Abstract
Microtubules (MTs) are key cellular components, long known to participate in morphogenetic events that shape the developing embryo. However, the links between the cellular functions of MTs, their effects on cell shape and polarity, and their role in large‐scale morphogenesis remain poorly understood. Here, these relationships were examined with respect to two strategies for generating the vertebrate neural tube: bending and closure of the mammalian neural plate; and cavitation of the teleost neural rod. The latter process has been compared with ‘secondary’ neurulation that generates the caudal spinal cord in mammals. MTs align along the apico‐basal axis of the mammalian neuroepithelium early in neural tube closure, participating functionally in interkinetic nuclear migration, which indirectly impacts on cell shape. Whether MTs play other functional roles in mammalian neurulation remains unclear. In the zebrafish, MTs are important for defining the neural rod midline prior to its cavitation, both by localizing apical proteins at the tissue midline and by orienting cell division through a mirror‐symmetric MT apparatus that helps to further define the medial localization of apical polarity proteins. Par proteins have been implicated in centrosome positioning in neuroepithelia as well as in the control of polarized morphogenetic movements in the neural rod. Understanding of MT functions during early nervous system development has so far been limited, partly by techniques that fail to distinguish ‘cause’ from ‘effect’. Future developments will likely rely on novel ways to selectively impair MT function in order to investigate the roles they play.
Keywords: Microtubules; neural tube; neurulation; morphogenesis; mouse, chick; amphibia, zebrafish, cell polarity
Introduction
The microtubule (MT) cytoskeleton plays a key role in many aspects of cellular function, including mitosis, centrosome dynamics and cargo transport within cells (Hancock, 2014; Venghateri et al. 2015; Yount et al. 2015). However, it has long been known that MTs also participate in more large‐scale morphogenetic events that shape the developing embryo. A model for understanding this relationship is the well‐studied process of neurulation, by which the early CNS takes on a hollow, tubular form during vertebrate embryogenesis. The role of MTs in the specification of cell polarity appears vital to this morphogenetic function, for example in enabling lumen formation during neural tube cavitation. Here, the experimental evidence underpinning the main cellular functions of MTs in relation to two different strategies for generating a luminal CNS primordium was reviewed: closure of the mammalian neural tube; and cavitation of the teleost neural rod. In both cases, MTs play a key role in the generation of a fully‐formed neural tube, with research in the mammalian and teleost systems highlighting different facets of this crucial MT function.
Strategies of neurulation
Neural tube closure, or primary neurulation, is a fundamental event of early neural development that generates the anlage of the CNS in most vertebrate classes. The early nervous system arises in the dorsal midline of the embryo as the neural plate, a thickened pseudostratified epithelium that is a specialization of the ectodermal germ layer and overlies the notochord in the midline and paraxial mesoderm bilaterally. At the junction between the neural plate and the contiguous non‐neural (‘surface’) ectoderm, neural folds form bilaterally and proceed to elevate dorsally, creating the neural groove that runs along the rostro‐caudal axis of the embryo. Following apposition of the tips of the neural folds, epithelial fusion produces a closed neural tube, which comes to underlie the future epidermis on the dorsal surface of the embryo (Fig. 1a–d). This process is initiated de novo at distinct ‘closure points’, the number and location of which vary in different vertebrates. Closure progresses from these points in a ‘zipper‐like’ manner, in both rostro‐caudal and caudo‐rostral directions, depending on the species and level of the neuraxis (Karfunkel, 1974; Copp et al. 2003; Greene & Copp, 2014). Failure of neural tube closure results in open neural tube defects (NTDs), such as myelomeningocele and anencephaly, congenital malformations that in humans cause severe disability or are lethal (Copp et al. 2013). However, despite a long‐standing research interest in neural tube closure from developmental biologists and clinicians alike, a full mechanistic understanding of the process remains to be achieved.
Figure 1.
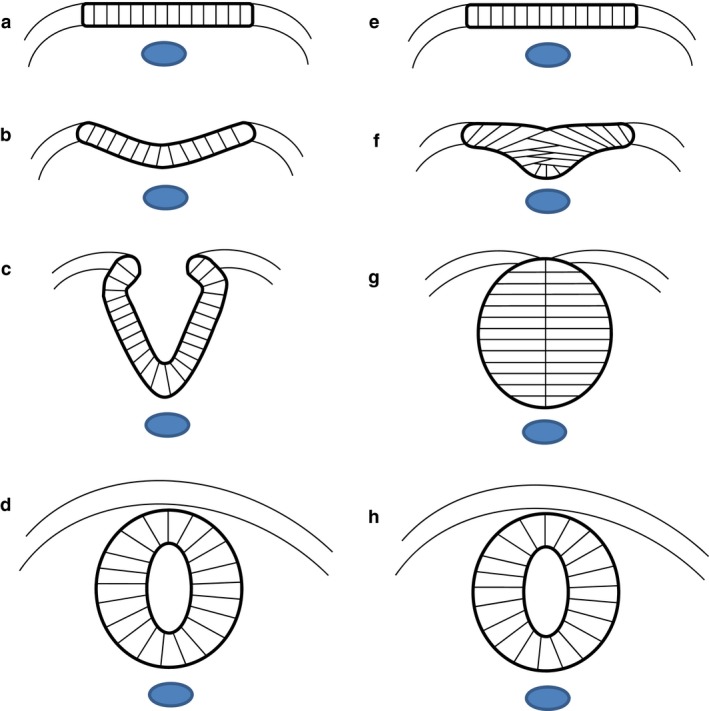
Two strategies of neurulation. (a–d) In most vertebrates, the flat neural plate (a) bends causing the neural folds to elevate and approach each other in the dorsal midline (b, c), where they fuse to form the closed neural tube (d). (e–h) In teleosts, such as the zebrafish, the cells of the neural plate coalesce to form the neural keel (e, f). This structure re‐organizes into the neural rod (g) before cavitating to form the neural tube (h). Images are schematic transverse sections. Blue: notochord.
A variant strategy for neural tube formation is seen in teleosts, such as the zebrafish. The cells of the teleost neural plate initially form a columnar epithelium, as in other vertebrates, then the cells converge and coalesce into a solid mass termed the neural keel, which subsequently develops into the neural rod, separate from the overlying epidermis. The final neural tube is generated by cavitation of the neural rod to produce a central lumen, termed the neurocoel (Papan & Campos‐Ortega, 1994; Fig. 1e–h).
A parallel is often drawn between teleost neural tube development and secondary neurulation, the process that generates the caudal‐most portion of the neural tube in birds and mammals. In the latter, a neural lumen forms by so‐called ‘canalization’ within a solid neural tube precursor, termed the ‘medullary cord’, that is located in the embryonic tail bud. This process is of clinical relevance, as its failure is thought to produce a range of skin‐covered ‘dysraphic’ conditions in human infants, which can cause significant morbidity resulting from terminal spinal cord tethering and dysfunction (Copp & Greene, 2013).
Both teleost neurulation and higher vertebrate secondary neurulation culminate in formation of a neural tube, without prior neural plate bending or closure. However, significant differences have been suggested between these two processes: for example, the secondary neural tube of chick and mouse embryos arises from a population of mesenchymal cells in the tail bud, whereas the substrate of zebrafish neural tube formation is epithelial (Lowery & Sive, 2004). Having said this, experimental studies now show that the caudal extremity of all post‐gastrulation vertebrate embryos, including fish, amphibia, birds and mammals, contains a population of bi‐potential neuro‐mesodermal progenitors, that generate both the neuroepithelium and mesoderm of the caudal trunk region (Tzouanacou et al. 2009; Wilson et al. 2009; Kimelman & Martin, 2012; Kondoh & Takemoto, 2012; Beck, 2015). Cells destined for a neural fate condense in the dorsal part of the tail‐bud and undergo a mesenchyme‐to‐epithelium transformation (MET), to form the trunk neural tube. In avian and mammalian secondary neurulation, this post‐MET phase involves establishment of epithelial cell polarity followed by lumen formation (Schoenwolf & Powers, 1987). It remains to be determined whether the molecular events recently identified for zebrafish neurulation, as reviewed below, will prove applicable also to secondary neurulation, but this certainly represents an attractive challenge for future research.
Hence, although mechanistically different methods of forming a neural tube exist among vertebrates, there are striking parallels between the phylogenetic groups. Commonalities exist both in the final neural tube structure that is achieved, in the origin of the trunk neural tube from a population of multipotential neuromesodermal progenitors, and in the cellular polarization that precedes neural tube morphogenesis. It is these cellular events, and their underlying molecular mechanisms, that form the subject of the remainder of this article.
MTs
Microtubules are dynamic, polarized intracellular filaments that are part of the cytoskeleton. They are formed by the heterodimerization and polymerization of the globular proteins α‐ and β‐tubulin, the ‘seed’ for which is generated by nucleation, a process that normally requires γ‐tubulin and occurs at a MT‐organizing centre such as the centrosome (Kollman et al. 2011). During polymerization, the ‘minus’ end of the MT is anchored to the MT‐organizing centre, whilst the ‘plus’ end grows with the addition of α‐/β‐tubulin dimers. MTs therefore have structural polarity, a feature integral to their cellular functions (Wade, 2009). By virtue of these processes, alongside their capacity to disassemble by depolymerization, populations of MTs can undergo rapid spatial re‐organization (Valiron et al. 2001).
MTs in neuroepithelial cell shape change
Microtubules have long been proposed to play a role in mediating cell shape change during neural tube closure. Half a century ago, Perry & Waddington (1966) found that the blastopore cells of gastrulating amphibia, which develop a high columnar wedge‐like shape, contain cytoplasmic MTs aligned parallel to their long axis, suggesting that MTs might be responsible for cellular apico‐basal elongation (Fig. 2a, b). They tentatively suggested that these principles might translate to the cells of the neural plate, which undergo similar morphological changes early in neurulation (Waddington & Perry, 1966). Various studies subsequently provided electron‐microscopic evidence for this view, describing the growth and so‐called ‘paraxial’ alignment of MTs in the elongating neuroepithelial cells of chick and amphibian embryos (Messier, 1969; Burnside, 1971). Disruption of MT integrity using depolymerizing agents such as vinblastine and colchicine abolished cell elongation in the closing primary and cavitating secondary neural tube (Schoenwolf & Powers, 1987), and caused neural tube closure to fail (Karfunkel, 1971, 1972). This role of MT function in cellular elongation has been confirmed in zebrafish neuroepithelia (Picone et al. 2010). The early studies also provided evidence that circumferential bands of contractile microfilaments, now known to be composed of actomyosin, serve to constrict the apical portion of the cell, producing a wedge‐like shape that could drive epithelial bending. This led to a model in which both MT‐mediated apico‐basal elongation and microfilament‐mediated apical constriction are required for the initiation and maintenance of neural fold elevation.
Figure 2.
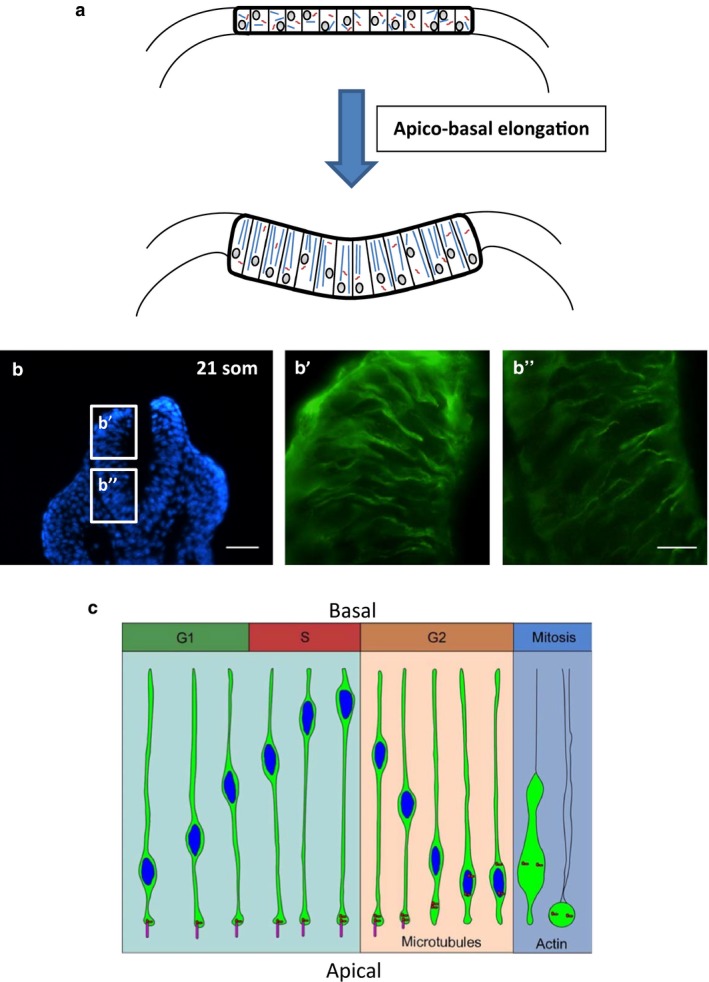
Role of MTs in apico‐basal elongation and INM, in the vertebrate neural plate. (a) Paraxial alignment of MTs causes epiblast cells to undergo apico‐basal elongation, producing the high columnar shape that characterizes cells of the early bending neural plate in birds, amphibia and mammals. Images are schematic transverse sections. (b) Immunohistochemistry (green) for α‐tubulin in transverse sections of the closing mouse spinal neural plate at the 21 somite stage. Apico‐basally aligned MTs are visible in both the dorsal (b') and ventral (b'') regions. Blue: DAPI‐stained nuclei. Scale bars: 80 μm (b); 20 μm (b', b''). (c) During INM, nuclei (blue) move basally during the G1‐phase and remain at the basal neuroepithelial surface during the S‐phase. During the S‐to‐G2 transition, dynein is activated and moves the nucleus toward the minus end of MTs at the centrosome (red), which is rooted in an apical cilium (pink). During the G2‐phase, the cilium disassembles, allowing the newly‐untethered centrosome to relocate to the nucleus, where it initiates mitosis. (c) Reproduced with permission from fig. 6 of Spear & Erickson, 2012, Developmental Biology 370: 33–41.
Role of Shroom3, the neogenin/repulsive guidance molecule (RGM)a system and the MID proteins
Our understanding of cytoskeletal regulation during neural tube morphogenesis has come a long way since those early studies. Work in Xenopus showed that paraxial MT alignment correlates temporally with a broad apical accumulation of the MT regulator γ‐tubulin (Lee et al. 2007). This γ‐tubulin redistribution was found to depend on the actin‐binding protein Shroom3, the knockdown of which caused paraxial MT arrays to be lost, and both apico‐basal elongation and apical constriction to fail. Shroom3 is also involved in the apical recruitment within the neuroepithelium of actin, myosin II (Hildebrand, 2005) and Rho‐kinase (Nishimura & Takeichi, 2008; Das et al. 2014). This is suggested to produce an apical molecular scaffold that drives tissue bending by inducing the wedge‐like shape of cells in the amphibian neural plate. Hence, Shroom3 may be a key regulator of cell shape change during epithelial morphogenesis, linking MT‐dependent apico‐basal elongation with microfilament‐dependent apical constriction.
Whilst it appears that Shroom3 can alter the distribution of cellular machinery and thereby drive polarized morphological changes, the initial establishment of cell polarity may not be Shroom3‐dependent. During Xenopus gastrulation, superficial cells are polarized whilst deeper blastomeres are not. Misexpression of Shroom3 mRNA was found to induce apical actin accumulation and apical constriction in superficial cells only (Haigo et al. 2003). Furthermore, whilst Shroom3 knockdown causes defective apico‐basal elongation and apical constriction in superficial cells of the neural plate, it does not impair the localization of apical markers ZO‐1 and Par3 (Lee et al. 2007). These findings suggest that a cell must become polarized, independently of Shroom3, before cell shape changes can be driven.
One candidate for this early establishment of apico‐basal polarity in the Xenopus neural plate is the axon guidance receptor neogenin and one of its ligands, RGMa. Expression analysis and knockdown studies have revealed that this ligand–receptor interaction is necessary for apico‐basal elongation of cells in the deep layer, regulating their polarity by organizing the MT network (Kee et al. 2008). Furthermore, knockdown of neogenin prevents radial intercalation of the deep cells with the superficial layer, resulting in a failure of apical constriction in the superficial cells and ultimately a failure of neural fold elevation. This suggests that superficial cell polarity is induced by radial intercalation, and hence Shroom3‐mediated apical constriction is dependent on the integrity of the deep cell MT network. RGMa‐neogenin could therefore represent the apico‐basal polarity regulator required for endogenous Shroom3 efficacy: a system by which cells must become epithelialized before they can play a role in tissue bending. This idea is consistent with findings that both Shroom3 and RGMa are required for neural tube closure in mice: cranial and spinal NTDs result from Shroom3 loss of function, while RGMa null embryos develop exencephaly (Hildebrand & Soriano, 1999; Niederkofler et al. 2004).
MID1 and MID2 are additional MT‐associated proteins implicated in embryonic cell shape change. MID1 gene mutations cause X‐linked Opitz G/BBB syndrome, which is characterized by congenital midline defects including cleft lip, hypospadias and agenesis of the corpus callosum (Quaderi et al. 1997). Depleting the orthologues of human MID1 and MID2 in Xenopus embryos destabilized paraxial MT arrays with a concomitant failure of cell elongation and apical constriction, ultimately resulting in defective closure (Suzuki et al. 2010). A further finding of this study was a reduction in apical actin accumulation in the neural plate of MID1/2‐depleted embryos, raising the question of whether MTs might play a role in apical constriction, which failed in the absence of functional MID1/2.
MTs in apical constriction
Apical constriction was first proposed as a mechanism of cell shape change well over a century ago (Rhumbler, 1902), and a recent focus has been on understanding its regulation and the role played by an apically localized actomyosin network. While many authors cite active actomyosin contraction as the driving force of apical constriction (Sawyer et al. 2010; Suzuki et al. 2012), leading to morphogenetic events such as neural tube closure, recent studies reveal that actomyosin contraction is not required for mouse spinal neurulation; rather, precisely regulated assembly–disassembly of apical actomyosin, downstream of Rho kinase/cofilin function, has proven essential for neural tube closure (Escuin et al. 2015). Evidence for a role of MTs in apical constriction comes from studies of Xenopus gastrulation, where intact MTs are required for efficient apical constriction of bottle cells, a requirement that appears independent of actin and phosphorylated myosin light chain localization, which were unaffected by pharmacological MT depolymerization (Lee & Harland, 2007). By contrast, in the morphogenetic furrow of the developing Drosophila eye, apical accumulation of these proteins is MT‐dependent, as is apical constriction (Corrigall et al. 2007). RhoGEF2, which induces Rho1‐mediated myosin‐II contractility, is distributed within Drosophila S2 cells by growing MTs. By interaction with the plus end‐tracking protein EB1, it is transported to the cell cortex where it plays a role in mediating cell shape change (Rogers et al. 2004).
Interkinetic nuclear migration (INM)
A further feature of neurulation that regulates cell shape, and has been long thought to depend on functional MTs, is INM. In the neuroepithelium, nuclei migrate apically in the G2‐phase of the cell cycle and basally in the G1‐phase such that cytokinesis occurs at the apical surface and S‐phase occurs basally (Sauer, 1935; Langman et al. 1966). MT disruption inhibits INM, producing a neural plate in which nuclei are distributed throughout the full thickness of the neuroepithelium (Messier, 1978). In the chick embryo, apical migration of G2 nuclei was found to be MT‐dependent, with blockade by the MT inhibitor colcemid (Fig. 2c; Spear & Erickson, 2012). In mouse brain slices, G2 nuclear movement was found to require Tpx2, a MT‐nucleating/bundling protein that dissociates from the nucleus and localizes in the apical portion of the neuroepithelial cell during apical nuclear movement. In contrast, migration of nuclei basally during G1 appears to be a largely passive process (Kosodo et al. 2011). However, studies of INM in the zebrafish retina have come to somewhat different conclusions: INM was found to depend largely on actomyosin‐generated forces, with only a minor role for MTs (Norden et al. 2009).
It is important to note that conventional MT‐depolymerizing agents, as used in many of the studies discussed above, adversely affect mitotic spindle dynamics (Jordan et al. 1992). Given the importance of a tightly‐controlled cell cycle in neural tube closure (Copp & Greene, 2010), the ability to draw conclusions about MT function in neurulation, independently of cell cycle effects, may be questioned. In future, alternative approaches to experimental disruption of paraxial MT arrays may prove beneficial: for example, depleting molecular determinants of paraxial MT alignment such as MID1/2 could provide a means of uncoupling the roles of the cell cycle in neural tube closure from other MT functions.
MTs in epithelial cell polarization
The events of neurulation in all vertebrates occur in highly specific planes within the neuroepithelium and require the co‐ordination of many individual cells. It follows that the spatial organization of subcellular machineries responsible for cell shape change must be tightly controlled, and MTs might be well‐positioned to enable this through intracellular transport. This idea is not new: following careful electron‐microscopic study of urodele neurulation over 40 years ago, Burnside (1971) proposed that the transport of cytoplasmic constituents along paraxially aligned MTs might explain apico‐basal cell elongation.
Lumen formation in cell cultures
A more recent example is in the generation of polarized surface domains in relation to epithelial lumen formation. In cells of the neural plate, as in all non‐stratified columnar epithelia undergoing morphogenesis, distinct apical and basal surface domains form, characterized by different patterns of protein expression. This occurs in tandem with paraxial MT alignment in polarizing epithelial cells, and in vitro evidence indicates that plus end‐ and minus end‐directed transport processes underlie the sorting of newly‐synthesized proteins, hence determining lumen position (Müsch, 2004). EMK1, which regulates MT stability by phosphorylation of MT‐associated proteins (Drewes et al. 1998; Müsch, 2004), is essential not only for paraxial MT alignment in polarizing Madin‐Darby canine kidney (MDCK) cells, but also for apico‐basal elongation of these cells and apical lumen positioning, as measured by localization of the luminal marker gp135 (Cohen et al. 2004). More recently it was found that kinesin‐2 is required for generation of a central lumen in vitro (Boehlke et al. 2013; Li et al. 2014). These findings highlight the requirement for MT organization in apical protein targeting, cell elongation and lumen positioning.
Cell polarization in neural tube closure
Despite this research focus on lumen formation in cultured cell lines, understanding of the corresponding process in neural tube morphogenesis remains limited. Kinesin heavy chain, kif5c, is expressed at the mRNA level in the chick neuroepithelium throughout neural tube closure (Dathe et al. 2004), consistent with a possible role for the plus end‐directed transport of basal surface proteins. Moreover, in the study of MID1/2 proteins, neuroepithelial MT organization was required for correct protein localization in the neural plate (Suzuki et al. 2010). Knockdown of MID1/2 function impaired the apical localization of several cell–cell adhesion molecules, as well as the localization of laminin to the basal lamina. This could suggest a role for both plus end‐ and minus end‐directed mechanisms in polarized protein distribution during neural tube closure, although whether MT‐mediated protein localization is functionally required for neural tube closure remains unclear.
In addition to considering the mechanisms by which MTs may promote cellular polarization in neural tube morphogenesis, it is also important to ask whether cell polarization is an all‐or‐none event, or alternatively a progressive, or step‐wise process. The latter concept is supported by the finding of two distinct phases of neuroepithelial polarization in zebrafish, frog and chick embryos (Yang et al. 2009). Early apico‐basal polarity was indicated by expression of the markers ZO‐1 and N‐cadherin, whereas a later phase of polarization was marked by the appearance of the Lin7c/Nok protein complex. In zebrafish, loss of either N‐cadherin or Lin7c disrupted neural tube formation, while precocious Lin7c overexpression induced multiaxial mirror symmetry. It was argued that complete epithelialization may be incompatible with the extent of morphological change required during neural tube morphogenesis. Vertebrate neuroepithelia may need to balance their level of polarization in order to retain sufficient plasticity to develop. Interestingly, however, the entire process of neural tube closure, including neural fold bending and midline fusion, was accomplished in amphibian and chick embryos during the ‘early’ (ZO‐1/N‐cadherin) phase, whereas Lin7c/Nok expression coincided with the opening of the neural tube lumen following closure (Yang et al. 2009). It will be interesting to determine how these progressive steps of neuroepithelial polarization relate to MT function during neurulation.
Oriented cell division and midline positioning in zebrafish neurulation
Because teleost neuroepithelial cells undergo a clear progressive epithelialization during neurulation (Hong et al. 2010), the zebrafish has proven a good model for studying mechanisms of cell polarization, and how they contribute to the generation and positioning of a central lumen in vivo. Recent evidence suggests multiple roles for MTs in these processes, which can be broadly attributed to their involvement in cell cycle mechanics and to their intracellular transport functions.
A cellular mechanism long considered to influence embryonic tissue morphogenesis in invertebrates and vertebrates alike is oriented cell division, by which cells undergo cytokinesis along a specific plane, depositing daughter cells in locations that affect the shape of the developing tissue (Ségalen & Bellaïche, 2009). In the neural plate of the zebrafish, cells divide preferentially along the long axis of the embryo; however, by neural keel stages, divisions are orthogonal to both rostro‐caudal and dorso‐ventral axes (Kimmel et al. 1994; Concha & Adams, 1998). Time‐lapse imaging has confirmed that a 90 ° rotation of the mitotic spindle is responsible for this change (Geldmacher‐Voss et al. 2003). In fact, this rotation of the MT spindle apparatus enables a mitotic division that deposits the two daughter cells to opposite sides of the midline; this midline‐crossing division (C‐division) is specific to neural keel/rod stages, at which it is widely regarded to exert powerful effects on the cellular organization of this developing structure (Tawk et al. 2007).
Partitioning‐defective‐3 (Par3, also referred to as Pard3), the vertebrate orthologue of a Caenorhabditis elegans polarity protein (Izumi et al. 1998), is localized symmetrically to the cleavage furrow of cells undergoing the C‐division (Tawk et al. 2007). As a result, the daughter cells appear to inherit mirror‐image polarity (i.e. medially positioned Par3) as they separate to opposite sides of the tissue midline. Remarkably, when ectopic C‐divisions occur lateral to the midline, in embryos with delayed neural plate convergence, mirror‐symmetric and apico‐basally polarized daughter cells form, and subsequently bilateral lumens are generated in a cell division‐dependent manner in these ectopic locations. This suggests roles for spindle rotation and the C‐division in orchestrating morphogenetic movements in the neural keel/rod, as well as in establishing apico‐basal polarity of the daughter cells with respect to the midline position of the future neurocoel (Clarke, 2009).
It is therefore at odds with this view that inhibition of cell division, both in embryos with impaired neural convergence and in other mutants with spindle orientation defects, rescues the neural rod midline, allowing cell polarization and subsequent lumen formation (Ciruna et al. 2006; Tawk et al. 2007; Quesada‐Hernández et al. 2010; Žigman et al. 2011). Considering that under these conditions the C‐division is inhibited, it can only be interpreted that other mechanisms are at play in lumen positioning. Indeed, recent evidence from zebrafish neurulation sheds light on a novel, MT‐dependent mechanism of cell polarization at the neural rod midline (Fig. 3) that occurs prior to and independently of the C‐division. Analysis of the MT plus end‐tracking protein EB3 reveals that in C‐division‐inhibited neuroepithelial cells, a mirror‐symmetric MT apparatus assembles and polymerizes from the point at which the cell intersects the midline, where the centrosome has become localized (Buckley et al. 2013; Compagnon & Heisenberg, 2013). Furthermore, pharmacological MT depolymerization reveals that apical localization of Par3 and another lumen‐organizing protein, Rab11a, is dependent on this MT system. Medial Par3 positioning during the C‐division itself, therefore, can be considered a product of this initial midline‐defining event, serving to maintain its effects throughout the cell movements that follow. These findings suggest that a minus end‐directed protein trafficking mechanism is at least partially responsible for apico‐basal polarization; demonstration of a functional requirement for dynein and associated molecules would thus prove useful in supporting this view. These observations have been recently corroborated by a study of Drosophila tracheal development in which Rab11 apical enrichment was found to depend on MT dynein motor transport (Le Droguen et al. 2015). Based on the above findings from zebrafish, alongside analysis of neurocoel morphology with and without inhibition of cell division, Buckley et al. (2013) proposed a revised interpretation of the C‐division: rather than generating the apical domain in its daughter cells, it is seen as orchestrating a series of cell movements that clears the neural rod midline of cells interdigitating across it. Because apical domain generation and therefore Par3 localization is at the point of intersection with the midline, cells are rendered able to orientate the cleavage plane of C‐division about this point, which ensures that their daughters do not bridge the midline. This may support more efficient cavitation of the neural rod thereafter.
Figure 3.
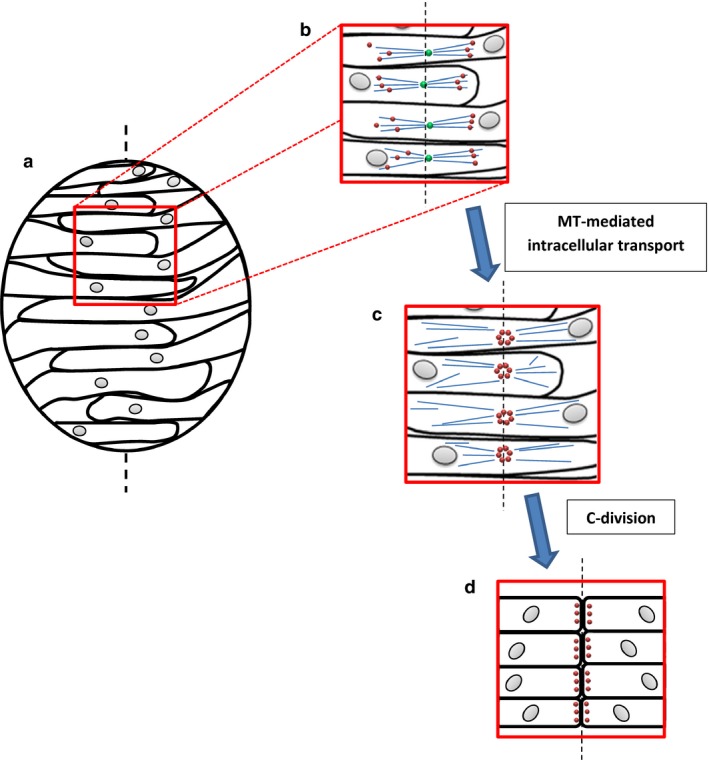
Medial Par3 localization by a mirror‐symmetric MT apparatus at the midline, and during subsequent C‐division. (a) In the zebrafish neural keel, cells interdigitate across the midline (black dotted line). (b) The centrosome localizes to the midline and organizes a mirror‐symmetric apparatus of MTs. (c) This enables the midline localization of Par3 and other polarity proteins by intracellular transport. (d) During the C‐division, each cell divides in a plane that deposits its daughter cells to opposite sides of the now well‐established midline. This produces good midline clearance for later cavitation of the neural rod and leaves Par3 localized to the apical surface. Blue: MTs; green: centrosomes; red: Par3. Figure based on data in Buckley et al. (2013).
Par3 and the molecular regulation of polarizing processes
How are the MT‐dependent processes of zebrafish neural tube development regulated at the molecular level? Several studies suggest a key role for Par3 (Fig. 4) and the proteins with which it forms a complex, namely Par6 and atypical protein kinase C (aPKC). The highly conserved Par complex has well‐documented functions in various contexts of cell polarity, including the C. elegans first zygotic division, cell fate determination, mammalian epithelial polarization and neuronal development (for reviews, see Goldstein & Macara, 2007; St Johnston & Ahringer, 2010). One process in which it has been implicated in various model systems is orientation of the mitotic spindle. In Drosophila neuroblasts, the Par complex triggers a signalling pathway that ultimately results in dynein‐mediated pulling forces on astral MTs that anchor the spindle to the cell cortex, allowing it to rotate relative to cortical molecular cues (for review, see Morin & Bellaïche, 2011; Peyre & Morin, 2012; Lu & Johnston, 2013). The basic principles of this pathway are evolutionarily conserved in C. elegans and mammals (Goldstein & Macara, 2007). Silencing of Par3 and aPKC in MDCK cells impairs spindle orientation and produces a multi‐lumen phenotype (Hao et al. 2010). There is also evidence that spindle orientation in the developing zebrafish neural tube depends on the Par complex, as it is impaired in Par6 mutant embryos and this results in the generation of multiple lumens (Munson et al. 2008). On the other hand, both knockdown and overexpression of Par3 cause relatively weak perturbations of spindle orientation in the neural rod (Geldmacher‐Voss et al. 2003; von Trotha et al. 2006).
Figure 4.
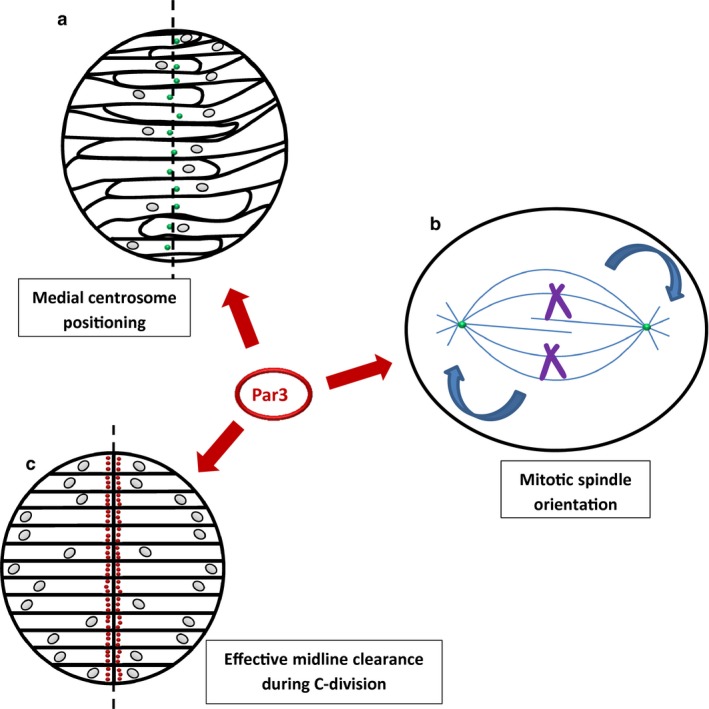
Roles of Par proteins in the developing zebrafish neural tube as revealed by morpholino‐mediated knockdown. (a) Par3 has been implicated in midline positioning of the centrosome in the neural keel, required for formation of the mirror‐symmetric MT apparatus. (b) Par proteins are important for orientation/rotation of the mitotic spindle in various models including the neural keel, in which this process is required for successful C‐divisions. (c) Normal clearance of the midline by dividing cells in the neural keel, which is important for neurocoel positioning, is Par3‐dependent as indicated by morpholino knockdown. Green: centrosomes; red: Par3. Figure based on data in Tawk et al. (2007); Munson et al. (2008); Hao et al. (2010); Hong et al. (2010); Buckley et al. (2013).
To further complicate this issue, C‐divisions in the neural rod, as well as spindle orientation during zebrafish gastrulation, depend on the Frizzled‐7 receptor, a component of the non‐canonical Wnt/planar cell polarity (PCP) signalling pathway, although a complete mechanism for this requirement has not been elucidated (Quesada‐Hernández et al. 2010; Ségalen et al. 2010). A further regulator of spindle orientation in the zebrafish neural keel appears to be Scribble, a polarity protein that has roles as a tumour suppressor, an apico‐basal polarity determinant and a component of PCP signalling (Žigman et al. 2011). Although Scribble has been implicated in orientating Drosophila neuroblast mitoses (Albertson & Doe, 2003), its mechanism of action is unclear. Interestingly, however, its role in orientating the axis of cell division in the zebrafish is independent of its role in PCP signalling (Žigman et al. 2011). Whilst it is clear, therefore, that correct spindle orientation is essential for successful C‐divisions, neural rod midline formation and neurocoel positioning, various lines of evidence implicate different molecular mechanisms, and we have yet to reach a unified model. The current understanding of spindle orientation regulators is derived predominantly from studies of invertebrate models and cultured cell lines. However, during zebrafish neurulation the mitotic spindle undergoes a 90 ° rotation (Geldmacher‐Voss et al. 2003); this is a special case of spindle orientation that warrants in vivo study of the underlying regulatory mechanisms. It will be interesting to see if the various molecules discussed above form partially redundant pathways regulating spindle orientation in the zebrafish neural rod, reflecting the evolutionary requirement for precise spindle orientation during such processes of embryonic development.
Aside from spindle orientation, Par3 seems to be involved in other aspects of zebrafish neural tube development (Fig. 4). During C‐division, midline crossing of daughter cells is significantly reduced not only by Par3 knockdown, but also by expression of a mutant version that fails to localize to the cleavage furrow during cytokinesis (Tawk et al. 2007). This suggests that the morphogenetic movements responsible for neurocoel positioning are dependent on the medial localization of Par3, which is effected prior to the C‐division by mirror‐symmetrically assembled centrosomal MTs (Buckley et al. 2013). The regulation of this MT network, however, requires further elucidation. In zebrafish, morpholino‐mediated Par3 knockdown and MT depolymerization using nocodazole disrupt normal midline positioning of the centrosome, which is thought to be upstream of mirror‐symmetric MT assembly (Hong et al. 2010; Buckley et al. 2013). This suggests, somewhat counter‐intuitively, that Par3 and cytoplasmic MTs in vertebrates may control apical positioning of the centrosome, which in turn establishes a MT system to localize Par3 to the midline. The link between Par3 function and centrosome apical positioning has also been reported in C. elegans: after cell division, the centrosome relocalizes γ‐tubulin and another MT‐nucleating protein, CeGrip‐1, to the apical membrane, reassigning it as the new MT‐organizing centre (Feldman & Priess, 2012). Deeper analysis of the mechanism by which Par3 controls centrosome positioning in zebrafish, with consideration of its intracellular localization and molecular interactions throughout this process, is required to provide clarity on this issue.
A further question is how cells of the neural rod ‘sense’ the midline, around which they base mirror‐symmetric MT assembly. Buckley et al. (2013) demonstrated that nascent cadherin‐based adhesions form between interdigitating cells from opposite sides of the neural rod, and suggest that this underlies midline formation by counteracting a default state in which cells position their apical domain at their most anti‐basal extremity. There is evidence that Scribble establishes nascent cadherin‐based adhesions responsible for co‐ordinating spindle orientation between adjacent neuroepithelial cells, providing a link between subcellular control mechanisms and more global tissue morphogenesis (Žigman et al. 2011). Could a similar mechanism allow cells to establish a midline? Scribble might control spindle orientation by tethering astral MTs to the site of cell–cell adhesion, a process that could be explained by various Scribble‐mediated mechanisms (Žigman et al. 2011). In this way, Scribble could underlie the MT‐dependent midline localization of the centrosome required for subsequent mirror‐symmetric MT assembly. It will be important, therefore, to establish what regulates cadherin‐based cell–cell adhesion in the zebrafish neural rod; why, for example, do adhesions form at the interdigitation of cells on contralateral sides (i.e. at the midline), and not at points where adjacent cells on one side of the neural rod interact? Understanding what determines midline assembly of the mirror‐symmetric MT network, and how this is co‐ordinated at the molecular level with 90 ° spindle rotation and cell polarization, are important goals for research in zebrafish nervous system development.
Conclusions and future prospects
Evidence from various model systems, both in vivo and in vitro, suggests that MTs play vital roles in regulating cell shape, cell polarity, subcellular organization, mitotic division and cell movements during development and three‐dimensional morphogenesis of the neural tube. Even before neurulation begins, MTs participate in regulating the cell movements that comprise convergent extension (Kwan & Kirschner, 2005). Then, during the onset of neurulation, MTs are required for the apico‐basal elongation of neuroepithelial cells, as the neural plate forms. MT regulation may also be required for bending of the neural plate and elevation of the neural folds in amphibia, birds and mammals, although this needs further research. In teleost fish, midline formation and subsequent lumen development in the neural tube depend on cell polarization and oriented cell division, with an essential role for MT regulation. It is clear, therefore, that MT function is vital for cell polarization in several aspects of vertebrate neurulation, irrespective of the precise morphology of the neural tube‐forming process. Moreover, it seems likely that MTs will prove to be of importance in epithelial morphogenesis more generally, for example during kidney, vascular and glandular development, as well as in pathological processes such as tumour angiogenesis.
A continuing challenge in MT research is to distinguish ‘cause’ from ‘effect’ in the relationship between MT dynamics and tissue morphogenesis. Cytoplasmic MTs have been known to align paraxially in the vertebrate neural plate for over 40 years – but is this synchronized intracellular MT re‐organization a cause of neural plate morphogenesis or a response to co‐existing influences? The paraxial alignment of MTs could be a response to tension generated by other subcellular or supracellular machineries, within or outside the neural plate. Subcellular forces generated by apical constriction during Drosophila mesoderm invagination are integrated across the tissue by supracellular actomyosin networks coupled through adherens junctions (Martin et al. 2010). Moreover, in the zebrafish neural rod, a defective cytoskeletal organization/cell shape phenotype in mutant cells is rescued when they are surrounded by wild‐type cells in mosaic embryos, suggesting that polarity is determined not just by cell‐autonomous processes, but also by tissue‐level feedback mechanisms (Žigman et al. 2011). The influence of such mechanisms is made possible by the close association between the MT cytoskeleton and cell–cell adhesion systems, though the complex interplay here also contri‐butes to the challenge of distinguishing ‘cause’ from ‘effect’.
A factor that has constrained progress in this field is the limited range of tools available to experimentally alter MT function. Drugs that destabilize MTs, including paclitaxel and nocodazole, are widely employed, but these agents can have multiple cellular effects, such as arresting cells in mitosis, which can cause more generalized consequences for embryonic systems. The increasing availability of selective means of interfering with MT function, for example through use of mutant forms of MT‐binding proteins like CLIP‐170 that specifically destabilize MTs (Nakano et al. 2010), may offer more specific routes to manipulation of the cell polarization functions of MTs in the future. Such tools will be most effective when used to experimentally manipulate MT sub‐populations in vivo, alongside high‐resolution imaging methods to record and quantify MT dynamics. Such advances promise to enable a better understanding of the contributions of these cytoskeletal elements to the processes of vertebrate neural tube morphogenesis.
Acknowledgement
This work was supported by the Wellcome Trust, grant number 087525.
References
- Albertson R, Doe CQ (2003) Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol 5, 166–170. [DOI] [PubMed] [Google Scholar]
- Beck CW (2015) Development of the vertebrate tailbud. Wiley Interdiscip Rev Dev Biol 4, 33–44. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Buchholz B, et al. (2013) Kif3a guides microtubular dynamics, migration and lumen formation of MDCK cells. PLoS ONE 8, e62165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CE, Ren X, Ward LC, et al. (2013) Mirror‐symmetric microtubule assembly and cell interactions drive lumen formation in the zebrafish neural rod. EMBO J 32, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside B (1971) Microtubules and microfilaments in newt neuralation. Dev Biol 26, 416–441. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, et al. (2006) Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J (2009) Role of polarized cell divisions in zebrafish neural tube formation. Curr Opin Neurobiol 19, 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Brennwald PJ, Rodriguez‐Boulan E, et al. (2004) Mammalian PAR‐1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J Cell Biol 164, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnon J, Heisenberg CP (2013) Neurulation: coordinating cell polarisation and lumen formation. EMBO J 32, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha ML, Adams RJ (1998) Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time‐lapse analysis. Development 125, 983–994. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND (2010) Genetics and development of neural tube defects. J Pathol 220, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene ND (2013) Neural tube defects‐disorders of neurulation and related embryonic processes. Wiley Interdiscip Rev Dev Biol 2, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN (2003) The genetic basis of mammalian neurulation. Nat Rev Genet 4, 784–793. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Stanier P, Greene ND (2013) Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol 12, 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall D, Walther RF, Rodriguez L, et al. (2007) Hedgehog signaling is a principal inducer of Myosin‐II‐driven cell ingression in Drosophila epithelia. Dev Cell 13, 730–742. [DOI] [PubMed] [Google Scholar]
- Das D, Zalewski JK, Mohan S, et al. (2014) The interaction between shroom3 and Rho‐kinase is required for neural tube morphogenesis in mice. Biol Open 3, 850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dathe V, Pröls F, Brand‐Saberi B (2004) Expression of kinesin kif5c during chick development. Anat Embryol (Berl) 207, 475–480. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Mandelkow EM (1998) MAPs, MARKs and microtubule dynamics. Trends Biochem Sci 23, 307–311. [DOI] [PubMed] [Google Scholar]
- Escuin S, Vernay B, Savery D, et al. (2015) Rho kinase‐dependent actin turnover and actomyosin disassembly are necessary for mouse spinal neural tube closure. J Cell Sci 128, 2468–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Priess JR (2012) A role for the centrosome and Par‐3 in the hand‐off of MTOC function during epithelial polarisation. Curr Biol 22, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher‐Voss B, Reugels AM, Pauls S, et al. (2003) A 90‐degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development 130, 3767–3780. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG (2007) The par proteins: fundamental players in animal cell polarization. Dev Cell 13, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene ND, Copp AJ (2014) Neural tube defects. Annu Rev Neurosci 37, 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, et al. (2003) Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol 13, 2125–2137. [DOI] [PubMed] [Google Scholar]
- Hancock WO (2014) Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol 15, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Du Q, Chen X, et al. (2010) Par3 controls epithelial spindle orientation by aPKC‐mediated phosphorylation of apical Pins. Curr Biol 20, 1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD (2005) Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci 118(Pt 22), 5191–5203. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P (1999) Shroom, a PDZ domain‐containing actin‐binding protein, is required for neural tube morphogenesis in mice. Cell 99, 485–497. [DOI] [PubMed] [Google Scholar]
- Hong E, Jayachandran P, Brewster R (2010) The polarity protein pard3 is required for centrosome positioning during neurulation. Dev Biol 341, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, et al. (1998) An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of caenorhabditis elegans polarity protein par‐3. J Cell Biol 143, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L (1992) Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles: implications for the role of microtubule dynamics in mitosis. J Cell Sci 102(Pt 3),401–416. [DOI] [PubMed] [Google Scholar]
- Karfunkel P (1971) The role of microtubules and microfilaments in neurulation in xenopus. Dev Biol 25, 30–56. [DOI] [PubMed] [Google Scholar]
- Karfunkel P (1972) The activity of microtubules and microfilaments in neurulation in the chick. J Exp Zool 181, 289–301. [DOI] [PubMed] [Google Scholar]
- Karfunkel P (1974) The mechanisms of neural tube formation. Int Rev Cytol 38, 245–271. [DOI] [PubMed] [Google Scholar]
- Kee N, Wilson N, De Vries M, et al. (2008) Neogenin and RGMa control neural tube closure and neuroepithelial morphology by regulating cell polarity. J Neurosci 28, 12 643–12 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D, Martin BL (2012) Anterior‐posterior patterning in early development: three strategies. Wiley Interdiscip Rev Dev Biol 1, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Kane DA (1994) Cell cycles and clonal strings during formation of the zebrafish central nervous system. Development 120, 265–276. [DOI] [PubMed] [Google Scholar]
- Kollman JM, Merdes A, Mourey L, et al. (2011) Microtubule nucleation by γ‐tubulin complexes. Nat Rev Mol Cell Biol 12, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Takemoto T (2012) Axial stem cells deriving both posterior neural and mesodermal tissues during gastrulation. Curr Opin Genet Dev 22, 374–380. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Suetsugu T, Suda M, et al. (2011) Regulation of interkinetic nuclear migration by cell cycle‐coupled active and passive mechanisms in the developing brain. EMBO J 30, 1690–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW (2005) A microtubule‐binding Rho‐GEF controls cell morphology during convergent extension of Xenopus laevis. Development 132, 4599–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman J, Guerrant RL, Freeman BG (1966) Behavior of neuro‐epithelial cells during closure of the neural tube. J Comp Neurol 127, 399–411. [DOI] [PubMed] [Google Scholar]
- Le Droguen PM, Claret S, Guichet A, et al. (2015) Microtubule‐dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Development 142, 363–374. [DOI] [PubMed] [Google Scholar]
- Lee JY, Harland RM (2007) Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Dev Biol 311, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Scherr HM, Wallingford JB (2007) Shroom family proteins regulate gamma‐tubulin distribution and microtubule architecture during epithelial cell shape change. Development 134, 1431–1441. [DOI] [PubMed] [Google Scholar]
- Li D, Kuehn EW, Prekeris R (2014) Kinesin‐2 mediates apical endosome transport during epithelial lumen formation. Cell Logist 4, e28928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Sive H (2004) Strategies of vertebrate neurulation and a re‐evaluation of teleost neural tube formation. Mech Dev 121, 1189–1197. [DOI] [PubMed] [Google Scholar]
- Lu MS, Johnston CA (2013) Molecular pathways regulating mitotic spindle orientation in animal cells. Development 140, 1843–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez‐Gonzalez R, et al. (2010) Integration of contractile forces during tissue invagination. J Cell Biol 188, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier PE (1969) Effects of beta‐mercaptoethanol on the fine structure of the neural plate cells of the chick embryo. J Embryol Exp Morphol 21, 309–329. [PubMed] [Google Scholar]
- Messier PE (1978) Microtubules, interkinetic nuclear migration and neurulation. Experientia 34, 289–296. [DOI] [PubMed] [Google Scholar]
- Morin X, Bellaïche Y (2011) Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev Cell 21, 102–119. [DOI] [PubMed] [Google Scholar]
- Munson C, Huisken J, Bit‐Avragim N, et al. (2008) Regulation of neurocoel morphogenesis by pard6 gamma b. Dev Biol 324, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A (2004) Microtubule organization and function in epithelial cells. Traffic 5, 1–9. [DOI] [PubMed] [Google Scholar]
- Nakano A, Kato H, Watanabe T, et al. (2010) AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP‐170 phosphorylation. Nat Cell Biol 12, 583–590. [DOI] [PubMed] [Google Scholar]
- Niederkofler V, Salie R, Sigrist M, et al. (2004) Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci 24, 808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M (2008) Shroom3‐mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development 135, 1493–1502. [DOI] [PubMed] [Google Scholar]
- Norden C, Young S, Link BA, et al. (2009) Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell 138, 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papan C, Campos‐Ortega JA (1994) On the formation of the neural keel and neural tube in the zebrafish Danio (Brachydanio) rerio . Roux's Arch Dev Biol 203, 178–186. [DOI] [PubMed] [Google Scholar]
- Perry MM, Waddington CH (1966) Ultrastructure of the blastopore cells in the newt. J Embryol Exp Morphol 15, 317–330. [PubMed] [Google Scholar]
- Peyre E, Morin X (2012) An oblique view on the role of spindle orientation in vertebrate neurogenesis. Dev Growth Differ 54, 287–305. [DOI] [PubMed] [Google Scholar]
- Picone R, Ren X, Ivanovitch KD, et al. (2010) A polarised population of dynamic microtubules mediates homeostatic length control in animal cells. PLoS Biol 8, e1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaderi NA, Schweiger S, Gaudenz K, et al. (1997) Opitz G/BBB syndrome, a defect of midline development, is due to mutations in a new RING finger gene on Xp22. Nature Genet 17, 285–291. [DOI] [PubMed] [Google Scholar]
- Quesada‐Hernández E, Caneparo L, Schneider S, et al. (2010) Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr Biol 20, 1966–1972. [DOI] [PubMed] [Google Scholar]
- Rhumbler L (1902) Zur Mechanik des Gastrulationsvorganges, insbesondere der Invagination. Arch Entw Mech 14, 401–476. [Google Scholar]
- Rogers SL, Wiedemann U, Häcker U, et al. (2004) Drosophila RhoGEF2 associates with microtubule plus ends in an EB1‐dependent manner. Curr Biol 14, 1827–1833. [DOI] [PubMed] [Google Scholar]
- Sauer FC (1935) Mitosis in the neural tube. J Comp Neurol 62, 377–405. [Google Scholar]
- Sawyer JM, Harrell JR, Shemer G, et al. (2010) Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol 341, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf GC, Powers ML (1987) Shaping of the chick neuroepithelium during primary and secondary neurulation: role of cell elongation. Anat Rec 218, 182–195. [DOI] [PubMed] [Google Scholar]
- Ségalen M, Bellaïche Y (2009) Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol 20, 972–977. [DOI] [PubMed] [Google Scholar]
- Ségalen M, Johnston CA, Martin CA, et al. (2010) The Fz‐Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell 19, 740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PC, Erickson CA (2012) Apical movement during interkinetic nuclear migration is a two‐step process. Dev Biol 370, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J (2010) Cell polarity in eggs and epithelia: parallels and diversity. Cell 141, 757–774. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hara Y, Takagi C, et al. (2010) MID1 and MID2 are required for Xenopus neural tube closure through the regulation of microtubule organization. Development 137, 2329–2339. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Morita H, Ueno N (2012) Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev Growth Differ 54, 266–276. [DOI] [PubMed] [Google Scholar]
- Tawk M, Araya C, Lyons DA, et al. (2007) A mirror‐symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature 446, 797–800. [DOI] [PubMed] [Google Scholar]
- von Trotha JW, Campos‐Ortega JA, Reugels AM (2006) Apical localization of ASIP/PAR‐3:EGFP in zebrafish neuroepithelial cells involves the oligomerization domain CR1, the PDZ domains, and the C‐terminal portion of the protein. Dev Dyn 235, 967–977. [DOI] [PubMed] [Google Scholar]
- Tzouanacou E, Wegener A, Wymeersch FJ, et al. (2009) Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell 17, 365–376. [DOI] [PubMed] [Google Scholar]
- Valiron O, Caudron N, Job D (2001) Microtubule dynamics. Cell Mol Life Sci 58, 2069–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venghateri JB, Jindal B, Panda D (2015) The centrosome: a prospective entrant in cancer therapy. Expert Opin Ther Targets 19, 957–972. [DOI] [PubMed] [Google Scholar]
- Waddington CH, Perry MM (1966) A note on the mechanisms of cell deformation in the neural folds of the Amphibia. Exp Cell Res 41, 691–693. [DOI] [PubMed] [Google Scholar]
- Wade RH (2009) On and around microtubules: an overview. Mol Biotechnol 43, 177–191. [DOI] [PubMed] [Google Scholar]
- Wilson V, Olivera‐Martinez I, Storey KG (2009) Stem cells, signals and vertebrate body axis extension. Development 136, 1591–1604. [DOI] [PubMed] [Google Scholar]
- Yang X, Zou J, Hyde DR, et al. (2009) Stepwise maturation of apicobasal polarity of the neuroepithelium is essential for vertebrate neurulation. J Neurosci 29, 11 426–11 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount AL, Zong H, Walczak CE (2015) Regulatory mechanisms that control mitotic kinesins. Exp Cell Res 334, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žigman M, Trinh lA, Fraser SE, et al. (2011) Zebrafish neural tube morphogenesis requires Scribble‐dependent oriented cell divisions. Curr Biol 21, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


