Abstract
Notable findings point to the significance of the dynorphin peptide neurotransmitter in chronic pain. Spinal dynorphin neuropeptide levels are elevated during development of chronic pain. Importantly, knockout of the dynorphin gene prevents development of chronic pain in mice, but acute nociception is unaffected. Intrathecal (IT) administration of opioid and non-opioid dynorphin peptides initiate allodynia through a non-opioid receptor mechanism; furthermore, anti-dynorphin antibodies administered by the IT route attenuate chronic pain. Thus, this review presents the compelling evidence in the field supporting the role of dynorphin in facilitating the development of a persistent pain state. These observations raise the question of the control mechanisms responsible for the upregulation of spinal dynorphin leading to chronic pain development. Also, spinal dynorphin regulation of downstream signaling molecules may be implicated in hyperpathic states. Therapeutic strategies to reduce spinal dynorphin may provide a non-addictive approach to improve the devastating condition of chronic pain that occurs in numerous human diseases.
Keywords: Dynorphin, chronic pain, neuron, glia, spinal cord, secretory vesicles, spinal ganglia
I. INTRODUCTION
An acute, high intensity, potentially tissue injuring stimulus will activate well-characterized afferent circuits to generate a pain state. This pain state is characterized by a constellation of physiological responses (autonomic, hormonal and motor withdrawal/escape), and accompanied by a psychological state possessed of a negatively reinforcing component, revealed by escape behavior and verbal report. In the face of tissue injury and inflammation, there is the development of an ongoing pain, which typically resolves over days to weeks in parallel with wound healing. It is increasingly appreciated that in many instances, resolution of injury and inflammation may not lead to a resolution of the pain state (1, 2). In this manner there appears to be a functional similarity of persistent inflammation with nerve injury, which leads to enduring changes in function and a persistent or chronic pain state.
This development of a chronic pain state is a matter of major public health concern. There is a high prevalence of chronic pain in the population with estimates ranging from 11% to over 50% (3, 4) and a higher instance among the economically disadvantaged and women (3, 5). Decades of studies have led to the conclusion that chronic pain is not simply a persistence of the nociceptive processing that occurs with an acute high intensity stimulus (Fig. 1, 2), but arises from secondary adaptations in the somatosensory system that persist after the initiating nerve injury or inflammatory condition resolves (6, 7) (Figure 3). Rodent models have been established to mimic the underlying pathologies arising secondary to persistent inflammatory conditions and nerve injury, and to define these changes in functional systems responsible for behavioral manifestations that characterize the pain state observed in humans (8, 9).
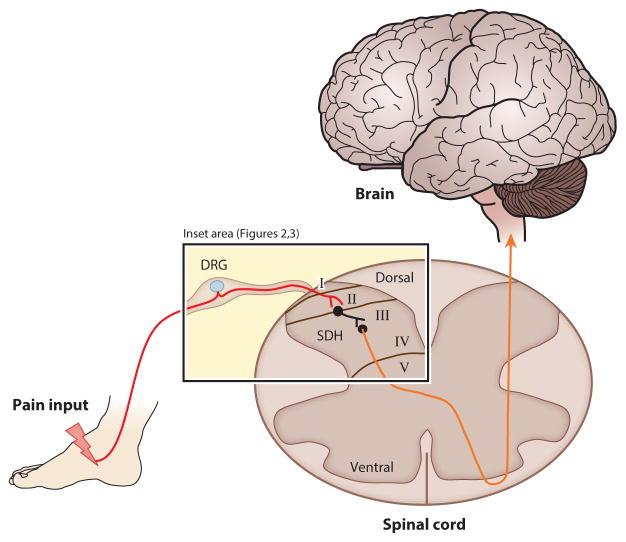
Figure 1. Anatomical regions of ascending pain processing via the spinal cord.
Pain information from a noxious peripheral stimulus travels along the nociceptor afferent (red line) into the primary sensory neuron soma, located in the Dorsal Root Ganglion (DRG) adjacent to the spine. The primary sensory neuron then synapses onto the sensory lamina (I/II) of the Spinal Dorsal Horn (SDH). Here, interneurons will modulate pain signals and synapse onto projection neurons in lamina III. This neuron integrates information from spinal cells in lamina I and II, decussates in the spinal cord to the contralateral spinothalamic tract (orange line) and sends pain information to somatosensory areas of the brain. Details of the inset area in short-term and chronic pain states are illustrated in Figures 2 and 3.
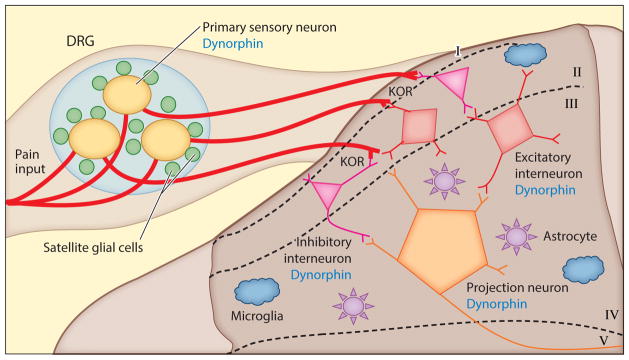
Figure 2. Short-term post-injury pain processing involves spinal glial and neuronal cells.
In acute pain processing, nociceptive information (red line) travels from the peripheral nerve ending into the primary sensory neuron cell soma (yellow) in the DRG. Excitatory factors (e.g. K+, ATP, substance P) are released from the cell soma, and the immediate area is modulated by chains of Satellite Glial Cells (SGCs, green) surrounding the neuron cell soma, which responds to these factors through cognate receptors. In turn, SGCs can further release excitatory factors (e.g. IL-1β, TNFα) propagating primary sensory neuron transmission of glutamate, dynorphin, and other transmitters (147) into the sensory lamina of SDH of the spinal cord. Endogenous opioid peptides are released from inhibitory interneurons (pink) and excitatory interneurons (red). Primary afferents are modulated presynaptically through inhibitory opioid receptors, including KOR (86, 148). GABAergic interneurons and glutamatergic interneurons express dynorphin (86, 88, 148). These interneurons will integrate signals onto the lamina III projection neurons (orange). Astrocytes (purple) express KOR and modulate the synaptic environment, maintaining homeostasis. Microglia (blue) are ramified and surveillant in the SDH, with no critical role in acute pain processing established to date.
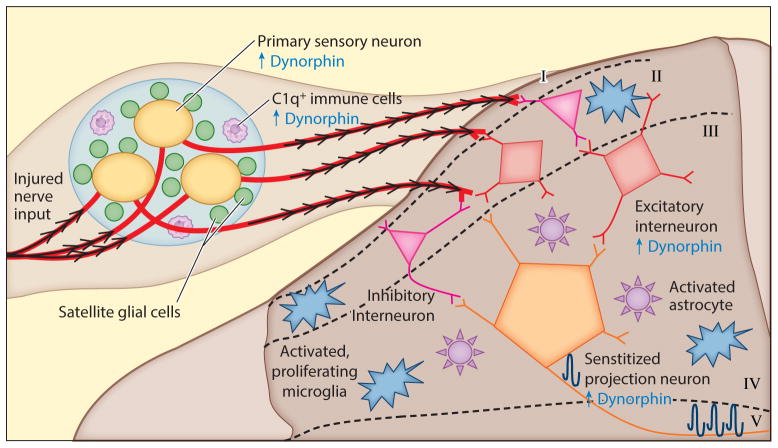
Figure 3. Chronic pain processing involving spinal glial and neuronal cells.
In chronic pain processing, activated glia and neurons release excitatory and inflammatory factors into the extracellular environment of the DRG and SDH causing “central sensitization,” persistent hyperexcitability of neurons (BDNF, substance P, nitric oxide, glutamate, CGRP, altered ion channel composition on neurons leading to facilitated depolarization) (14, 15). Continued input from the injured nerve activates DRG neurons (yellow), which increase synaptic release of excitatory and inflammatory transmitters (ATP, substance P, nociceptin, cytokines, chemokines, nitric oxide) (147). SGCs (green) persistently release excitatory neuropeptides (substance P, CGRP) and inflammatory molecules (e.g. IL-1β) (147) and have altered membrane conductance. Together, this produces an allodynic phenotype. C1q+ cells (white) proliferate in the DRG and induce substantial dynorphin upregulation (39). In the SDH, synaptic inhibition by inhibitory interneurons (pink) is additionally lost (149), and excitatory interneurons (red) increase glutamate release onto projection neurons (orange) (16). Glial cells play a strong role in chronic pain processing by proliferating) and releasing inflammatory chemokines, cytokines and excitatory neurotransmitters which affect synaptic activity of spinal neurons (15). The altered extracellular environment in chronic pain processing in the sensory lamina leads to central sensitization of lamina III projection neurons, amplifying pain signals (16). These findings demonstrate that spinal neurons and glia participate in chronic pain processing.
This review compiles evidence that dynorphin, an endogenous opioid peptide neurotransmitter (neuropeptide), participates as a key factor in the transition from the acute pain condition to the chronic pain state through unique non-opioid spinal mechanisms. Findings in the field that illustrate the role of spinal dynorphin in chronic pain development have been under-appreciated. This review, therefore, highlights the salient scientific data that provide strong support for involvement of dynorphin in chronic pain. The observed upregulation of spinal dynorphin in chronic pain indicates that that dynorphin biosynthesis intervenes critically in the neural circuitry of chronic pain, together with proinflammatory and related factors in chronic pain. The current knowledge about the neurobiology of spinal dynorphin in chronic pain supports a dynorphin targeted gene silencing strategy to block dynorphin upregulation as a rational drug approach for the treatment of chronic pain, without addiction.
II. SPINAL NEUROBIOLOGY OF CHRONIC PAIN IS DISTINCT FROM ACUTE PAIN
Heuristically, a pain state, as defined by the behavior of the organism, may be generated by three general classes of events: (1) events evoked by an acute and potentially injurious high intensity stimulus, (2) events secondary to tissue injury and inflammation and (3) events secondary to nerve injury. In the following paragraphs, we will provide a brief overview of the associated mechanisms.
1. Acute pain
An acute high intensity stimulus will generate an acute pain state, the magnitude and duration of which coincides with the site, time course, and intensity of the stimulus. The essential underlying mechanism is the activation of populations of high threshold sensory afferents (A∂/C afferent) with the frequency of discharge increasing monotonically with increasing stimulus intensity. This acute afferent traffic provides excitatory input mediated by the release of glutamate in populations of dorsal horn neurons lying superficial in the dorsal horn (lamina I: marginal layer) and the distal dendrites of more deeply lying dorsal horn neurons (Lamina V) (figures 1 and 2). These deep neurons also receive convergent input from large low threshold afferents. Thus, these cells respond with increasing frequency over a wide range from low (innocuous) to high intensity (noxious) and are referred to as wide dynamic range neurons. (10, 11).
2. Post tissue injury pain
Should the application of the stimulus persist, tissue injury will occur. The associated pain states display a characteristic behavioral phenotype: i) an ongoing pain state in the absence of the initiating stimulus (dysesthesia); ii) a low intensity stimulus (allodynia); iiii) an enhancement of the pain behavior initiated by a moderately intense stimulus (hyperalgesia); iv) pain referred to dermatomal areas distant to the injury (extraterritorial pain). The associated pain state will parallel the time-course of stimulus application and wound healing, often days to weeks. (9, 12). Mechanistically, the ongoing sensation following the injury reflects ongoing afferent input generated by the release of proinflammatory products proximal to the site of injury, which act upon eponymous receptors present on the terminals of the small high threshold afferents (13). The enhanced response of the organism to a stimulus applied to the injury site (1° hyperalgesia) is a result of the phosphorylation of channels and receptors by kinases at the afferent nerve terminal and the initiation of a variety of subsequent adaptive changes that facilitate neural responsiveness to subsequent stimulation. These adaptations involve neuronal and glia reorganization of spinal circuits in the spinal dorsal horn (SDH) and the dorsal root ganglia (DRG), and have been termed “central sensitization” (14, 15). Central sensitization leads to a long-term potentiation (LTP)-like synaptic strengthening leading to an enhanced activation of spinal sensory projection neurons (16). This central sensitization state involves activation of spinal glia and neurons to release excitatory and neuroinflammatory factors into the DRG and SDH (illustrated in figure 3). As primary afferents display extrasegmental collateral projections in to the dorsal horn (17), enhanced excitability of 2nd order neurons in a given segment may increase the responsiveness of these neurons to primary collateral inputs. In this case, these neurons may now be driven by afferent input from adjacent, non injured dermatomes and provide a mechanisms of a pain state being driven by input from an adjacent non-injured region (e.g. 2nd hyperalgesia or allodynia) (18).
3. Nerve injury evoked pain
Anomalously, injury to the nerve (as in a mononeuropathy) or the distal terminals (as in a polyneuropathy) can lead to chronic changes characterized by an ongoing pain state (dysesthesia) and an enhanced response to an otherwise innocuous, typically mechanical, stimulus (e.g tactile allodynia) (6). The underlying mechanisms of these changes in processing may be broadly summarized in terms of the appearance of ongoing afferent traffic arising from the neuroma formed at the nerve injury site and from the dorsal root ganglion of the injured axon. The mechanical sensitivity is believed to be mediated by large low threshold mechanically sensitive primary afferents (Aβ). The appearance of ectopic activity in the afferent after injury reflects the large scale increase in the expression of channels (e.g. sodium and calcium) and receptors (e.g., adrenergic, TNF) in the neuroma and DRG and the migration of inflammatory cells into the DRG and dorsal horn (19, 20). In the spinal dorsal horn, massive and persistent activation of glia (astrocytes and microglia) are noted (21, 22). Clinically, the neuropathic state displays important differences in their pharmacological management as compared to inflammatory pain states (23).
4. Chronic pain states - A convergence of tissue and nerve injury mechanisms
In contrast to the acute phase after tissue injury and inflammation, evidence has evolved suggesting that pain states may evolve that persist after the resolution of the original injury state (1, 2). Thus, in animal models of persistent inflammation (24, 25) there is a hyperpathic state that parallels the inflammation. However after resolution of the inflammation, the hyperpathia remains. Importantly, the early, but not late phase hyperpathia, is sensitive to a variety of anti-inflammatory agents. In the late phase however, agents with efficacy in the neuropathic facilitated state remain active. This observation corresponds with the lack of covariance in arthritic patients between pain and the state of joint inflammation (26, 27). There is now a growing appreciation that in the face of injury states, such as in arthritic pain (28), reorganization of mechanisms leading to a chronic pain state occur in combination with mechanisms that converge with those observed in the face of nerve injury.
III. EVIDENCE FOR THE KEY ROLE OF SPINAL DYNORPHIN IN THE DEVELOPMENT OF CHRONIC PAIN
Extending from the general principles outlined above, we now consider the potential role played by dynorphin in the systems underlying the evolution of a persistent pain state.
Upregulation of spinal dynorphin
Findings in the pain research field have demonstrated strong evidence supporting the hypothesis that upregulation of dynorphin occurs in spinal pain processing cells as a critical event in the transition to chronic pain. First, increases in spinal dynorphin occur in chronic pain models of nerve injury and inflammation (29–32) (discussed in more detail below), consistent with elevated dynorphin in CSF of cohorts of chronic pain patients (33, 34) and in polyarthritic rats (35, 36). During elevated spinal dynorphin in rodents caused by nerve injury and peripheral inflammation, elevation of spinal preprodynorphin (PPD) gene expression occurs approximately 4 days following injury, and persists while the chronic pain state is maintained from weeks to months after injury (29, 30). Increased spinal levels of the active dynorphins A and B occur in several chronic pain models of nerve injury and inflammation (Table 1) together with the adaptations leading to chronic pain, occurring 5–7 days following injury. The elevated dynorphins persist while chronic pain sensations are elevated, occurring up to several months (30, 37, 38). Elevated dynorphin expression is also reported in the DRG (39, 40). Because primary afferents innervate neurons and glia in Lamina I and II in the SDH, this source of spinal dynorphin may additionally contribute to the elevated levels of dynorphin neuropeptides in the SDH that cause phenotypes initiated by peripheral nerve injury and inflammation (Figure 3). Furthermore, a single intrathecal (IT) spinal delivery of opioid (e.g. Dyn 11–17) and non-opioid dynorphin (e.g. des-Tyr-Dyn 1-17) peptides in rodents induces a robust allodynia lasting more than 45 days ((41, 42), Table 2B), mimicking the behavioral phenotype of nerve injury and inflammation models. Thus, IT dynorphin increases extracellular spinal dynorphin and, thereby, facilitates chronic pain development (41, 43).
Table 1.
Upregulation of Spinal Dynorphins in Chronic Pain
| Pain Model | Phase I, Acute Pain (Prehyperpathic) | Phase II, Chronic Pain (Hyperpathic) | References |
|---|---|---|---|
| Nerve Injury Models | |||
| L5/L6 Spinal Nerve Ligation, mouse | No change compared to sham |
 Dyn A(1-17) Dyn A(1-17) Dyn B(1-13) Dyn B(1-13)Lamina I,II,V |
(31) (37) (32) |
| Chronic Constriction Injury of the Sciatic Nerve, rat | No change compared to sham |
 Dyn A(1-8) Dyn A(1-8) |
(38) |
| Sciatic Cryoneurolysis, rat | No change compared to sham |
 Dyn Dyn
 Dyn A(1-8) Dyn A(1-8)Lamina I, II, V |
(84) |
| Inflammation Models | |||
| Carrageenan in paw, rat | No change compared to sham |
 Dyn Dyn
 Dyn A(1-8) Dyn A(1-8)No change- Dyn B (1-13) |
(30) (32) |
| Freund’s Adjuvant in paw or tail, rat | No change compared to sham |
 Dyn Dyn
 Dyn A(1-8) Dyn A(1-8) Dyn A(1-17) Dyn A(1-17) |
(139) (140) |
| Mycobacterium in paw or tail, rat | No change compared to sham |
 Dyn Dyn
 Dyn A(1-8) Dyn A(1-8) Dyn A(1-17) Dyn A(1-17) Dyn A(1-13) Dyn A(1-13) Dyn A(1-17), Lam I/II Dyn A(1-17), Lam I/II αNeo-Endorphin αNeo-Endorphin |
(139) (140) (141) (142) (143) |
Levels of spinal dynorphin (Dyn) peptides were assessed in rodent models of acute and chronic pain in nerve injury and inflammatory states. Phase I acute pain (prehyperpathic) is described as pain responses that may be elevated due to nociceptor transmission at the injury or inflammation site, but is reduced as the injury site heals. Phase II chronic pain (hyperpathic) has an elevated pain threshold and abnormal pain states that persist after the injury or inflammation heals due to sensitization of central nervous system pain processing neurons. This phase develops about 4–7 days post-injury and lasts several months. In models of nerve injury and inflammation, dynorphin levels are consistently found to be elevated in Phase II chronic pain states.
Table 2.
Spinal Intrathecal (IT) Administration of Dynorphin Peptides: Effects on Short-Term Compared to Long-term Pain Responses in Rodents
| 2.A. Effects of IT Dynorphins on Short-term Pain Responses | |||||
|---|---|---|---|---|---|
| Receptor System | IT Dynorphin Peptide | Behavioral Test | Behavioral Effect of IT Dynorphin | Blocked by Receptor Antagonist | References |
| KOR | Dyn A (25 nmol), rat | Thermal Hyperalgesia (up to 1 h) | Analgesia | MR1452, Naloxone | (60) |
| KOR | Dyn A (25 nmol), rat | Pain Vocalization (up to 1 h) | Analgesia | MR1452, Naloxone | (60) |
| KOR | Dyn B (20 nmol), rat | Thermal Hyperalgesia (up to 1.5 h) | Analgesia | Naloxone | (56) |
| KOR | Dyn A (1-13) (0.47–4.7 nmol), rat | Thermal Hyperalgesia (up to 2 h) | Analgesia | Naloxone | (144) |
| KOR | Dyn A (1-8) (51 nmol), rat | Thermal Hyperalgesia (up to 2 h) | Analgesia | Naloxone | (144) |
| KOR | Dyn A (1-17) (4.65 nmol), rat | Thermal Hyperalgesia (up to 2 h) | Analgesia | Naloxone | (144) |
| KOR | Dyn A (1-17) (20 nmol), rat | Thermal Hyperalgesia (up to 2 h) | Analgesia | Naloxone (partial) | (145) |
| KOR | Dyn A (1-8) (2.3–9.3 nmol), rat | Thermal Hyperalgesia (up to 24 h) | Analgesia | U50488, Naloxone | (146) |
| KOR | Dyn A (1-13) (9.3 nmol), rat | Thermal Hyperalgesia (up to 24 h) | Analgesia | U50488, Naloxone | (146) |
| Bradykinin 2 Receptor | Dyn A (2-13) (3 nmol), rat | Thermal Hyperalgesia (up to 30 min) | Hyperalgesia | HOE140 | (45) |
| Bradykinin 1 Receptor | Dyn A (2-13) (3 nmol), rat | Thermal Hyperalgesia (up to 30 min) | Hyperalgesia | DALBK (partial) | (45) |
| Bradykinin 2 Receptor | Dyn A (2-13) (3 nmol), rat | Thermal Hyperalgesia (up to 2 h) | Hyperalgesia | des-Arg-Dyn A (4-11) | (47) |
| 2.B. Effects of IT Dynorphins on Long-term Pain Responses | |||||
|---|---|---|---|---|---|
| Receptor System | IT Dynorphin Peptide | Behavioral Test (up to 60 days) | Behavioral Effect of IT Dynorphin | Blocked by Receptor Antagonist | Reference |
| NMDA Receptor | Dyn A (1-17) (15 nmol), rat | Von Frey Assay for Tactile Allodynia | Allodynia | MK-801 | (41) |
| NMDA Receptor | Dyn A (1-13) (15 nmol), rat | Von Frey Assay for Tactile Allodynia | Allodynia | MK-801 | (41) |
| NMDA Receptor | Dyn A (2-17) (15 nmol), rat | Von Frey Assay for Tactile Allodynia | Allodynia | MK-801 | (41) |
| NMDA Receptor | Dyn A (2-13) (15 nmol), rat | Von Frey Assay for Tactile Allodynia | Allodynia | MK-801 | (41) |
| NMDA Receptor | Dyn A (1-17) (3 nmol, mouse) | Von Frey Assay for Tactile Allodynia | Allodynia | MK-801, LY235959 | (42) |
| NMDA Receptor | Dyn A (2-17) (3 nmol, mouse) | Von Frey Assay for Tactile Allodynia | Allodynia | MK-801, LY235959 | (42) |
| Bradykinin 1 Receptor | None, SNL mouse model of chronic pain, | Thermal hyperalgesia | Hyperalgesia | DALBK | (45) |
| Bradykinin 2 Receptor | None, SNL mouse model of chronic pain | Von Frey Assay for Tactile Allodynia | Allodynia | HOE140 | (45) |
| Bradykinin 2 Receptor | None, SNL mouse model of chronic pain | Thermal hyperalgesia | Hyperalgesia | HOE140 | (45) |
| Bradykinin 1 Receptor | None, CFA paw inflammation mouse model of chronic pain | Thermal hyperalgesia | Hyperalgesia | DALBK | (46) |
| Bradykinin 2 receptor | None, CFA paw inflammation mouse model of chronic pain | Thermal hyperalgesia | Hyperalgesia | HOE140 | (46) |
(A) In short-term pain responses (< 2 days), IT administration of dynorphin peptides resulted in analgesia through the KOR and bradykinin receptors. Dynorphin peptides were administered to rodents (rats or mice) by the spinal intrathecal (IT) route, and the effects of the IT dynorphins on pain responses in behavioral pain assays were compared to vehicle-IT animals at different times after peptide administration.
(B) In longer-term pain responses (> 2 days), IT dynorphin in naïve animals results in allodynia through the NMDA receptor, shown by reversibility by a NMDA-R antagonist (but not by an antagonist of KOR). Of note, Dyn A (2-17 or 2-13), have no activity at the KOR, but have activity at NMDA-R and bradykinin receptor supporting their roles in excitatory transmission in nociceptive processing. In mice subjected to chronic pain by L5 SNL (spinal nerve ligation) or CFA-induced paw inflammation, which result in elevated spinal dynorphin levels, the observed hyperalgesia or allodynia was blocked by bradykinin receptor antagonists (45, 46).
Reduction of dynorphin attenuates chronic pain development
Key experiments testing the hypothesis that spinal dynorphin upregulation is essential for development of chronic pain have used strategies to deplete dynorphin in vivo in animals prepared with a mononeuropathic injury. Knockout of the prodynorphin (PPD) gene results in mice that do not show chronic pain behaviors following nerve injury (> 7 days), but have similar acute pain responses to that in wild-type mice (31). Also, in nerve-injured rodent strains that lack dynorphin upregulation (44, 45), there is no chronic pain phenotype. In further agreement with these data, intrathecal (IT) delivery of anti-dynorphin A (Dyn A) antibodies diminishes pain hypersensitivity (37, 44). These findings demonstrate that elevation of spinal dynorphin is a key factor participating in the development of chronic pain.
IV. SPINAL INTRATHECAL DYNORPHIN PHARMACOLOGY DEMONSTRATES A NON-OPIOID MECHANISM IN CHRONIC PAIN
Intrathecal dynorphin displays a non-opioid mechanism in chronic pain development
Behavioral pharmacology investigations into spinal receptor systems of dynorphin peptides have revealed evidence for multiple receptors through which dynorphin mediates spinal nociceptive processing. These studies were conducted at times corresponding to short-term acute (< 2 days) and long-term chronic pain (> 2 days) (Table 2). In studies of short-term pain responses, intrathecal (IT) spinal administration of dynorphin peptides during thermal hyperalgesia resulted in modulated nociceptive responses involving the κ-opioid receptor (KOR) and the bradykinin (BK) receptor (45–47) (Table 2A). It is of interest to note that Dyn A and Dyn B produced analgesia through KOR based on blockade by the antagonist naloxone. But IT administration of the N-terminally modified des-Tyr-Dyn A-(2-13) resulted in hyperalgesia that was blocked by an antagonist to the BK receptor (45–47).
In long-term studies, the effects of a single IT administration of dynorphin peptides on von Frey induced tactile allodynia was assessed in naïve mice and mice subjected to chronic pain (Table 2B). In naïve rats, IT Dyn A(1-17) and Dyn A(1-13) produced allodynia that was blocked by an antagonist to the NMDA receptor. These long-term effects of IT dynorphins were not affected by naloxone, indicating a non-opioid mechanism that does not involve KOR (41, 42). In mouse chronic pain models (spinal nerve ligation (SNL) and CFA paw inflammation) the developed hyperalgesia was blocked by antagonists to the bradkykinin receptor when there is a sustained elevation of spinal dynorphin during maintenance of neuropathic pain (45, 46). These findings suggest non-opioid dynorphin mechanisms occurring in chronic pain development.
Dynorphin pharmacology: variant sensitivities and effects
Dynorphin was first identified by Goldstein and colleagues (48, 49) as a novel porcine pituitary peptide containing the sequence for Leu-enkephalin (Figure 4A) (50). Dynorphin interacted with a naloxone-sensitive opioid receptor having κ-opioid characteristics (51). Early work showed κ-opioid receptor (KOR) activation by various benzomorphans suppressed contraction in ex vivo bioassays (52) and reduced nociceptive processing in vivo after systemic (53) and spinal (54, 55) delivery.
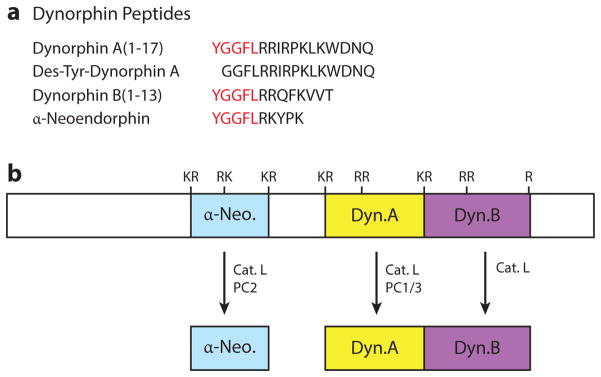
Figure 4. Bioactive dynorphin peptides are derived from the prodynorphin precursor.
(A) Dynorphin neuropeptides. The primary sequences of the dynorphin neuropeptides are shown. They share the sequence of Leu-enkephalin (red).
(B) Protease processing of prodynorphin to generate active dynorphins. Proteases that participate in converting prodynorphin to generate dynorphin A, dynorphin B and α-neoendorphin are illustrated, based largely on protease gene knockout studies (74, 76, 150). The differential utilization of cathepsin L and the proprotein convertases (PC1/3 and PC2) to generate each of the dynorphin species – dynorphin A, dynorphin B, and α-neoendorphin – are illustrated.
In spite of evidence supporting the association of dynorphin as an agonist at the KOR (56, 57) (i.e. reversible by naloxone and KOR specific antagonists, Table 2A) associated with the actions of non-peptides delivered IT (e.g. U50,488, MR 1452, or ethylkeotcyclazocine), spinal IT delivery of dynorphins has complex effects (54, 55). Among these findings, interpretation of analgesic actions of spinal IT delivered dynorphin peptides in behavioral pain assays relying on reflexive movement away from a noxious stimulus are confounded by reports of prolonged motor dysfunction (58, 59). Two studies utilized vocalization in response to an electrical shock applied to the tail as an alternative assessment of Dyn A(1-13)-mediated analgesia that does not rely on movement (60, 61). These studies reported a transient Dyn A-mediated analgesia up to one hour that was reversible by a KOR antagonist (60). But studies of IT des-Tyr-Dyn A showed short-term hyperalgesic responses that were not naloxone reversible (Table 2A) (62, 63).
Excitatory actions of dynorphins
Single unit recording in the dorsal horn frequently showed both excitation and inhibition with dynorphin that were naloxone insensitive, in contrast to suppressant effects produced by κ-opioid agonists (64). These observations suggested excitatory effects of spinal dynorphin, leading to an apparent neurotoxicity (65, 66), which supported the notion that the dynorphin peptide could exert a paradoxical excitatory effect upon neuronal function in general and spinal activity in particular (46, 67, 68). Similar findings in other studies (Table 2) showed that spinal IT delivery of des-Tyr-dynorphins caused acute hyperalgesia through NMDA-R (67), and these actions were not reversible by an antagonist of KOR. Surprisingly, big dynorphin, which is an intermediate precursor of Dyn A and Dyn B containing the entire sequence for both peptides, has potent excitatory activity at the NMDA-receptor but is not naloxone reversible (62, 63).
Potential mechanisms mediating paralysis and neurotoxicity have also suggested paradoxical excitatory effects through KOR and NMDA-R (69). Dyn A(1-13) causes extracellular release of excitatory amino acids through both receptor systems, although a more prominent mechanism of dynorphin-induced neurotoxicity and necrosis in dorsal horn neurons appears to be mediated through NMDA-R mediated release of aspartate in particular (69).
An additional excitatory component by both Tyr- and des-Tyr-dynorphin species is through NMDA-R mediated release of prostaglandins through COX-2 activation (70). These results indicate that the acute neurotoxic, paralytic and excitatory effects of dynorphin are complex, and may be mediated by both KOR and NMDA-R receptor systems. Downstream effects of IT dynorphin-induced hyperpathias are not well defined, but involve elevation of nitric oxide (71) and proinflammatory cytokines through NF-κB transcription in persistent allodynia (72). Furthermore, if IT dynorphin causes neuronal necrosis, and the behavioral output is hyperalgesic, one could hypothesize that the neurons affected by elevated spinal dynorphin are involved in an inhibitory mechanism of nociceptive processing.
It now seems apparent that this excitatory component of the effects of endogenous dynorphins may play an important role in the evolution of the chronic pain state (39, 73). Studies investigating the spinal receptor systems of dynorphin in chronic pain behavior show that a single spinal IT injection of Dyn A or des-Tyr-Dyn A produces allodynia that lasts over 45 days and which is reversed by NMDA receptor antagonists, but not by pan opioid or KOR preferring antagonists (41, 42). Therefore, exogenous spinal delivery of dynorphin causes an analogous profile of chronic pain behavior as assessed in most nerve injury and inflammation models that also show elevated spinal dynorphin levels in chronic pain (findings summarized in Table 1), and upon which expression of dynorphin is dependent (31). The observation that persistent abnormal pain caused by IT dynorphin is NMDA-R antagonist reversible would support post-synaptic excitatory effects of dynorphin acting through NMDA-R in chronic pain models. The fact that a single dose of dynorphin is sufficient to produce long-lasting allodynia suggests the short-term release of excitatory amino acids (69) and prostaglandins (70) may be involved in the ability of dynorphin to cause chronic pain states after a single injection, and suggests a role for dynorphin as an initiator of LTP-like central sensitization in chronic pain.
V. DYNORPHIN BIOSYNTHESIS BY PROTEASES IN SPINAL NEURONS AND GLIA CELLS
Proteases for production of dynorphins
The upregulation of spinal dynorphin in chronic pain development suggests that increases in the biosynthesis of dynorphins occur to result in elevated spinal levels. However, studies investigating regulation of the prodynorphin processing proteases for the production of spinal dynorphins during chronic pain development have not yet been conducted. Current understanding of dynorphin production in brain is considered below. Spinal dynorphin neuropeptides are present in neurons and glia cells, but the roles of neurons and/or glia cells in producing spinal dynorphins have yet to be characterized. In addition, proteolytic degradation may also participate in regulating the increased levels of dynorphins during chronic pain.
Dynorphin neuropeptides are derived from the prodynorphin precursor (PDYN) by proteolytic processing occurring largely in secretory vesicles (74–76). Dynorphins A and B, and α–neo-endorphin (α–NE) contain the peptide sequence for the endogenous opioid Leu-enkephalin, a δ-opioid receptor (DOR) agonist (Figure 4A). Proteases responsible for production of dynorphin peptides from PDYN have been defined largely by protease gene knockout studies in mice (74, 76). The cysteine protease cathepsin L and the subtilisin-like proprotein convertases (PC1/3 and PC2) are two protease pathways (77) involved in the production of Dyn A, Dyn B and α–NE (74, 76). The data suggest that different groups of proteases participate in generating each of the distinct Dyn A, DynB, and α-NE neuropeptides. In particular, production of Dyn A utilizes cathepsin L and PC1/3, whereas Dyn B production involves primarily cathepsin L alone, and α–NE production utilizes both cathepsin L and PC2 (74, 75) (Figure 4B). In addition, N-truncated species of Dyn A, particularly Dyn A (2-17) (78) have activity in chronic pain development (41, 42); however, the identity of the aminopeptidase protease(s) that remove N-terminal Tyr is not known. Investigation of the proteolytic processing mechanisms for generation of dynorphin neuropeptides, as well as des-Tyr dynorphin, will provide understanding of how dynorphin levels are increased during chronic pain development. These efforts may lead to the utility of protease inhibitors as drug targets.
Degradation of dynorphins by proteases
Neuropeptide activities are also regulated by the proteases responsible for their degradation (79, 80). Proteolytic degradation of dynorphins into inactive peptides may also contribute to the chronic pain state. In the inflamed peripheral nerve in rat, inhibition of neprilysin/neutral endopeptidase E (NPE) and aminopeptidase N (AP-N) elevated Dyn A (1-17) levels and alleviated pain (81). Furthermore, in dorsal horn neurons, inhibition of these proteases preserved Dyn A bioactivity at opioid receptors (82).
Dynorphin production in neuronal and glial cells
Studies have indicated that dynorphin is produced in both neuronal and glial cells within the brain. However the relative contribution of each cell type to the synthesis of spinal dynorphins continues to be explored.
Spinal cord tissue at the nerve injury site shows dynorphin immunoreactivity in neurons to be upregulated at chronic pain time points (83, 84), and a time-course analysis shows increased immunolabeling from 1–7 d.p.i. (days post injury) (85). In uninjured tissue, prodynorphin immunoreactivity has been shown in galanin-positive, GABAergic interneurons in lamina I/II (86), and glutamatergic (VGLUT2+) interneurons that contact projection neurons in lamina III that also express dynorphin (87). Recent evidence confirms the role of dynorphinergic inhibitory interneurons in the SDH in chronic pain processing (88).
Spinal astrocytes and microglia demonstrate expression of dynorphin A, dynorphin B, and α-neoendorphin that are released upon stimulation with agonists of purinergic and toll receptors (89) and Wahlert, Yaksh, & Hook unpublished data). Such receptors have been implicated in chronic pain signaling (90, 91). Participation of glia type cells in chronic pain is suggested by the finding that minocycline, an inhibitor of microglial activation (92) substantially attenuates the upregulation of spinal prodynorphin and DRG prodynorphin following nerve injury. This suggests that microglia activation following injury participates in the up-regulation of dynorphin (39, 40).
Future investigation of the biosynthetic mechanisms of dynorphins by spinal neurons and glia will reveal how dynorphin biosynthesis and metabolism may be regulated in chronic pain development.
VI. HUMAN CHRONIC PAIN AND DYNORPHIN
Differential spinal cell-cell communication distinguishes the quality of pain experienced by chronic pain patients compared to acute nociception. In acute injury pain (Figure 2), tissue injury or inflammation lowers nociceptive threshold because of the presence of inflammatory and excitatory factors released from responding cells at the injury site, but the pain threshold is restored as the injury heals. Persistent, chronic pathological pain transmission involves long-lasting alterations in primary sensory neurons, DRG, and spinal cord neurons and glia that continue to transmit a pain signal in absence of a nociceptive stimulus (Figure 3). Changes that occur among spinal pain processing cells of human tissues are investigated in detail in animal models of nerve injury and peripheral inflammation (93). Combined with discovery of genetic susceptibilities to chronic pain in humans and analysis of potential fluid biomarkers, modern drug discovery approaches hold promise for the development of new treatments for chronic pain that circumvent the addictive potential of many current analgesics. Investigations of chronic pain in patients and in animal models are providing insight into new drug targets and the underlying mechanisms of chronic pain treatment, including the conributions of dynorphin.
Dynorphins in human chronic pain
Thus far, two studies in cohorts of cancer pain (34) and fibromyalgia (33) have identified elevated dynorphin in lumbar CSF. This observation is consistent with the observations of elevated dynorphin in a mouse model of chronic pain (spinal nerve ligation, SNL, model) described in this review. These data suggest animal model studies to be representative of human chronic pain development. More detailed studies of dynorphin regulation in human chronic pain will be needed understand its human neurobiology.
One important question to assess is the role of human proteases for producing human dynorphins from prodynorphin. The mouse protease gene knockout studies confirm the involvement of cathepsin L in the production of dynorphin peptides (74). But analysis of the human genome revealed that cathepsin V and cathepsin L are closely related homologues, suggesting a potential role for cathepsin V in the biosynthesis of human dynorphins (94, 95). The cathepsin V gene is present only in humans and no orthologues have been found in other mammalian or other species. Notably, human cathepsin V is capable of processing proenkephalin to active enkephalin neuropeptides in human neural cells (96). It will, therefore, be important to define the roles of human cathepsin V compared to human cathepsin L in processing prodynorphin to generate active dynorphin neuropeptides.
Transient potential receptors (TRP)
The pronociceptive action of dynorphin A has been found to involve the transient receptor potential ankyrin channel (97), and such ion channels have been investigated in human pain conditions. Human nerve biopsies show changes in cation channel expression in injured nerves. The heat and pain-sensing channel TRPV1 is upregulated in such patients (98). Deletion of the TRPV1 gene in mice reduces spinal glia activation related to pathological pain (99); a receptor antagonist produced similar effects (100). The heat-sensing cation channel TRPV3 is upregulated in human neuropathic nerves, but down-regulated in DRG (98). TRPV3 knockout mice show diminished heat sensation (101), suggesting a role for TRPV3 in abnormal thermal sensation in chronic pain (102). TRPM8, a cold and touch-sensing channel is decreased in neuropathic human nerves (98). TRPM8 knockout mice show decreased cold sensitivity (103).. These data demonstrate the role of TRP ion channels in human nerve injury and chronic pain.
Regulation of inflammatory responses
Dynorphin induced allodynia involves cytokines in rodent models of neuropathic pain (72, 104). Notably, evidence for cytokine mechanisms in human pain conditions has also been found. IL-1β, a proinflammatory cytokine, is elevated in patients with neuropathic pain (105). IL-6 is upregulated in human and rat neuropathic nerves (105, 107), and in CSF of human CPRS (106) (complex regional pain syndrome) as well as in serum of nerve-injured rats (108);, gene knockout of IL-6 attenuates allodynia in the SNL mouse model (109). Monocyte chemoattractant protein-1 (MCP-1), a biomarker for human CRPS, is also upregulated in rats (108, 110). The cytokine TNFα is elevated in spinal cord of human inflammatory neuropathy (105). Such increases in TNFα are also found in nerve-ligated rats (111, 112). These findings support the role of inflammatory factors in chronic pain.
Spinal glia cells
Glia cells have classically been assessed for adopting an “activated” state, producing and releasing proinflammatory and excitatory factors that are correlated with expression with cell-specific protein “markers.” GFAP is classically used to identify activated astrocytes, and it is upregulated in the SDH of neuropathic pain in humans (113) and rat (114). The microglia marker CD68 is upregulated in human and rat DRG (115, 116), supporting the contribution of microglia to neuropathic pain. Following SNL in rats, minocycline, which prevents activation of microglia to the proinflammatory state, results in attenuation of chronic pain (117).
Cyclooxygenase-2 (COX-2) and enzymes that produce proinflammatory prostaglandins are elevated in human spinal microglia in the nerve injury and in the persistent pain condition (118). Moreover, COX-2 knockout mice show decreased allodynia and hyperalgesia (119). COX-2 inhibitors have been utilized to treat mild to moderate pain and inflammation. These findings indicate that COX-2 inhibitors may reduce the spinal glia inflammatory response in chronic pain.
Purinergic receptors are ligand-gated ion channels that have a substantial role in chronic pain development and are linked to release of IL-1β (120). In human DRG, P2X7 receptor levels are elevated and co-localizes with satellite glia cells and Schwann cells (121). Furthermore, knockout of the P2X7 gene in mice abolishes chronic neuropathic pain (121). Activation of the P2X receptor stimulates the secretion of dynorphins from spinal astrocytes in primary culture (74). It will be of interest to investigate the role of P2X7 receptors in dynorphin regulation in human chronic pain
Single nucleotide polymorphisms (SNPs)
SNPs identified by genomic association studies in chronic pain patients are contributing to understanding molecular mechanisms of chronic pain. Two SNPs in genes encoding a voltage-gated sodium channel elevating nociceptor transmission are associated with chronic widespread pain syndrome (CWPS (122)) and erythromelalgia (123). Antagonist of Na(v)1.7 studies in nerve-injured mice showed reversal of allodynia (124). A SNP in the KCNJ6 (GIRK2) gene that encodes in inward rectifying potassium channel that couples with μ-OR is associated with chronic low back pain.
Monoamine and catecholamine transmission is linked to enhanced descending facilitation of chronic pain (125). A SNP in the HTR2A serotonin receptor is associated with human CWPS (126) indicating a higher risk with the HTR2A gene variant. Catechol-O-Methyl Transferase (COMT) is an enzyme that inactivates dopamine, epinephrine, and norepinephrine. A SNP in COMT, linked to fibromyalgia (127; 128), results in lower activity, increasing the half-life of catecholamines. In the rat SNL mode, a COMT inhibitor reduces development of neuropathic (129).
While dynorphin levels are elevated in CSF in chronic pain patients (33, 34), so far no SNPs in PDYN have been identified. But PDYN SNPs have been identified that are associated with addiction (130), and schizophrenia (131).
Chronic pain in male compared to female patients
Gender differences in chronic pain have long been reported in human patients (133), with women experiencing chronic pain at a higher rate than men. Thus, there is Increasing emphasis on the inclusion of both females and males in animal studies of chronic pain models (132). Interestingly, female rodents show lower pain thresholds to noxious stimuli and lower stress-induced analgesia than male rodents (134). Estrogen derivatives modulate CNS processing of chronic pain (135, 136), and female mice have slower nerve regeneration at the injury site and more reactive gliosis in the SDH than male mice. Furthermore, the administration of sex steroids in studies of pain responses can influence levels of spinal dynorphin (135, 137). Women respond differently to opioid treatments for chronic pain conditions, experiencing higher rates of opioid-induced hyperalgesia (OIH) than men (134). Evidence for dynorphin modulation of the gender-specific responses to morphine has been provided as part of a growing body of studies describing gender differences in pain and analgesia (138; 138)
VII. THERAPEUTIC PERSPECTIVE FOR DYNORPHIN IN THE TREATMENT OF CHRONIC PAIN
The evidence presented here support a key role for dynorphin in chronic pain development. Because prodynorphin gene knockout mice do not develop hyperpathic pain responses during the chronic pain phase after nerve injury (31), and spinal IT delivered anti-dynorphin antibodies attenuate allodynia and hyperalgesia in chronic pain models (31, 37), lowering dynorphin levels is a logical approach for development of drugs to treat chronic pain. The clinical detection of elevated dynorphin levels in CSF of chronic pain patient cohorts, fibromyalgia (33) and cancer pain (34), supports the hypothesis for targeting dynorphin to ameliorate chronic pain development. Therefore, strategies to block spinal dynorphin upregulation will be important for development of effective therapeutic agents to alleviate chronic pain without the addictive liability of current medications used for chronic pain treatment.
Targeting dynorphin by gene silencing approaches for alleviation of chronic pain has several predicted advantages that differ from existing clinical drugs, as well as those in clinical trials, for treating chronic pain. The opiates, NSAIDS, and anticonvulsant drugs can relieve pain for short periods of time, but they are considerably less effective for chronic pain treatment which requires frequent medication beset with the problems of addiction and severe side effects of GI distress and respiratory problems.
Among patients with severe chronic pain, morphine and ziconotide medications are given by intrathecal (IT) administration into the spinal cord. But these pain medications have limited efficacy and cause significant side effects. A further issue and limitation in the use of the spinal route for administration of morphine and ziconotide is that continued analgesia requires continuous delivery of these agents, meaning that the patient must endure a chronic catheter and pump implant. This clearly restricts the population to be aided by the IT route with morphine or ziconotide.
Two agents currently in clinical trials are resniferotoxin (targeting the TRPV1 receptor) and substance P saporin (targeting the NK1 bearing spinal pain transmitting neuron). Both candidate agents are neurolytic and lead to an irreversible loss of neurons. While this may be appropriate for late stage terminal patients, the issue of neuronal cell death will restrict use of these agents in chronic pain patients.
These drugs are in contrast to a dynorphin gene silencing approach which may provide long durations of actions (e.g., weeks to months) without loss of neuronal cells necessary for normal functions (151, 152). This feature is relevant to broad populations of chronic pain patients for long-term treatment.
In summary, future discovery of anti-dynorphin targeted strategies will be of significant interest for development of efficacious agents, without addiction, for the treatment of chronic pain.
Summary Points.
Spinal dynorphin is a key peptide neurotransmitter (neuropeptide) responsible for development of chronic pain.
Spinal dynorphin is upregulated during the development of chronic pain, and chronic pain is blocked by mouse gene knockout of prodynorphin, the pro-neuropeptide precursor of active dynorphin neuropeptides.
Acute pain processing is not affected by prodynorphin gene knockout in mice, indicating the distinct role of dynorphin in chronic pain but not acute pain.
Elevated cerebrospinal fluid levels of dynorphin are found in human chronic pain patients.
Upregulation of dynorphin is accompanied by a change in receptor pharmacology, switching from an inhibitory, endogenous opioid role to an excitatory non-opioid role.
Elevated dynorphin during chronic pain is hypothesized to involve protease biosynthetic mechanisms to convert prodynorphin into active dynorphin neuropeptides.
A rational therapeutic approach for the treatment and likely prevention of chronic pain is to reduce spinal dynorphin neuropeptides.
Future Issues List.
Design therapeutic strategies to reduce spinal dynorphin levels to prevent chronic pain development, for a non-addictive approach in the treatment of chronic pain.
Evaluate prodynorphin gene knockout in multiple animal models of chronic pain development.
Determine protease mechanisms for upregulation of spinal dynorphin in neurons and/or glia cells during chronic pain.
Determine whether endogenous spinal des-Try-dynorphin participates in chronic pain, since IT des-Tyr induces chronic pain.
Evaluate prodynorphin gene knockout and spinal dynorphin during chronic pain in male and female mice.
Acknowledgments
This work was supported by NIH grants to V. Hook (R01 MH077305 and T32 DA07315), and to T. Yaksh (DA02110, DA15353) S. Podvin was supported on NIH T32 DA07315 training grant.
LITERATURE CITED
- 1.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert review of neurotherapeutics. 2009;9:723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology. 2010;112:514–5. doi: 10.1097/ALN.0b013e3181cf423d. [DOI] [PubMed] [Google Scholar]
- 3.Harstall C, Ospina M. Pain clinical updates. Seattle, WA: International Association for the Study of Pain; 2003. pp. 1–4. [Google Scholar]
- 4.Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354:1248–52. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 5.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 6.Baron R. Neuropathic pain: a clinical perspective. Handbook of experimental pharmacology. 2009:3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]
- 7.Voscopoulos C, Lema M. When does acute pain become chronic? British journal of anaesthesia. 2010;105(Suppl 1):i69–85. doi: 10.1093/bja/aeq323. [DOI] [PubMed] [Google Scholar]
- 8.Sorkin LS, Yaksh TL. Behavioral models of pain states evoked by physical injury to the peripheral nerve. Neurotherapeutics : the journal of the American Society for Experimental Neuro Therapeutics. 2009;6:609–19. doi: 10.1016/j.nurt.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvino B, Crepon-Bernard MO, Le Bars D. Parallel clinical and behavioural studies of adjuvant-induced arthritis in the rat: possible relationship with ‘chronic pain’. Behavioural brain research. 1987;24:11–29. doi: 10.1016/0166-4328(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 10.Millan MJ. The induction of pain: an integrative review. Progress in neurobiology. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 11.Willis WD., Jr The somatosensory system, with emphasis on structures important for pain. Brain research reviews. 2007;55:297–313. doi: 10.1016/j.brainresrev.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 13.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain : official journal of the American Pain Society. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain practice : the official journal of World Institute of Pain. 2010;10:167–84. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science (New York, NY) 2006;312:1659–62. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 17.Shortland P, Wall PD. Long-range afferents in the rat spinal cord. II. Arborizations that penetrate grey matter. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1992;337:445–55. doi: 10.1098/rstb.1992.0120. [DOI] [PubMed] [Google Scholar]
- 18.Schaible HG. Peripheral and central mechanisms of pain generation. Handbook of experimental pharmacology. 2007:3–28. doi: 10.1007/978-3-540-33823-9_1. [DOI] [PubMed] [Google Scholar]
- 19.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nature reviews. Drug discovery. 2014;13:533–48. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carozzi VA, Canta A, Chiorazzi A, Cavaletti G. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neuroscience letters. 2014 doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Svensson CI, Brodin E. Spinal astrocytes in pain processing: non-neuronal cells as therapeutic targets. Molecular interventions. 2010;10:25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- 22.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2010;16:519–31. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2014;19:328–35. doi: 10.1155/2014/754693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bas DB, Su J, Sandor K, Agalave NM, Lundberg J, et al. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis and rheumatism. 2012;64:3886–96. doi: 10.1002/art.37686. [DOI] [PubMed] [Google Scholar]
- 25.Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh TL, Svensson CI. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain. 2010;151:394–403. doi: 10.1016/j.pain.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. The Journal of rheumatology. 2007;34:1674–83. [PubMed] [Google Scholar]
- 27.Taylor P, Manger B, Alvaro-Gracia J, Johnstone R, Gomez-Reino J, et al. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. The Journal of international medical research. 2010;38:1213–24. doi: 10.1177/147323001003800402. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis research & therapy. 2012;14:R101. doi: 10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ. Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain research. 1991;560:186–92. doi: 10.1016/0006-8993(91)91231-o. [DOI] [PubMed] [Google Scholar]
- 30.Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–26. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1779–86. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen A, Lundeberg T, Bytner B, Nylander I. Central changes in nociceptin dynorphin B and Met-enkephalin-Arg-Phe in different models of nociception. Brain research. 2000;857:212–8. doi: 10.1016/s0006-8993(99)02432-4. [DOI] [PubMed] [Google Scholar]
- 33.Vaeroy H, Nyberg F, Terenius L. No evidence for endorphin deficiency in fibromyalgia following investigation of cerebrospinal fluid (CSF) dynorphin A and Met-enkephalin-Arg6-Phe7. Pain. 1991;46:139–43. doi: 10.1016/0304-3959(91)90068-9. [DOI] [PubMed] [Google Scholar]
- 34.Samuelsson H, Ekman R, Hedner T. CSF neuropeptides in cancer pain: effects of spinal opioid therapy. Acta anaesthesiologica Scandinavica. 1993;37:502–8. doi: 10.1111/j.1399-6576.1993.tb03755.x. [DOI] [PubMed] [Google Scholar]
- 35.Pohl M, Ballet S, Collin E, Mauborgne A, Bourgoin S, et al. Enkephalinergic and dynorphinergic neurons in the spinal cord and dorsal root ganglia of the polyarthritic rat - in vivo release and cDNA hybridization studies. Brain research. 1997;749:18–28. doi: 10.1016/s0006-8993(96)01161-4. [DOI] [PubMed] [Google Scholar]
- 36.Ballet S, Mauborgne A, Hamon M, Cesselin F, Collin E. Altered opioid-mediated control of the spinal release of dynorphin and met-enkephalin in polyarthritic rats. Synapse (New York, NY) 2000;37:262–72. doi: 10.1002/1098-2396(20000915)37:4<262::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, et al. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–94. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 38.Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11:719–28. doi: 10.1016/0196-9781(90)90187-a. [DOI] [PubMed] [Google Scholar]
- 39.Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience. 2010;165:1420–8. doi: 10.1016/j.neuroscience.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 40.Calza L, Pozza M, Zanni M, Manzini CU, Manzini E, Hokfelt T. Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocytochemical and in situ hybridization study. Neuroscience. 1998;82:575–89. doi: 10.1016/s0306-4522(97)00272-8. [DOI] [PubMed] [Google Scholar]
- 41.Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, et al. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68:275–81. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, et al. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–60. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 43.Laughlin TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent nociception by dynorphin. The Journal of pharmacology and experimental therapeutics. 2001;299:6–11. [PubMed] [Google Scholar]
- 44.Gardell LR, Ibrahim M, Wang R, Wang Z, Ossipov MH, et al. Mouse strains that lack spinal dynorphin upregulation after peripheral nerve injury do not develop neuropathic pain. Neuroscience. 2004;123:43–52. doi: 10.1016/j.neuroscience.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 45.Lai J, Luo MC, Chen Q, Ma S, Gardell LR, et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nature neuroscience. 2006;9:1534–40. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- 46.Luo MC, Chen Q, Ossipov M, Rankin DR, Porreca F, et al. Spinal dynorphin and bradykinin receptors maintain inflammatory hyperalgesia. J Pain. 2008;9:1096–1105. doi: 10.1016/j.jpain.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YS, Muthu D, Hall SM, Ramos-Colon C, Rankin D, et al. Discovery of amphipathic dynorphin A analogues to inhibit the neuroexcitatory effects of dynorphin A through bradykinin receptors in the spinal cord. Journal of the American Chemical Society. 2014;136:6608–16. doi: 10.1021/ja501677q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:6666–70. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7219–23. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chavkin C. Dynorphin--still an extraordinarily potent opioid peptide. Molecular pharmacology. 2013;83:729–36. doi: 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oka T, Negishi K, Suda M, Sawa A, Fujino M, Wakimasu M. Evidence that dynorphin-(1-13) acts as an agonist on opioid kappa-receptors. European journal of pharmacology. 1982;77:137–41. doi: 10.1016/0014-2999(82)90008-5. [DOI] [PubMed] [Google Scholar]
- 52.Hutchinson M, Kosterlitz HW, Leslie FM, Waterfield AA. Assessment in the guinea-pig ileum and mouse vas deferens of benzomorphans which have strong antinociceptive activity but do not substitute for morphine in the dependent monkey. British journal of pharmacology. 1975;55:541–6. doi: 10.1111/j.1476-5381.1975.tb07430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin WR. History and development of mixed opioid agonists, partial agonists and antagonists. British journal of clinical pharmacology. 1979;7(Suppl 3):273S–9S. doi: 10.1111/j.1365-2125.1979.tb04700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tung AS, Yaksh TL. In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain research. 1982;247:75–83. doi: 10.1016/0006-8993(82)91029-0. [DOI] [PubMed] [Google Scholar]
- 55.Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. The Journal of pharmacology and experimental therapeutics. 1984;228:1–12. [PubMed] [Google Scholar]
- 56.Han JS, Xie GX, Goldstein A. Analgesia induced by intrathecal injection of dynorphin B in the rat. Life sciences. 1984;34:1573–9. doi: 10.1016/0024-3205(84)90612-x. [DOI] [PubMed] [Google Scholar]
- 57.Przewlocki R, Stala L, Greczek M, Shearman GT, Przewlocka B, Herz A. Analgesic effects of mu-, delta- and kappa-opiate agonists and, in particular, dynorphin at the spinal level. Life sciences. 1983;33(Suppl 1):649–52. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- 58.Stevens CW, Weinger MB, Yaksh TL. Intrathecal dynorphins suppress hindlimb electromyographic activity in rats. European journal of pharmacology. 1987;138:299–302. doi: 10.1016/0014-2999(87)90449-3. [DOI] [PubMed] [Google Scholar]
- 59.Faden AI, Jacobs TP. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. British journal of pharmacology. 1984;81:271–6. doi: 10.1111/j.1476-5381.1984.tb10074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spampinato S, Candeletti S. Characterization of dynorphin A-induced antinociception at spinal level. European journal of pharmacology. 1985;110:21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- 61.Caudle RM, Isaac L. Intrathecal dynorphin(1-13) results in an irreversible loss of the tail-flick reflex in rats. Brain research. 1987;435:1–6. doi: 10.1016/0006-8993(87)91579-4. [DOI] [PubMed] [Google Scholar]
- 62.Tan-No K, Esashi A, Nakagawasai O, Niijima F, Tadano T, et al. Intrathecally administered big dynorphin, a prodynorphin-derived peptide, produces nociceptive behavior through an N-methyl-D-aspartate receptor mechanism. Brain research. 2002;952:7–14. doi: 10.1016/s0006-8993(02)03180-3. [DOI] [PubMed] [Google Scholar]
- 63.Isaac L, Van Zandt O’Malley T, Ristic H, Stewart P. MK-801 blocks dynorphin A (1-13)-induced loss of the tail-flick reflex in the rat. Brain research. 1990;531:83–7. doi: 10.1016/0006-8993(90)90760-9. [DOI] [PubMed] [Google Scholar]
- 64.Knox RJ, Dickenson AH. Effects of selective and non-selective kappa-opioid receptor agonists on cutaneous C-fibre-evoked responses of rat dorsal horn neurones. Brain research. 1987;415:21–9. doi: 10.1016/0006-8993(87)90265-4. [DOI] [PubMed] [Google Scholar]
- 65.Gaumann DM, Yaksh TL, Post C, Wilcox GL, Rodriguez M. Intrathecal somatostatin in cat and mouse studies on pain, motor behavior, and histopathology. Anesthesia and analgesia. 1989;68:623–32. [PubMed] [Google Scholar]
- 66.Gaumann DM, Grabow TS, Yaksh TL, Casey SJ, Rodriguez M. Intrathecal somatostatin, somatostatin analogs, substance P analog and dynorphin A cause comparable neurotoxicity in rats. Neuroscience. 1990;39:761–74. doi: 10.1016/0306-4522(90)90259-7. [DOI] [PubMed] [Google Scholar]
- 67.Shukla VK, Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends in pharmacological sciences. 1994;15:420–4. doi: 10.1016/0165-6147(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L, Peoples RW, Oz M, Harvey-White J, Weight FF, Brauneis U. Potentiation of NMDA receptor-mediated responses by dynorphin at low extracellular glycine concentrations. Journal of neurophysiology. 1997;78:582–90. doi: 10.1152/jn.1997.78.2.582. [DOI] [PubMed] [Google Scholar]
- 69.Skilling SR, Sun X, Kurtz HJ, Larson AA. Selective potentiation of NMDA-induced activity and release of excitatory amino acids by dynorphin: possible roles in paralysis and neurotoxicity. Brain research. 1992;575:272–8. doi: 10.1016/0006-8993(92)90090-v. [DOI] [PubMed] [Google Scholar]
- 70.Koetzner L, Hua XY, Lai J, Porreca F, Yaksh T. Nonopioid actions of intrathecal dynorphin evoke spinal excitatory amino acid and prostaglandin E2 release mediated by cyclooxygenase-1 and -2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1451–8. doi: 10.1523/JNEUROSCI.1517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu WH, Li F, Qiang WA, Liu N, Wang GQ, et al. Dual role for nitric oxide in dynorphin spinal neurotoxicity. Journal of neurotrauma. 1999;16:85–98. doi: 10.1089/neu.1999.16.85. [DOI] [PubMed] [Google Scholar]
- 72.Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin-induced allodynia. Pain. 2000;84:159–67. doi: 10.1016/s0304-3959(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 73.Mika J, Rojewska E, Makuch W, Korostynski M, Luvisetto S, et al. The effect of botulinum neurotoxin A on sciatic nerve injury-induced neuroimmunological changes in rat dorsal root ganglia and spinal cord. Neuroscience. 2011;175:358–66. doi: 10.1016/j.neuroscience.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 74.Minokadeh A, Funkelstein L, Toneff T, Hwang SR, Beinfeld M, et al. Cathepsin L participates in dynorphin production in brain cortex, illustrated by protease gene knockout and expression. Molecular and cellular neurosciences. 2010;43:98–107. doi: 10.1016/j.mcn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Funkelstein L, Beinfeld M, Minokadeh A, Zadina J, Hook V. Unique biological function of cathepsin L in secretory vesicles for biosynthesis of neuropeptides. Neuropeptides. 2010;44:457–66. doi: 10.1016/j.npep.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berman Y, Mzhavia N, Polonskaia A, Furuta M, Steiner DF, et al. Defective prodynorphin processing in mice lacking prohormone convertase PC2. Journal of neurochemistry. 2000;75:1763–70. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- 77.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annual review of pharmacology and toxicology. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young EA, Walker JM, Houghten R, Akil H. The degradation of dynorphin A in brain tissue in vivo and in vitro. Peptides. 1987;8:701–7. doi: 10.1016/0196-9781(87)90046-5. [DOI] [PubMed] [Google Scholar]
- 79.Roques BP, Fournie-Zaluski MC, Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nature reviews. Drug discovery. 2012;11:292–310. doi: 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- 80.Maldonado R, Valverde O, Turcaud S, Fournie-Zaluski MC, Roques BP. Antinociceptive response induced by mixed inhibitors of enkephalin catabolism in peripheral inflammation. Pain. 1994;58:77–83. doi: 10.1016/0304-3959(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 81.Schreiter A, Gore C, Labuz D, Fournie-Zaluski MC, Roques BP, et al. Pain inhibition by blocking leukocytic and neuronal opioid peptidases in peripheral inflamed tissue. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:5161–71. doi: 10.1096/fj.12-208678. [DOI] [PubMed] [Google Scholar]
- 82.Song B, Marvizon JC. Peptidases prevent mu-opioid receptor internalization in dorsal horn neurons by endogenously released opioids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:1847–58. doi: 10.1523/JNEUROSCI.23-05-01847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–98. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 84.Wagner R, DeLeo JA, Coombs DW, Willenbring S, Fromm C. Spinal dynorphin immunoreactivity increases bilaterally in a neuropathic pain model. Brain research. 1993;629:323–6. doi: 10.1016/0006-8993(93)91339-t. [DOI] [PubMed] [Google Scholar]
- 85.Zhu X, Vincler MA, Parker R, Eisenach JC. Spinal cord dynorphin expression increases, but does not drive microglial prostaglandin production or mechanical hypersensitivity after incisional surgery in rats. Pain. 2006;125:43–52. doi: 10.1016/j.pain.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 86.Sardella TC, Polgar E, Garzillo F, Furuta T, Kaneko T, et al. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Molecular pain. 2011;7:76. doi: 10.1186/1744-8069-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nahin RL, Hylden JL, Humphrey E. Demonstration of dynorphin A 1-8 immunoreactive axons contacting spinal cord projection neurons in a rat model of peripheral inflammation and hyperalgesia. Pain. 1992;51:135–43. doi: 10.1016/0304-3959(92)90254-9. [DOI] [PubMed] [Google Scholar]
- 88.Duan B, Cheng L, Bourane S, Britz O, Padilla C, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–32. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wahlert A, Funkelstein L, Fitzsimmons B, Yaksh T, Hook V. Spinal astrocytes produce and secrete dynorphin neuropeptides. Neuropeptides. 2013;47:109–15. doi: 10.1016/j.npep.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. Journal of neuroinflammation. 2013;10:148. doi: 10.1186/1742-2094-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain research reviews. 2010;63:222–32. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiological reviews. 2009;89:707–58. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 94.Bromme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38:2377–85. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 95.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. The EMBO journal. 2001;20:4629–33. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Funkelstein L, Lu WD, Koch B, Mosier C, Toneff T, et al. Human cathepsin V protease participates in production of enkephalin and NPY neuropeptide neurotransmitters. The Journal of biological chemistry. 2012;287:15232–41. doi: 10.1074/jbc.M111.310607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei H, Saarnilehto M, Falck L, Viisanen H, Lasierra M, et al. Spinal transient receptor potential ankyrin 1 channel induces mechanical hypersensitivity, increases cutaneous blood flow, and mediates the pronociceptive action of dynorphin A. J Physiol Pharmacol. 2013;64:331–40. [PubMed] [Google Scholar]
- 98.Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC neurology. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Experimental neurology. 2009;220:383–90. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watabiki T, Kiso T, Tsukamoto M, Aoki T, Matsuoka N. Intrathecal administration of AS1928370, a transient receptor potential vanilloid 1 antagonist, attenuates mechanical allodynia in a mouse model of neuropathic pain. Biological & pharmaceutical bulletin. 2011;34:1105–8. doi: 10.1248/bpb.34.1105. [DOI] [PubMed] [Google Scholar]
- 101.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science (New York, NY) 2005;307:1468–72. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 102.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7723–30. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PloS one. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rojewska E, Makuch W, Przewlocka B, Mika J. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology. 2014;86:301–10. doi: 10.1016/j.neuropharm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta neuropathologica. 2003;105:593–602. doi: 10.1007/s00401-003-0689-y. [DOI] [PubMed] [Google Scholar]
- 106.Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–9. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Arruda JL, Colburn RW, Rickman AJ, Rutkowski MD, DeLeo JA. Increase of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: potential role of IL-6 in neuropathic pain. Brain Research Molecular Brain Research. 1998;62:228–35. doi: 10.1016/s0169-328x(98)00257-5. [DOI] [PubMed] [Google Scholar]
- 108.Xu Y, Zhang X, Pu S, Wu J, Lv Y, Du D. Circulating microRNA expression profile: a novel potential predictor for chronic nervous lesions. Acta biochimica et biophysica Sinica. 2014;46:942–9. doi: 10.1093/abbs/gmu090. [DOI] [PubMed] [Google Scholar]
- 109.Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain. 1998;78:115–21. doi: 10.1016/S0304-3959(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 110.Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, et al. MicroRNA modulation in complex regional pain syndrome. Journal of translational medicine. 2011;9:195. doi: 10.1186/1479-5876-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim CF, Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain research. 2011;1405:95–108. doi: 10.1016/j.brainres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 112.Liang Y, Jiang W, Zhang Z, Yu J, Tao L, Zhao S. Behavioral and morphological evidence for the involvement of glial cells in the antinociceptive effect of najanalgesin in a rat neuropathic pain model. Biological & pharmaceutical bulletin. 2012;35:850–4. doi: 10.1248/bpb.35.850. [DOI] [PubMed] [Google Scholar]
- 113.Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:10833–40. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- 115.Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain, behavior, and immunity. 2009;23:85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 116.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain, behavior, and immunity. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 117.Mei XP, Xu H, Xie C, Ren J, Zhou Y, et al. Post-injury administration of minocycline: an effective treatment for nerve-injury induced neuropathic pain. Neuroscience research. 2011;70:305–12. doi: 10.1016/j.neures.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 118.Durrenberger PF, Facer P, Gray RA, Chessell IP, Naylor A, et al. Cyclooxygenase-2 (Cox-2) in injured human nerve and a rat model of nerve injury. Journal of the peripheral nervous system : JPNS. 2004;9:15–25. doi: 10.1111/j.1085-9489.2004.09104.x. [DOI] [PubMed] [Google Scholar]
- 119.Chillingworth NL, Morham SG, Donaldson LF. Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;291:R327–34. doi: 10.1152/ajpregu.00901.2005. [DOI] [PubMed] [Google Scholar]
- 120.Magni G, Ceruti S. The purinergic system and glial cells: emerging costars in nociception. BioMed research international. 2014;2014:495789. doi: 10.1155/2014/495789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–96. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Holliday KL, McBeth J, Macfarlane G, Huhtaniemi IT, Bartfai G, et al. Investigating the role of pain-modulating pathway genes in musculoskeletal pain. European journal of pain. 2013;17:28–34. doi: 10.1002/j.1532-2149.2012.00163.x. [DOI] [PubMed] [Google Scholar]
- 123.Dib-Hajj SD, Rush AM, Cummins TR, Hisama FM, Novella S, et al. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain : a journal of neurology. 2005;128:1847–54. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- 124.McGowan E, Hoyt SB, Li X, Lyons KA, Abbadie C. A peripherally acting Na(v)1.7 sodium channel blocker reverses hyperalgesia and allodynia on rat models of inflammatory and neuropathic pain. Anesthesia and analgesia. 2009;109:951–8. doi: 10.1213/ane.0b013e3181b01b02. [DOI] [PubMed] [Google Scholar]
- 125.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Current opinion in supportive and palliative care. 2014;8:143–51. doi: 10.1097/SPC.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicholl BI, Holliday KL, Macfarlane GJ, Thomson W, Davies KA, et al. Association of HTR2A polymorphisms with chronic widespread pain and the extent of musculoskeletal pain: results from two population-based cohorts. Arthritis and rheumatism. 2011;63:810–8. doi: 10.1002/art.30185. [DOI] [PubMed] [Google Scholar]
- 127.Martinez-Jauand M, Sitges C, Rodriguez V, Picornell A, Ramon M, et al. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. European journal of pain. 2013;17:16–27. doi: 10.1002/j.1532-2149.2012.00153.x. [DOI] [PubMed] [Google Scholar]
- 128.Matsuda JB, Barbosa FR, Morel LJ, de Franca SC, Zingaretti SM, et al. Serotonin receptor (5-HT 2A) and catechol-O-methyltransferase (COMT) gene polymorphisms: triggers of fibromyalgia? Revista brasileira de reumatologia. 2010;50:141–9. [PubMed] [Google Scholar]
- 129.Kambur O, Mannisto PT, Pusa AM, Kaenmaki M, Kalso EA, Kontinen VK. Nitecapone reduces development and symptoms of neuropathic pain after spinal nerve ligation in rats. European journal of pain. 2011;15:732–40. doi: 10.1016/j.ejpain.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 130.Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1185–97. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang CS, Tan Z, Lu L, Wu SN, He Y, et al. Polymorphism of Prodynorphin promoter is associated with schizophrenia in Chinese population. Acta pharmacologica Sinica. 2004;25:1022–6. [PubMed] [Google Scholar]
- 132.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Progress in brain research. 2010;186:141–57. doi: 10.1016/B978-0-444-53630-3.00009-9. [DOI] [PubMed] [Google Scholar]
- 134.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gender medicine. 2005;2:137–45. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 135.Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain. 2000;85:93–9. doi: 10.1016/s0304-3959(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 136.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 137.Gutierrez S, Hayashida K, Eisenach JC. The puerperium alters spinal cord plasticity following peripheral nerve injury. Neuroscience. 2013;228:301–8. doi: 10.1016/j.neuroscience.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu NJ, von Gizycki H, Gintzler AR. Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. The Journal of pharmacology and experimental therapeutics. 2007;322:654–60. doi: 10.1124/jpet.107.123620. [DOI] [PubMed] [Google Scholar]
- 139.Iadarola MJ, Douglass J, Civelli O, Naranjo JR. Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain research. 1988;455:205–12. doi: 10.1016/0006-8993(88)90078-9. [DOI] [PubMed] [Google Scholar]
- 140.Millan MJ, Millan MH, Pilcher CW, Czlonkowski A, Herz A, Colpaert FC. Spinal cord dynorphin may modulate nociception via a kappa-opioid receptor in chronic arthritic rats. Brain research. 1985;340:156–9. doi: 10.1016/0006-8993(85)90786-3. [DOI] [PubMed] [Google Scholar]
- 141.Millan MJ, Millan MH, Czlonkowski A, Hollt V, Pilcher CW, et al. A model of chronic pain in the rat: response of multiple opioid systems to adjuvant-induced arthritis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:899–906. doi: 10.1523/JNEUROSCI.06-04-00899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Millan MJ, Czlonkowski A, Morris B, Stein C, Arendt R, et al. Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain. 1988;35:299–312. doi: 10.1016/0304-3959(88)90140-6. [DOI] [PubMed] [Google Scholar]
- 143.Weihe E, Millan MJ, Hollt V, Nohr D, Herz A. Induction of the gene encoding pro-dynorphin by experimentally induced arthritis enhances staining for dynorphin in the spinal cord of rats. Neuroscience. 1989;31:77–95. doi: 10.1016/0306-4522(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 144.Przewlocki R, Shearman GT, Herz A. Mixed opioid/nonopioid effects of dynorphin and dynorphin related peptides after their intrathecal injection in rats. Neuropeptides. 1983;3:233–40. doi: 10.1016/0143-4179(83)90019-7. [DOI] [PubMed] [Google Scholar]
- 145.Herman BH, Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. The Journal of pharmacology and experimental therapeutics. 1985;232:27–32. [PubMed] [Google Scholar]
- 146.Jhamandas K, Sutak M, Lemaire S. Comparative spinal analgesic action of dynorphin1-8, dynorphin1-13, and a kappa-receptor agonist U50,488. Canadian journal of physiology and pharmacology. 1986;64:263–8. doi: 10.1139/y86-042. [DOI] [PubMed] [Google Scholar]
- 147.Ohara PT, Vit JP, Bhargava A, Romero M, Sundberg C, et al. Gliopathic pain: when satellite glial cells go bad. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2009;15:450–63. doi: 10.1177/1073858409336094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baseer N, Polgar E, Watanabe M, Furuta T, Kaneko T, Todd AJ. Projection neurons in lamina III of the rat spinal cord are selectively innervated by local dynorphin-containing excitatory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:11854–63. doi: 10.1523/JNEUROSCI.2707-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeilhofer HU. Loss of glycinergic and GABAergic inhibition in chronic pain—contributions of inflammation and microglia. International Immunopharmacology. 2008;8:182–7. doi: 10.1016/j.intimp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 150.Day R, Lazure C, Basak A, Boudreault A, Limperis P, et al. Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. The Journal of biological chemistry. 1998;273:829–36. doi: 10.1074/jbc.273.2.829. [DOI] [PubMed] [Google Scholar]
- 151.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–44. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith RA, Miller TM, Yamanaka K, Monia BP, Condon TP, et al. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116:2290–6. doi: 10.1172/JCI25424. [DOI] [PMC free article] [PubMed] [Google Scholar]