Abstract
Prenatal maternal mood may explain the adverse obstetric outcomes seen in disadvantaged populations yet the effects of trauma history are not well studied. We examined the impact of trauma exposure and mood symptoms on obstetric outcomes in 358 women. Women with antecedent trauma were more likely to have a history of depression χ2(1, N = 358) = 19.2, p =.001; OR = 2.83, 95% CI [1.81, 4.42], were younger at their first pregnancy t(356) = −2.97, p = .003 and had a higher number of previous pregnancies t(356) = 2.77, p = .011 compared to those with no trauma exposure. Women with prenatal anxiety had significantly smaller babies than nonanxious women F(1, 322) = 5.32, p = .024. Trauma history magnified the effects of maternal prenatal mood on birth weight; the moderating effect was limited to those who first experienced a trauma under 18 years of age F(14, 320) = 2.44, p =.005. Childhood trauma exposure increases vulnerability for low birthweight delivery associated with prenatal mood disturbance. Screening pregnant women for trauma history and current mood symptoms is indicated.
Preterm birth and low birthweight are significant public health concerns with well-documented socioeconomic disparities; however, the risk factors underlying these disparities are unclear (Martin et al., 2012; Spong, Iams, Goldenberg, Hauck & Willinger, 2011). Maternal mental health has been proposed as a potential risk factor because there are compelling biological mechanisms linking depression and anxiety to poor obstetric outcomes (Wadhwa, Entringer, Buss & Lu, 2011), and mental health conditions covary with low socioeconomic status. Empirical support for this hypothesis is widespread, as several studies assessing diverse samples demonstrate robust links between maternal prenatal depression or anxiety and higher rates of preterm birth and lower birthweight (Ding et al, 2014; Grigoriadis et al, 2013; Grote et al, 2010; Ibanez et al., 2012; Wadhwa et al., 2011). One important limitation of many studies in this area is that they do not systematically assess traumatic life events. This is significant given the high rates of trauma history, particularly in low income and high psychosocial risk women (Putnam, Harris & Putnam, 2013; Putnam, 2003), the increasing awareness of trauma history in pregnant populations (Onoye, Goebert, Morland, Matsu & Wright, 2009; Smith, Poschman, Cavaleri, Howell & Yonkers, 2006), and research documenting that depression and anxiety are comorbid with, and may be a consequence of, trauma exposure (Putnam, 2003). Large-scale epidemiological studies have shown that maternal exposure to severe life events, just prior to conception or during pregnancy, is associated with significantly lower birthweight (Class, Lichtenstein, Langstrom & D’Onofrio, 2011; Khashan et al, 2008) and preterm birth (Khashan et al.,2008). Examining the impact of stress and trauma exposures that pre-date pregnancy is consistent with a life-course approach, which is gaining support for understanding the multiple influences of maternal biology on birth outcomes (Wadhwa et al.,2011). Indeed, there may be specific biological effects of early trauma (Heim, Newport, Mletzko, Miller & Nemeroff, 2008; Bublitz et al.,2014) that may be relevant for pregnancy and delivery.
There is a limited literature on trauma exposure, pregnancy health and delivery complications. Trauma exposed women are more likely to engage in poor antenatal health behaviors such as smoking, drinking, and illicit drug use (Chung et al., 2010; Morland et al., 2007) that could explain shorter gestation or preterm birth (Seng, Low, Sperlich, Ronis & Liberzon, 2011; Yonkers et al, 2014). The possibility that trauma history may compound other risks for poor obstetric outcomes was recently suggested by Yonkers and colleagues (2014) who found a 4-fold risk of preterm birth in women with both posttraumatic stress disorder (PTSD) and depression. Furthermore, experiencing childhood trauma may have a more deleterious effect on adverse obstetric outcomes (Bublitz et al., 2014; Chung et al., 2010; Heim et al., 2008; Putnam, 2003; Seng et al., 2011).
Limitations of previous studies include a focus on a specific type of trauma (e.g. intimate partner violence, disasters), use of cross-sectional designs, or lack of control for confounding factors including depression and anxiety symptoms. The current study advances research in this area by using a longitudinal design, assessing multiple trauma types and their onset, and considers confounding and compounding risk factors in a prediction model. We hypothesized that women with a history of trauma would be more likely to experience an adverse obstetric outcome defined as lower birthweight, earlier gestational age, and delivery complication. The study also examined if trauma severity, defined as the number of traumatic events experienced, and age of first trauma exposure improved prediction of obstetric outcome.
Method
Participants and Procedure
The data are derived from two related prospective, longitudinal cohort studies that used parallel clinical protocols. Because we were interested in affective symptoms in medically normal-risk women, we excluded women with significant compromising medical conditions (e.g., autoimmune disorders) and women at high obstetric risk defined as an increased likelihood of maternal or fetal complications due to young or old maternal age, carrying more than one fetus, experiencing problems in a previous pregnancy (e.g., multiple miscarriages), or prenatal drug or alcohol use.
The sample was obtained from pregnant women receiving obstetrical care from a hospital-based practice serving a predominantly low-income, inner-city population. Ethical approval was obtained from the University of Rochester Research Subjects Review Board and all participants provided written informed consent. Data collection occurred between May 2007 and May 2012.
The inclusion criteria were a confirmed singleton pregnancy of less than 18 weeks gestation, aged 19–34 years old, considered low to medium obstetric risk by the medical team, fluent in English, and able to provide informed consent. The exclusion criteria were presenting for obstetrical care at >18 weeks gestation, current substance or alcohol abuse, past or current diagnosis of bipolar disorder or schizophrenia or presence of psychotic features. We included only pregnancies resulting in live births.
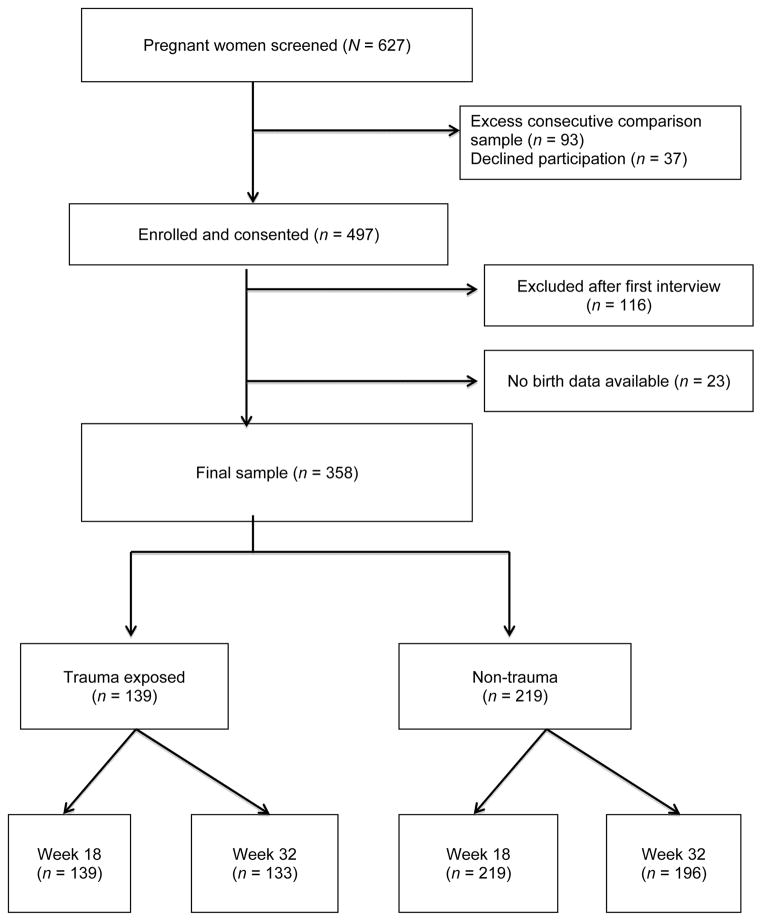
Nursing staff in the clinic provided an overview of the study to attendees who met inclusion criteria and asked if they were interested in finding out more about the study. The primary aim of both studies was to examine correlates of maternal depression and anxiety across the perinatal period. Eligible women were screened using the Edinburgh Postnatal Depression Scale (EPDS; Cox, Holden & Sagovsky, 1987) and Penn State Worry Questionnaire (PSWQ) (Meyer, Miller, Metzger & Borkovec, 1990). The nonprobability sampling technique for this study enrolled all women who scored above the established cut-offs on the EPDS (≥13) or PSWQ (>45) and utilized consecutive sampling to enroll and obtain women who scored below the cut-off scores. The consecutive sampling strategy created a comparison stratum that was approximately equal to the target sample size of women with affective symptoms and significant mood disturbance. Figure 1 shows the recruitment flow for the study; of 627 women screened 497 (79.3%) were subsequently consented and 130 (20.7%) were excluded or exceeded the comparison sample size. A further 116 women were excluded after the first interview as they had at least one exclusion criteria that was not previously detected, suffered a perinatal loss, changed obstetrics provider, or were lost to contact prior to the clinical interview. Obstetric data were unavailable on 23 women who gave birth at a different hospital or out of state. The final sample was 358 women, of which 139 (38.8%) were trauma exposed and 219 (61.2%) were not trauma exposed.
Figure 1.
Participants were assessed by a trained interviewer twice during pregnancy, at approximately 18 (N = 358) and 32 weeks gestation (n = 348). At each assessment, women completed a battery of health and psychosocial questionnaires as well as a psychiatric clinical diagnostic interview (Structured Clinical Interview for DSM, SCID; First, 2002). All questionnaires were read aloud to participants to pre-empt problems in literacy and to ensure understanding of each question. Detailed medical, clinical, and sociodemographic data were collected via interview and medical records.
Table 1 depicts the demographic and clinical characteristics of the sample according to trauma exposure. The majority of the sample were young, single, low-income women, most of whom had high school education or less. Two thirds were multigravid and 43.6% (n = 156) were under 18 years old when they first became pregnant. Of the total sample, 139 (38.8%) reported experiencing at least one traumatic event in their lifetime. Although the two groups did not differ on sociodemographic variables, there were significant differences in mental health, pregnancy history, and reproductive outcomes. Women with antecedent trauma were significantly more likely to have experienced miscarriage OR = 1.89, 95% CI [1.15, 3.09], or a previous depression OR = 2.83, 95% CI [1.81, 4.42]; they were significantly younger at their first pregnancy mean age 18.86 vs. 20.10 years, t(356) = −2.97, p = .003 and had a higher number of previous pregnancies mean 2.01 vs 1.54, t(356) = 2.77, p = .011. They were also more likely to have experienced depression (antenatal depression) OR = 2.27, 95% CI [1.36, 3.80] or anxiety OR = 1.97, 95% CI [1.27, 3.05] in the index pregnancy.
Table 1.
Sociodemographic and Clinical Characteristics of Pregnant Sample by Trauma Exposure Status.
| Total (N = 358) | Exposed (n = 139) | Not exposed (n = 219) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | M or n | SD or % | M or n | SD or % | M or n | SD or % |
| Maternal age (years) | 24.47a | 3.75 | 24.42b | 3.74 | 24.50c | 3.76 |
| Ethnicity | ||||||
| Caucasian | 107 | 29.9 | 39 | 28.1 | 68 | 31.1 |
| African American | 178 | 49.7 | 71 | 51.1 | 107 | 48.9 |
| Biracial | 20 | 5.6 | 13 | 9.4 | 7 | 3.2 |
| Hispanic/Latina | 48 | 13.4 | 14 | 10.1 | 34 | 15.5 |
| Other | 5 | 1.4 | 2 | 1.4 | 3 | 1.4 |
| Education | ||||||
| < High school | 79 | 22.1 | 33 | 23.7 | 46 | 21.0 |
| High school | 136 | 38.0 | 49 | 35.3 | 87 | 39.7 |
| Some college | 111 | 31.0 | 46 | 33.1 | 65 | 29.7 |
| College | 32 | 8.9 | 11 | 7.9 | 21 | 9.6 |
| Marital status | ||||||
| Single | 199 | 55.6 | 82 | 59.0 | 117 | 53.4 |
| Cohabiting/Married | 159 | 44.4 | 57 | 41.0 | 102 | 46.6 |
| Receiving Medicaid | 250 | 69.8 | 103 | 74.1 | 147 | 67.1 |
| BMI 18 weeks gestation | ||||||
| Underweight (<18.5) | 12 | 3.4 | 8 | 5.8 | 4 | 1.8 |
| Normal (18.5–24.9) | 135 | 37.7 | 46 | 33.1 | 89 | 40.6 |
| Overweight (25.0–29.9) | 81 | 22.6 | 33 | 23.7 | 48 | 21.9 |
| Obese (>30) | 130 | 36.3 | 52 | 37.4 | 78 | 35.6 |
| Smoking during | 34 | 9.5 | 18 | 12.9 | 16 | 7.3 |
| Alcohol during | 61.7 | 2 | 1.4 | 4 | 1.8 | |
| Age at first pregnancy | 19.59d | 3.84 | 18.86e | 3.78 | 20.05f | 3.81 |
| n previous pregnancies | 1.71g | 1.61 | 1.97h | 1.64 | 1.54i | 1.57 |
| History of miscarriage | 85 | 26.3 | 43 | 33.6 | 42 | 21.5 |
| Birth weight (g) | 3272.54j | 558.35 | 3265.53k | 514.56 | 3276.99l | 585.57 |
| Gestational age (days) | 274.71m | 13.51 | 275.49n | 10.34 | 274.26o | 15.23 |
| Obstetric complications | 124 | 34.6 | 45 | 32.4 | 79 | 36.1 |
| Mode of delivery | ||||||
| Vaginal | 239 | 66.8 | 95 | 68.3 | 144 | 65.8 |
| Vaginal assisted | 14 | 4.0 | 4 | 2.9 | 10 | 4.6 |
| Emergent C-section | 56 | 15.6 | 24 | 17.3 | 32 | 14.6 |
| Scheduled C-section | 49 | 13.7 | 16 | 11.5 | 33 | 15.1 |
| C-section (yes/no) | 105 | 29.3 | 40 | 28.8 | 65 | 29.7 |
| History of depression | 187 | 52.2 | 94 | 67.6 | 93 | 42.5 |
| Antenatal depression | 77 | 21.5 | 42 | 30.2 | 35 | 16.0 |
| Antenatal anxiety | 135 | 37.7 | 66 | 47.5 | 69 | 31.5 |
Note. BMI = body mass index; c-section = Cesarean section. Antenatal depression defined as meeting SCID diagnosis for major, minor or not otherwise specified (NOS) at 18 or 32 weeks gestation. Antenatal anxiety defined as meeting SCID diagnosis for Generalized Anxiety Disorder (GAD) or GAD NOS at 18 or 32 weeks gestation.
Range = 19–34.
Range = 19–34.
Range = 20–34.
Range = 11–34.
Range = 12–31.
Range -11–34.
Range = 0–9.
Range = 0–7.
Range = 0–9.
Range = 1110.9–4735.0.
Range = 1370.0–4680.0.
Range = 1110.0–4735.0.
Range =178–293.
Range = 196–288.
Range = 178–293.
Measures
Traumatic events were elicited through the PTSD section of the SCID interview (First, 2002). A description of each event that a woman had experienced and the age at which it occurred were recorded. We defined a traumatic event as meeting criterion A1 of the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev; DSM-IV-TR; American Psychiatric Association, 2000) diagnostic criteria for PTSD.
The individual traumatic events were grouped into six types based on conceptual and empirical grounds (see Table 2). Lifetime trauma exposure was defined in three ways: a dichotomous measure of trauma history (yes/no), the severity or frequency of traumas experienced (coded 0, 1, 2, 3+ traumas) and the age at first trauma exposure (no trauma, <18 years, ≥18 years).
Table 2.
Type, Onset, and Recency of Trauma Exposure in Total Sample
| Variable | %or M |
|---|---|
| History of TE | 38.8 |
| Mean age (years) at first TEa | 14.59 |
| First TE prior to 18 years old | 71.9 |
| Frequency of TE | |
| 1 | 37.4 |
| 2 | 32.4 |
| 3+ | 30.2 |
| Mean years since last TEb | 5.45 |
| TE during current pregnancy | 23.0 |
| Type of TE | |
| Sudden death/illness someone close | 38.1 |
| Family/friend victim of any violence | 31.7 |
| Childhood sexual abuse | 30.9 |
| Adult physical/sexual violence | 28.8 |
| Serious MVA/natural disaster | 28.1 |
| Childhood neglect/physical abuse | 11.5 |
Note. N = 358. TE = trauma exposure; MVA = motor vehicle accident.
Range = 1–30.
Range = 1 month–29 years.
Continuous symptoms of depression were derived from the EPDS, the most widely used and validated screening tool for depressive symptoms in pregnant and postpartum women (Cox et al., 1987; α = .79). Anxiety symptoms were assessed using the PSWQ, a 16-item self-report questionnaire of anxiety with demonstrated reliability and validity that has been used in the perinatal period (α = .87; Meyer et al., 1990). Clinical diagnoses of current and past history of depression and Generalized Anxiety Disorder (GAD) were made from the mood episodes section of the SCID.
Obstetric outcomes were defined according to standard definitions and maternal and infant case notes reviewed by a maternal fetal specialist (EKP). Birth weight and gestational age were examined as both continuous (grams [g] and days, respectively) and dichotomous variables (<2500g and <37 weeks, respectively). The obstetric complication variable was a composite measure and included any medical complication in pregnancy or delivery (e.g., preterm premature rupture of membranes, preeclampsia, intrauterine infection, gestational diabetes).
Data Analysis
Based on previous research, covariates that could influence obstetric outcomes were included in analyses: ethnicity (African-American versus other), smoking (any prenatal smoking, coded no/yes), maternal body mass index (BMI) at 18 weeks gestation, alcohol (any prenatal alcohol use, coded no/yes), maternal age, pregnancy history (primigravid no/yes), and socioeconomic status indexed by years of education, marital status (single vs. married/cohabiting) and Medicaid status. When predicting birthweight we included mode of delivery as a covariate (Cesarean vs. vaginal delivery).
Pearson’s χ2 analysis was used for categorical variables, and ANOVA and t tests were conducted for continuous variables. Logistic regression was used to predict dichotomous outcome variables; hierarchical regression analysis was used to predict continuous birthweight and gestational age. For our analysis, we first adjusted for the effects of sociodemographic variables and then included trauma exposure and depression or anxiety as predictors; we examined trauma as a moderator of mood symptoms in predicting outcome in a subsequent step. Analyses were completed using SPSS 21.0.
Results
Of the 358 deliveries, 8.1% (n = 29) were considered low birthweight (< 2500g) and 5.3% (n = 19) were premature (<37 weeks delivery). The frequency of traumatic exposures was significantly associated with experiencing antenatal depression OR = 1.33, 95% CI [1.13, 1.56]; χ2(3, N = 358) = 20.97, p < .001 but not postpartum depression OR = 1.18, 95% CI [0.99, 1.41]; χ2(3, N = 358) = 1.701, p > . 637 or having a diagnosis of GAD or GAD NOS χ2(3, N = 358) = 3.62, p = .306. Table 2 describes the type and frequency of traumas experienced, age of onset and recency of exposure.
Univariate analysis showed that birthweight was not significantly associated with either history r = −.10, p = .850 or frequency of traumatic events r = .00, p = .936 but was significantly predicted by symptom measures of anxiety at the 2nd (PSWQ r = −.12, p = .034) and 3rd trimester assessments (PSWQ r = −.16, p = .005). Women with a clinical diagnosis of anxiety during pregnancy had babies who were 116.1g smaller on average than women without an anxiety diagnosis (anxiety diagnosis mean weight 3,313.17 g, SD = 441.58 vs. 3,429.27 g, SD = 437.82; F(1, 322) = 5.32, p = .022. Similarly, birthweight was significantly associated with depressive symptoms at both the 2nd (EPDS r = −.12, p = .034) and 3rd trimester assessments (EPDS r = −.15, p = .012). Women with a diagnosis of depression at any point in their pregnancy delivered babies who were on average 115.3g smaller than babies of non-depressed women (3,294.69 g, SD = 414.73 vs. 3,409.92 g, SD = 446.91, respectively; F(1, 321) = 0.24, p = .503).
Table 3 shows the results from a hierarchical regression analysis predicting birthweight from sociodemographic covariates, anxiety and trauma exposure, and the interaction of trauma and anxiety. Results showed that sociodemographic characteristics accounted for 6.5% of the variability in birthweight, and the addition of the mental health factors significantly improved the predictive power of the model to 7.7%. The interaction of the trauma and anxiety symptoms significantly improved the model to account for 9.4% of the variance. The coefficients show that birthweight was significantly predicted from being African American, higher BMI, being single, positive lifetime trauma history and higher anxiety in late pregnancy. The interaction result shows prediction from anxiety levels was significantly stronger in women with a trauma history.
Table 3.
Hierarchical Regression Predicting Birth Weight from Sociodemographics, TE, and Anxiety.
| Variable | b |
|---|---|
| Model 1a | |
| Maternal age | −.10 |
| African American ethnicity | −.13 |
| BMI | .11 * |
| Education (years) | −.03 |
| Single | .14* |
| Primigravid | −.08 |
| Medicaid recipient | .06 |
| C-section | .09 |
| Smoking in pregnancy | −.05 |
| Model 2b: Model 1, TE, anxiety at 32 weeks | |
| TE | .33 * |
| Anxiety at 32 weeks | .02 |
| Model 3c: Model 2, TE * anxiety | |
| TE *anxiety levels | −.41* |
Note. TE = trauma exposure; BMI = body mass index; c-section = Cesarean section.
Model 1 R2 = .07, F(9, 319) = 2.49**.
Model 2 R2 = .08, F(2, 317) = 2.44**.
Model 3 R2 = .09, F(3, 314) = 2.76**.
p < .01.
Table 4 reports a regression analysis that tested the hypothesis that age at first trauma predicted birthweight or moderated the effect of other predictors of birthweight. The results provided an important qualification to the results in Table 3. They showed that the moderating effect of trauma on anxiety was limited to those women who experienced a trauma under 18 years of age (overall model F(14, 320) = 2.44, p = .005). The models in Tables 3 and 4 were then re-analyzed using depression symptoms instead of anxiety. Results obtained were essentially identical (Supplementary Tables 1 and 2).
Table 4.
Hierarchical Regression Predicting Birth Weight from Sociodemographics, Age at First TE, and Anxiety.
| Variable | b |
|---|---|
| Model 1a | |
| Maternal age | −.07 |
| African American ethnicity | −.11* |
| BMI | .12* |
| Education (years) | −.04 |
| Single | .15** |
| Primigravid | −.05 |
| Medicaid recipient | .07 |
| C-section | .06 |
| Smoking in pregnancy | −.05 |
| Model 2b: Model 1, first TE < 18 years and >18 years | |
| First TE ≥18 years | −.01 |
| First TE <18 years | .31* |
| Model 3c: Model 2, first TE <18 years* anxiety at 32 weeks gestation | |
| First TE <18 years * anxiety at 32 weeks gestation | −.34** |
Note. BMI = body mass index; TE = trauma exposure; c-section = Cesarian section.
Model 1 R2 = .06, F(9, 318) = 2.51**.
Model 2 R2 = .06, F(2, 316) = 2.05*.
Model 3 R2 = .08, F(3, 313) = 2.44**.
p < .05.
p < .01.
A comparable analytic approach was applied to gestational age. Bivariate analyses showed that neither depression (r = .06, p = .230), anxiety (r = .02, p = .671), trauma history (r = .05, p = .336) nor frequency of traumatic events (r = .04, p = .430) was significantly associated with gestational age. Primigravidity was the only significant predictor of gestational age in the final models. In the anxiety model B = 2.745, SE = .994, p = .006; in the depression model B = 2.819, SE = .987, p = .005. There was not evidence that trauma history moderated the effect of prenatal maternal mood on gestational age.
Bivariate analyses showed that broad diagnosis of GAD (including NOS) χ2(1, N = 358) = .205, p = .651, trauma history χ2(1) = .514, p = .473 nor frequency of traumas χ2(3, N = 358) = 3.486, p = .323 was significantly associated with obstetric complications. Experiencing antenatal depression (yes/no) was significantly associated with obstetric complication χ2(1, N = 358) = 5.94, p = .019, however inspection of the data showed that the association was in the non-depressed group (76.6% vs. 23.4%). In the prediction models BMI was the only significant predictor of obstetric complication b = .053, SE = .017, p = .002, adjusted OR = 1.05, 95% CI [1.02, 1.09]. There was not evidence that trauma moderated the effect of prenatal maternal mood on obstetric complication. We then examined the effect of trauma exposure during the index pregnancy (n = 32) as opposed to at another time and found that women exposed during pregnancy had babies who were on average 75g (3,207.7 vs. 3,282.8g), smaller than trauma exposed women but the difference was not significant t(137) = −0.72, p = .474. Similarly, there was no difference in gestational age; 276.1 days pregnancy exposed versus 275.2 days at other times, t(137) = 0.45, p = .658.
Discussion
The rate of trauma reported here was similar to previous studies of low-income pregnant women (29% to 61%) (Rich-Edwards, James-Todd, Mohllajee, Kleinman, Burke, Gillman & Wright, 2011; Mezey, Bacchus, Bewley & White, 2005; Smith et al., 2006), providing further support that trauma history is common among high psychosocial risk perinatal samples. The findings suggested that lifetime trauma history and, in particular, childhood trauma exposure, magnified the prediction of low birthweight from maternal anxiety and depression. Our findings also supported previous work showing that lifetime trauma history, particularly childhood trauma, was associated with adverse or potentially adverse reproductive outcomes, including younger age at first pregnancy (Onoye et al., 2009), higher number of pregnancies (Onoye et al., 2009) and history of miscarriage (Rogal et al., 2007). Trauma significantly increased the risk of lifetime and antenatal depression and anxiety, which are associated with a range of adverse biological and psychiatric maternal and child outcomes. Trauma, however, did not have a direct effect on birthweight, gestational age, or obstetric complication in the current study.
The finding that trauma history magnified the prediction of both anxiety and depression on birthweight supports a life course perspective to obstetric health and suggests several possible mechanisms. Within the trauma group, the majority of women were first trauma exposed during childhood. Data on the psychobiology of trauma suggested that these women may have entered pregnancy with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Heim et al., 2008) and increased inflammation (O’Donovan, Neylan, Metzler & Cohen, 2012); both of these have been linked with the predictors of BMI, anxiety and depression (Raison & Miller, 2011) and may constitute pathways for increased vulnerability to adverse birth outcomes (Wadhwa et al., 2011). Furthermore, birthweight and neonatal weight gain are established predictors of adverse metabolic outcomes in adult life (Kerkhof & Hokken-Koelega, 2012) and it may be that cumulative life experiences prior to pregnancy have a psychological and biological influence on subsequent obstetric and infant outcomes (Kerkhof & Hokken-Koelega, 2012; Rogal et al., 2007; Seng et al., 2011; Yonkers et al., 2014).
The finding that anxious and depressive symptoms predicted lower birthweight but not gestational age or delivery complications adds to a growing literature that shows a modest effect of mood symptoms on obstetric outcomes in some, but not all, studies (Ding et al., 2014; Grigoriadis et al., 2013; Grote et al., 2010; Ibanez et al., 2012; Wadhwa et al., 2011). The present study is novel in examining both trauma history and mood symptoms and finding that the effects of anxiety and depression on birthweight were moderated by trauma, suggesting that symptom-based predictions of birth outcomes need to consider a broader risk phenotype.
The study had several limitations. The key limitation was that the sample, although high psychosocial risk, was obstetrically low to normal risk. In addition, the longitudinal design required that women present for obstetric care in their first trimester; the findings may not generalize to trauma-exposed women who present late for prenatal care or receive minimal prenatal care. In addition, we excluded women with psychotic features and oversampled women with depression and anxiety at baseline and so the rates of perinatal affective disorders in the sample are much higher than those reported in community samples. Our rates, however, of trauma and depression are comparable to those reported in previous samples of low income, high psychosocial risk pregnant women (49% and 22% respectively; Rich-Edwards et al.,2011). These limitations were offset by several strengths of the study, including a diverse sample of psychosocially disadvantaged women, a longitudinal design with a life course approach to trauma, and consideration of several risk phenotypes including the administration of gold-standard clinical diagnostic interviews.
Patients with a history of trauma and without significant psychiatric or medical diagnosis comprise a much larger percentage of the female population, with one study reporting a prevalence of 22%, and are more likely to be undiagnosed (McCauley et al., 1997). The findings of this study suggest that health providers need to consider lifetime trauma history, as well as symptoms of anxiety and depression, as a risk factor for lower birthweight. There are several screening tools for depression and anxiety that are readily available and have been validated for use in perinatal women (ACOG, 2015). As trauma has not been widely studied as a potential predictor of low birth weight, there are no specific guidelines for screening for prior trauma in pregnancy, although screening for intimate partner violence is recommended and guidelines developed (ACOG, 2012). Guidelines for perinatal mental health emphasize that screening techniques are not designed to diagnose depressive disorders, but aim to identify women for whom further comprehensive psychosocial and clinical assessment is needed. Appropriate training is therefore necessary for health professionals to ensure sensitive and thorough psychosocial assessment with appropriate referral and care pathways for identified risk factors. Appropriate referral strategies and treatment approaches, however, need to be in place for women who screen positive and to improve women’s mental health; this remains a challenge for universal screening.
There is now considerable interest in prenatal interventions to benefit the health of the mother and baby (O’Connor, Monk & Fitelson, 2014) and growing interest in the range of affective symptoms that may be particular to the perinatal period and require intervention. Preventive interventions that exist for low birth weight aim to control underlying medical conditions such as hypertension and diabetes, discontinuing smoking and drinking alcohol and illicit drug use, and encouraging appropriate nutrition and weight gain. It is possible that identifying prior trauma and providing treatment for perinatal depression for high-risk women (Grote et al., 2012) could be another method for promoting healthy obstetric outcomes. Early identification of women at increased risk of lower birth weight babies may help to reduce disparities in maternal and child health outcomes.
Supplementary Material
Acknowledgments
This work was supported by NIMH grants K23MH080290 (ERB), R01MH073019 (TO’C); University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH; and the Brain & Behavior Research Foundation (ERB). The funding organizations had no role in the design and conduct of the study; collection, analysis or preparation of the data; or preparation, review or approval of the manuscript. Emma Robertson Blackmore had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. Committee Opinion No. 630. Obstetrics and Gynecology. 2015;125:1268–1271. doi: 10.1097/01.AOG.0000465192.34779.dc. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Intimate partner violence. Committee Opinion No. 518. Obstetrics and Gynecology. 2012;119:412–417. doi: 10.1097/AOG.0b013e318249ff74. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Bublitz MH, Rodriguez D, Gobin AP, Waldemore M, Magee S, Stroud LR. Maternal history of adoption or foster care placement in childhood: A risk factor for preterm birth. American Journal of Obstetrics & Gynecology. 2014;210:e1–e6. doi: 10.1016/j.ajog.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Lichtenstein P, Langstrom N, D’Onofrio BM. Timing of Prenatal Maternal Exposure to Severe Life Events and Adverse Pregnancy Outcomes: A Population Study of 2.6 Million Pregnancies. Psychosomatic Medicine. 2011;73:234–241. doi: 10.1097/PSY.0b013e31820a62ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EK, Nurmohamed L, Mathew L, Elo IT, Coyne JC, Culhane JF. Risky Health Behaviors among Mothers-to-Be: The Impact of Adverse Childhood Experiences. Academic Pediatrics. 2010;10:245–251. doi: 10.1016/j.acap.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Ding XX, Wu YL, Xu SJ, Zhu RP, Jia XM, Zhang SF, Tao FB. Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. Journal of Affective Disorders. 2014;159:103–110. doi: 10.1016/j.jad.2014.02.027. [DOI] [PubMed] [Google Scholar]
- First M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Grigoriadis S, Vonder Porten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, … Ross LE. The Impact of Maternal Depression During Pregnancy on Perinatal Outcomes: A Systematic Review and Meta-Analysis. Journal of Clinical Psychiatry. 2013;74:e321–e341. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- Grote NK, Spieker SJ, Lohr MJ, Geibel SL, Swartz HA, Frank E, … Katon W. Impact of childhood trauma on the outcomes of a perinatal depression trial. Depression & Anxiety. 2012;29:563–573. doi: 10.1002/da.21929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. Meta-analysis of depression during pregnancy and the risk of preterm birth, low birthweight, and intrauterine growth restriction. Archives of General Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Ibanez G, Charles MA, Forhan A, Magnin G, Thiebaugeorges O, Kaminski M, Saurel-Cubizolles MJ The EDEN Mother-Child Cohort Study Group. Depression and anxiety in women during pregnancy and neonatal outcome: Data from the EDEN mother-child cohort. Early Human Development. 2012;88:643–649. doi: 10.1016/j.earlhumdev.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Kerkhof GF, Hokken-Koelega ACS. Rate of neonatal weight gain and effects on adult metabolic health. Nature Reviews Endocrinology. 2012;8:689–692. doi: 10.1038/nrendo.2012.168. [DOI] [PubMed] [Google Scholar]
- Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, … Baker PN. Reduced Infant Birthweight Consequent Upon Maternal Exposure to Severe Life Events. Psychosomatic Medicine. 2008;70:688–694. doi: 10.1097/PSY.0b013e318177940d. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Wilson EC, Mathews TJ. Births: final data for 2010. National Vital Statistics Reports. 2012;61(1):1–72. [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, … Bass EB. Clinical Characteristics of Women with a History of Childhood Abuse: Unhealed Wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mezey G, Bacchus L, Bewley S, White S. Domestic violence, lifetime trauma and psychological health of childbearing women. BJOG: an international journal of obstetrics and gynaecology. 2005;112:197–204. doi: 10.1111/j.1471-0528.2004.00307.x. [DOI] [PubMed] [Google Scholar]
- Morland L, Goebert D, Onoye J, Frattarelli L, Derauf C, Herbst M, … Friedman M. Posttraumatic stress disorder and pregnancy health: preliminary update and implications. Psychosomatics. 2007;48:304–308. doi: 10.1176/appi.psy.48.4.304. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Monk C, Fitelson EM. Practitioner Review: Maternal mood in pregnancy and child development: Implications for child psychology and psychiatry. Journal of Child Psychology and Psychiatry. 2014;55:99–111. doi: 10.1111/jcpp.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behavior & Immunity. 2012;26:642–649. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoye JM, Goebert D, Morland L, Matsu C, Wright T. PTSD and postpartum mental health in a sample of Caucasian, Asian, and Pacific Islander women. Archives of Womens Mental Health. 2009;12:393–400. doi: 10.1007/s00737-009-0087-0. [DOI] [PubMed] [Google Scholar]
- Putnam KT, Harris WH, Putnam FW. Synergistic Childhood Adversities and Complex Adult Psychopathology. Journal of Traumatic Stress. 2013;26:435–442. doi: 10.1002/jts.21833. [DOI] [PubMed] [Google Scholar]
- Putnam FW. Ten-year research update review: child sexual abuse. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:269–278. doi: 10.1097/00004583-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Current Psychiatry Reports. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, James-Todd T, Mohllajee A, Kleinman K, Burke A, Gillman MW, Wright RJ. Lifetime maternal experiences of abuse and risk of pre-natal depression in two demographically distinct populations in Boston. International Journal of Epidemiology. 2011;40:375–84. doi: 10.1093/ije/dyq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogal SS, Poschman K, Belanger K, Howell HB, Smith MV, Medina J, Yonkers KA. Effects of posttraumatic stress disorder on pregnancy outcomes. Journal of Affective Disorders. 2007;102:137–143. doi: 10.1016/j.jad.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Low LK, Sperlich M, Ronis DL, Liberzon I. Post-traumatic stress disorder, child abuse history, birthweight and gestational age: a prospective cohort study. British Journal of Obstetrics & Gynaecology. 2011;118:1329–1339. doi: 10.1111/j.1471-0528.2011.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MV, Poschman K, Cavaleri MA, Howell HB, Yonkers KA. Symptoms of posttraumatic stress disorder in a community sample of low-income pregnant women. American Journal of Psychiatry. 2006;163:881–884. doi: 10.1176/ajp.2006.163.5.881. [DOI] [PubMed] [Google Scholar]
- Spong CY, Iams J, Goldenberg R, Hauck FR, Willinger M. Disparities in Perinatal Medicine. Preterm birth, Stillbirth, and Infant Mortality. Obstetrics & Gynecology. 2011;117:948–955. doi: 10.1097/AOG.0b013e318211726f. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C, Lu MC. The Contribution of Maternal Stress to Preterm Birth: Issues and Considerations. Clinics in Perinatology. 2011;38:351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Smith MV, Forray A, Epperson CN, Costello D, Lin H, Belanger K. Pregnant women with Posttraumatic Stress Disorder and Risk of Preterm Birth. JAMA Psychiatry. 2014;71:897–904. doi: 10.1001/jamapsychiatry.2014.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.