Abstract
Type 1 diabetes (T1D) is an autoimmune disease in which progressive loss of self-tolerance, evidenced by accumulation of auto-antibodies and auto-reactive T cells that recognize diverse self-proteins, leads to immune-mediated destruction of pancreatic beta cells and loss of insulin secretion. In this review, we discuss antigens and epitopes in T1D and the role that post-translational modifications play in circumventing tolerance mechanisms and increasing antigenic diversity. Emerging data suggest that, analogous to other autoimmune diseases such as rheumatoid arthritis and celiac disease, enzymatically modified epitopes are preferentially recognized in T1D. Modifying enzymes such as peptidyl deiminases and tissue transglutaminase are activated in response to beta cell stress, providing a mechanistic link between post-translational modification and interactions with the environment. Although studies of such responses in the at-risk population have been limited, current data suggests that breakdown in tolerance through post-translational modification represents an important checkpoint in the development of T1D.
Keywords: Type 1 diabetes, Post-translational, Modified epitope, Autoimmune, T cell, HLA
Introduction
Type 1 diabetes (T1D) is recognized as an autoimmune-mediated disease [1]. The major immunological hallmarks of T1D include association with specific susceptible HLA class II haplotypes and the development of islet cell autoantibodies [2, 3]. In all likelihood, the major contribution of susceptible HLA-DQ and HLA-DR molecules is their role in selecting a potentially autoreactive CD4+ T cell repertoire. For example, it has been demonstrated using rigorous tetramer-based assays that auto-reactive T cells are present in healthy subjects who have autoimmune-susceptible HLA haplotypes [4]. This selection of a potentially autoreactive repertoire occurs in spite of tolerance mechanisms in the thymus that normally direct naïve T cells with strongly self-reactive receptors (TCR) toward deletion or conversion to a regulatory phenotype. Although there is little direct evidence to document T cell responses at the earliest stages of T1D development, ample data from longitudinal studies of at-risk subjects (such as TEDDY) illustrate that the development of T1D is marked by a sequential accumulation of auto-antibodies [5–7]. The appearance of these high affinity antibodies implies active recognition of beta cell antigens by auto-reactive CD4+ T cells that provide help to auto-reactive B cells. Indeed, a growing body of experimental evidence from studies of pancreatic tissue samples demonstrates that auto-reactive CD4+ and CD8+ T cells infiltrate pancreatic islets, where they likely contribute to beta cell death through direct cytotoxicity and secretion of inflammatory cytokines. It has been shown that auto-reactive T cells recognize diverse self-proteins in subjects with T1D and that such auto-reactive T cells occur at higher frequencies and have a more inflammatory phenotype in subjects with T1D than in healthy subjects [8, 9]. However, fundamental questions remain about the earliest events that lead to loss of tolerance to beta cell antigens. Post-translational modification (PTM) represents one means through which the expected deletion of self-reactive T cells can be circumvented. Such modifications alter the primary sequence of self-peptides. These alterations have the potential to increase the affinity of HLA/peptide interactions or HLA/peptide-TCR interactions depending on the positioning of the affected residue in relation to other HLA-anchoring residues along the peptide sequence. In this review, we discuss the diversity of antigens that are recognized in T1D and the increase in antigenic diversity through PTM. We further discuss current evidence demonstrating the recognition of modified epitopes in subjects with T1D and the mechanistic role that modifying enzymes and the epitopes that they generate may play in the initiation and amplification of autoimmunity. Finally, we address the overall implications of our current knowledge in this area and discuss key unanswered questions that are ripe for further investigation.
Antigenic and Epitope Diversity in T1D
A wealth of data affirms that diverse antigens and epitopes are relevant components of autoimmune responses in T1D. Table 1 provides a summary of various beta cell antigens that have been confirmed to be immunogenic and disease relevant by more than one independent study. At the level of islet cell antibodies (ICA), multiple antigens are recognized, and among these, multiple specificities have utility as diagnostic indicators of risk, including insulin, GAD65, IA-2, and ZNT8. ICA that recognize these diverse specificities emerge sequentially, with insulin and GAD65 autoantibodies typically appearing at early time points (in some cases, within the first year of life) and other specificities tending to appear at later times [34]. The numbers of biochemically defined ICA that are present in at-risk subjects directly mirror the probability of developing disease, in that subjects who are positive for multiple autoantibodies are more likely to develop diabetes, tend to have an earlier age of onset, evidence of aggressive beta cell destruction, and require more exogenous insulin [35]. This pattern suggests a sequential loss of tolerance to beta cell antigens, which could be the consequence either of continuous inflammation and auto-reactivity or multiple bursts of autoimmune activity that are separated by periods of quiescence. The predictive correlation between ICA and risk of progression to develop T1D suggests an important role for B cells that recognize and present beta cell antigens in T1D pathogenesis. This is indirectly confirmed by the efficacy of rituximab (anti-CD20) in delaying c-peptide loss in patients with new-onset T1D and other supporting data from mouse models. However, as reviewed elsewhere, the precise mechanisms by which B cells contribute to disease are incompletely characterized in the pathogenesis of T1D [36•].
Table 1.
Antigenic diversity in T1D
| Antigen | Recognized by | Relevant PTM | Key references |
|---|---|---|---|
| Insulin/proinsulin | Ab, CD4, CD8 | Oxidation, deamidation | [10–16] |
| GAD65 | Ab, CD4, CD8 | Deamidation, Citrullination | [17–19, 20•] |
| IA-2 | Ab, CD4, CD8 | Deamidation | [21, 22] |
| ZnT8 | Ab, CD4, CD8 | Phosphorylation | [23, 24] |
| IGRP | CD4, CD8 | Citrullination | [25–27] |
| Chromogranin A | CD4, CD8 | Cross-linked peptide | [28, 29, 30•, 31] |
| IAPP | CD4, CD8 | Citrullination | [32•] |
| GRP78 | Ab, CD4 | Citrullination | [33] |
Given the diagnostic importance of ICA and the strong genetic association between T1D and a relatively small number of HLA class II alleles, it stands to reason that CD4+ T cells play a mechanistic role in the disease. Based on the observation that detailed studies of viral immunity have demonstrated an overlap between the antigens targeted by B cells (antibodies) and CD4+ T cells [37], many studies have investigated CD4+ T cell responses to self-proteins that are targeted by auto-antibodies. Studies utilizing samples from human subjects and the nonobese diabetic (NOD) mouse model clearly indicate that such overlap in antigenic recognition is seen in T1D (Table 1). Early studies supported the view that self-reactive CD4+ T cells recognize a modest number of immunodominant epitopes within a limited number of “primary” target antigens [10, 17]. As a corollary, it was thought that these epitopes reflect peptide specificities for which self-reactive T cells are more easily selected and/or reactivated in the periphery; to some extent, this may be true. Strong data from the NOD mouse model implicate insulin as a primary antigen and demonstrate that responses to the Ins B9–23 epitope in particular are crucial for disease development [11]. However, recent studies illustrate the importance of responses to additional antigens, including islet-specific glucose 6 phosphatase catalytic subunit-related protein (IGRP), chromogranin A, and IAPP, both in the NOD model and in human diabetes [25–29, 32•]. Subsequent studies of human T cell responses affirm the relevance not only of the insulin B9–23 epitope, but also of additional insulin epitopes (most notably proinsulin 76–90) and epitopes derived from numerous other antigens. In particular, multiple studies have demonstrated CD4+ T cell reactivity toward insulin, GAD65, IA-2, IGRP, and ZNT8 [12–14, 18, 21, 23, 24]. As such, it is increasingly evident that diverse antigens and epitopes are recognized by autoreactive CD4+ T cells in T1D and that the most prevalent specificities vary for different individuals. For example, in our own work, we observed a diversity of epitopes derived from GAD65 that are naturally processed and presented in the context of HLA-DRB1*04:01 [19]. Among these, one specificity (GAD65 113–132) was consistently immunogenic in most subjects, but multiple GAD65 epitopes were required in order to visualize a response in every subject.
Although there is compelling circumstantial evidence that autoantibodies and CD4+ T cell responses play an important role in autoimmune responses in T1D, CD8+ T cells are of particular interest, as it is these cells that are most abundant in inflamed islets in pancreata with T1D [38••]. Genetic association of HLA class I genes is generally weaker than that of HLA class II; only one allele (B*3906, odds ratios=10.31) is strongly associated to the risk of developing T1D, but several other alleles have weak associations, including A*2402 (odds ratio=1.71), A*0201 (odds ratio=1.35), B*1801 (odds ratio=2.05), and C*0501 (odds ratio=1.56) [39]. Among the susceptible HLA class I alleles, there has been considerable effort to characterize islet cell-derived epitopes restricted by HLA A*0201, mainly due to its wide prevalence in most populations and availability of effective HLA class I tetramer reagents and prediction algorithms to facilitate experimental work [40, 41]. Studies using tetramers and other epitope-specific methodologies have demonstrated the importance of CD8+ T cell responses to epitopes such as Ins B10–18, PPI15–24, GAD114–123, and, more recently, ZnT8 [15, 23, 42, 43]. While not yet validated by T cell cloning or tetramer data, chromogranin A was also recently implicated as an antigen for auto-reactive CD8+ T cells in patients and NOD mice [29]. A recent study of beta cell-specific CD8+ T cells demonstrated that, in patients with recent-onset T1D, these cells had a surface phenotype and a limited TCR profile that suggested repeated antigen-driven expansion in vivo [44]. Studies in the NOD mouse model have demonstrated that adoptive transfer of beta cell-specific CD8+ T cells is sufficient to elicit T1D and that tetramer-based measures of the frequency of such cells can predict the onset of disease in mice [45]. Recent studies in human subjects affirm that autoreactive CD8+ T cell frequencies correlate with clinical outcome in islet cell-transplanted patients [43]. Although it is probable that CD8+ T cells that recognize beta cell epitopes are responsible for beta cell destruction, the activity of these cells is likely preceded by and mobilized by autoreactive CD4+ T cells. In this scenario, CD4+ T cell responses would be of primary importance during the initiation stage of disease development, recognizing an increasing diversity of antigens and epitopes (perhaps presented by different subsets of antigen-presenting cells at different times). Their activity would then provide help to successive waves of self-reactive B cells that produce an increasing diversity of ICA and to autoreactive CD8+ T cells which secrete inflammatory cytokines and directly mediate beta cell killing.
PTM as a Means for Increasing Antigenic Diversity
Given that the risk for developing T1D increases substantially with the absolute number of antigens targeted [6, 7], a key question becomes apparent: what are the mechanisms that lead to the initial loss of tolerance and to increasing antigenic diversity over the course of progression to develop autoimmune diabetes? PTMs are a clear means through which antigenic diversity is increased in autoimmune disease and also represent one plausible mechanism through which initial tolerance could be lost. As reviewed by Doyle and Mamula [46••], diverse PTMs, including phosphorylation, citrullination, acetylation, carbamylation, deamidation, and oxidation, have been documented in human disease. As discussed in that informative review, PTM can contribute to autoimmune etiology at many levels. At the level of peptides, PTM leads to altered sequences that are recognized with increased affinity. At the level of proteins, PTM can alter structure and subsequent processing by antigen-presenting cells. At the level of cellular processes, PTM can alter signaling pathways, leading to modified biological function.
For T cell-mediated human autoimmune diseases, there is a growing body of data supporting a role for increased immune recognition of altered peptide sequences. In particular, two modifications have emerged that appear to have particular relevance in the etiology of autoimmunity. One clearly understood example of this phenomenon comes from celiac disease, where disease-relevant gliadin epitopes are deamidated by the enzyme tissue transglutaminase 2 (TG2). Conversion of glutamine into its negatively charged analog glutamic acid has been shown to greatly enhance peptide binding to disease-associated HLA DQ2 and DQ8 proteins, creating stable MHC/peptide complexes that elicit robust T cell activation [47]. Such T cells not only can provide the inflammatory signals necessary to drive further TG2 upregulation and activation leading to continued protein modification, but also cross-react with the unmodified epitope, leading to sustained immune activation even in the absence of TG2 activity [48]. The accumulated result is a greatly broadened antigenic repertoire and increased autoimmune activity.
A second type of PTM that has well documented importance is protein and peptide citrullination. In this instance, peptidyl arginine deiminase (PAD) enzymes convert positively charged arginine into the polar citrulline residue. Elimination of that positive charge at key protein residues has been shown to elicit citrulline-specific antibodies, the presence of which is highly correlated with the risk of developing rheumatoid arthritis (RA), particularly in subjects who have HLA-DRB1*04:01 haplotypes [49, 50]. A recent crystal structure indicates that citrulline residues are more flexible than arginine, allowing that amino acid side chain to bend and adopt a more favorable orientation within the HLA-DR binding cleft [51•]. In addition, conversion of arginine to citrulline modulates electrostatic interactions between the peptide and amino acid side chains within certain HLA class II binding pockets (especially binding pocket 4, which contains multiple amino acids with positively charged side chains), thereby increasing overall peptide-binding affinity [52, 53]. Peptides derived from joint-associated proteins such as vimentin, fibrinogen, and filaggrin bind disease-associated HLA molecules with high affinity only in their citrullinated forms [52, 53]. Correspondingly, citrulline-specific CD4+ T cells are found at elevated frequencies in patients with recent-onset RA [54•]. Such cells are antigen experienced and have a predominantly Th1 phenotype and are therefore likely important players in the autoimmune processes that lead to tissue destruction in the joint. Citrullination of self-proteins has further been implicated in other autoimmune disorders including multiple sclerosis and Alzheimer's disease [55]. Thus, citrullination may be common in settings of sustained inflammation and relevant in a number of autoimmune conditions.
PTM of self-proteins provides a mechanism that can plausibly drive the break in tolerance and activation of autoreactive T cells. Because the activity of modifying enzymes such as PADs and TG2 is expected to be minimal in healthy tissues and during thymic selection (in comparison with inflamed tissues, in which these enzymes are upregulated and highly activated), CD4+ T cells capable of responding to modified self-epitopes would not typically encounter extensively modified self-antigens during thymic development and therefore could evade negative selection and deletion. Once a T cell encounters its cognate neo-antigen in inflamed peripheral tissue, the modified peptide is recognized as a neo-epitope and adaptive immunity is elicited accordingly. Given the established role of PTMs in human autoimmune diseases such as RA and celiac disease and shared genetic susceptibility between T1D and these diseases (including coinciding HLA class II susceptibility haplotypes), research has recently begun to address the contribution of modified beta cell antigens in the pathogenesis of T1D.
Recognition of Modified CD4+ T Epitopes in T1D
The earliest published evidence documenting a role for PTM in directly altering epitope recognition in T1D was provided by Mannering et al. in 2005 [16]. Working with proinsulin-reactive T cell clones of unknown specificity, this study demonstrated that an immunogenic peptide derived from the insulin A chain contained a vicinal disulfide bond that arose from spontaneous oxidation of adjacent cysteine residues. This modification was crucial for T cell recognition as replacement of either cysteine with a serine ablated T cell responses. This study concluded that the unique three-dimensional conformation of these disulfide cross-linked residues was uniquely recognized by these auto-reactive TCR and could not be mimicked by other amino acids. Mannering et al. went on to show that T cells isolated from an autoantibody-positive at-risk subject proliferated in response to the modified peptide whereas T cells isolated from healthy individuals did not.
Building on this early discovery of a modified neo-epitope that arose due to a spontaneous PTM, subsequent research has focused on enzymatically catalyzed modifications. Recent work by Delong et al. highlighted the drastic effect that enzymatic PTM can have on immune recognition [30•]. The NOD mouse-derived BDC 2.5 CD4+ T cell clone was shown to be specific for the secretory granule protein chromogranin A; however, very high concentrations of the suspected cognate peptide (WE14) were required to activate these cells. Delong et al. went on to demonstrate that treatment of the WE14 peptide with TG2 increased T cell recognition up to 40-fold, displaying enhanced proliferation and IFN-γ production upon stimulation. This particular effect was not due to deamidation, as synthesized peptides containing glutamic acid substitutions failed to recapitulate the TG2 effect. Rather, the observed increase in immunogenicity resulted from TG2's ability to covalently cross-link peptides, mediating increased recognition through the formation of elongated peptide aggregates produced by the enzyme. While the exact mechanism is unknown, it is hypothesized that peptide aggregates may alter HLA-binding affinity or be preferentially taken up by antigen-presenting cells (APCs) and presented to autoreactive T cells. The relevance of this modification was extended to human T1D with the demonstration of enhanced recognition of TG2-treated WE14 antigen in recent-onset subjects [31]. Thus, covalent cross-linking of self-proteins provides a general mechanism for greatly broadening the number of target antigens and warrants further investigation.
As previously mentioned, TG2 also mediates a deamidation reaction where glutamine is converted to negatively charged glutamic acid, which has the potential to alter peptide interaction with HLA. It was recently demonstrated in a study by van Lummel et al. that deamidated islet peptides can be eluted from disease-associated HLA-DQ8cis and HLA-DQ8trans molecules and identified by mass spectrometry [56•]. This study went on to demonstrate that T cell response rates to a particular proinsulin peptide increased dramatically in patients with recent-onset T1D when both native and deamidated versions of the peptide were utilized. Our own recently published work identified a deamidated GAD65 epitope that binds with high affinity to HLA-DRB1*04:01 [20•]. CD4+ T cells specific for this epitope were present at significantly higher frequencies in patients with T1D as compared to HLA-matched healthy controls and exhibited an antigen-experienced Th1 phenotype. Intriguingly, the unmodified version of this GAD65 peptide is also known to be antigenic [19, 33]; however, we demonstrated that the two epitopes were found to be recognized by completely distinct populations of T cells. These examples establish a precedent that protein deamidation and peptide cross-linking by TG2 generate neoepitopes that diversify the autoreactive T cell repertoire in T1D. Our more recent work has identified multiple peptides from the N-terminal domain of IA-2 antigen that bind with high affinity to HLA-DQ8 and are preferentially (or exclusively) recognized in their deamidated form. T cells specific for these epitopes are present at significantly higher frequencies in patients with T1D as compared to HLA-matched healthy controls and exhibited an antigen-experienced phenotype.
Given the importance of citrullinated epitopes in the pathogenesis of RA and the significant overlap in genetic risk between RA and T1D, recent studies have also sought to identify citrullinated beta cell antigens and epitopes that are relevant in autoimmune diabetes. One recent compelling study by Rondas et al. [57•] demonstrated that citrullinated glucose-regulated protein 78 (GRP78), an ER chaperone protein, is recognized as an autoantigen in the NOD model of diabetes. Splenocytes isolated from NOD mice produced IFN-γ in response to citrullinated, but not native, GRP78. In addition, NOD mice had elevated autoantibody titers against the modified protein. The PAD enzymes that mediate citrullination were found to be specifically upregulated and activated in islets of NOD mice, and inflammatory signaling in stressed beta cells was determined to induce citrullination. Given the inflammatory nature of the insulitic process in the T1D pancreas, it is likely that such events also occur in human T1D, though this remains to be proven. In addition to citrullinated GRP78, our group has also reported a GAD65-derived epitope that binds DRB1*04:01 with high affinity in both unmodified and citrullinated forms [20•]. Citrullinated GAD65-specific T cells were found at elevated frequencies in diabetic subjects and were also found to be antigen experienced. Much like the transglutaminated GAD65 epitope described above, this peptide has previously been identified as an antigen in its unmodified form [19, 58]. Yet, unlike the previous example where no cross-recognition was observed, antigen-specific T cell clones could be stained with both unmodified and citrullinated tetramer and proliferated in response to both forms of peptide. However, T cells always preferentially responded to the citrullinated epitope as evidenced by higher mean fluorescence intensity (MFI) of tetramer staining and a higher stimulation index for proliferative responses. This scenario may be analogous to the cross-reactivity that has been observed in celiac disease, in which deamidated gliadin peptides elicit T cell responses that also respond to unmodified peptides [48]. Alternatively, it is possible that CD4+ T cells are activated in response to initiating epitopes from unmodified antigens whose activity generates the inflammatory environment necessary for enzymatic modifications to occur.
Recognition of Modified CD8+ T Epitopes in T1D
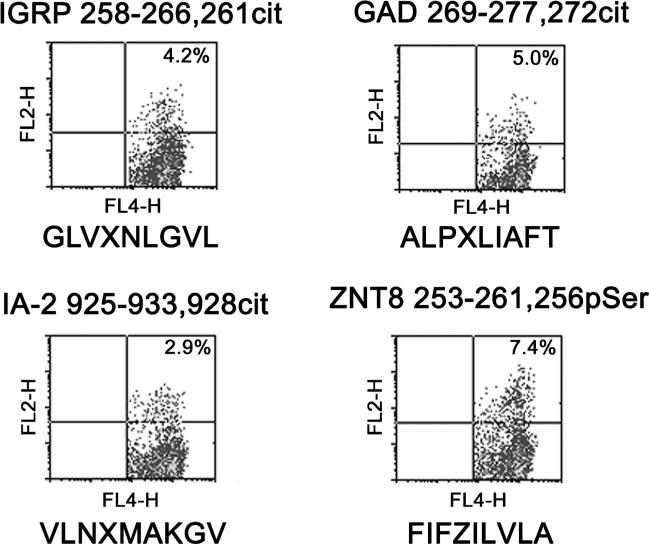
While significantly less studied, there is some evidence to suggest that recognition of beta cell antigens by CD8+ T cells is also modulated by PTMs. However, the most relevant modifications that confer improved binding to HLA class I may differ from those that are most important for HLA class II. For example, Gianfrani et al. [59] demonstrated the recognition of A-gliadin 123–132 by CD8+ T cells. However, the immunogenicity of this peptide was completely abolished when the glutamate residue at position 123 of this peptide was converted to glutamic acid. In our own work, we recently demonstrated that various amino acid modifications influence peptide binding to HLA-A2 in ways that are distinct from HLA class II proteins. As summarized in Table 2, citrullination predominantly increases peptide binding to HLA-DR proteins (especially DR0401), but this modification can also increase peptide binding to HLA-A2 albeit with a more modest increase in magnitude. Deamidation predominantly increases peptide binding to HLA-DQ proteins, but this modification can also modestly increase peptide binding to HLA-A2 and to DR0401 (at pocket 4). In contrast, phosphorylation reduces peptide binding to HLA-DR and HLA DQ proteins but increases peptide binding to HLA-A2. Utilizing this information, we assessed HLA-A2 binding (using a cell-based assay) and antigenicity (using a CD137 upregulation assay) of citrullinated, deamidated, and phosphorylated peptides derived from beta cell antigens. Through these efforts, we identified three citrullinated and one phosphorylated beta cell peptides that elicited CD137 upregulation in CD8+ T cells from HLA-A2+ subjects with T1D (Fig. 1). These findings suggest that autoreactive CD8+ T cells recognize modified peptides in subjects with T1D and that recognition of phosphorylated peptides may be uniquely important in the context of HLA class I-mediated recognition.
Table 2.
Modulation of peptide binding through amino acid modification
| HLA allele | Modification | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 |
|---|---|---|---|---|---|---|---|---|---|---|
| HLA-A2 | Arg → Cit | NT | ND | + | ND | + | ND | – | + | ND |
| Gln → Glu | NT | ND | ND | + | + | – | ND | + | + | |
| Ser → pSer | NT | ND | + | + | ND | + | + | ND | + | |
| HLA-DQ8 | Arg →Cit | ND | NT | NT | ND | ND | – | ND | – | – |
| Gln → Glu | + | NT | NT | ND | ND | + | ND | – | + | |
| Ser → pSer | ND | NT | NT | – | ND | ND | – | ND | – | |
| HLA-DQ2 | Arg →Cit | – | NT | NT | – | – | – | – | – | ND |
| Gln → Glu | – | NT | NT | – | ND | + | ND | + | + | |
| Ser → pSer | – | NT | NT | ND | ND | ND | – | – | – | |
| HLA-DR0301 | Arg →Cit | + | NT | NT | ND | NT | – | + | NT | – |
| Gln → Glu | ND | NT | NT | ND | NT | – | – | NT | ND | |
| Ser → pSer | ND | NT | NT | ND | NT | – | – | NT | – | |
| HLA-DR0401 | Arg →Cit | ND | NT | NT | + | NT | ND | + | NT | + |
| Gln → Glu | ND | NT | NT | + | NT | ND | ND | NT | – | |
| Ser → pSer | ND | NT | NT | ND | NT | – | NT | NT | – |
+ a significant increase in binding affinity, – a significant decrease in binding affinity, ND no difference in binding affinity, NT not tested
Fig. 1.
CD8+ T cell responses to modified beta cell peptides. CD8+ T responses to modified peptides derived from IGRP, GAD65, IA-2, and ZNT8 were assessed using an overnight CD137 upregulation assay utilizing T cell lines that were expanded from the peripheral blood of HLA-A2+ T1D patients. For analysis, cells were co-stained using anti-CD4 APC (FL-4 channel) and anti-CD137 PE (FL-2 channel). The amino acid sequence of each peptide (corresponding to the label above each panel) is indicated below each graph. In these sequences, X indicates citrulline and Z indicates phosphoserine. Quadrant boundaries were defined using unstimulated cells of the corresponding cell line
Mechanistic Importance of PTM Responses
As we have described here, many excellent studies have demonstrated an important role for PTM in generating antigenic diversity in T1D. However, it is important to consider the cellular processes by which beta cell proteins become modified. Generation of beta cell neo-antigens requires activation of the PTM enzymes that mediate the modifications described above, such as TG2 and PAD. TG2 and PAD are calcium-dependent enzymes that reside in the cytosol [60, 61]. To become activated, these enzymes require cytosolic calcium concentrations to be raised significantly higher than those found under conditions of normal cellular physiology. Indeed, these enzymes are most often activated under conditions of severe or prolonged cellular stress, such as endoplasmic reticulum (ER) stress [62–67]. Therefore, the modifications of beta cell proteins by TG2 or PAD would likely only occur under conditions of ER stress in beta cells.
Beta cells, as professional secretory cells, are uniquely susceptible to ER stress as a result of their normal physiology [68–77]. Secretory cells must translate and properly fold not only the proteins necessary for normal cellular maintenance, but also the proteins intended for export. For example, in response to increased blood glucose concentrations, beta cells increase the translation and folding of preproinsulin by 50-fold [78]. These dynamic increases in the demand for insulin production heavily burden the ER and cause heightened ER stress. Indeed, high levels of ER stress are observed in beta cells during post-prandial glucose-stimulated insulin synthesis [72, 73]. Therefore, normal insulin-secreting physiology alone significantly increases ER stress in beta cells. This physiological ER stress is observed in the pancreas from an early age. In transgenic ER stress reporter mice, the pancreas was the first tissue to exhibit high ER stress. This stress became evident as early as 16 days old [79]. Since TG2 and PAD become activated during ER stress, these enzymes may be more activated in beta cells than in nonsecretory cells. Once activated, these enzymes may modify beta cell proteins to generate the neo-antigens described in the studies reviewed above.
In addition to the high levels of inherent ER stress, the environmental triggers proposed to be associated with T1D onset further enhance beta cell ER stress. First, viral infection disrupts the gradient of calcium across the ER membrane [80–82] increasing cytosolic calcium concentrations. Second, chemicals such as streptozotocin and alloxan cause protein ADP-ribosylation [83] and reactive oxygen species (ROS) generation [84–86], both of which cause protein misfolding and increase cytosolic calcium [87, 88]. Third, ROS from either extracellular or intracellular sources releases calcium from the ER lumen into the cytosol [89–91]. As beta cell function decreases in T1D, dysglycemia leads to increased glucose sensing that, as discussed above, significantly increases insulin production and secretion [76]. Finally, pancreatic inflammation and cytokine production activate c-Jun N-terminal (JNK) mitogen-activated protein (MAP) kinase signaling pathways [92, 93]. Each of these mechanisms initiated by T1D environmental triggers increases beta cell ER stress. Therefore, although these environmental triggers may accelerate T1D through different mechanisms, all these factors exacerbate beta cell ER stress above the normal, physiological levels. This heightened ER stress may further activate TG2 and PAD enzymes, leading to modification of endogenous beta cell proteins and the generation of neo-antigens for the autoimmune response in T1D.
The ER stress-induced modification of beta cell proteins may also initiate a positive feedback loop. In celiac disease, the inflammatory environment established by the immune response to TG2-modified gliadin increases TG2 expression. Increased TG2 expression and activation in turn lead to the PTM of additional gliadin molecules, enhancing the immune response and inflammatory environment. Similarly, the generation of beta cell neo-antigens through PTM and the subsequent autoimmune response to these neo-antigens may exacerbate the local inflammatory environment. Local inflammation would cause higher beta cell ER stress and higher activation of TG2 and PAD. Active TG2 and PAD would modify additional beta cell proteins, generating neo-antigens to exacerbate the autoimmune response. The autoimmune cells that respond to the newly generated neo-antigens would again increase the local inflammatory environment, perpetuating the inflammatory-ER stress-PTM cycle until insufficient beta cell mass remains and T1D onset occurs.
Future Prospects and Important Unanswered Questions
Based on all that we have discussed, it is clear that increasing evidence supports the relevance and potential pathogenic role of T cell responses to PTM epitopes in T1D. A major question that remains unanswered is at what stage of the disease process T cells and autoantibodies that are specific for modified antigens appear. Conceptually, it could be argued that responses to modified self-antigens could be a very early initiating event in the loss of tolerance. In that scenario, modifying enzymes could be upregulated and activated through infection, ER stress, or other environmental factors leading to generation of modified self-proteins and autoimmune recognition. Alternatively, the earliest break in tolerance could involve unmodified primary antigens such as insulin and a first wave of autoimmune inflammation that subsequently triggers upregulation and activation of modifying TG2 and PAD enzymes that fuel further waves of autoimmune attack, epitope spreading, and disease progression. Differentiating between these two models will require longitudinal study of at-risk individuals carrying disease-associated HLA alleles. Such series of samples are difficult to obtain given the typically young age of the subjects, the low rate of disease incidence in the general population, and the volumes of blood required to detect rare antigen-specific T cells. However, efforts are currently underway to design and carry out such studies. In the meantime, we were able to perform an informative pilot study utilizing “long-term nonprogressing” subjects who are positive for one or more autoantibodies for many years. Intriguingly, when assessing the frequency of PTM-specific CD4+ T cells, these subjects represented an intermediate state between healthy antibody-negative controls and T1D subjects. Such individuals have experienced some loss of tolerance without progressing to develop diabetes or impaired glucose tolerance, implying a halting of autoimmune progression, perhaps due to effective immune regulation or the absence of a crucial environmental triggering event. Although further study of at-risk and recent-onset subjects is clearly needed, these observations raise the possibility that T cells recognizing modified self-epitopes may serve as an informative biomarker for disease progression. If this is indeed the case, interrupting the processes that lead to the upregulation of modifying enzymes and their subsequent activity could be relevant pathways for therapeutic intervention.
Conclusions
In T1D, there is clear evidence that self-tolerance is progressively lost, leading to an accumulation of autoantibodies and auto-reactive T cells that recognize increasing numbers of self-proteins. These autoreactive immune cells lead to either sustained inflammation or successive rounds of immune-mediated destruction that overcome the regulatory networks that would otherwise halt immune-mediated damage to pancreatic beta cells. In this review, we have presented a cross-section of emerging experimental evidence that underscores the important role that PTMs play in circumventing tolerance mechanisms in T1D. In this emerging paradigm, it is apparent that many different types of modification which affect multiple classes of antigens lead to preferential recognition of neo self-antigens by antibodies, CD4+ T cells, and CD8+ T cells. Although mechanistic studies of the at-risk population have been limited, current data support the contention that loss of tolerance due to PTMs represents an important checkpoint in the development of T1D. This current knowledge of the proteins, epitopes, and modifications that is relevant in T1D develops a framework for further study and provides an arsenal of effective tools for monitoring humoral and cellular responses to PTM epitopes during the development of T1D.
Footnotes
This article is part of the Topical Collection on Pathogenesis of Type 1 Diabetes
Compliance with Ethics Guidelines
Conflict of Interest John W. McGinty, Meghan L. Marré, and Veronique Bajzik declare that they have no conflict of interest.
Eddie A. James and Jon D. Piganelli report grants from the Juvenile Diabetes Research Foundation.
Human and Animal Rights and Informed Consent Informed consent was obtained from all individual participants included in the study.
This article does not contain any studies with animals performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
•• Of major importance
- 1.Eisenbarth GS. Type I, diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–8. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11:533–42. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S25–31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 4.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–72. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 5.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81:4264–7. doi: 10.1210/jcem.81.12.8954025. [DOI] [PubMed] [Google Scholar]
- 7.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the diabetes prevention trial-type 1. Diabetes Care. 2009;32:2269–74. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death. Diabetes. 2011;60:2112–9. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tree TI, Lawson J, Edwards H, Skowera A, Arif S, Roep BO, et al. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes. 2010;59:1451–60. doi: 10.2337/db09-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schloot NC, Willemen S, Duinkerken G, de Vries RR, Roep BO. Cloned T cells from a recent onset IDDM patient reactive with insulin B-chain. J Autoimmun. 1998;11:169–75. doi: 10.1006/jaut.1997.0183. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Chow IT, Sosinowski T, Torres-Chinn N, Greenbaum CJ, James EA, et al. Autoreactive T cells specific for insulin B:11-23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc Natl Acad Sci U S A. 2014;111:14840–5. doi: 10.1073/pnas.1416864111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durinovic-Belló I, Schlosser M, Riedl M, Maisel N, Rosinger S, Kalbacher H, et al. Pro- and anti-inflammatory cytokine production by autoimmune T cells against preproinsulin in HLA-DRB1*04, DQ8 type 1 diabetes. Diabetologia. 2004;47:439–50. doi: 10.1007/s00125-003-1315-1. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Danke N, Roti M, Huston L, Greenbaum C, Pihoker C, et al. CD4+ T cells from type 1 diabetic and healthy subjects exhibit different thresholds of activation to a naturally processed proinsulin epitope. J Autoimmun. 2008;31:30–41. doi: 10.1016/j.jaut.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Skowera A, Ellis RJ, Varela-Calviño R, Arif S, Huang GC, Van-Krinks C, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannering SI, Harrison LC, Williamson NA, Morris JS, Thearle DJ, Jensen KP, et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med. 2005;202:1191–7. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohmann T, Leslie RD, Hawa M, Geysen M, Rodda S, Londei M. Immunodominant epitopes of glutamic acid decarboxylase 65 and 67 in insulin-dependent diabetes mellitus. Lancet. 1994;343:1607–8. doi: 10.1016/s0140-6736(94)93061-9. [DOI] [PubMed] [Google Scholar]
- 18.Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, et al. GAD65-specific CD4+ T-cells with high antigen avidity are prevalent in peripheral blood of patients with type 1 diabetes. Diabetes. 2004;53:1987–94. doi: 10.2337/diabetes.53.8.1987. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, James EA, Sanda S, Greenbaum C, Kwok WW. CD4+ T cells recognize diverse epitopes within GAD65: implications for repertoire development and diabetes monitoring. Immunology. 2013;138:269–79. doi: 10.1111/imm.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes. 2014;63:3033–40. doi: 10.2337/db13-1952. [Demonstrates by direct ex vivo tetramer analysis and T cell cloning that citrullinated and deamidated self-epitopes are preferentially recognized by T cells from subjects with type 1 diabetes. Documents elevated frequencies and a Th1-like phenotype for Tcells that recognize modified epitopes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog BA, Ott PA, Dittrich MT, Quast S, Karulin AY, Kalbacher H, et al. Increased in vivo frequency of IA-2 peptide-reactive IFNgamma+/IL-4-T cells in type 1 diabetic subjects. J Autoimmun. 2004;23:45–54. doi: 10.1016/j.jaut.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Peakman M, Stevens EJ, Lohmann T, Narendran P, Dromey J, Alexander A, et al. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest. 1999;104:1449–57. doi: 10.1172/JCI7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scotto M, Afonso G, Larger E, Raverdy C, Lemonnier FA, Carel JC, et al. Zinc transporter (ZnT)8(186–194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia. 2012;55:2026–31. doi: 10.1007/s00125-012-2543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang M, Rockell J, Wagner R, Wenzlau JM, Yu L, Hutton JC, et al. Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol. 2011;186:6056–63. doi: 10.4049/jimmunol.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100:8384–8. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee R, Wagar D, Stephens TA, Lee-Chan E, Singh B. Identification of CD4+ T cell-specific epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related protein: a novel beta cell autoantigen in type 1 diabetes. J Immunol. 2005;174:5306–15. doi: 10.4049/jimmunol.174.9.5306. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Danke NA, Berger D, Reichstetter S, Reijonen H, Greenbaum C, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol. 2006;176:2781–9. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 28.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–31. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhou L, Li Y, Zhang J, Guo B, Meng G, et al. Identification of autoreactive CD8(+) T cell responses targeting chromogranin A in humanized NOD mice and type 1 diabetes patients. Clin Immunol. 2015;159:63–71. doi: 10.1016/j.clim.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 30•.Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes. 2012;61:3239–46. doi: 10.2337/db12-0112. [Provides the first evidence that antigen cross linking alters the antigenicity of self-peptides.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottlieb PA, Delong T, Baker RL, Fitzgerald-Miller L, Wagner R, Cook G, et al. Chromogranin A is a T cell antigen in human type 1 diabetes. J Autoimmun. 2014;50:38–41. doi: 10.1016/j.jaut.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Delong T, Baker RL, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Islet amyloid polypeptide is a target antigen for diabetogenic CD4+ T cells. Diabetes. 2011;60:2325–30. doi: 10.2337/db11-0288. [Presents the first direct evidence that IAPP is targeted by CD4+ T cells in autoimmune diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wicker LS, Chen SL, Nepom GT, Elliott JF, Freed DC, Bansal A, et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J Clin Invest. 1996;98:2597–603. doi: 10.1172/JCI119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L, Dong F, Miao D, Fouts AR, Wenzlau JM, Steck AK. Proinsulin/Insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care. 2013;36:2266–70. doi: 10.2337/dc12-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabbah E, Savola K, Kulmala P, Veijola R, Vähäsalo P, Karjalainen J, et al. Diabetes-associated autoantibodies in relation to clinical characteristics and natural course in children with newly diagnosed type 1 diabetes. The childhood diabetes in Finland study group. J Clin Endocrinol Metab. 1999;84:1534–9. doi: 10.1210/jcem.84.5.5669. [DOI] [PubMed] [Google Scholar]
- 36•.Hinman RM, Cambier JC. Role of B lymphocytes in the pathogenesis of type 1 diabetes. Curr Diab Rep. 2014;14:543–8. doi: 10.1007/s11892-014-0543-8. [Useful review discussing potential roles for B cells in the initiation of autoimmune diabetes.] [DOI] [PubMed] [Google Scholar]
- 37.Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28:847–58. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [Presents direct visual evidence that autoreactive Tcells infiltrate the pancreatic islets of patients with type 1 diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noble JA, Valdes AM, Varney MD, Carlson JA, Moonsamy P, Fear AL, et al. Type 1 diabetes genetics consortium. HLA class I and genetic susceptibility to type 1 diabetes: results from the type 1 diabetes genetics consortium. Diabetes. 2010;59:2972–9. doi: 10.2337/db10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–9. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 41.Peters B, Tong W, Sidney J, Sette A, Weng Z. Examining the independent binding assumption for binding of peptide epitopes to MHC-I molecules. Bioinformatics. 2003;19:1765–72. doi: 10.1093/bioinformatics/btg247. [DOI] [PubMed] [Google Scholar]
- 42.Knight RR, Dolton G, Kronenberg-Versteeg D, Eichmann M, Zhao M, Huang GC, et al. A distinct immunogenic region of glutamic acid decarboxylase 65 is naturally processed and presented by human islet cells to cytotoxic CD8 T cells. Clin Exp Immunol. 2015;179:100–7. doi: 10.1111/cei.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velthuis JH, Unger WW, Abreu JR, Duinkerken G, Franken K, Peakman M, et al. Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes. 2010;59:1721–30. doi: 10.2337/db09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skowera A, Ladell K, McLaren JE, Dolton G, Matthews KK, Gostick E, et al. β-cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes. 2015;64:916–25. doi: 10.2337/db14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111:217–23. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol. 2012;24:112–8. doi: 10.1016/j.coi.2011.12.003. [Useful review summarizing current knowledge about a variety of post-translational modifications that modulate immune recognition of self-proteins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Wal Y, Kooy Y, Van Veelen VP, Pena S, Mearin L, Papadopoulos G, et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–8. [PubMed] [Google Scholar]
- 48.Hovhannisyan Z, Weiss A, Martin A, Wiesner M, Tollefsen S, Yoshida K, et al. The role of HLA-DQ8 beta57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature. 2008;56:534–8. doi: 10.1038/nature07524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snir O, Widhe M, von Spee C, Lindberg J, Padyukov L, Lundberg K, et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLADRB1 alleles. Ann Rheum Dis. 2009;68:736–43. doi: 10.1136/ard.2008.091355. [DOI] [PubMed] [Google Scholar]
- 50.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 51•.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med. 2013;210:2569–82. doi: 10.1084/jem.20131241. [Presents a crystal structure that depicts the altered presentation of citrullinated peptides by HLA-DR4 proteins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–41. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 53.James EA, Moustakas AK, Bui J, Papadopoulos GK, Bondinas G, Buckner JH, et al. HLA-DR1001 presents “altered-self” peptides derived from joint-associated proteins by accepting citrulline in three of its binding pockets. Arthritis Rheum. 2010;62:2909–18. doi: 10.1002/art.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.James EA, Rieck M, Pieper J, Gebe JA, Yue BB, Tatum M, et al. Arthritis Rheum. 2014;66:1712–22. doi: 10.1002/art.38637. [Presents the first direct ex vivo analysis of citrulline specific T cells in arthritis. Documents a Th1 memory phenotype for these T cells and presents cross-sectional data indicating that the frequency of citrulline-specific T cells varies with disease duration and treatment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–77. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 56•.van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A, et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–47. doi: 10.2337/db12-1214. [Provides mass spectrometry data verifying that deamidated peptides can be eluted from human antigen presenting cells. Further demonstrates that such peptides are recognized by T cells in the context of DQ8 and the DQ2/8 transdimer.] [DOI] [PubMed] [Google Scholar]
- 57•.Rondas D, Crèvecoeur I, D'Hertog W, Ferreira GB, Staes A, Garg AD, et al. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes. 2015;64:573–86. doi: 10.2337/db14-0621. [Demonstrates selective recognition of citrullinated GRP78 by antibodies and autoreactive T cells in NOD mice.] [DOI] [PubMed] [Google Scholar]
- 58.Patel SD, Cope AP, Congia M, Chen TT, Kim E, Fugger L, et al. Identification of immunodominant T cell epitopes of human glutamic acid decarboxylase 65 by using HLA-DR(alpha1*0101, beta1*0401) transgenic mice. Proc Natl Acad Sci U S A. 1997;94:8082–7. doi: 10.1073/pnas.94.15.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gianfrani C, Troncone R, Mugione P, Cosentini E, De Pascale M, Faruolo C, et al. Celiac disease association with CD8+ T cell responses: identification of a novel gliadin-derived HLA-A2-restricted epitope. J Immunol. 2003;170:2719–26. doi: 10.4049/jimmunol.170.5.2719. [DOI] [PubMed] [Google Scholar]
- 60.Lesort M, Attanavanich K, Zhang J, Johnson GV. Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem. 1998;273:11991–4. doi: 10.1074/jbc.273.20.11991. [DOI] [PubMed] [Google Scholar]
- 61.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays. 2003;25:1106–18. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 62.Lentile R, Caccamo D, Griffin M. Tissue transglutaminase and the stress response. Amino Acids. 2007;33:385–94. doi: 10.1007/s00726-007-0517-0. [DOI] [PubMed] [Google Scholar]
- 63.Kojima S, Kuo TF, Tatsukawa H, Hirose S. Induction of cross-linking and silencing of Sp1 by transglutaminase during liver injury in ASH and NASH Via different ER stress pathways. Dig Dis. 2010;28:715–21. doi: 10.1159/000324278. [DOI] [PubMed] [Google Scholar]
- 64.Kuo TF, Tatsukawa H, Matsuura T, Nagatsuma K, Hirose S, Kojima S. Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J Cell Physiol. 2012;227:1130–7. doi: 10.1002/jcp.22833. [DOI] [PubMed] [Google Scholar]
- 65.Takahara H, Okamoto H, Sugawara K. Calcium-dependent properties of peptidylarginine deiminase from rabbit skeletal muscle. Agric Biol Chem. 1986;50:2899–904. [Google Scholar]
- 66.Verhaar R, Drukarch B, Bol JG, Jongenelen CA, Musters RJ, Wilhelmus MM. Increase in endoplasmic reticulum-associated tissue transglutaminase and enzymatic activation in a cellular model of Parkinson’s disease. Neurobiol Dis. 2012;45:839–50. doi: 10.1016/j.nbd.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Wilhelmus MM, Verhaar R, Andringa G, Bol JG, Cras P, Shan L, et al. Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson’s disease brain. Brain Pathol. 2011;21:130–9. doi: 10.1111/j.1750-3639.2010.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern Med. 2003;42:7–14. doi: 10.2169/internalmedicine.42.7. [DOI] [PubMed] [Google Scholar]
- 69.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 70.Fonseca SG, Lipson KL, Urano F. Endoplasmic reticulum stress signaling in pancreatic beta-cells. Antioxid Redox Signal. 2007;9:2335–44. doi: 10.1089/ars.2007.1790. [DOI] [PubMed] [Google Scholar]
- 71.Kim MK, Kim HS, Lee IK, Park KG. Endoplasmic reticulum stress and insulin biosynthesis: a review. Exp Diabetes Res. 2012:509437. doi: 10.1155/2012/509437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–54. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Lipson KL, Fonseca SG, Urano F. Endoplasmic reticulum stress-induced apoptosis and auto-immunity in diabetes. Curr Mol Med. 2006;6:71–7. doi: 10.2174/156652406775574613. [DOI] [PubMed] [Google Scholar]
- 74.Ortsater H, Sjoholm A. A busy cell—endoplasmic reticulum stress in the pancreatic beta-cell. Mol Cell Endocrinol. 2007;277:1–5. doi: 10.1016/j.mce.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Teodoro T, Odisho T, Sidorova E, Volchuk A. Pancreatic beta-cells depend on basal expression of active ATF6alpha-p50 for cell survival even under nonstress conditions. Am J Physiol Cell Physiol. 2012;302:C992–1003. doi: 10.1152/ajpcell.00160.2011. [DOI] [PubMed] [Google Scholar]
- 76.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic beta-cell. Diabetes Obes Metab. 2010;12:48–57. doi: 10.1111/j.1463-1326.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- 77.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–84. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 78.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–33. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 80.van Kuppeveld FJ, de Jong AS, Melchers WJ, Willems PH. Enterovirus protein 2B Po(U)Res out the calcium: a viral strategy to survive? Trends Microbiol. 2005;13:41–4. doi: 10.1016/j.tim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 81.van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, et al. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–32. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Kuppeveld FJ, Melchers WJ, Willems PH, Gadella Jr TW. Homomultimerization of the coxsackievirus 2B protein in living cells visualized by fluorescence resonance energy transfer microscopy. J Virol. 2002;76:9446–56. doi: 10.1128/JVI.76.18.9446-9456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandler S, Swenne I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia. 1983;25:444–7. doi: 10.1007/BF00282526. [DOI] [PubMed] [Google Scholar]
- 84.Bedoya FJ, Solano F, Lucas M. N-monomethyl-arginine and nicotinamide prevent streptozotocin-induced double strand DNA break formation in pancreatic rat islets. Experientia. 1996;52:344–7. doi: 10.1007/BF01919538. [DOI] [PubMed] [Google Scholar]
- 85.Heikkila RE, Winston B, Cohen G. Alloxan-induced diabetes-evidence for hydroxyl radical as a cytotoxic intermediate. Biochem Pharmacol. 1976;25:1085–92. doi: 10.1016/0006-2952(76)90502-5. [DOI] [PubMed] [Google Scholar]
- 86.Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. Streptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets. H2O2 as mediator for DNA fragmentation. Diabetes. 1991;40:1141–5. doi: 10.2337/diab.40.9.1141. [DOI] [PubMed] [Google Scholar]
- 87.Kim HR, Rho HW, Park BH, Park JW, Kim JS, Kim UH, et al. Role of Ca2+ in alloxan-induced pancreatic beta-cell damage. Biochim Biophys Acta. 1994;1227:87–91. doi: 10.1016/0925-4439(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 88.Park BH, Rho HW, Park JW, Cho CG, Kim JS, Chung HT, et al. Protective mechanism of glucose against alloxan-induced pancreatic beta-cell damage. Biochem Biophys Res Commun. 1995;210:1–6. doi: 10.1006/bbrc.1995.1619. [DOI] [PubMed] [Google Scholar]
- 89.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–63. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 90.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 91.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 92.Lee H, Park MT, Choi BH, Oh ET, Song MJ, Lee J, et al. Endoplasmic reticulum stress-induced JNK activation is a critical event leading to mitochondria-mediated cell death caused by beta-lapachone treatment. PLoS One. 2011;6:e21533. doi: 10.1371/journal.pone.0021533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Q, Zhang H, Zhao B, Fei H. IL-1beta caused pancreatic beta-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the C-Jun N-terminal kinase pathway. Mol Cell Biochem. 2009;324:183–90. doi: 10.1007/s11010-008-9997-9. [DOI] [PubMed] [Google Scholar]