Abstract
By employing DNAzyme as a recognition group and amplifier, and DNA-stabilized silver nanoclusters (DNA/AgNCs) as signal reporters, we for the first time reported a label-free catalytic and molecular beacon as an amplified biosensing platform for highly selective detection of cofactors such as Pb2+ and L-histidine.
We developed a label-free catalytic and molecular beacon as a universal amplified biosensing platform for highly selective detection of cofactors.
DNAzymes are a class of functional nucleic acids, which show high protein enzyme-like catalytic hydrolytic cleavage activities toward specific substrates by employing specific metal ions or neutral molecules as cofactors.1-3 Compared to protein enzyme, DNAzymes are cheaper, more stable, easier modified, and can be denatured and renatured many times without losing catalytic activities. These unique advantages make DNAzymes particularly suitable for developing biosensors for detecting analytes such as Pb2+, 4 Cu2+, 5 UO22+, 6 Zn2+, 7 Mg2+, 8 and neutral L-histidine9 with high specificity. By combining traditional DNAzyme with molecular beacon (MB), our group previously reported an amplified biosensing strategy termed catalytic and molecular beacons (CAMB), which realized multiple turnover catalysis of DNAzyme.10 With the advantages including simple operator, high sensitivity, high specificity and highly efficient quenching, CAMB has been used for signal amplification to develop a series of biosensors.11-14 However, CAMB required modifications of both fluorophore and quencher that are synthesis complicated, technically demanding and expensive.15, 16 Therefore, development of a simple, inexpensive and label-free CAMB biosensor should be more attractive.
Since they were first reported by Dickson et al.,17 silver nanoclusters (AgNCs) stabilized by oligonucleotides have attracted increasing interest due to their excellent performance including facile synthesis, extreme brightness, photo-stability, tunable fluorescence emission and low-toxicity. They have been employed to construct biosensors for the detection of various analytes, such as thiol compounds,18 metal ions,19, 20 and single-nucleotide mutation.21 Particularly, Werner et al. reported a new AgNCs-based strategy for target DNA detection.22 They found that after forming AgNCs on C-rich DNA sequence (DNA template), the proximity of a guanine-rich DNA can trigger reversible transformation of NCs between a dark species and a bright red-emitting species with a 500-fold fluorescence enhancement.
Based on the amplified recognition of CAMB, in this work, we employed AgNCs as fluorescent reporters to develop a label-free CAMB amplified sensing platform for the detection of L-histidine and Pb2+. DNAzyme used in this strategy could recognize target molecules and act as an amplifier to afford improved sensitivity by multiple enzymatic turnovers. Such design allows the sensing platform a high sensitivity for the detection of L-histidine and Pb2+. In addition, since various recognition units might be fused into the sensing system, the new platform can be employed to design label-free biosensors for detection of various targets.
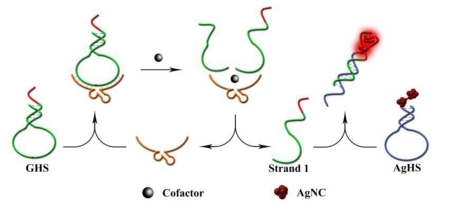
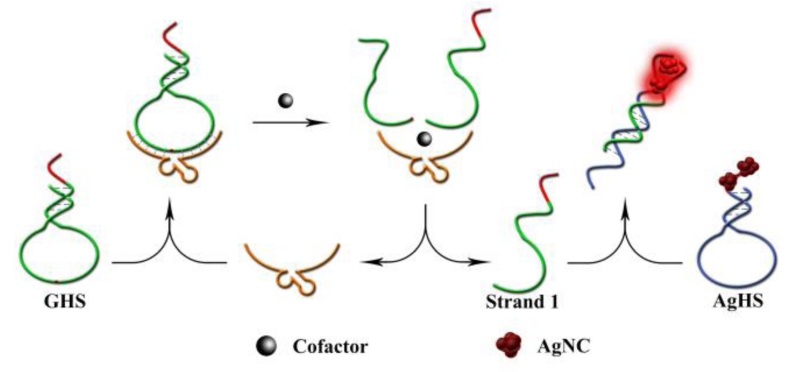
The label-free CAMB biosensor is composed of two hairpin-structured molecules. As show in Scheme 1, the first one is an unmodified G-rich hairpin structure (GHS), which together with DNAzyme strand serve as molecular recognition unit and amplifier for design Pb2+ or L-histidine biosensors (sequences see Supporting Information). GHS contains two components. The green portion is a hairpin-structured substrate strand of DNAzyme, and the red portion is a G-rich sequence which extended on the terminal of the substrate strand. The other hairpin-structure containing dark AgNCs (AgHS) is designed to be complementary to the cleaved G-rich product of GHS (called strand 1, see Scheme 1), and such a hybridization can form bright red-emitting AgNCs to act as signal reporter. In the absence of target, strand 1 is caged in the GHS, which make it difficult to hybridize with AgHS with no enhanced-fluorescence, thus affording low background fluorescence. However, in the present of target, the enzyme sequence catalyzes the cleavage of the substrate strand GHS, and liberates free strand 1 from the GHS caged structure to hybridize with AgHS. As a consequence, G-rich sequence was placed in proximity to the dark AgNCs, and produced an obvious increase of the fluorescent signal. At the same time, the detached DNAzyme strand could bind another GHS and trigger another cycle of digestion, providing more strands 1 and achieving an amplified detection signal for the target. To obtain the best response, we found that 2 equiv of substrates are necessary for 1 equiv of DNAzyme strand (Fig. S1) (ESI†), which make it possible to take advantage of DNAzymes as catalysts for amplified sensing through multiple turnover reactions.
Scheme 1.
Schematic illustration of the fluorescence biosensor for Pb2+ and L-histidine.
To verify the feasibility of our design, the fluorescence changes of AgHS under different conditions were first investigated (Fig. S2a) (ESI†). In the absence of strand 1, AgHS exhibited no fluorescence emission peak (curve a). After incubation of AgHS with strand 1 for 30 min, a bright redemitting fluorescence was observed (curve c). One could also find that Pb2+ exhibited no effect on the fluorescence of AgHS (curve b and d). These results indicate that fluorescence of AgHS is modulated only by strand 1, demonstrating the success of our design.
In order to achieve highest sensitivity, the length of “caged” sequence on AgHS was optimized. The effect of the length of the caged sequence on AgHS was examined through measuring the fluorescence intensities of AgNCs in the absence and presence of target. We synthesized four different hairpin probes (Ag-1, Ag-2, Ag-3, and Ag-4) that included 11, 10, 9, and 8 base pairs in the stem, respectively. 10 base-pairs for the stem of AgHS were found to exhibit a largest signal-to background ratio (SBR) (showed in Fig. S3a) (ESI†). More caged bases could lead to more difficult strand-displacement between GHS and AgHS, resulting in lower background signal, however, it might results in a high melting temperature between strand 1 and AgHS and making a low fluorescence signal. But, fewer “caged” bases may form an erratic hairpin of AgHS, increasing background fluorescence. In order to obtain a maximum SBR, the incubation time of strand 1 with AgHS was optimized (showed in Fig. S3b) (ESI†). The fluorescent intensity increased with increasing reaction time up to 45 min then reaching a balance. In order to obtain a reliable result, the incubation time of 45 min was adopted as the optimum and employed for all other investigations.
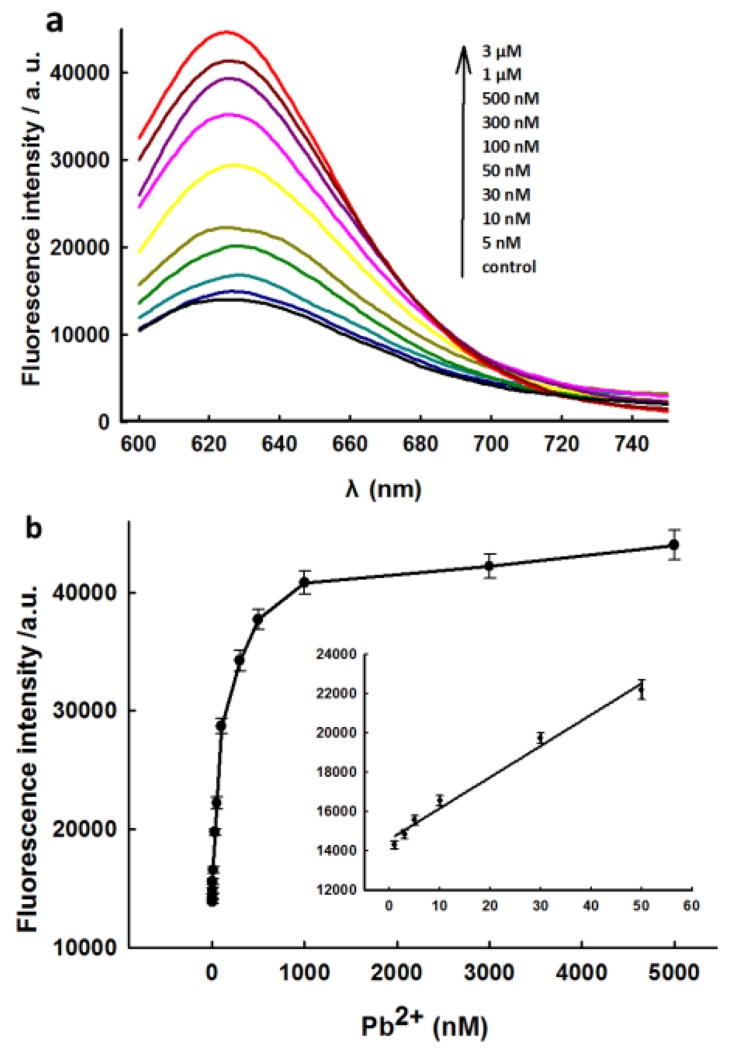
The analytical performance for Pb2+ was then investigated under optimized conditions. Fig. 1a depicts the fluorescence-emission spectra of the sensing system upon the addition of Pb2+ at different concentrations. The addition of increasing concentrations of Pb2+ could lead more substrate strand being cleaved and liberate more strands 1 to afford a dramatic fluorescence signal enhancement. The large fluorescence enhancement is mainly benefit from the fact that GR-5 possesses multiple turnover catalytic activity for GHS to release strand 1. Fig. 1b shows the relationship between the fluorescence intensity and the concentrations of Pb2+. It shows a dynamic range from 5 nM to 3μM, and a linear relationship range from 5 nM to 50 nM with a low detection limit of 1 nM for Pb2+.
Fig.1.
(a) Fluorescence spectra of sensing system at various concentrations of Pb2+ corresponding to data in the graph. (b) The relationship of the fluorescence enhancement with the Pb2+ concentration. Inset shows the responses of sensing system to Pb2+ at low concentration. The error bars indicated the standard deviations of three independent experiments.
A high specificity for target analyte is necessary for a new designed biosensor with potential applications in complicated samples. To investigate the selectivity of the DNAzyme-AgNCs based sensing system for Pb2+, the fluorescence responses of the sensor to various metal ions which are potential interferences were recorded. As shown in Fig. 2, Pb2+ could induce a significant fluorescence enhancement of the sensing system, while other metal ions did not give an obvious fluorescence change, indicating that our sensing system exhibits a high selectivity to Pb2+ over other metal ions. This result is consistent with the previously reported GR-5 DNAzyme sensors, and could meet the selective requirements for environmental and biomedical applications.7
Fig.2.
Selectivity of DNAzyme-AgNCs based detection system. The degree of signal enhancement is defined as (FI-F0)/F0, where F0 and FI are the fluorescence intensities at 635 nm in the absence and presence of Pb2+ and other kinds of metal ions, respectively. The concentrations for Pb2+ and Cu2+ ions were 1 μM, and 1 mM for other metal ions.
In order to evaluate the practical application of the new sensor for detection of Pb2+, the recovery experiments with spiked Pb2+ in river water samples were carried out. The river water samples were obtained from the Xiang River (Changsha, China), which were simply filtered and showed that no Pb2+ was present. The analytical results are shown in Table S1 (ESI†). One observed that the results obtained in real water samples showed good recovery values, which confirmed that the proposed sensor was applicable for practical Pb2+ detection in real samples with other potentially competing species coexisting.
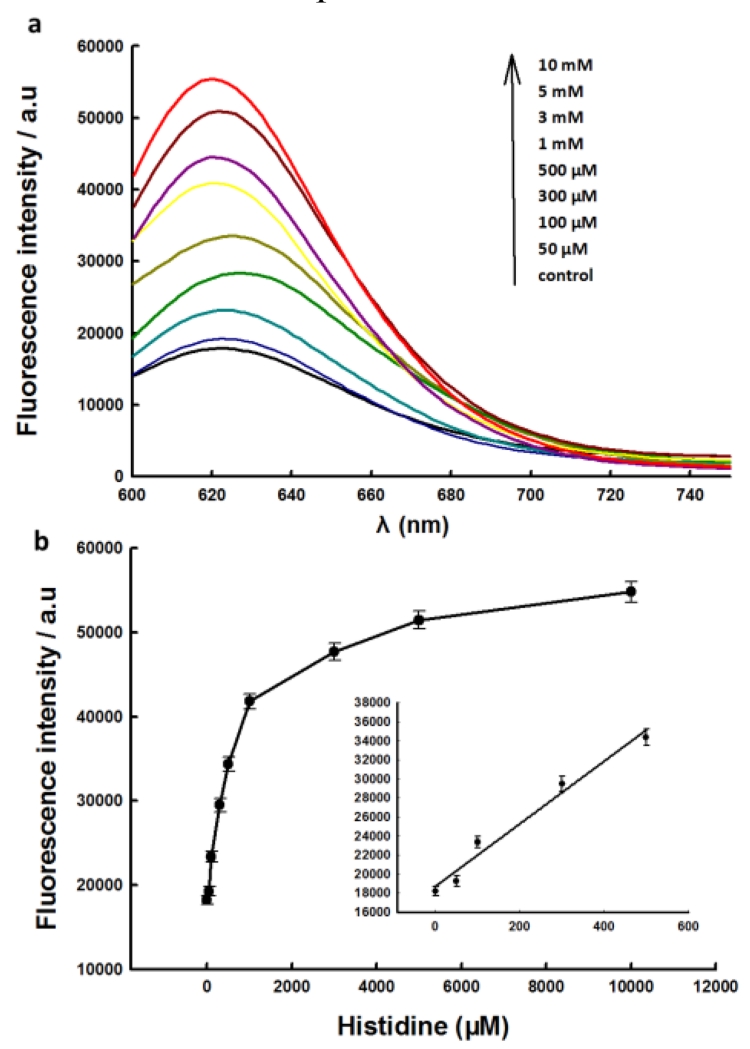
To verify the universality of our label-free CAMB strategy, it was further adopted to construct catalytic biosensor for L-histidine by choosing L-histidine-dependent DNAzyme as recognition unit. As L-histidine plays a significant role in the growth and repair of tissues, as well as controls the transmission of metal elements in biological bases.23 L-histidine itself has not effect on the fluorescence of AgNCs (Fig. S2b) (ESI†). The procedure for detection of L-histidine is similar to that of Pb2+. After incubating with different concentration of L-histidine and then mixing with AgNCs, the fluorescence spectra of the sensing solution were recorded (Fig. 3). Based on the fact that the fluorescence intensity of the biosensor is increased with the addition of increasing concentrations of L-histidine, one can come to the conclusion that the biosensor is feasible for turn on detection of L-histidine. The calibration curve of the biosensor for L-histidine was shown in Fig. 3b. Due to the enhanced effect of G-rich sequences to AgNCs, the L-histidine-dependent DNAzyme based catalytic biosensor also exhibits a satisfactory sensitivity to L-histidine, and it exhibited a linear response concentration range from 50 μM to 500 μM, with a LOD of 17 μM. The proposed catalytic biosensor also shows high selectivity to L-histidine over other potential interferences. As shown in Fig. S4 (ESI†), 1 mM of L-histidine could induce obvious fluorescence enhancement of the biosensor, while no obvious fluorescence change were observed upon the addition of 1 mM of other compounds.
Fig.3.
(a) Fluorescence spectra of sensing systems at various concentrations of L-histidine corresponding to data in the graph. (b) The relationship of the fluorescence enhancement with the L-histidine concentration.Inset shows the responses of sensing system to L-histidine at low concentration.
In conclusion, we for the first time reported a label-free catalytic and molecular beacon as an amplified biosensing platform for detection of DNAzyme cofactors such as Pb2+ and L-histidine. The introduction of target cofactor could trigger enhanced fluorescence of AgNCs to afford a “turn-on” fluorescence response to target biomolecule. By employing the multiple enzymatic turnover of DNAzyme for cycle amplifying, the proposed sensing system shows highly sensitive response to target cofactor. In addition, the design is simple, easy operation, and inexpensive. Since numerous DNAzymes have been selected to bind a wide range of targets, the label-free catalytic and molecular beacon provides a new general platform for sensitive detection of various targets and could find wide applications in the environmental and biomedical fields.
Supplementary Material
Acknowledgments
This work was supported by the National Key Scientific Program of China (2011CB911000), the National Key Basic Research Program of China (No. 2013CB932702), NSFC (Grants 21405100, 21275044, 21325520, 21327009, J1210040, 21177036), the Foundation for Innovative Research Groups of NSFC (Grant 21221003), National Instrumentation Program (2011YQ030124), and Hunan Provincial Natural Science Foundation (Grant 2013WK3015, 11JJ1002).
Notes and references
- 1.Liu J, Cao Z, Lu Y. Chem. Rev. 2009;109:1948–1998. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X-B, Kong R-M, Lu Y. Annu. Rev. Anal. Chem. 2011;4:105–128. doi: 10.1146/annurev.anchem.111808.073617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong L, Zhao Z, Lv Y-F, Huan S-Y, Fu T, Zhang X-B, Shen G-L, Yu R-Q. Chem. Commun. 2015;51:979–995. doi: 10.1039/c4cc06855f. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Lu Y. J. Am. Chem. Soc. 2000;122:10466–10467. [Google Scholar]
- 5.Liu J, Lu Y. J. Am. Chem. Soc. 2007;129:9838–9839. doi: 10.1021/ja0717358. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2056–2061. doi: 10.1073/pnas.0607875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X-H, Kong R-M, Zhang X-B, Meng H-M, Liu W-N, Tan W, Shen G-L, Yu R-Q. Anal. Chem. 2011;83:5062–5066. doi: 10.1021/ac200843x. [DOI] [PubMed] [Google Scholar]
- 8.Breaker RR, Joyce GF. Chem. Biol. 1995;2:655–660. doi: 10.1016/1074-5521(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 9.Kong R-M, Zhang X-B, Chen Z, Meng H-M, Song Z-L, Tan W, Shen G-L, Yu R-Q. Anal. Chem. 2011;83:7603–7607. doi: 10.1021/ac2018926. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Wang Z, Xing H, Xiang Y, Lu Y. Anal. Chem. 2010;82:5005–5011. doi: 10.1021/ac1009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L-M, Zhang X-B, Kong R-M, Yang B, Tan W. J. Am. Chem. Soc. 2011;133:11686–11691. doi: 10.1021/ja203693b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X-H, Gong L, Zhang X-B, Yang B, Fu T, Hu R, Tan W, Yu R. Anal. Chem. 2013;85:3614–3620. doi: 10.1021/ac303457u. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, He Y, Yang X, Wang K, Quan K, Lin X. Analyst. 2014;139:2994–2997. doi: 10.1039/c4an00454j. [DOI] [PubMed] [Google Scholar]
- 14.Kong R-M, Fu T, Sun N-N, Qu F-L, Zhang S-F, Zhang X-B. Biosens. Bioelectron. 2013;50:351–355. doi: 10.1016/j.bios.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet G, Tyagi S, Libchaber A, Kramer FR. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6171–6176. doi: 10.1073/pnas.96.11.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CJ, Lin H, Tan W. J. Am. Chem. Soc. 2005;127:12772–12773. doi: 10.1021/ja053482t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petty JT, Zheng J, Hud NV, Dickson RM. J. Am. Chem. Soc. 2004;126:5207–5212. doi: 10.1021/ja031931o. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Pu F, Lin Y, Ren J, Qu X. Chem. Commun. 2011;47:3487–3489. doi: 10.1039/c0cc05651k. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Yuan J, Wang E. Chem. Commun. 2009:3395–3397. doi: 10.1039/b821518a. [DOI] [PubMed] [Google Scholar]
- 20.Lan G-Y, Huang C-C, Chang H-T. Chem. Commun. 2010;46:1257–1259. doi: 10.1039/b920783j. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Yuan J, Dong Q, Wang E. J. Am. Chem. Soc. 2009;132:932–934. doi: 10.1021/ja907075s. [DOI] [PubMed] [Google Scholar]
- 22.Yeh H-C, Sharma J, Han JJ, Martinez JS, Werner JH. Nano Lett. 2010;10:3106–3110. doi: 10.1021/nl101773c. [DOI] [PubMed] [Google Scholar]
- 23.Fu T, Zhao X-H, Bai H-R, Zhao Z-L, Hu R, Kong R-M, Zhang X-B, Tan W, Yu R-Q. Chem. Commun. 2013;49:6644–6646. doi: 10.1039/c3cc43054e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.