained with DHE (final concentration 5 μM) at 37 °C in the dark for 30 min, and then washed twice with PBS. The fluorescence signals were detected at 510 nm (excitation at 488 nm) for eGFP and at 610 nm (excitation at 520 nm) for DHE using an Olympus Fluoview scanning laser confocal microscopy system. DHE fluorescence intensity was measured in ImageJ. The background subtracted fluorescence values from at least 30 cells per condition were plotted as bar diagrams.
Results
Subcellular localization of Grx3 in mammalian cells
To investigate the subcellular localization of Grx3 in mammalian cells, Grx3 fusion constructs were made, in which Grx3 was fused with red fluorescent protein (RFP). When expressed in HeLa cells, Grx3–RFP displayed uniform expression patterns in transfected cells and were diffuse in the cytosol with weak signals in the nucleus (Fig. 1). In agreement with this finding, cytoplasmic and nuclear fractionation and Western blot analysis demonstrated that endogenous Grx3 was predominately localized in the cytoplasmic fraction, with a small portion in the nuclear fraction under the normal growth conditions (Fig. 2 and Supplementary Fig. S1).
Fig. 1.
The subcellular localization of mammalian Grx3 fusion proteins in HeLa cells. HeLa cells were transfected with Grx3–RFP plasmid DNAs and fluorescence images were taken by confocal microscopy 48 h after transfection. Yellow arrowheads indicate nuclei. Scale bars, 20 μm.
Fig. 2.
Grx3 nuclear accumulation under oxidative stress. (A) HeLa cells were treated with 0, 0.3, 0.7, and 1.0 mM diamide for 30 min. (B) HeLa cells were treated with 1.0 mM diamide for 0, 5, 15, and 30 min. Cells were harvested after treatment and nuclear and cytoplasmic fractions were extracted. Western blot analysis of cytosolic and nuclear Grx3 was conducted using an anti-Grx3 monoclonal antibody (1:1000). The same blot of either cytosolic or nuclear fraction was reprobed with an anti-β-actin (1:2000) or anti-lamin A/C (1:1000) antibody as control for the cytoplasmic and nuclear fraction, respectively. The densitometry intensity of Western blots was calculated and quantified. Student’s t test, n=3; *p<0.05, **p<0.01, ***p<0.001 diamide- vs vehicle-treated cells.
Nuclear accumulation of Grx3 under oxidative stress
Whereas Grx3 is predominantly located in the cytoplasm under the normal growth conditions, we were curious to know whether changes in the cellular redox balance resulted in redistribution of Grx3. Therefore, we performed cellular fractionation of HeLa cells treated with various concentrations of diamide. As shown in Fig. 2A, the amount of Grx3 proteins in the nuclear fraction was significantly increased in a dose-dependent manner. Similarly, Grx3 accumulated in the nucleus under diamide treatment in a time-dependent manner (Fig. 2B). To determine whether Grx3 nuclear accumulation is a general response to oxidative stress, mouse 3T3 L1 fibroblasts and MEFs were tested with various concentrations of diamide. Indeed, without stress, Grx3 was primarily localized in the cytoplasm, whereas under oxidative stress the protein significantly accumulated in the nuclei (Supplementary Figs. S1A and S1B). These data suggest that Grx3 can move from the cytoplasm into the nucleus under stress.
To determine whether Grx3 translocation to the nucleus is a response to general oxidative stress, we evaluated Grx3 distribution in response to two other oxidants, diethyl maleate (DEM) and 1-chloro-2,4-dinitrobenzene (CDNB). Similar to diamide treatment, both DEM and CDNB stimulated Grx3 nuclear accumulation in both HeLa cells and MEFs (Fig. 3A and B). We further investigated whether nuclear accumulation of Grx3 occurs under irradiation, which condition is known to generate oxidative stress in the tissues and cells. As shown in Fig. 3C, Grx3 levels in the nuclear fraction from the cells treated with γ-irradiation were higher than those without treatment. Taken together, these results demonstrate nuclear accumulation of Grx3 under oxidative stress.
Fig. 3.
Effects of various oxidants and irradiation on nuclear accumulation of Grx3. (A) HeLa cells and (B) MEFs were treated with 0.7 mM diamide for 20 min, 0.5 and 1.0 mM DEM for 30 and 120 min, and 5 and 10 μM CDNB for 30 and 120 min. MEFs were also treated with 1 mM buthionine sulfoximine (BSO) for 18 h. (C) Human breast cancer cells (MCF7 and MDA-MB-231) were treated with irradiation as indicated. Western blot analysis was done as indicated in the Fig. 2 legend except anti-histone H3 (1:2000) and anti-PCNA antibody (1:2000) were used as controls for the nuclear fraction, and anti-Gapdh (1:2000) was used as a control for the cytosolic fraction. Shown is one representative of two independent experiments with consistent results.
Grx3 is critical for protection of cells against oxidative stress-induced cell death
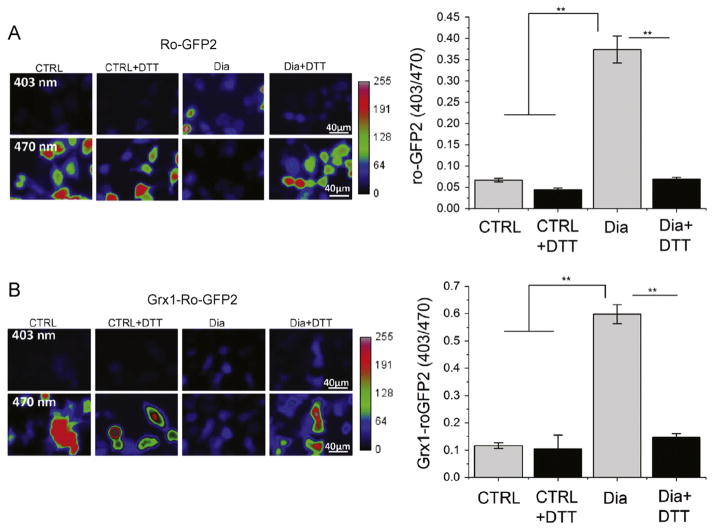
To measure the intracellular oxidative stress caused by diamide treatment, a redox-sensitive fluorescent protein (Ro-GFP2) was used to monitor the changes in ROS levels. As shown in Fig. 4A, HeLa cells treated with diamide displayed a significant increase in Ro-GFP2 fluorescence, indicating increased cellular oxidative stress, compared to controls without diamide treatment. Furthermore, a Grx1–Ro-GFP2 reporter, a translational fusion between Grx1 and Ro-GFP2, was used to monitor cellular glutathione redox potential. Diamide shifted the glutathione redox potential to a more oxidizing state as measured by an increase in the 403/470 ratio of Grx1–Ro-GFP2 (Fig. 4B). To determine whether diamide-induced cellular oxidative stress could be reduced by reducing agents or antioxidant enzymes, such as catalase, HeLa cells were treated with both diamide and DTT or PEGylated catalases before diamide stimulation. As shown in Fig. 4 and Supplementary Fig. S2, both DTT and PEGylated catalase could significantly prevent cells from diamide-induced cellular oxidative stress.
Fig. 4.
ROS and GSH potential measurement. HeLa cells were treated with diamide (0.7 mM) for 15 min alone or plus dithiothreitol (DTT; 10 mM) for 2 min and then imaged with (A) Ro-GFP2 for ROS levels or (B) Grx1–Ro-GFP2 for glutathione-mediated redox potentials. Student’s t test, n≥30; **p<0.01.
Diamide-induced cell death was measured by cell viability assay. HeLa cells were treated with increasing concentrations of diamide (Fig. 5A) or with 1 mM diamide for up to 30 min (Fig. 5B), resulting in a significant decrease in cell viability. Most interestingly, Grx3-KD cells were more sensitive to diamide treatment, with significantly increased cell death in comparison to wild-type HeLa cells (Fig. 5C). These results indicate that Grx3 plays an important role in counteracting oxidative stress-induced cell death.
Oxidative stress-induced nuclear accumulation of Grx3 is reversible
To further study whether changes in cellular redox states could affect Grx3 nuclear accumulation, HeLa cells and MEFs were treated with reducing reagents before diamide stress was applied. As shown in Fig. 6A and B, there was much less nuclear Grx3 accumulated in cells first treated with NAC and then with diamide compared to those treated with diamide alone. These results suggest that oxidative stress-induced nuclear accumulation of Grx3 could be blocked by reducing reagents. In addition, when HeLa cells were first treated with diamide for 20 min followed by incubation in culture medium containing NAC, the Grx3 nuclear accumulation induced by oxidative stress was significantly reversed by NAC treatment (Fig. 6C), suggesting that this nuclear accumulation of Grx3 is reversible and dependent on the cellular redox state.
Fig. 6.
N-acetylcysteine (NAC) is able to prevent and reverse oxidative stress-induced nuclear accumulation of Grx3. (A) HeLa cells and (B) MEFs were treated with various concentrations of NAC for 3 h before diamide treatment for 20 min as indicated. (C) HeLa cells were treated with 0.7 mM diamide for 20 min and then incubated in the medium containing 5 mM NAC for 3 h as indicated. Nuclear and cytosolic fractions were extracted and subjected to Western blot analysis as described in the Fig. 2 legend. Shown is one representative of two independent experiments with consistent results.
Effect of Grx3 domains on its nuclear accumulation under oxidative stress
Grx3 contains an N-terminal Trx-like domain (called Grx3-N) and a C-terminal region with two Grx domains (called Grx3-G1, Grx3-G2, and Grx3-G1G2) (Fig. 7A). Grx3 also consists of five cysteine residues (C1, Cys48; C2, Cys148; C3, Cys161; C4, Cys231; and C5, Cys263) of which C3 and C5 are located in the CGFS motifs (Fig. 7A). To understand whether different regions of Grx3 contribute to the nuclear accumulation of Grx3 under oxidative stress, truncated Grx3 with either a single or two Grx domains was generated and tested for Grx3 nuclear accumulation. Compared to full-length Grx3, Grx3-N and Grx3-G2 displayed similar localization upon diamide treatment. Interestingly, both Grx3-G1 and Grx3-G1G2 show significant accumulation in the nucleus (Fig. 7B), suggesting that the Grx3-G1 domain is more responsive to diamide-induced nuclear accumulation compared to the Grx3-N and Grx3-G2 domains.
Fig. 7.

Effects of Grx3 domains on Grx3 nuclear accumulation. (A) Diagram of Grx3 structure with the N-terminal Trx-like domain, C-terminal Grx G1 and G2 domains, five Cys residues, and the putative leucine-rich region. (B) HeLa cells were transfected with Flag-Grx3, Flag-Grx3-N (Trx-like domain), Flag-Grx3-G1G2 (C-terminal region without the N-terminus), Flag-Grx3-G1 (the single Grx domain 1), or Flag-Grx3-G2 (the single Grx domain 2) and treated with NAC or diamide alone or in combination as indicated. Nuclear and cytosolic fractions were extracted and subjected to Western blot analysis as described in the Fig. 2 legend except using an anti-Flag antibody. Shown is one representative of two independent experiments with consistent results.
To determine whether the Cys residue in the CGFS motif contributes to its nuclear accumulation, Cys161 (C3) and Cys263 (C5) were replaced by Ser residues. Both Grx3-C161S and Grx3-C263S, similar to Grx3, were significantly accumulated in the nuclei under oxidative stress (Supplementary Fig. S3A).
Computational analysis indicates that Grx3 does not contain an authentic nuclear localization signal peptide, but it does contain a putative nuclear export signal with multiple Leu residues that are located in the Grx3-G1 domain (Fig. 7A). Leu139 and Leu142 in Grx3 are predicted to be critical for the putative protein export signal (Fig. 7A). To determine if a protein export mechanism contributes to Grx3 nuclear accumulation (retention), HeLa cells were treated with leptomycin B, a potent inhibitor of the Crm1-dependent nuclear protein export pathway [52]. Western blot analysis indicated that no significant Grx3 nuclear accumulation (retention) was found compared to controls (Supplementary Fig. S3B). In agreement with this finding, the Grx3-L139A single and Grx3-L139142A double mutants were made and showed that the mutations did not affect Grx3 nuclear accumulation, either (Supplementary Fig. S3C). These results suggest that Grx3 nuclear accumulation (retention) may not be mediated by the putative nuclear export signal in the Grx3-G1 domain.
Nuclear Grx3 is sufficient for protecting cell survival against oxidative stress
Whereas Grx3 nuclear accumulation is significant, whether it is important for cell survival under oxidative stress is still unclear. A nuclear Grx3 (NuGrx3) was generated by tagging Grx3 with the SV40WT nuclear localization signal at its C-terminus and with eGFP at its N-terminus. eGFP–NuGrx3 was expressed exclusively in the nuclei (Fig. 8A). To test if NuGrx3 could rescue the sensitivity of Grx3-KD HeLa cells, eGFP and eGFP–NuGrx3 were expressed in Grx3-KD cells; NuGrx3-expressing Grx3-KD cells grew better than eGFP-expressing Grx3-KD cells under normal growth medium (Fig. 8B). Under diamide treatment, the proportion of viable vs dead cells was significantly decreased in eGFP-expressing cells, whereas the viability of NuGrx3-expressed cells was increased compared to that of eGFP-expressing cells (Fig. 8B). These findings suggest that nuclear Grx3 is sufficient for protecting cells and that Grx3 nuclear accumulation is required for cell survival under oxidative stress. To determine whether NuGrx3 could reduce cellular ROS, both NuGrx3- and non-NuGrx3-transfected Grx3-KD cells were stained with fluorescent dye, DHE. In NuGrx3-expressing cells, the fluorescence intensity was significantly reduced compared to non-NuGrx3-transfected cells (Fig. 8C, D, and E), indicating that expression of nuclear Grx3 is able to protect cells from ROS production and oxidative stress.
Fig. 8.
Nuclear-targeting Grx3 is sufficient to suppress the sensitivity of Grx3-KD cells to oxidative stress. (A) The subcellular localization of nuclear-targeted Grx3 fusion proteins in HeLa cells. HeLa cells were transfected with eGFP–NuGrx3 and the nuclei were stained with Sytox orange dye. The fluorescence images were taken by confocal microscopy 24 h after transfection. (B) Cell viability assay. Grx3-KD cells (shRNA 2 line) were transfected with eGFP as controls or eGFP–NuGrx3 and then seeded at 1×105 cells into each well (triplicates for each treatment) and grown for 24 h with normal growth medium. Cell viability assay was conducted after being treated with various concentrations of diamide as indicated for 14 h. Shown is one representative of two independent experiments with consistent results. Statistical analysis using a two-way ANOVA, n=3; *p<0.05. Consistent results were obtained by neutral red uptake assay (data not shown). (C) DHE staining (red) in NuGrx3-transfected cells (green, arrowheads) and untransfected (arrows) cells. Scale bars, 30 μm. (D and E) Quantification of DHE fluorescence intensity in NuGrx3- and non-NuGrx3-transfected cells (D) without and (E) with diamide treatment. Each bar represents the mean±SD for NuGrx3- vs non-NuGrx3-transfected cells. Student’s t test, n≥30; *p<0.05, **p<0.01.
Discussion
In this study, we have shown that mammalian Grx3 is predominately localized in the cytoplasm under normal conditions, whereas under oxidative stress Grx3 is able to translocate into and accumulate in the nucleus. Our findings further demonstrate that the nuclear–cytosolic shuttling of Grx3 is reversible depending on the cellular redox potential, which is correlated to the sensitivity of cells to external oxidants. In addition, forced expression of nuclear-targeted Grx3 is sufficient to protect cells against oxidative stress. Therefore, our results provide insights into Grx3 regulation and its role in counteracting environmental insults.
Mammalian Grx3 was evenly distributed in the cytoplasm when Grx3 fusion proteins were expressed in the resting cell (Fig. 1), which is consistent with the result from Western blot analysis of cellular fractionations (Fig. 2 and Supplementary Fig. S1). Within the cytosol, Grx3 appears to have different subcellular localizations depending on the cell type or tissue. A previous study indicates that in unstimulated T cells Grx3/PICOT is localized in a distinct cytoplasmic area under the plasma membrane, where it is able to associate with protein kinase θ isoform [32]. Under phorbol myristate acetate stimulation, Grx3/PICOT is able to translocate to a more extended membrane area [32], suggesting that Grx3, under certain conditions and along with its interacting partner, could be associated with the plasma membrane. Furthermore, in heart muscle cells, Grx3/PICOT is targeted to the sarcomeric Z-disc, where it is associated with the muscle LIM protein [40].
Under oxidative stress, a significant proportion of Grx3 is accumulated in the nucleus (Figs. 2 and 3 and Supplementary Fig. S1). This nuclear accumulation of Grx3 is correlated well to increased cellular ROS production and depletion of reduced glutathione (GSH) (Fig. 4). Interestingly, oxidants such as diamide, DEM, and CDNB that can rapidly deplete cellular GSH have stronger effects on Grx3 nuclear accumulation than does buthionine sulfoximine, which slowly decreases cellular GSH levels (Fig. 3) [53]. In addition, our findings also imply that both reducing agents, such as DTT, and antioxidant enzymes, such as catalase, are able to reduce diamide-induced cellular oxidative stress (Fig. 4 and Supplementary Fig. S2). Under our current test conditions, hydrogen peroxide, hypoxia (CoCl2 treatment), and nutrient deprivation have little to no effect on Grx3 translocation. On the other hand, irradiation triggers Grx3 nuclear accumulation (Fig. 3C).
A previous study reported that tyrosine phosphorylation of Grx3/PICOT is required for Grx3/PICOT translocation in T cells under oxidative stress [54]. There are seven tyrosine residues in Grx3, but only the tyrosine residue at position 82 (Tyr82) is predicted to be possibly phosphorylated by tyrosine kinase. To further test if Tyr82 is involved in oxidative stress-induced Grx3 nuclear accumulation, Grx3-Y82A was generated and analyzed by nuclear/cytoplasmic fractionation (Supplementary Fig. S2D). The results indicate that this tyrosine-to-alanine mutation does not alter the Grx3 distribution under oxidative stress. How tyrosine phosphorylation of Grx3 affects its nuclear translocation remains to be investigated. Analysis of domain truncation and mutation of cysteine residues in CGFS motifs also shows that the Grx3 Trx-like domain, G2 domain, and two catalytic Cys residues are not involved in Grx3 nuclear accumulation in response to oxidative stress (Fig. 7 and Supplementary Fig. S3A). In contrast, the nuclear translocation of yeast ScGrx3 is mediated by its N-terminal Trx-like domain [55], even though the full-length ScGrx3 is predominantly localized in the cytoplasm [56]. Our results suggest that the Grx3 G1 domain may be responsible for its nuclear accumulation through a mechanism independent of the Crm1-mediated nuclear export pathway (Fig. 7 and Supplementary Figs. S3B and S3C). Although Grx3 does not contain an authentic nuclear localization signal sequence, interestingly, several nuclear import inhibitors, such as mifepristone, ivermectin, and importazole [57,58], could partially block diamide-mediated Grx3 nuclear accumulation (Supplementary Fig. S4; data not shown), suggesting that protein import pathways may be involved in Grx3 nuclear accumulation under oxidative stress.
Grx3 nuclear accumulation is reversible and dependent on cellular redox states (Figs. 4 and 6), suggesting that Grx3, like thioredoxin 1, may play distinct roles in the cytoplasm and in the nucleus [59]. Grx3 is an iron–sulfur-binding protein [36]. Under reducing conditions, it can form [2Fe–2S] bridged complexes with Bol2 protein in an in vitro assay [60]. Although the nature of this binding in vivo has not been determined yet, this finding does suggest that cytosolic Grx3 may function in Fe–S cluster delivery and iron regulation in the cytoplasm [37,38,61,62]. Under oxidative stress, Grx3 is presumably no longer binding an iron–sulfur cluster as a dimer; rather it could be a monomer and act as a thioreductase to protect S-glutathionylation of target proteins. This notion is supported by our previous findings that both RelA and RelB of the NF-κB pathway are glutathionylated by cellular oxidation when Grx3 is absent from the cell [45]. It is possible that nuclear Grx3 could prevent the transcriptional machinery from damage and regulate gene expression in response to oxidative stress. Interestingly, in yeast, under iron-deplete condition, ScGrx3 can physically interact with an iron-regulatory transcription factor, Aft1, and translocate into the nucleus for regulating gene expression related to iron uptake [26]. Previous studies indicate that Grx3 can interact with PKC θ isoform and muscle LIM protein to modulate stress-related transcription factors, such as AP1 and NFAT [32,40], but whether those interactions are redox dependent remains to be addressed. In this study, our findings suggest that Grx3 is critical for protecting cells from oxidative stress-induced cell death, as the absence of Grx3 renders cells susceptible to oxidized conditions, whereas overexpressing nuclear Grx3 in Grx3-KD cells is able to reduce cellular ROS production and sufficient to resist environmental insults.
Supplementary Material
Acknowledgments
This work is supported by the U.S. Department of Agriculture/Agricultural Research Service under Cooperation Agreement 6250–51000-054 (N.H.C.). Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award R01 AR061370 to G.G.R and by National Institutes of Health (CA151610), the Avon Foundation (02-2014-063), David Salomon Translational Breast Cancer Research Fund, and the Fashion Footwear Charitable Foundation of New York, Inc. and the Margie and Robert E. Petersen Foundation to Y.Q. and X.C.
Abbreviations
- anti-PCNA
antibody against proliferating cell nuclear antigen
- AP1
activator protein 1
- CDNB
1-chloro-2,4-dinitrobenzene
- CS
calf serum
- DEM
diethyl maleate
- DHE
dihydroethidium
- DMEM
Dulbecco’s modified Eagle medium
- DTT
dithiothreitol
- eGFP
enhanced green fluorescent protein
- FBS
fetal bovine serum
- Grx3
glutaredoxin 3
- GSH
glutathione
- KD
knockdown
- MEF
mouse embryonic fibroblast
- NAC
N-acetylcysteine
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor κB
- PICOT
protein kinase C-interacting cousin of thioredoxin
- PKC θ isoform
protein kinase C θ isoform
- PMSF
phenyl-methanesulfonyl fluoride
- RFP
red fluorescent protein
- Ro-GFP2
redox-sensitive fluorescent protein 2
- ROI
regions of interest
- ROS
reactive oxygen species
- Trx
thioredoxin
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.freeradbiomed.2015.05.003.
References
- 1.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Reactive species and antioxidants: redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signaling. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 5.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signaling. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longevity. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176–184. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 8.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 11.Holmgren A, Aslund F. Glutaredoxin. Methods Enzymol. 1995;252:283–292. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 12.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Gallogly MM, Shelton MD, Qanungo S, Pai HV, Starke DW, Hoppel CL, Lesnefsky EJ, Mieyal JJ. Glutaredoxin regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2 and Bcl-xL: implications for cardiac aging. Antioxid Redox Signaling. 2010;12:1339–1353. doi: 10.1089/ars.2009.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anathy V, Aesif SW, Hoffman SM, Bement JL, Guala AS, Lahue KG, et al. Glutaredoxin-1 attenuates S-glutathionylation of the death receptor Fas and decreases resolution of Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2014;189:463–474. doi: 10.1164/rccm.201310-1905OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolin JD, Tully JE, Hoffman SM, Guala AS, van der Velden JL, Poynter ME, et al. The glutaredoxin/S-glutathionylation axis regulates interleukin-17A-induced proinflammatory responses in lung epithelial cells in association with S-glutathionylation of nuclear factor κB family proteins. Free Radic Biol Med. 2014;73:143–153. doi: 10.1016/j.freeradbiomed.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aesif SW, Anathy V, Kuipers I, Guala AS, Reiss JN, Ho YS, et al. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol. 2011;44:491–499. doi: 10.1165/rcmb.2009-0136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonn ME, Hudemann C, Berndt C, Cherkasov V, Capani F, Holmgren A, Lillig CH. Expression pattern of human glutaredoxin 2 isoforms: identification and characterization of two testis/cancer cell-specific isoforms. Antioxid Redox Signaling. 2008;10:547–557. doi: 10.1089/ars.2007.1821. [DOI] [PubMed] [Google Scholar]
- 19.Lillig CH, Lonn ME, Enoksson M, Fernandes AP, Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci USA. 2004;101:13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Lin L, Giblin F, Ho YS, Lou MF. Glutaredoxin 2 knockout increases sensitivity to oxidative stress in mouse lens epithelial cells. Free Radic Biol Med. 2011;51:2108–2117. doi: 10.1016/j.freeradbiomed.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrero E, de la Torre-Ruiz MA. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci. 2007;64:1518–1530. doi: 10.1007/s00018-007-6554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng NH, Hirschi KD. Cloning and characterization of CXIP1, a novel PICOT domain-containing Arabidopsis protein that associates with CAX1. J Biol Chem. 2003;278:6503–6509. doi: 10.1074/jbc.M210883200. [DOI] [PubMed] [Google Scholar]
- 23.Lopreiato R, Facchin S, Sartori G, Arrigoni G, Casonato S, Ruzzene M, Pinna LA, Carignani G. Analysis of the interaction between piD261/Bud32, an evolutionarily conserved protein kinase of Saccharomyces cerevisiae, and the Grx4 glutaredoxin. Biochem J. 2004;377:395–405. doi: 10.1042/BJ20030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng NH, Liu JZ, Brock A, Nelson RS, Hirschi KD. AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J Biol Chem. 2006;281:26280–26288. doi: 10.1074/jbc.M601354200. [DOI] [PubMed] [Google Scholar]
- 25.Ojeda L, Keller G, Muhlenhoff U, Rutherford JC, Lill R, Winge DR. Role of glutaredoxin-3 and glutaredoxin-4 in the iron regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae. J Biol Chem. 2006;281:17661–17669. doi: 10.1074/jbc.M602165200. [DOI] [PubMed] [Google Scholar]
- 26.Pujol-Carrion N, Belli G, Herrero E, Nogues A, de la Torre-Ruiz MA. Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J Cell Sci. 2006;119:4554–4564. doi: 10.1242/jcs.03229. [DOI] [PubMed] [Google Scholar]
- 27.Rouhier N, Couturier J, Johnson MK, Jacquot JP. Glutaredoxins: roles in iron homeostasis. Trends Biochem Sci. 2010;35:43–52. doi: 10.1016/j.tibs.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, Lillig CH, Lill R. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron–sulfur cluster. Cell Metab. 2010;12:373–385. doi: 10.1016/j.cmet.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Mapolelo DT, Dingra NN, Keller G, Riggs-Gelasco PJ, Winge DR, Johnson MK, Outten CE. Histidine 103 in Fra2 is an iron–sulfur cluster ligand in the [2Fe–2S] Fra2–Grx3 complex and is required for in vivo iron signaling in yeast. J Biol Chem. 2011;286:867–876. doi: 10.1074/jbc.M110.184176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann B, Uzarska MA, Berndt C, Godoy JR, Haunhorst P, Lillig CH, Lill R, Muhlenhoff U. The multidomain thioredoxin–monothiol glutaredoxins represent a distinct functional group. Antioxid Redox Signaling. 2011;15:19–30. doi: 10.1089/ars.2010.3811. [DOI] [PubMed] [Google Scholar]
- 31.Isakov N, Witte S, Altman A. PICOT-HD: a highly conserved protein domain that is often associated with thioredoxin and glutaredoxin modules. Trends Biochem Sci. 2000;25:537–539. doi: 10.1016/s0968-0004(00)01685-6. [DOI] [PubMed] [Google Scholar]
- 32.Witte S, Villalba M, Bi K, Liu Y, Isakov N, Altman A. Inhibition of the c-Jun N-terminal kinase/AP-1 and NF-kappaB pathways by PICOT, a novel protein kinase C-interacting protein with a thioredoxin homology domain. J Biol Chem. 2000;275:1902–1909. doi: 10.1074/jbc.275.3.1902. [DOI] [PubMed] [Google Scholar]
- 33.Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, Levi S, Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110:1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- 34.Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, Rouault TA. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J Clin Invest. 2010;120:1749–1761. doi: 10.1172/JCI40372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linares GR, Xing W, Govoni KE, Chen ST, Mohan S. Glutaredoxin 5 regulates osteoblast apoptosis by protecting against oxidative stress. Bone. 2009;44:795–804. doi: 10.1016/j.bone.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haunhorst P, Berndt C, Eitner S, Godoy JR, Lillig CH. Characterization of the human monothiol glutaredoxin 3 (PICOT) as iron–sulfur protein. Biochem Biophys Res Commun. 2010;394:372–376. doi: 10.1016/j.bbrc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Outten CE. Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis. Biochemistry. 2012;51:4377–4389. doi: 10.1021/bi300393z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haunhorst P, Hanschmann EM, Brautigam L, Stehling O, Hoffmann B, Muhlenhoff U, Lill R, Berndt C, Lillig CH. Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation. Mol Biol Cell. 2013;24:1895–1903. doi: 10.1091/mbc.E12-09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong D, Cha H, Kim E, Kang M, Yang DK, Kim JM, Yoon PO, Oh JG, Bernecker OY, Sakata S, Le TT, Cui L, Lee YH, do Kim, Woo H, Liao SH, Hajjar R, Park RJ, PICOT WJ. inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ Res. 2006;99:307–314. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- 40.Jeong D, Kim JM, Cha H, Oh JG, Park J, Yun SH, Ju ES, Jeon ES, Hajjar RJ, Park WJ. PICOT attenuates cardiac hypertrophy by disrupting calcineurin-NFAT signaling. Circ Res. 2008;102:711–719. doi: 10.1161/CIRCRESAHA.107.165985. [DOI] [PubMed] [Google Scholar]
- 41.Kato N, Motohashi S, Okada T, Ozawa T, Mashima K. PICOT, protein kinase C theta-interacting protein, is a novel regulator of FcepsilonRI-mediated mast cell activation. Cell Immunol. 2008;251:62–67. doi: 10.1016/j.cellimm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Oh JG, Jeong D, Cha H, Kim JM, Lifirsu E, Kim J, Yang DK, Park CS, Kho C, Park S, Yoo YJ, do Kim, Kim H, Hajjar J, Park RJ, PICOT WJ. increases cardiac contractility by inhibiting PKCzeta activity. J Mol Cell Cardiol. 2012;53:53–63. doi: 10.1016/j.yjmcc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Cha H, Kim JM, Oh JG, Jeong MH, Park CS, Park J, Jeong HJ, Park BK, Lee YH, Jeong D, Yang DK, Bernecker OY, Kim do H, Hajjar RJ, Park WJ. PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility. J Mol Cell Cardiol. 2008;45:796–803. doi: 10.1016/j.yjmcc.2008.09.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng NH, Zhang W, Chen WQ, Jin J, Cui X, Butte NF, Chan L, Hirschi KD. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J. 2011;278:2525–2539. doi: 10.1111/j.1742-4658.2011.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, Bukoye BA, Liu B, Lee AV, Lin X, Huang P, Martens JW, Giuliano AE, Zhang N, Cheng NH, Cui X. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-kappaB signaling. J Clin Invest. 2011;121:212–225. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun N, Kim C, Cha H, Park WJ, Shibayama H, Park IS, Oh YJ. Caspase-3-mediated cleavage of PICOT in apoptosis. Biochem Biophys Res Commun. 2013;432:533–538. doi: 10.1016/j.bbrc.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;3B doi: 10.1002/0471142735.ima03bs21. Appendix 3: Appendix. [DOI] [PubMed] [Google Scholar]
- 48.Gao LP, Cheng ML, Chou HJ, Yang YH, Ho HY, Chiu DT. Ineffective GSH regeneration enhances G6PD-knockdown of Hep G2 cell sensitivity to diamide-induced oxidative damage. Free Radic Biol Med. 2009;47:529–535. doi: 10.1016/j.freeradbiomed.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Cheng NH. AtGRX4, an Arabidopsis chloroplastic monothiol glutaredoxin, is able to suppress yeast grx5 mutant phenotypes and respond to oxidative stress. FEBS Lett. 2008;582:848–854. doi: 10.1016/j.febslet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Pal R, Basu Thakur P, Li S, Minard C, Rodney GG. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PLoS One. 2013;8:e63989. doi: 10.1371/journal.pone.0063989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pal R, Monroe TO, Palmieri M, Sardiello M, Rodney GG. Rotenone induces neurotoxicity through Rac1-dependent activation of NADPH oxidase in SHSY-5Y cells. FEBS Lett. 2014;588:472–481. doi: 10.1016/j.febslet.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krance SM, Keng PC, Palis J, Ballatori N. Transient glutathione depletion determines terminal differentiation in HL-60 cells. Oxid Med Cell Longevity. 2010;3:53–60. doi: 10.4161/oxim.3.1.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babichev Y, Isakov N. Tyrosine phosphorylation of PICOT and its translocation to the nucleus in response of human T cells to oxidative stress. Adv Exp Med Biol. 2001;495:41–45. doi: 10.1007/978-1-4615-0685-0_6. [DOI] [PubMed] [Google Scholar]
- 55.Molina MM, Belli G, de la Torre MA, Rodriguez-Manzaneque MT, Herrero E. Nuclear monothiol glutaredoxins of Saccharomyces cerevisiae can function as mitochondrial glutaredoxins. J Biol Chem. 2004;279:51923–51930. doi: 10.1074/jbc.M410219200. [DOI] [PubMed] [Google Scholar]
- 56.Kumanovics A, Chen OS, Li L, Bagley D, Adkins EM, Lin H, Dingra NN, Outten CE, Keller G, Winge D, Ward DM, Kaplan J. Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron–sulfur cluster synthesis. J Biol Chem. 2008;283:10276–10286. doi: 10.1074/jbc.M801160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara-Bingen M, Weis K, Heald R. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem Biol. 2011;6:700–708. doi: 10.1021/cb2000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 60.Li H, Mapolelo DT, Randeniya S, Johnson MK, Outten CE. Human glutaredoxin 3 forms [2Fe–2S]-bridged complexes with human BolA2. Biochemistry. 2012;51:1687–1696. doi: 10.1021/bi2019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Philpott CC. Coming into view: eukaryotic iron chaperones and intracellular iron delivery. J Biol Chem. 2012;287:13518–13523. doi: 10.1074/jbc.R111.326876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paul VD, Lill R. SnapShot: eukaryotic Fe–S protein biogenesis. Cell Metab. 2014;20:384. doi: 10.1016/j.cmet.2014.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.