Abstract
A large proportion of individuals with schizophrenia show an inadequate response to treatment with antipsychotics. It can be unclear whether this is secondary to subtherapeutic antipsychotic plasma levels or to medication ineffectiveness. The purpose of the present study was to determine the extent of subtherapeutic antipsychotic plasma levels in a group of patients clinically identified as treatment-resistant. In addition we investigated the frequency of antipsychotic plasma level monitoring in standard clinical practice.
Antipsychotic plasma levels were measured in 36 patients identified as having treatment-resistant schizophrenia by their treating clinicians. 16 (44%) patients showed either undetectable (19%) or subtherapeutic levels (25%), and 20 (56%) had levels in the therapeutic range. Subtherapeutic plasma levels were significantly associated with black ethnicity, shorter duration of current treatment, and antipsychotics other than olanzapine and amisulpride. Antipsychotic plasma levels had been measured in only one patient in the year prior to our study.
We found over one-third of patients identified as treatment-resistant have subtherapeutic antipsychotic levels. This indicates that they may be under-treated rather than treatment-resistant, and thus should receive different management. Currently the measurement of antipsychotic levels may be under-utilised.
Keywords: schizophrenia, treatment resistant, therapeutic drug monitoring, antipsychotics, adherence
Introduction
About one third of patients with schizophrenia are treatment resistant (Mortimer et al., 2010). Definitions of treatment resistance in schizophrenia vary in the stringency of their criteria (Suzuki et al., 2012). A universally necessary condition is that patients should have not responded satisfactorily to adequate treatment. Adequate treatment typically refers to taking antipsychotic medication at a therapeutic dose for a sufficient duration (Taylor et al., 2012; Suzuki et al., 2012; National Institute for Health and Care Excellence, 2014). The rationale for this specification is to ensure that patients receive sufficient exposure to an antipsychotic prior to it being deemed ineffective. This inference is unlikely to be valid, however, if the antipsychotic plasma level is sub-therapeutic, be that secondary to poor-adherence or to pharmacokinetic factors.
Multiple factors can contribute to sub-therapeutic drug plasma levels. These include the rapid metabolism of antipsychotics due to genetic factors (Nikisch et al., 2011) or the use of substances that induce metabolic enzymes, such as smoking in the case of olanzapine (Patel et al., 2011). Poor absorption of the drug from the gastrointestinal tract could also play a role (Gandelman et al., 2009). Adherence also has a major impact, and is recognised as a problematic issue throughout medicine (Stephenson et al., 1993). Within psychiatry, adherence can become especially relevant due both to the high side effect burden associated with many psychotropic medications (Lambert et al., 2004), and also as a result of the lack of insight that frequently accompanies psychotic disorders (Bartkó et al., 1988). The concept of adherence occupies a spectrum and there is no universal definition (Velligan et al., 2006). In both research and clinical practice, patient self-report and clinician opinion are the most common methods used for identifying non-adherence. These, however, are often unreliable when compared to more objective measures such as medication blood plasma levels, pill counts or direct observations (Velligan et al., 2007).
The monitoring of antipsychotic plasma levels is recommended when adherence is in doubt or response is inadequate (Velligan et al., 2009; Taylor et al., 2012). However, apart from clozapine, this is rarely performed in clinical practice (Conca et al., 2011). Current guidelines (Gardner et al., 2010; Taylor et al., 2012; National Institute for Health and Care Excellence, 2014) do not state whether plasma level monitoring should be used in assessing treatment resistance, but local guidelines recommend their use (Beck et al., 2014). Notwithstanding this, accurately determining plasma levels in this population is of major importance as a diagnosis of treatment resistance implies the patient should be offered clozapine whereas subtherapeutic antipsychotic plasma levels will be managed quite differently (National Institute for Health and Care Excellence, 2014). However, it is not known how common subtherapeutic plasma levels of antipsychotic treatment are in patients who are identified as treatment-resistant. Our primary aim was therefore to determine the extent of subtherapeutic antipsychotic levels in a group of patients who had been designated as treatment-resistant by their treating community mental health team.
Secondary aims were to examine how frequently plasma level monitoring is used in clinical practice when evaluating treatment resistance and to identify potential risk factors associated with subtherapeutic plasma levels in this patient population. We also investigated the relationship between antipsychotic plasma levels and inpatient admission rates.
Materials and Methods
Participants
Potential participants were all patients referred to an outpatient service for the assessment and management of treatment-resistant schizophrenia between January 2012 and September 2013. Referrals are accepted from all the community mental health teams in Lambeth and Southwark in South East London (total population 591,400) (Office of National Statistics, 2011). The most common reason for referral is for consideration of clozapine initiation. As such referring clinicians were of the opinion that the patient had had an adequate trial of at least two antipsychotics. To be included in the study patients needed to be referred for a treatment-resistant psychotic disorder, to be taking an oral antipsychotic other than clozapine, and to consent to give a blood sample for antipsychotic plasma level testing. Patients taking clozapine were excluded from the study. The South London & Maudsley audit committee approved the study.
Measurements
36 patients met inclusion criteria and were included in the study. As part of their assessment the Positive and Negative Syndrome Scale (PANSS) was completed and treatment history was recorded. The dose of each drug was converted into chlorpromazine equivalents (using the method specified in Gardner et al.(2010)). Further details including ethnicity, diagnosis, current medication dose and length of current treatment were obtained during the assessment and from patient notes. In addition patient notes were later reviewed to see if there had been any inpatient admissions during the period following the initial assessment up until February 2014. In order to determine the extent of plasma level monitoring in standard clinical practice, patient notes and laboratory records were reviewed to see if antipsychotic plasma levels had been measured by the patient’s referring team in the year prior to the assessment.
Blood samples were taken at the time of initial assessment (ethylenediaminetetraacetic acid–anticoagulated whole blood). The samples were analysed using liquid chromatography-tandem mass spectrometry at the local hospital laboratory (Fisher et al., 2013). Lower limits of quantification were as follows: aripiprazole and dehydroaripiprazole 5 μg/L; amisulpride, and quetiapine 2 μg/ L; olanzapine, risperidone, and 9-hydroxyrisperidone, 1 μg/L. Coefficients of variation were 8% or less across the assay ranges.
If a participant was on multiple antipsychotics only the medication taken at the highest dose (expressed as chlorpromazine equivalents) was included in the analysis. To be classified as therapeutic an individual’s plasma level had to be above a minimum target concentration as specified in the Maudsley Prescribing Guidelines (Taylor et al., 2012). For amisulpride this was 200μg/l, for aripiprazole 150 μg/l, for olanzapine 20 μg/l, for quetiapine 100μg/l, and for risperidone 20μg/l (total risperidone and 9-hydroxyrisperidone) (Taylor et al., 2012). Levels were classified as subtherapeutic if the level was measurable but below the minimum, and as undetectable if no antipsychotic was detected.
Statistical analysis
Analyses were carried out using commercial software (IBM SPSS Statistics for Macintosh, Version 21.0. Armonk, NY: IBM Corp). Normally distributed continuous variables were compared using the independent samples t-test. Mann–Whitney U tests were used to compare groups on any continuous variable with a significant deviation from normality at p<0.05 as measured using the Shapiro–Wilks test. Categorical variables were compared Fisher’s exact test. Survival curves were estimated using the Kaplan-Meier procedure. The hazard ratio was calculated using Cox’s regression model and the significance of differences between the two groups was measured by the Mantel–Cox log-rank test. All tests were two tailed with p<0.05 identified as significant.
Results
Demographics
Demographic details of the 36 participants for whom plasma levels were obtained are shown in Table 1. PANSS scores were not available for 2 of the participants with subtherapeutic levels and for 7 of the therapeutic level group.
Table 1. Demographic and Clinical Data.
| N | 36 | |
| Age in years (S.D.) | 40.6 (11.1) | |
| Male gender, n,(%) | 28 (78) | |
| Ethnicity, n(%) | Black | 19 (53) |
| White British | 11 (31) | |
| Other | 6 (17) | |
| Diagnosis, n(%) | Schizophrenia | 27 (75) |
| Schizoaffective | 7(19) | |
| Other | 2(6) | |
| Antipsychotic, n(%) | Olanzapine | 19 (53) |
| Amisulpride | 6 (17) | |
| Aripiprazole | 5 (14) | |
| Quetiapine | 2(6) | |
| Risperidone | 3 (8) | |
| Haloperidol | 1 (3) | |
| Average dose, CPZ equiv12 mg/day (S.D.) | 586 (251) | |
| Length of treatment with current antipsychotic in months (SD) | 57 (49) | |
| PANSS, mean (S.D.) | Positive | 21.7 (9.9) |
| Negative | 16.7 (6.9) | |
| General | 39.1 (13.8) | |
| Total | 77.1 (25.2) | |
Antipsychotic plasma levels
Of the 36 participants included in the study 20 (56%) were classified as having a therapeutic plasma level, 16 (44%) were classified as subtherapeutic, and of these 7 had no antipsychotic detectable in their plasma sample. The plasma levels classified as therapeutic had a mean (S.D.) concentration of 354 (232)% of the minimum target concentration, whereas those classified as subtherapeutic had a mean of 37 (25)% (excluding those with an undetectable level).
3 participants were prescribed multiple antipsychotics. One was prescribed aripiprazole 30mg and olanzapine 10mg, another amisulpride 900mg and olanzapine 20mg, and another was receiving a pipotiazine depot and also prescribed olanzapine 20mg. Of these, three had levels above the minimum target concentration while one was below.
Only one participant had had antipsychotic plasma levels measured by their treating clinical team in the year prior to their assessment.
The relationship between plasma levels and various clinical and demographic factors are shown in Table 2. Individuals with subtherapeutic plasma levels were significantly more likely to be of black ethnicity, to be taking an antipsychotic other than olanzapine or amisulpride and to have been treated with their current antipsychotic for a shorter duration.
Table 2. Relationship Between Plasma levels and Demographic/ Clinical Factors.
| Therapeutic Level | Subtherapeutic/ Undetectable | p | ||
| N, % | 20 (56) | 16 (44) | ||
| Age in years (S.D.) | 43 .1 (11.0) | 37.6 (10.8) | 0.15a | |
| Male gender, n(%) | 16 (80) | 12 (75) | 0.51b | |
| Ethnicity, n | White British/other | 12 | 4 | 0.02b |
| Black | 7 | 13 | ||
| Diagnosis, n | Schizophrenia | 15 | 12 | 0.29b |
| Schizoaffective | 5 | 2 | ||
| Other | 0 | 2 | ||
| Antipsychotic, n | Olanzapine or Amisulpride | 17 | 8 | 0.03b |
| Other | 3 | 8 | ||
| Length of treatment with current antipsychotic in months (S.D.) | 79 (48) | 30 (32) | 0.001c | |
| Average dose, CPZ equiv12 mg/day (S.D.) | 664 (294) | 487 (138) | 0.06a | |
| Average level, %min range(S.D.) | 354 (232) | 37 (25)* | ||
| PANSS, mean (SD) | Positive | 20.2.4 (9.9) | 23.1 (10.0) | 0.38c |
| Negative | 17.4 (7.1) | 16.1 (6.9) | 0.63a | |
| General | 36.2 (12.2) | 41.7 (15.0) | 0.24c | |
| Total | 73.8 (22.8) | 80.3 (27.8) | 0.58c | |
| Admission, n (%) | 1 (5) | 3 (19) | 0.19d | |
| Antipsychotic plasma levels measured in the previous year | 0 | 1 | ||
Independent samples t-test
Fisher’s Exact test
Mann-Whitney
Mantel-Cox log rank
undetectable values not included in calculation
Relapse
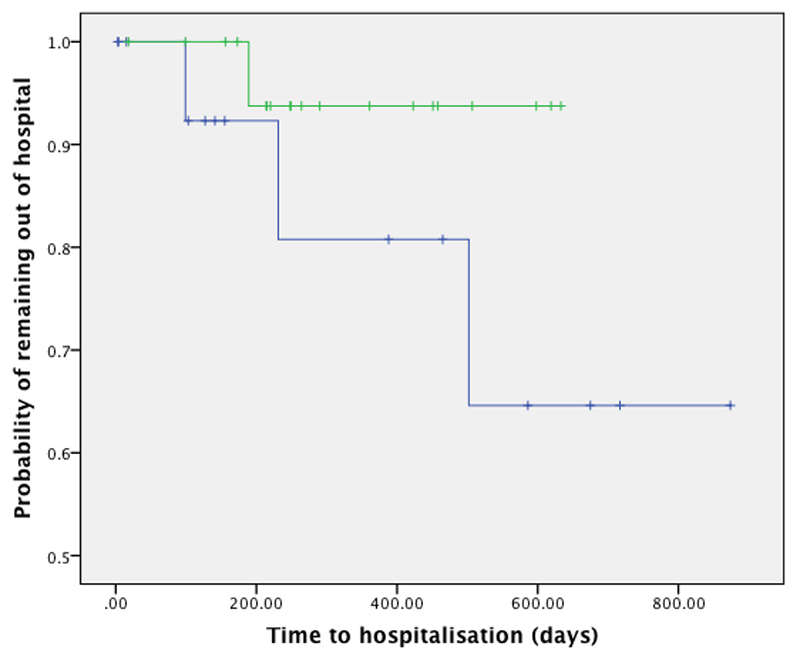
Hospitalisation rates in the period following assessment were lowest in the group with therapeutic plasma levels (5% of patients were hospitalised) compared to the subtherapeutic group (19%), though this did not reach statistical significance (P=0.18, log rank test, hazard ratio 4.22, 95% CI 0.43-41.4). Survival curves are shown in Figure 1.
Figure 1. Time to hospitalisation for patients with therapeutic plasma levels (green line) and those with levels below the minimum therapeutic range (blue line).
Discussion
Our main finding is that 44% of patients on oral antipsychotics who were identified by their treating team as having treatment-resistant schizophrenia were found to have subtherapeutic antipsychotic plasma levels. To our knowledge this is the first study to have examined antipsychotic plasma levels in this population.
The proportion of patients with undetectable antipsychotic plasma levels was 19%. For comparison purposes, out of 2929 routine clinical samples sent for antipsychotic plasma level measurement for a range of clinical indications, at the same drug monitoring service, only 6 % had undetectable levels (Fisher et al., 2013).
Black participants were significantly (p=0.02) more likely to show subtherapeutic plasma levels, with only 35% of black individuals having adequate levels compared to 75% of individuals from non-black ethnic backgrounds. This result is in accordance with previous research suggesting that black patients are more likely to be non-adherent (Valenstein et al., 2004). Other factors may also contribute, for example there is some evidence that black ethnicity is associated with greater olanzapine clearance than other ethnic backgrounds (Bigos et al., 2008).
A significantly higher proportion of participants taking olanzapine and amisulpride had therapeutic plasma levels (68% therapeutic) compared to other medications (27% therapeutic). Although there is the possibility this finding could have been amplified by a lack of correspondence between various therapeutic ranges; this is in agreement with evidence suggesting these drugs are the most effective and least discontinued of non-clozapine antipsychotics (Leucht et al., 2013). Participants with subtherapeutic plasma levels had on average been taking their current antipsychotic for a significantly (P=0.001) shorter period (30 months) than those with adequate levels (79 months).
The association between poor adherence and increased rates of hospital admission is well established (Weiden et al., 2004; Law et al., 2008). Possibly due to the small sample size the rate of hospital admission was not significantly different between individuals with adequate plasma levels and those with subtherapeutic levels.
The fact that only one participant had had plasma levels monitored in the previous year suggests this may be an underutilised resource is assessing adherence, particularly when determining whether a patient is treatment-resistant. These findings have relevance for current guidelines (Taylor et al., 2012; National Institute for Health and Care Excellence, 2014) on the assessment and management of treatment resistance, which at present do not explicitly refer to measuring antipsychotic plasma levels.
Strengths of the current study
While previous research has suggested that non-adherence with medication is a risk factor for later development of treatment resistance (Huber et al., 2008) this is the first study to specifically examine anti-psychotic plasma levels in a group of patients believed to be treatment-resistant. The broad inclusion criteria mean the study sample is representative of a general clinical population. Although a treatment responsive control group is not included, the findings still have relevance for clinical practice as they show a proportion of patients considered to be treatment resistant may have not had sufficient exposure to antipsychotics for this conclusion to be drawn.
Limitations of the current study
Antipsychotic plasma levels can be seen as a gold standard in terms of objectivity. In isolation, however, they are unable to indicate the reasons for a low level. A subtherapeutic plasma level could be due to an individual’s rapid metabolism, reduced drug absorption or poor adherence.
In addition levels are dependent on the length of time since the last dose, and samples were not taken at a set time. When viewed as a marker of adherence, plasma levels only reflect the patient’s most recent behaviour and so can both over or underestimate the general pattern of adherence. For the majority of antipsychotics, reference ranges refer to trough samples so our results may in fact overestimate the number of patients with a therapeutic level. The one exception in the study is olanzapine where a 20-40 μg/l range is for a 12h post dose sample (Perry, 2001), and so the classification of some levels as subtherapeutic could be related to timings of samples if they were taken late in the day. This is unlikely to have had a major impact though seeing as patients taking olanzapine were some of the most likely to have a therapeutic level. Furthermore, the long plasma half-life of olanzapine means sample timing is less likely to have a significant effect on the measured plasma concentration (Kassahun et al., 1997).
While one can conclude that a patient with undetectable plasma levels has been non-adherent for a period, the dividing line between full and partial adherence is more arbitrary, and the reference ranges for different antipsychotics are not absolutely equivalent. That being said the average level was markedly higher in the therapeutic level group. In addition, measuring trough levels could have allowed conclusions to be drawn about what proportion of patients may be exposed to overly high plasma levels of anti-psychotics.
As only 36 participants were included in the study the power to detect statistically significant risk factors for non-adherence was limited. While a number of statistically significant findings were observed, only the finding relating to differences in length of current treatment would survive a Bonferroni correction for multiple comparisons. Future studies should include a control group of treatment-responsive patients to enable comparisons as to the prevalence of subtherapeutic levels in each group.
When should plasma levels be monitored?
Guidelines for therapeutic drug monitoring encourage its use in cases of suspected non-adherence or a lack of treatment response (Hiemke et al., 2011). This recommendation, however, is not present in guidelines for the assessment of treatment resistance. With the exception of clozapine, testing of antipsychotic plasma levels is not part of routine clinical practice. This was the case in our study with only one participant having had antipsychotic plasma levels monitored in the previous year. The rationale for this is often that it is better practice to titrate medication dose against clinical response than a plasma level. This is an appropriate strategy when clinical response is satisfactory.
However if response is unsatisfactory, assuming medication ineffectiveness without further inquiry may be premature. Partial or non-adherence occurs in close to half of patients (Lacro et al., 2002), and patient self-report or clinician opinion tends to markedly overestimate adherence compared to more objective measures (Velligan et al., 2007; Velligan et al., 2009). Accurately determining adherence, or investigating pharmacokinetic factors, may be of lesser importance if a patient remains relatively well. However, where a patient is considered treatment-resistant this is clearly not the case and establishing that the patient has received adequate exposure to an antipsychotic is critical to their on-going management, at least until a diagnostic test for resistant schizophrenia is developed (Demjaha et al., 2012). While the relationship between plasma level and clinical response is not well defined for all antipsychotics (Mauri et al., 2007), a trial of an antipsychotic cannot be considered adequate if the plasma level is well below the suggested minimum level.
Monitoring may not be suitable in all situations. Cost and lack of availability may be significant barriers. Whether these obstacles are insurmountable will likely depend on the location and funding of the clinical service. At the South London and Maudsley NHS foundation trust, plasma level monitoring is available for about £35 per test at the Toxicology Unit, Department of Clinical Biochemistry, King’s College Hospital, London, SE5 9RS. This cost needs to be weighed against the potential cost saving of identifying medication that is prescribed but not taken as well as the potential clinical benefit. Given that costs for new antipsychotics are £150-250/month (Taylor et al., 2012), potential cost savings may be large. Lack of access to plasma level monitoring may also be a problem for patients taking typical antipsychotics, as the availability of assays for these drugs is limited (Flanagan, 2006).
Monitoring can have disadvantages. Plasma levels provide only a snapshot in time, and in chronic psychiatric conditions adherence tends to decline with time (Kane, 1985; Razali & Yahya, 1995). In addition there are common risk factors for non-adherence (Higashi et al., 2013) and treatment resistance (Huber et al., 2008), and medication ineffectiveness is one of the primary reasons for non-adherence (Adams & Howe, 1993; Velligan et al., 2009). As such, a patient who has a subtherapeutic level may well be treatment-resistant. There are routinely significant delays in patients receiving treatment with clozapine, with underuse more of a concern than overuse (Howes et al., 2012). Furthermore, there is evidence that clozapine may improve adherence (Valenstein et al., 2004). It could be argued that the use of plasma level monitoring risks further prolonging ineffective treatments. However if it is felt that further time is needed to address the cause of a subtherapeutic level the additional delay in starting clozapine will be insignificant compared to the average delay of 4 years seen in clinical practice (Howes et al., 2012). There is an argument for plasma levels to be used early in treatment when poor response is first apparent – thereby potentially clarifying adherence, pharmacokinetic factors and accelerating the route to clozapine.
Source of Funding
This study was funded by a Medical Research Council (UK) grant to Dr Howes (grant number: MC-A656-5QD30), the National Institute of Health Research Biomedical Research Council grant to King’s College London and a grant from the Maudsley Charity (Grant number 666). Dr Reis Marques’ research is supported by NARSAD.
Footnotes
Conflicts of Interest
Dr Howes has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organised by Astra-Zeneca, Eli Lilly, Jansenn, Lundbeck, Lyden-Delta, Servier, and Roche. Neither Dr Howes or his family have been employed by or have holdings/ a financial stake in any biomedical company. Drs Beck, Bloomfield, McCutcheon, Marques and Rogdaki report no conflicts of interest.
References
- Adams SG, Howe JT. Prediciting medication compliance in a psychotic population. J Nerv Ment Dis. 1993;181:558–560. doi: 10.1097/00005053-199309000-00005. [DOI] [PubMed] [Google Scholar]
- Bartkó G, Herczeg I, Zádor G. Clinical symptomatology and drug compliance in schizophrenic patients. Acta Psychiatr Scand. 1988;77:74–6. doi: 10.1111/j.1600-0447.1988.tb05080.x. [DOI] [PubMed] [Google Scholar]
- Beck K, McCutcheon R, Bloomfield Ma, et al. The practical management of refractory schizophrenia–the Maudsley Treatment REview and Assessment Team service approach. Acta Psychiatr Scand. 2014:1–12. doi: 10.1111/acps.12327. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Coley KC, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48:157–65. doi: 10.1177/0091270007310385. [DOI] [PubMed] [Google Scholar]
- Conca a, Schmidt E, Pastore M, et al. Therapeutic drug monitoring in Italian psychiatry. Pharmacopsychiatry. 2011;44:259–62. doi: 10.1055/s-0031-1286281. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Murray RM, McGuire PK, et al. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- Fisher D, Partridge S, Handley SA, et al. LC–MS/MS of some atypical antipsychotics in human plasma, serum, oral fluid and haemolysed whole blood. Forensic Sci Int. 2013;229:145–150. doi: 10.1016/j.forsciint.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Flanagan R. Therapeutic Monitoring of Antipsychotic Drugs. CPD Clin Biochem. 2006;7:3–18. [Google Scholar]
- Gandelman K, Alderman J, Glue P, et al. The impact of calories and fat content of meals on oral ziprasidone absorption: a randomized, open-label, crossover trial. J Clin. 2009;70:59–63. doi: 10.4088/jcp.08m04104. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44:195–235. doi: 10.1055/s-0031-1286287. [DOI] [PubMed] [Google Scholar]
- Higashi K, Medic G, Littlewood KJ, et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3:200–18. doi: 10.1177/2045125312474019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201:481–5. doi: 10.1192/bjp.bp.111.105833. [DOI] [PubMed] [Google Scholar]
- Huber C, Naber D, Lambert M. Incomplete remission and treatment resistance in first-episode psychosis: definition, prevalence and predictors. Expert Opin Pharmacother. 2008;9:2027–2038. doi: 10.1517/14656566.9.12.2027. [DOI] [PubMed] [Google Scholar]
- Kane J. Compliance issues in outpatient treatment. J Clin Psychopharmacol. 1985;5:22–27. doi: 10.1097/00004714-198506001-00005. [DOI] [PubMed] [Google Scholar]
- Kassahun K, Mattiuz E, Nyhart E, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab. 1997;25 [PubMed] [Google Scholar]
- Lacro J, Dunn L, Dolder C, et al. Prevalence of and Risk Factors for Medication Nonadherence in Patients With Schizophrenia: A Comprehensive Review of Recent Literature. J Clin Psychiatry. 2002;63:893–910. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- Lambert M, Conus P, Eide P, et al. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry. 2004;19:415–22. doi: 10.1016/j.eurpsy.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69:47–53. doi: 10.4088/jcp.v69n0107. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;6736:1–12. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- Mauri MC, Volonteri LS, Colasanti A, et al. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46:359–88. doi: 10.2165/00003088-200746050-00001. [DOI] [PubMed] [Google Scholar]
- Mortimer A, Singh P, Shepherd C, Puthiryackal J. Clozapine for Treatment-Resistant Schizophrenia: National Institute of Clinical Excellence (NICE) Guidance in the Real World. Clin Schizophr Relat Psychoses. 2010;4:49–55. [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Psychosis and schizophrenia: treatment and management. Clinical guideline 178. 2014 [PubMed] [Google Scholar]
- Nikisch G, Baumann P, Oneda B, et al. Cytochrome P450 and ABCB1 genetics: association with quetiapine and norquetiapine plasma and cerebrospinal fluid concentrations and with clinical response in patients suffering from schizophrenia. A pilot study. J Psychopharmacol. 2011;25:896–907. doi: 10.1177/0269881110389208. [DOI] [PubMed] [Google Scholar]
- Office of National Statistics. Digitised Boundary Data (England and Wales) UK Data Service Census Support; 2011. [Google Scholar]
- Patel MX, Bowskill S, Couchman L, et al. Plasma olanzapine in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1999-2009. J Clin Psychopharmacol. 2011;31:411–7. doi: 10.1097/JCP.0b013e318221b408. [DOI] [PubMed] [Google Scholar]
- Perry PJ. Therapeutic drug monitoring of antipsychotics. Psychopharmacol Bull. 2001;35:19. [PubMed] [Google Scholar]
- Razali M, Yahya H. Compliance with treatment in schizophrenia: a drug intervention program in a developing country. Acta Psychiatr Scand. 1995:331–335. doi: 10.1111/j.1600-0447.1995.tb09790.x. [DOI] [PubMed] [Google Scholar]
- Stephenson BJ, Rowe BH, Haynes RB, et al. Is this patient taking the treatment as prescribed? JAMA. 1993;269:2779–2781. [PubMed] [Google Scholar]
- Suzuki T, Remington G, Mulsant BH, et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 2012;197:1–6. doi: 10.1016/j.psychres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. John Wiley & Sons; 2012. [Google Scholar]
- Valenstein M, Blow FC, Copeland LA, et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull. 2004;30:255–264. doi: 10.1093/oxfordjournals.schbul.a007076. [DOI] [PubMed] [Google Scholar]
- Velligan D, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58:1187–1192. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- Velligan D, Weiden P, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70:1–46. [PubMed] [Google Scholar]
- Velligan DI, Lam Y-WF, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32:724–42. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55:886–91. doi: 10.1176/appi.ps.55.8.886. [DOI] [PubMed] [Google Scholar]