Abstract
Insufficient sleep and circadian rhythm disturbances have been each associated with adverse cardiovascular outcomes in epidemiologic studies, but experimental evidence for a causal link is scarce. The present study compares the impact of circadian misalignment (CM) to circadian alignment (CA) on human autonomic function using a nonrandomized parallel group design to achieve the same total sleep time in both conditions. Following baseline assessments (three days with 10h bedtimes), 26 healthy young adults were assigned to sleep restriction (SR; eight 5h bedtimes) with either fixed nocturnal bedtimes (CA; n=13) or bedtimes delayed by 8.5h on 4 of the 8 days (CM; n=13). Daytime ambulatory blood pressure (BP) and heart rate (HR, CA: n=11, CM: n=10), and 24-hour urinary norepinephrine levels (NE, CA: n=13, CM: n=13) were assessed at baseline and the end of SR. Nocturnal HR and heart rate variability (HRV) were analyzed during sleep at baseline and during the 4th and 7th nights of SR (CA: n=8, CM: n=12). SR resulted in a significant increase in daytime HR in both groups, without changes in BP. SR increased 24-hour urinary NE in the CM group (30±4 vs. 21±2 μg), but not in the circadian alignment group (group×condition, p=0.005). In contrast to the lack of detectable impact of CM on daytime autonomic function, SR with CM elicited greater increases in nocturnal HR, as well as greater reductions in vagal indices of HRV, than SR without CM (group×condition, p<0.05). In conclusion, SR and CM both result in impaired autonomic function that could lead, under chronic conditions, to enhanced cardiovascular risk.
Keywords: Sleep, circadian rhythm, autonomic nervous system, norepinephrine, blood pressure, heart rate, shift work
INTRODUCTION
The proportion of short sleepers in the adult U.S. population has increased significantly over the past few decades.1 A recent review2 highlights that the majority of epidemiological studies report a significant association between short sleep and hypertension. A number of these epidemiological studies have been prospective in nature, and a recent meta-analysis of longitudinal cohort studies reported that short sleep was associated with increased risk for hypertension.3 In contrast, data from well-controlled laboratory studies, thus far, have failed to support a cause and effect relationship between short sleep and hypertension. Meier-Ewert et al.4 and Sauvet et al.5 found that 10 and 6 days, respectively of experimental sleep restriction did not change daytime arterial blood pressure (BP). Additionally, experimental sleep restriction has been shown to impair daytime heart rate (HR) or heart rate variability (HRV) in some,4, 6 but not all,5, 7 studies.
Circadian misalignment (CM) is a condition typically associated with sleep restriction8 and occurs when endogenous circadian rhythms are desynchronized from the sleep-wake and feeding cycles. During CM, a number of rhythms of autonomic activity and endocrine release remain locked to the master circadian pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus, while multiple circadian oscillators located in peripheral tissues, including cardiac tissue and vascular smooth muscle, are partly synchronized by the timings of activity, sleep and/or food intake.9 It is well recognized that shift work and jet lag are both associated with CM, but other factors such as chronotype, feeding habits, technological devices, and psychiatric health can influence CM.10 As such, the prevalence of acute, recurrent or chronic CM is growing 11 and this has been suggested to have adverse cardiovascular consequences. Forced desynchrony, the gold-standard laboratory approach to examine CM, has been shown to increase daytime BP in humans.8 However, this protocol involves reductions in total sleep time and thus the role of sleep loss in causing the cardiovascular alterations could not be fully elucidated.8
The present study aims to determine the impact of CM on autonomic nervous system (ANS) control of cardiovascular function. To this effect, we designed a sleep restriction protocol comparing healthy adults under conditions of circadian alignment (CA) versus CM, keeping the amount of daily sleep restriction identical. We hypothesized that sleep restriction would impair autonomic function, and that these impairments would be potentiated by CM when compared to CA.
METHODS
Protocol and Participants
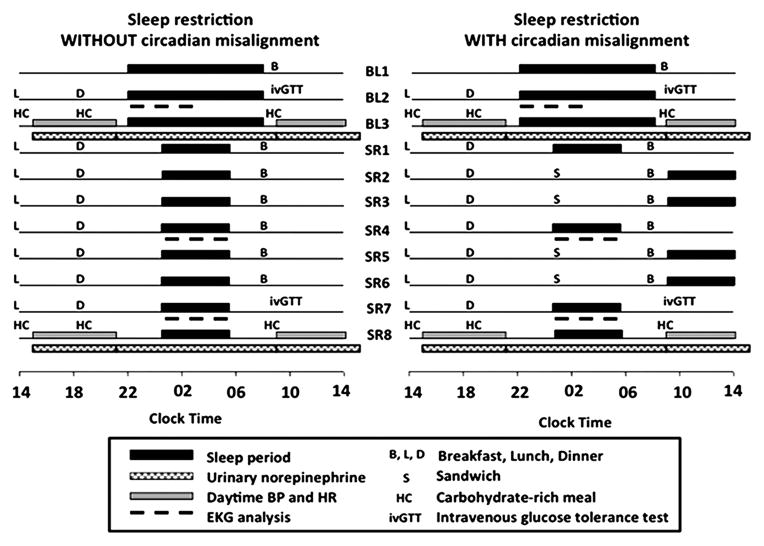
The study complied with the Declaration of Helsinki, all participants signed a written informed consent and the Institutional Review Board of the University of Chicago approved the protocol (Figure 1).
Figure 1.
Schematic representation of the study protocol.
Participants included 26 young healthy men (n=19) and women (n=7) aged 20–39 years who have been described in a previous article that focused on glucose metabolism.12 The current manuscript focuses on autonomic and cardiovascular outcomes that have not been reported. All subjects were free of endocrine, cardiovascular, psychiatric and sleep disorders. Other exclusion criteria included medication use, smoking, excessive alcohol (> 2 drinks/day) or caffeine consumption (> 300 mg caffeine/day), shift work or travelling across time zones during the past 2 months. Self-reported habitual sleep duration was 7.5h to 8.5h. Participants in each group were recruited from the community around the University of Chicago campus using the same advertisement materials. They were not pair-matched or group-matched. The CM and CA groups did not differ in sex distribution, age, or BMI.12
Prior to the study, participants underwent a full in-laboratory polysomnography to exclude sleep apnea (apnea/hypopnea index>5 events/hour) and periodic leg movement during sleep (>5 events/hour).13 Participants were asked to follow standardized schedules (23:00h–07:00h bedtimes) for a week before the study. Compliance was verified using wrist activity recordings (Actiwatch, MiniMitter Inc.). In women, the study was initiated during the early follicular phase of the menstrual cycle.
The study followed a nonrandomized, parallel group design comparing two interventions, each involving approximately 2 weeks of hospitalization. Both interventions (Figure 1) consisted of three days with 10-hour bedtimes (22:00h–08:00h: BL1-BL3; baseline rested condition), followed by 8 days with 5-hour bedtimes (SR1-SR8) resulting in a total of 24 hours of lost sleep opportunity over 8 days. Bedtimes were either always centered at 03:00h (00:30h–05:30h, CA) or delayed by 8.5 hours on 4 days (09:00h–14:00h on days SR2-SR3, SR5-SR6; CM). Participants abstained from caffeinated beverages throughout the protocol. On days BL3 and SR8, 24h blood sampling for hormonal and metabolic assessments was performed at 15- to 30-minute intervals (for more details, see Leproult et al.12).
Sleep
Sleep during each night was polygraphically recorded (Neurofax EEG-1100A, Nihon Kohden, Foothill Ranch, CA) using frontal, central and occipital electroencephalographic channels, electrooculograms, submental electromyogram, electrocardiogram, chest and abdominal belts. Sleep recordings were visually scored in 30-sec epochs in stages Wake, I, II, slow-wave sleep (SWS) and REM according to standardized criteria.14 Total sleep time was defined as the sum of the minutes of intra-sleep stages I, II, SWS and REM.
Ambulatory BP and HR
Blood pressure and HR measurements were obtained from the non-dominant arm at BL3 and SR8 while the subjects remained at bedrest. BP and HR were recorded at 15-minute intervals from 14:00h until 21:00h and again from 8:00h to 14:00h via an ambulatory monitoring device (Accutracker II, Suntech Medical Instruments, Morrisville, NC). The first hour of each measurement was not included in the analysis to eliminate possible artifacts related to the beginning of the recording (Figure 1). Each isolated measurement that corresponded to a peak or a drop greater than 15 (mmHg or bpm) over or below the previous and next measurements was considered to be a technical artifact and was interpolated. In five subjects, the ambulatory blood pressure monitoring device failed to record multiple and consecutive measurements over ≥2 hours time frame. As such, these five individuals were excluded from the repeated-measure analysis, resulting in a total of 21 subjects with complete daytime ambulatory BP (CA, n=11; CM, n=10). Mean arterial pressure (MAP) was estimated using the standard calculation of one-third pulse pressure plus diastolic pressure.15
Urinary Norepinephrine
Urine norepinephrine (NE) was obtained over 3 time intervals (15:00h–21:00h, 21:00h–9:00h and 9:00h–15:00h) during BL3 and SR8 (Figure 1). Fractionated urinary NE was assayed using a high performance liquid chromatography with electrochemical detection system (Coulochem Electrode Array System (CEAS), ESA, Inc.). Twenty-four-hour NE excretion and urinary NE levels for the 3 time intervals normalized per hour were computed.
HRV during sleep
HRV analyses were performed during one baseline night (BL2) and two nights of nocturnal bedtime restriction (SR4 and SR7; Figure 1). In 3 participants, baseline HRV assessments were derived from night BL1 because recordings during BL2 were of poor quality (n=1 in CA and n=2 in CM).
HRV analyses focused on the first three cycles of sleep (i.e. approximately the first 5 hours of sleep). For each cycle, HRV analysis was performed on 5-minute periods during stage II, SWS and REM sleep. An experienced neurologist (DG), blinded to the intervention condition (i.e., CM vs CA), visually selected each 5-minute period to exclude portions of recording with breathing instabilities, arousals,16 body movements, ectopic beats and artifacts. To ensure a stationary signal,17 the same selection criteria were applied during the 30-sec epoch preceding and the 30-sec epoch following each 5-minute period.
HR and HRV were quantified during each 5-minute interval to derive time and frequency domain indices (PRANA software; Phitools, Strasbourg, France). For each sleep stage, results of the analysis of the 5-minute periods in the three sleep cycles were averaged. The time domain index of HRV is reported as the square root of the mean of the squares of the successive differences between adjacent R-R intervals (RMSSD), which is strongly correlated with the parasympathetic modulation of HR.17 Spectral analysis of HRV was performed by Fast Fourier Transform, and spectral power was calculated in the high frequency band (HF; 0.15–0.40 Hz) reflecting mostly vagal activity and in the low frequency band (LF: 0.04–0.14 Hz) reflecting a combination of vagal and sympathetic activities. Limitations associated with frequency domain analyses are acknowledged.18
Statistical Analysis
Results are expressed as mean (standard deviation) for normally distributed data, or as median (25th, 75th percentiles) for data not normally distributed. We maintained the use of SEM in figures to maximize legibility. Repeated measures ANOVA were performed including condition (baseline vs. day of sleep restriction) and time (time of day), when applicable, as the within-subject factors, and group (CA vs. CM) as the between-subject factor. During nocturnal HR and HRV analyses, each stage was analyzed independently. Age and BMI were included as covariates. Between-group differences in changes from baseline of HR and HRV indices were also analyzed using a generalized linear model for repeated measures. When not normally distributed, the variables were log-transformed to meet the requirements for linear model analysis. All statistical calculations were performed using JMP software (SAS Institute Inc., Cary, NC). Statistical significance was set at p < 0.05.
RESULTS
Participants
Demographics for the 26 participants have been previously reported.12 Daytime BP and HR were available for 21 participants, and nocturnal HR and HRV for 20 participants. There were no significant differences in demographic characteristics between the CA and CM groups in any of the analyses.
Polysomnography
The protocol was successful in achieving similar sleep durations in both the aligned and misaligned conditions as total sleep time during each night of the study (3 nights of baseline and 8 nights of sleep restriction) was nearly identical in the CM and CA groups, as previously reported.12 For the subset of 20 participants in whom we report nocturnal HR and HRV, total sleep time, as well as the amounts of stage II, SWS and REM sleep, were also similar in the two groups (see online Figure S1).
Daytime Ambulatory BP and HR
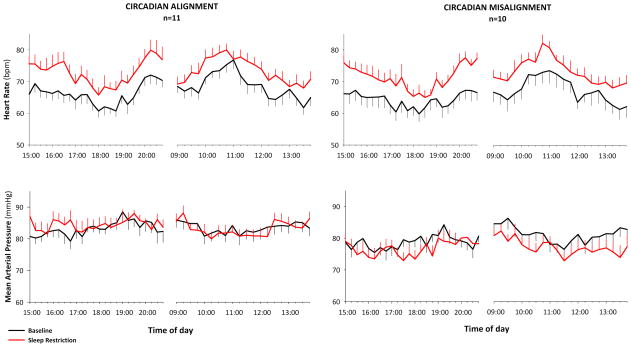
Figure 2 illustrates that sleep restriction significantly increased daytime HR from baseline by 5–10 bpm above baseline in both CM and CA groups (condition: p<0.001). These clinically relevant increases in daytime HR were not different between CM and CA groups (condition × group: p=0.46). Sleep restriction did not alter daytime MAP in either CM or CA groups (condition: p=0.14; condition x group: p= 0.52). Similarly, mean systolic and diastolic BP during evening and morning hours were not affected by sleep restriction in either group (data shown in Table S1).
Figure 2.
Mean (±SEM) heart rate (upper panels) and mean arterial pressure (lower panels) measured every 15 minutes during daytime hours at baseline (black) and after eight days of sleep restriction (red). Heart rate, but not mean arterial blood pressure, significantly increased after sleep restriction in both the circadian alignment and misalignment groups (condition, p < 0.001; condition x group, p= 0.46).
Daytime and Nocturnal Urinary NE
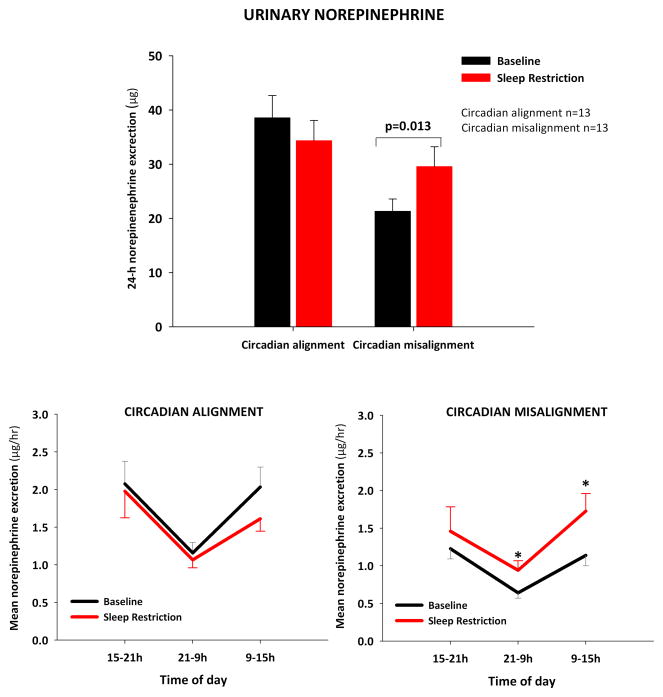
Baseline 24-hour NE levels were within the normal range for healthy adults (< 80 μg/24-hour) in all subjects.19 Baseline NE values were higher in subjects exposed to CA (group: p<0.01), and this difference was not explained by differences in demographic characteristics. Figure 3 (upper panel) illustrates that 24-hour urine NE increased significantly after 8 days of sleep restriction in subjects exposed to CM (baseline: 21±8 μg; SR: 30±13 μg; p=0.013), but not in participants exposed to sleep restriction alone (baseline: 39±15 μg; SR: 34±14 μg; p=0.26, condition x group: p= 0.009).
Figure 3.
Upper panel: mean (+SEM) 24h norepinephrine urine concentrations at baseline and at 8th day of sleep restriction (group x condition: p= 0.009). Lower panels: mean (± SEM) norepinephrine levels during daytime and nighttime periods (normalized per hour) (group x condition: p=0.005). * p ≤ 0.05 vs. corresponding baseline.
When urinary NE levels were examined during the 3 time intervals of urine collection, there was a significant group x condition interaction (p=0.005), and post-hoc analysis revealed that NE levels increased more during nocturnal and morning hours as compared to baseline in the CM group, while no significant changes in urinary NE were observed in the CA group (Figure 3, lower panels). Specifically, sleep restriction with CM increased urine NE by approximately 76% during the nighttime period (baseline: 0.6±0.3 μg/hr; intervention: 0.9±0.4 μg/hr; p=0.047) and by approximately 78% during the morning period (baseline: 1.1±0.5 μg/hr; intervention: 1.7±0.8 μg/hr; p=0.012).
Nocturnal HR and HRV
In the subset of participants in whom nocturnal HR and HRV could be analyzed, there were no significant group differences in sex distribution (CA: 3/8 women; CM: 4/12; p=0.85), BMI (CA: 23.1±2.4 kg/m2; CM: 22.1±2.5 kg/m2; p=0.19) and age (CA: 24.5±4.5 years; CM: 22.7±4.0 years; p=0.09). Nocturnal HR and HRV indices and respiratory frequency at baseline were not different between the two groups during all sleep stages (see online Table S2). Changes in respiratory frequencies during the intervention nights were comparable in the two groups in all sleep stages (group x condition: Stage II p=0.82; SWS p=0.28; REM p=0.51).
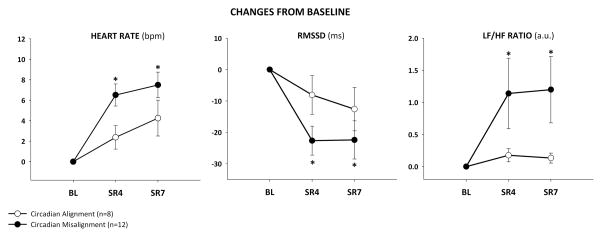
Figure 4 illustrates that during SWS, normally the most restorative state of sleep, sleep restriction increased HR, in both the CM and CA groups (condition: p=0.030), but these increases in HR were greater in the CM group (group × condition: p<0.001). During SWS, sleep restriction decreased RMSSD (condition: p<0.01) and increased LF/HF ratio (condition: p<0.01) in the CM group, but not in the CA group (group × condition: p=0.008 and p=0.045, respectively).
Figure 4.
Changes from baseline (± SEM) in heart rate (group x condition p= 0.016), root mean square of sequential differences of RR-intervals (RMSSD) (group x condition p= 0.008), and low frequency to high frequency (LF/HF) ratio (group x condition p= 0.045) in the 4th and 7th night of sleep restriction (SR4 and SR7) during slow wave sleep. bpm= beats per minute; a.u.= arbitrary units. * p ≤ 0.05 vs. corresponding baseline.
Similarly, sleep restriction increased HR during stage II in both CM and CA groups (condition: p=0.013), and these increases in HR were greater in the CM group (group × condition: p= 0.016). Sleep restriction increased LF/HF ratio during stage II (condition: p<0.01) in the CM group, but not in the CA group (group × condition: p=0.045). Sleep restriction tended to decrease RMSSD during stage II in the CM group (p=0.049), but not in the CA group (group × condition: p=0.082). Sleep restriction increased LF/HF ratio (p<0.01) during REM sleep in the CM group, but not in the CA group (group × condition: p= 0.038). Sleep restriction, with or without CM, did not change HR during REM sleep (condition: p= 0.921; group × condition: p=0.303) or REM RMSSD (condition: p= 0.887; group × condition: p=0.301).
DISCUSSION
This well-controlled laboratory study is the first to determine the impact of CM on autonomic and cardiovascular function in sleep restricted humans. Indeed, when controlling for the amount of sleep restriction, our data reveal three novel findings. First, sleep restriction with CM, but not with CA, significantly increased urinary NE and reduced nocturnal HRV. Second, sleep restriction with CM elicited greater increases in nocturnal HR when compared to sleep restriction alone. Third, these alterations in autonomic indices did not translate into an augmentation of arterial BP, suggesting that other compensatory mechanisms are engaged to offset the observed impairment in sympathetic and parasympathetic functions during sleep restriction with CM. Collectively, these findings demonstrate a clear adverse impact of CM on autonomic function in sleep-restricted healthy adults.
Several recent studies have suggested that CM can impair autonomic and cardiovascular function.20–22 Of particular interest, Scheer et al.8 utilized a forced desynchrony protocol to examine the impact of CM on autonomic and cardiovascular function. The protocol involved 10 days of forced desynchrony between endogenous circadian cycles and the imposed sleep-wake schedule. When participants slept and ate approximately 12 hours out of phase with their endogenous circadian rhythm, their BP was elevated during waking periods, while HR was unchanged. However, the authors report that total sleep time was reduced, and acknowledged that the role of this reduction of sleep duration in the observed cardiovascular alterations could not be fully determined.8 Accordingly, the present study used a novel protocol in which sleep was restricted to the same extent with or without CM to examine the impact of CM, in the presence of nearly identical levels of sleep loss. Our results reveal that this approach successfully increased sleep pressure, and yielded similar total sleep times throughout all baseline and sleep restriction nights in both the CM and CA groups. However, our findings do not demonstrate that an impact of CM could be detected in the absence of sleep loss.
Epidemiological studies have reported significant associations between sleep loss and hypertension.2 A recent meta-analysis of longitudinal epidemiological studies reports that short sleep is associated with increased risk for hypertension.3 Moreover, acute experimental models utilizing 24 to 88 consecutive hours of total sleep deprivation consistently report increases in arterial BP.4, 23–25 Taken together, these prospective epidemiological and acute total sleep deprivation studies hint towards a causal relationship between sleep loss and hypertension, but experimental data of well-controlled semi-chronic sleep restriction do not support this concept. Meier-Ewert et al.4 and Sauvet et al.5 both reported that experimental sleep restriction of, respectively, 10 and 6 days did not affect daytime arterial BP. Our findings advance this prior work in an important way. Indeed, these two previous studies obtained only a single time point “snapshot” of arterial BP, one using a 10-min period of calibrated Portapres recordings5 and the other using unspecified casual General Clinical Research Center (GCRC) BP measurements.4 In contrast, the present study utilized a carefully controlled ambulatory BP monitoring system providing BP values at 15-min intervals throughout waking hours. Our findings demonstrate unambiguously that one week of experimental bedtime restriction to 5h per night does not result in significant elevations of arterial BP at any time of the waking period in healthy young adults, even when sleep loss is combined with CM, thus confirming and greatly extending the previous studies.4–5
There are several possible explanations for the discrepancies between the prospective epidemiological studies and the laboratory sleep restriction studies. First, studies of experimental sleep restriction, including our own, have ranged in duration from 7 to 10 days, while prospective epidemiological studies have ranged from 2–12 years.2 Second, participants in laboratory studies were young and healthy while epidemiologic studies enrolled a subject population with a wider range of demographics. Thirdly, the vast majority of epidemiological studies were based on self-reported sleep duration, which is not strongly correlated with objectively-defined sleep duration. Lastly, it is possible that the well-controlled conditions of the laboratory studies eliminate effects of sleep restriction that could, under real life conditions, cause elevations in BP. For example, participants in our laboratory studies typically report low levels of stress on validated scales,6 while meeting professional and personal demands when being chronically sleep deprived may be much more stressful. In addition, in the laboratory, intake of caffeine, salt, and caloric-dense food is carefully controlled while, under real life conditions, insufficient sleep often results in increased caffeine, salt, and caloric intake that may in turn increase blood pressure.
Despite similar ambulatory BP responses to sleep restriction in CA and CM groups, our data reveal significant differences in several key autonomic variables between the 2 groups that may precede changes in ambulatory BP. Of particular interest, sleep restriction increased urinary NE levels only when combined with CM. NE baseline values were higher in subjects exposed to CA compared to those exposed to CM. However, all values were within the normal range for healthy adults 19 and the demographic characteristics of the two groups were similar. The difference in baseline NE levels therefore reflects random sampling within the wide physiologic range of basal NE levels. Of note, despite a wide inter-individual variability in 24h NE levels, intra-individual levels are relatively stable over time.26 Moreover, it is worth noting that Faraut et al. 27 recently reported that one-night of sleep deprivation increased urinary NE from ~40 mcg/day to more than 70 mcg/day. Our CA group displayed a similar baseline value to that of Faraut et al. (i.e., 39 mdg/day); thus, we are confident that our subjects were well within the range of being able to demonstrate further increases in urinary NE with an adequate stressor (i.e., not a “ceiling” effect). We acknowledge that the urinary NE baseline value for the CM group was on the lower side of the normative range (i.e., 15 – 80 μg/24 hr),19 and thus we cannot rule out regression to the mean as an alternative explanation for the increase in NE levels in the CM group.
Urinary NE provides a reliable marker of sympathetic function that is highly dependent upon glomerular filtration rate and renal function.28 The sympathetic nervous system is highly specific and regionalized, thus we do not infer that sleep restriction with CM elicits a global increase of sympathetic activation. However, the increase of urinary NE after sleep restriction with CM suggests it may be valuable to utilize more invasive and regionally specific techniques such as microneurography and norepinephrine spillover to increase our understanding of the impact of sleep restriction and circadian dysfunction on sympathetic function.
Consistent with prior work,4 sleep restriction increased daytime ambulatory HR. The present study advances this prior work by demonstrating that CM does not appear to exacerbate this tachycardia response to sleep restriction. However, in contrast to the daytime ambulatory HR, we observed a significant influence of CM on nocturnal HR and HRV responses to sleep restriction. During sleep, HRV analysis explores the integrity of ANS function in the absence of confounding factors such as physical activity and higher cortical functions. A reduction of HRV has been associated with an adverse prognosis in multiple cardiovascular diseases.17, 29 Previous studies that have explored the cardiovascular alterations associated with CM and sleep have primarily focused on assessments during daytime sleep when the circadian rhythm of ANS activity is in an opposite phase relative to nighttime sleep.21, 30 These prior studies 21, 30 report that HR and LF:HF ratio were significantly increased during daytime sleep compared to nighttime sleep, suggesting that daytime sleep cannot provide the same cardiovascular restorative effects as nighttime sleep. In contrast, we investigated the cardiovascular consequences of sleep restriction with CM during the first re-aligned night after daytime sleep, when the behavioral sleep-wake cycle was identical to that of the CA group. We found that sleep restriction with CM was associated with higher HR and lower HRV during non-REM stages (i.e., SWS and Stage II) as compared to sleep restriction with CA. Given the importance of SWS and non-REM sleep in cardiovascular restoration,31 these findings suggest that recurrent or chronic circadian misalignment has adverse cardiovascular effects that are not readily corrected as soon as a normal daytime schedule is re-instated. The rate of recovery of nocturnal autonomous nervous system activity following abrupt shifts of the sleep-wake cycle needs further study to improve our understanding of the adverse cardiovascular consequences of shift work and design preventive strategies.
Morris et al.32 recently reported that 3 days of CM (12-h inversion of the behavioral and environmental cycle) without bedtime restriction elicited a reduction of daytime vagal indices of HRV, an increase in 24-h BP and inflammatory markers, but no changes in 24-h urinary NE excretion. The reduction in diurnal vagal cardiac control described by Morris et al.32 corroborates our findings from nighttime HRV analysis indicating a reduction in vagal activity in subjects exposed to CM. There is, however, a discrepancy between our findings and those of Morris et al.32 regarding BP and urinary NE responses to CM. The differences in the two experimental approaches (i.e., CM and CA with similar levels of sleep restriction vs. CM via 12-h inversion of the behavioral and environmental cycle without control for sleep duration) likely explain these divergent findings. Indeed, Morris et al.32 report that sleep duration was different between their CM and CA groups. It is important to note that while these in-laboratory studies are well-controlled, they are still relatively short in duration (i.e., 3–8 days) compared to real-life CM. It remains possible that longer duration CM, with or without sleep restriction, would negatively impact both urinary NE and ambulatory BP. Nevertheless, when taken together these two studies complement one another in suggesting the possibility of an increased cardiovascular risk in subjects exposed to CM, and suggest that there may be a complex interaction between CM and sleep restriction that warrants further investigation.
We acknowledge some limitations in the present study. First, as previously reported,12 the two interventions could not be conducted simultaneously for logistic reasons and therefore assignment of the participants to the CA or CM group was not randomized in a traditional fashion. However, the same advertisement materials were used for both groups and the subjects were not aware that an alternate intervention existed. It is possible that the lack of randomization contributed to the differences in NE baseline values between the two groups, but we believe this is unlikely since baseline differences were not observed for other autonomic variables or for metabolic variables. Second, we introduced age and BMI as covariates in our analyses despite our limited sample size, and therefore there is a potential for statistical over-fitting. However, when we excluded age and BMI from our statistical models, the primary findings remained unchanged.
In conclusion, the present study reports that sleep restriction with CM, as typically occurs in shift workers, results in altered autonomic function during wakefulness and sleep. In particular, sleep restriction with CM increases norepinephrine secretion and reduces cardiac vagal modulation which could, under chronic conditions, lead to an increased risk of cardiovascular disease.
Perspectives
Our findings are of importance to a number of conditions associated with sleep restriction with CM. For example, insufficient sleep is particularly common in shift workers, who represent 15 to 30% of the working population.33–35 Irrespective of the schedule, shift work is typically associated with chronic CM. Prospective studies report adverse cardiovascular outcomes in shift workers,19–22 and a recent meta-analysis of cross-sectional studies found that shift work is associated with an increased risk for myocardial infarction and all coronary events.36 In healthy subjects, the highest vagal influence on HR occurs during NREM sleep, together with the highest feedback contribution of the baroreflex,37–38 consistent with a cardio-restorative role of NREM sleep. The results of this non randomized parallel group study suggest that shift workers might not benefit from the restorative cardiovascular effects of aligned nights of sleep following a shift work rotation, and may therefore not readily recover from their irregular sleep-wake pattern. Our findings hence suggest a mechanistic link between the reduction of cardiac vagal modulation during sleep found in our subjects exposed to CM, and the increased risk to develop adverse cardiac events observed in shift workers.
Evidence from rodent models has revealed that there are distinct phase relationships between rhythms of circadian gene expression in cardiac tissue, the liver and the SCN, and that these phase relationships need to be stable to achieve optimal cardio-metabolic function at each time of day.39–40 In addition to shift work, other factors such as jet lag, chronotype, feeding cycles, technological devices, and psychiatric health can influence CM.9 Therefore, the autonomic dysfunction reported during sleep restriction with CM in the present study is relevant to an increasingly wide-range of individuals.
Supplementary Material
Novelty and Significance.
1) What is New?
Sleep restriction with circadian misalignment increases urinary norepinephrine and nocturnal heart rate, and reduces nocturnal heart rate variability, when compared to sleep restriction with circadian alignment.
These altered autonomic indices do not translate into an augmentation of ambulatory daytime arterial blood pressure.
2) What is Relevant?
Sleep loss and circadian misalignment are common among shift workers. Our results suggest that shift workers might not benefit from the restorative cardiovascular effects of nighttime sleep following a shift work rotation.
Summary
This nonrandomized study is the first to determine the impact of CM on autonomic and cardiovascular function in sleep restricted humans. Our results demonstrate altered autonomic function during wakefulness and sleep, which under chronic conditions could lead to an increased risk of cardiovascular disease.
Acknowledgments
The authors thank Ulf Holmbäck, PhD, for his precious involvement in data collection, and the nursing and dietary staff of the University of Chicago General Clinical Resource Center and the team of polysomnography technicians of the Sleep, Metabolism and Health Center for their expert assistance.
Funding Sources: This research was supported by National Institutes of Health grants R01-HL72694, ULl-TR000430, P60-DK020595, P01-AG11412 and NIOSH R01-OH009482. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Rachel Leproult is a recipient of a grant “Brains Back to Brussels” from INNOVIRIS, the Brussels Institute for Research and Innovation, Région Bruxelles-Capitale, Belgium.
Footnotes
The study was conducted at the Sleep Metabolism and Health Center, Department of Medicine, The University of Chicago, Chicago, IL 60637, USA
Disclosures: None of the authors has financial conflicts of interest to declare.
Eve Van Cauter (EVC) and Rachel Leproult (RL) designed the protocol of the study. EVC obtained funding and RL directed data collection and management. Daniela Grimaldi, EVC, RL and Jason Carter participated in data analysis, interpretation, and drafting of the manuscript.
References
- 1.Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014;27:1235–1242. doi: 10.1093/ajh/hpu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: A meta-analysis of prospective cohort studies. Hypertens Res. 2013;36:985–995. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on c-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Sauvet F, Drogou C, Bougard C, Arnal PJ, Dispersyn G, Bourrilhon C, Rabat A, Van Beers P, Gomez-Merino D, Faraut B, Leger D, Chennaoui M. Vascular response to 1 week of sleep restriction in healthy subjects. A metabolic response? Int J Cardiol. 2015;190:246–255. doi: 10.1016/j.ijcard.2015.04.119. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 7.Muenter NK, Watenpaugh DE, Wasmund WL, Wasmund SL, Maxwell SA, Smith ML. Effect of sleep restriction on orthostatic cardiovascular control in humans. J Appl Physiol (1985) 2000;88:966–972. doi: 10.1152/jappl.2000.88.3.966. [DOI] [PubMed] [Google Scholar]
- 8.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challet E. Circadian clocks, food intake, and metabolism. Prog Mol Biol Transl Sci. 2013;119:105–135. doi: 10.1016/B978-0-12-396971-2.00005-1. [DOI] [PubMed] [Google Scholar]
- 10.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott SM, Reid KJ, Zee PC. Circadian Rhythm Sleep-Wake Disorders. Psychiatr Clin North Am. 2015;38:805–802. doi: 10.1016/j.psc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Icsd-2 international classification of sleep disorders: Diagnostic and coding manual. American Academy of Sleep Medicine. 2005 [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 15.Smulyan H, Safar ME. Blood pressure measurement: Retrospective and prospective views. Am J Hypertens. 2011;24:628–634. doi: 10.1038/ajh.2011.22. [DOI] [PubMed] [Google Scholar]
- 16.EEG arousals: Scoring rules and examples: A preliminary report from the sleep disorders atlas task force of the american sleep disorders association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 17.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 18.Eckberg DL. Sympathovagal balance: A critical appraisal. Circulation. 1997;96:3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 19.Rosano TG, Swift TA, Hayes LW. Advances in catecholamine and metabolite measurements for diagnosis of pheochromocytoma. Clin Chem. 1991;37:1854–1867. [PubMed] [Google Scholar]
- 20.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 21.Chung MH, Kuo TB, Hsu N, Chu H, Chou KR, Yang CC. Sleep and autonomic nervous system changes - enhanced cardiac sympathetic modulations during sleep in permanent night shift nurses. Scand J Work Environ Health. 2009;35:180–187. doi: 10.5271/sjweh.1324. [DOI] [PubMed] [Google Scholar]
- 22.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 23.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: Sex differences. Am J Physiol Heart Circ Physiol. 2012;302:H1991–1997. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–1175. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep. 2003;26:986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 26.Forsman L, Lundberg U. Consistency in catecholamine and cortisol excretion in males and females. Pharmacol Biochem Behav. 1982;17:555–562. doi: 10.1016/0091-3057(82)90318-5. [DOI] [PubMed] [Google Scholar]
- 27.Faraut B, Nakib S, Drogou C, Elbaz M, Sauvet F, De Bandt JP, Léger D. Napping reverses the salivary interleukin-6 and urinary norepinephrine changes induced by sleep restriction. J Clin Endocrinol Metab. 2015;100:E416–E412. doi: 10.1210/jc.2014-2566. [DOI] [PubMed] [Google Scholar]
- 28.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 29.Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis. 2012;55:321–331. doi: 10.1016/j.pcad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36:1919–1928. doi: 10.5665/sleep.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murali NS, Svatikova A, Somers VK. Cardiovascular physiology and sleep. Front Biosci. 2003;8:s636–652. doi: 10.2741/1105. [DOI] [PubMed] [Google Scholar]
- 32.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113:E1402–1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alterman T, Luckhaupt SE, Dahlhamer JM, Ward BW, Calvert GM. Prevalence rates of work organization characteristics among workers in the u.S.: Data from the 2010 national health interview survey. Am J Ind Med. 2013;56:647–659. doi: 10.1002/ajim.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sands MR, Lauderdale DS, Liu K, Knutson KL, Matthews KA, Eaton CB, Linkletter CD, Loucks EB. Short sleep duration is associated with carotid intima-media thickness among men in the coronary artery risk development in young adults (cardia) study. Stroke. 2012;43:2858–2864. doi: 10.1161/STROKEAHA.112.660332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male japanese workers. Obesity (Silver Spring) 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 36.Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG. Shift work and vascular events: Systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancia G. Autonomic modulation of the cardiovascular system during sleep. N Engl J Med. 1993;328:347–349. doi: 10.1056/NEJM199302043280511. [DOI] [PubMed] [Google Scholar]
- 38.Silvani A, Grimaldi D, Vandi S, Barletta G, Vetrugno R, Provini F, Pierangeli G, Berteotti C, Montagna P, Zoccoli G, Cortelli P. Sleep-dependent changes in the coupling between heart period and blood pressure in human subjects. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1686–1692. doi: 10.1152/ajpregu.00756.2007. [DOI] [PubMed] [Google Scholar]
- 39.van der Veen DR, Shao J, Xi Y, Li L, Duffield GE. Cardiac atrial circadian rhythms in period2::Luciferase and per1:Luc mice: Amplitude and phase responses to glucocorticoid signaling and medium treatment. PLoS One. 2012;7:e47692. doi: 10.1371/journal.pone.0047692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse period1 mrna expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.