Abstract
Sestrins are highly conserved, stress-inducible proteins that inhibit target of rapamycin complex 1 (TORC1) signaling. Following their transcriptional induction, both vertebrate and invertebrate Sestrins turn on the AMP-activated protein kinase (AMPK) which activates the tuberous sclerosis complex (TSC), a key inhibitor of TORC1 activation. However, Sestrin overexpression, on occasion, can result in TORC1 inhibition even in AMPK-deficient cells. This effect has been attributed to Sestrin’s ability to bind the TORC1-regulating GATOR2 protein complex, which was postulated to control trafficking of TORC1 to lysosomes. How the binding of Sestrins to GATOR2 is regulated and how it contributes to TORC1 inhibition is unknown. New findings suggest that the amino acid leucine specifically disrupts the association of Sestrin2 with GATOR2, thus explaining how leucine and related amino acids stimulate TORC1 activity. Here we discuss whether and how these findings fit what has already been learned about Sestrin-mediated TORC1 inhibition from genetic studies conducted in fruit flies and mammals.
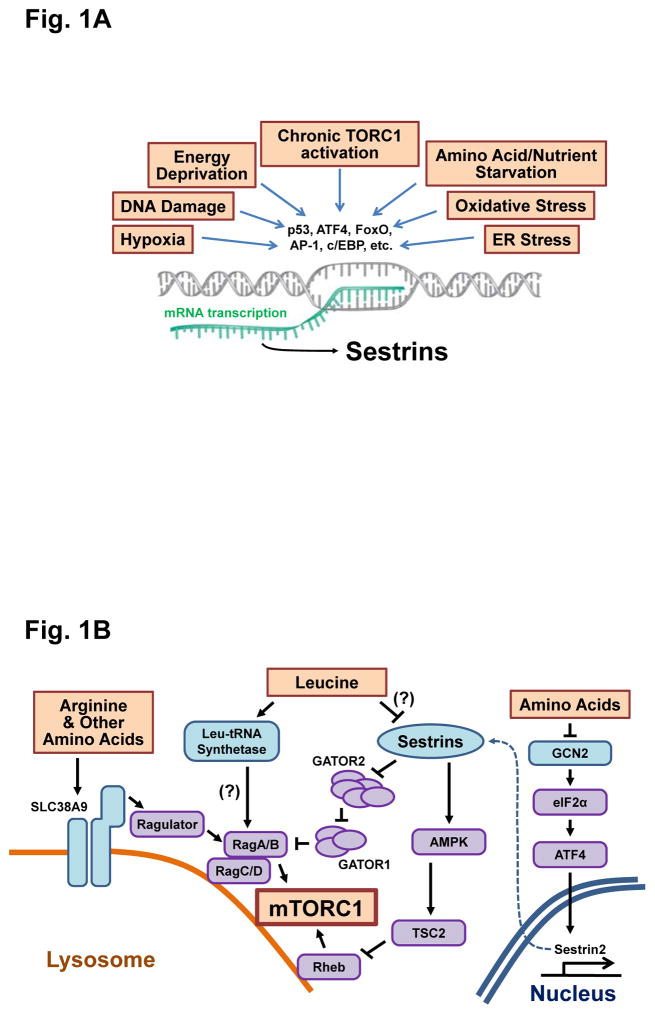
The Sestrins were discovered in 1999 through a differential display screen for new targets for the transcriptional activity of the tumor suppressor p53 (1). The three mammalian Sestrin isoforms, Sestrin 1–3, are encoded by three separate chromosomal loci, whereas Drosophila and Caenorhabditis elegans harbor only a single Sestrin gene. The abundance of Sestrins is relatively low in resting cells but environmental and metabolic stresses, including DNA damage, oxidative stress, hypoxia, endoplasmic reticulum (ER) stress, energy deprivation and amino acid starvation, induce their expression by activating several transcription factors, including p53, forkhead box O (FoxO), CCAAT-enhancer-binding protein (c/EBP), activating transcription factor 4 (ATF4) and activator protein 1 (AP-1) (Fig. 1A) (1–3). Although Sestrins have been assigned a number of distinct biological functions, their most important and least contentious function is regulation of the nutrient responsive AMPK-TORC1 axis, such that Sestrin accumulation stimulates AMPK and inhibits TORC1 (Fig. 1B) (3). Despite extensive investigation, the mechanism by which vertebrate or invertebrate Sestrins activate AMPK remains obscure, although it has been reported that the Sestrins can interact with AMPK or increase the abundance of AMPK subunits (4–6). Nonetheless, genetic experiments in both flies (7) and mice (8, 9) show that metabolic defects caused by Sestrin deficiencies can be corrected by expression of constitutively activated forms of AMPK or treatment with pharmacological AMPK activators. Moreover, AMPK activation accounts for most of the inhibition of TORC1 activity seen upon Sestrin induction, because restoration of AMPK activity results in suppression of dysregulated TORC1 in Sestrin-deficient fly and mouse mutants (7–9). Additional activities attributed to Sestrins include suppression of the accumulation of reactive oxygen species (ROS) (10–12), activation of TORC2 and AKT signaling (13) and inhibition of the cell survival factor X-linked inhibitor of apoptosis protein (XIAP) (14). All mammalian Sestrin isoforms, as well as invertebrate Sestrins, can regulate AMPK, TORC1 and ROS-mediated signaling (3). However, Sestrin overexpression in AMPK-deficient cells can on occasion still attenuate TORC1 activity (15–17). Despite their obscure physiological importance, these findings have led to an extensive search for additional Sestrin-related activities that can explain AMPK-independent inhibition of TORC1 activity.
Fig 1. The impact of Sestrins on amino acid-dependent TORC1 signaling.
(A) Sestrins are transcriptionally induced in response to diverse types of environmental stress. (B) Once induced, Sestrins inhibit TORC1 signaling through activation of AMPK and modulation of the GATOR complexes. Two papers (37, 38) suggest that amino acid leucine disrupts the Sestrin2-GATOR2 interaction, thereby modulating the ability of Sestrin2 to inhibit TORC1 signaling. Additional molecules, such as leucyl-tRNA synthetase, GCN2 and SLC38A9, also play a role in mediating amino acid-dependent TORC1 regulation.
TORC1 is a nutrient-responsive protein complex containing the protein kinase TOR which is inactivated in response to energy deprivation, in part through activation of AMPK triggered by increased AMP concentrations and insufficient ATP synthesis (18). In addition, specific amino acids, such as glutamine, arginine and leucine, can activate TORC1 through AMPK- and TSC-independent mechanisms. Although this response has been mainly observed in yeast and cultured cells but not in intact animals, a number of different proteins and pathways have been proposed to mediate amino acid-dependent regulation of TORC1 (19–21). The current consensus is that amino acid-generated signals somehow target the RagA/B GTPases that modulate the lysosomal localization and subsequent activation of TORC1. However, additional signaling through the GTPase Rheb (whose activity is inhibited upon AMPK activation) can be a prerequisite for amino acid-induced TORC1 activation (19). Furthermore, several studies have challenged this dogma because glutamine can activate TORC1 in the absence of RagA/B (22), and Rab1A, another GTPase located at the Golgi apparatus, can mediate amino acid sensing independently of RagA/B (23). Although RagA-null mouse embryonic fibroblasts (MEFs) do not display lysosomal localization of TORC1, they exhibit persistent TORC1 activity which is not inhibited by glucose or amino acid starvation (24). Depletion of both RagA and RagB in mouse cardiomyocytes also does not diminish TORC1 activity (25), suggesting that RagA/B may be dispensable for physiological TORC1 activation. There is also a conflicting report questioning whether amino acids can alter the GTP/GDP-loading status of RagA/B GTPases (26). Moreover, the physiological function of RagA/B is not confined to amino acid sensing and TORC1 regulation, because genetic studies in mice indicate that RagA/B have a critical role in maintaining lysosomal homeostasis and cardiac functionality independently of amino acids and TORC1 (25). Genetic studies in zebrafish have also concluded that RagA/B have TORC1-independent functions related to lysosomal proteolysis (27). In summary, the involvement of RagA/B in TORC1 regulation is rather contentious, remaining to be demonstrated in intact multicellular organisms.
Studies in isolated cells suggest that several molecules may play a role in direct sensing of amino acids and transmitting the signal to TORC1 (Fig. 1B). Three independent groups reported that the amino acid transporter SLC38A9 functions as an amino acid sensor at the lysosomal membrane (28–30). Although SLC38A9-deficient cells cannot sense arginine, they can still respond to other amino acids, including leucine, implicating the existence of a distinct leucine sensor. One such sensor is the leucyl-tRNA synthetase which functions as a GTPase-activating protein (GAP) for RagD, an antagonist of RagA/B activity (31). In this attractive model, leucine binding to leucyl-tRNA synthetase increases its GAP activity toward RagD, ultimately resulting in increased RagA/B activity. Although this model has been challenged because the RagD-GAP activity of leucyl-tRNA synthetase is relatively weak and another protein (folliculin) may be the preferred GAP for RagD (32), leucyl-tRNA synthetase remains a valid potential participant in leucine sensing because Cdc60, its yeast homolog, interacts with Rag GTPases in a leucine-dependent manner, and is genetically responsible for leucine-dependent activation of yeast TORC1 (33). As in every field, protein function needs to be validated biochemically, physiologically, and genetically.
GAP activity towards Rags (GATOR) is another evolutionarily conserved protein complex that controls TORC1 signaling (34). In mammals, GATOR is composed of two subcomplexes, GATOR1 and GATOR2. GATOR2 is composed of five proteins, WDR59, WDR24, MIOS, SEH1L and SEC13, whereas GATOR1 consists of three subunits, NPRL2, NPRL3 and DEPDC5 (35). GATOR1 functions as a GAP for the RagA/B GTPases, which activate TORC1, whereas GATOR2 is an inhibitor of GATOR1. Affinity purification experiments have shown that Sestrin1, 2, and 3 bind to the GATOR2 subcomplex (16, 17, 36), mainly through its WDR24 and SEH1L subunits (16). Although Sestrin2 does not affect the interaction between GATOR1 and GATOR2 when expressed at moderate amounts (16, 36), Sestrin2 overexpression can induce partial dissociation of GATOR2 from GATOR1, resulting in enhancement of GATOR1 GAP activity (17). The TORC1 inhibitory effect of Sestrin expression is diminished in GATOR1-mutated or silenced cells, suggesting that GATOR1 may have a role in Sestrin-dependent TORC1 inhibition (16, 17, 36). However, the biochemical mechanisms through which GATOR1 functions as a GAP for RagA/B, how GATOR2 inhibits GATOR1, or how Sestrin binding to GATOR2 affects its GATOR1-binding activity and TORC1 regulation is far from clear. Furthermore, the physiological importance of GATOR1/2 regulation by Sestrin2 remains to be determined through rigorous genetic analysis in intact animals. Despite these uncertainties and limitations, two studies by the Sabatini group suggest that Sestrin2 is a leucine sensor (37, 38).
One study shows that the Sestrin2-GATOR2 complex purified from amino acid-starved cells is destabilized upon in vitro incubation with leucine (38). Although Sestrin2 and GATOR2 were almost completely dissociated after addition of a subphysiological concentration (20 μM) of leucine, the GATOR2 subcomplex itself remained intact. Isoleucine and methionine, but not arginine, also disrupted the interaction of Sestrin2 with GATOR2. Considering that leucine, isoleucine and methionine are amphipathic molecules, their effect on Sestrin2-GATOR2 interaction may not be specific. However, the authors of that study showed that Sestrin1 and Sestrin2 themselves bind leucine, whereas no such binding was exhibited by GATOR2 or RagA/C (38). Leucine binding to Sestrin2 also alters its melting temperature, implicating a leucine-induced conformational change. Furthermore, the binding affinity (Kd) of Sestrin2 to leucine was determined to be around 20 μM, which is lower than that of leucyl-tRNA synthetase, whose Michaelis constant (KM) for leucine activation is around 45 μM (39). Based on these results, Sestrin2 was proposed to be a leucine sensor that binds GATOR2 only in the absence of leucine, an interaction postulated to inhibit TORC1 activity. To support this proposal, the authors generated Sestrin1, 2 and 3 triple-knockout (Sestrin-null) HEK293 cells using CRISPR-Cas9 methodology. Consistent with previous results (15), Sestrin-null cells did not efficiently shut down TORC1 activity after amino acid starvation. These Sestrin-null cells were reconstituted with various Sestrin2 mutants. The S190W substitution abolished the TORC1-inhibitory function of Sestrin2, whereas two other substitution mutations, L261A and E451A, prevented leucine binding to Sestrin2 and rendered the Sestrin2(L261A/E451A):GATOR2 complex insensitive to leucine. Furthermore, Sestrin2 (L261A/E451A) variants were unable to restore leucine-mediated TORC1 activation in Sestrin-null HEK293 cells (38).
These findings stand in contrast to several other reports, including one from the Sabatini lab itself (16, 17, 36), according to which the Sestrin2:GATOR2 complex is stable and can be isolated from cells cultured in conventional growth media, which contain 300–800 μM of leucine, a concentration that is 15–40 fold higher than its proposed dissociation constant from Sestrin2 (16, 17, 36). The interaction in leucine-rich environments can be reproducibly detected between endogenous Sestrin2 and GATOR2 in various cell types (16, 17, 36). Assuming that Sestrin2 does bind leucine, a more physiologically relevant scenario is that leucine weakens the binding of Sestrin2 to GATOR2, but does not disrupt it completely. Supporting this idea, two earlier papers showed that, although Sestrin2 binds quite well to GATOR2, the interaction becomes stronger upon amino acid starvation (16, 36). Because Wolfson et al. used a partially purified Sestrin2:GATOR2 immunocomplex (38), which may contain proteins other than the five GATOR2 components, it is plausible that other proteins or particular posttranslational modifications may further reduce the binding of Sestrin2 to GATOR2 in the presence of leucine.
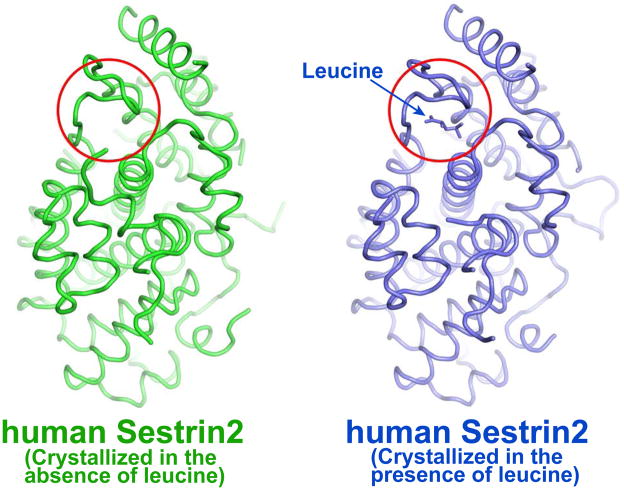
In the absence of strong in vivo genetic data, additional support for the role of leucine in control of Sestrin2 function came from structural studies showing that Sestrin2 contains a leucine binding pocket and that leucine is required for the proper folding of Sestrin2 (37). Using structural information, the authors identified two other residues, Arg390 and Trp444, as being involved in leucine binding. Curiously, however, one of the previously mutated residues, Leu261, does not seem to be part of the leucine binding pocket. Furthermore, two of us (JHL and USC) found that Sestrin2 crystallizes without leucine addition (12) and that the structure thus formed is largely identical to the one generated in the presence of leucine (Fig. 2). These results suggest that leucine binding does not induce a substantial conformational change in Sestrin2. Although we cannot rule out the possibility that the recombinant Sestrin2 protein isolated from E. coli and used in our structural studies was already saturated with leucine or a similar ligand, this seems a rather unlikely possibility because the affinity of leucine to Sestrin2 is relatively low (Kd = 20 μM), such that bound leucine should have readily dissociated from Sestrin2 during protein purification and dialysis. Since both of these structures were determined in the crystalline state (12, 37), which may not fully replicate the physiological dilute solution state, we also cannot rule out the possibility that leucine binding may produce effects in local structural dynamics of the leucine-binding pocket in solution. Undoubtedly, additional experiments are needed to rule out or validate an effect of leucine on Sestrin2 structure and function. Considering that leucine binding alters the melting temperature of Sestrin2 (38), it is plausible that leucine binding could affect Sestrin2 stability. In such a case, it seems likely that leucine should stabilize the protein and increase its abundance, thereby potentiating its effect on AMPK-dependent TORC1 inhibition, unless the leucine-bound form cannot activate AMPK. The effect of leucine binding on Sestrin2-mediated AMPK activation needs to be tested. Although Wolfson et al. found that leucine affected the binding of Sestrin2 to GATOR2, they noted that the binding of leucine to Sestrin3 was too weak to have any effect on its interaction with GATOR2 (38). Likewise, the binding of leucine to Drosophila Sestrin was also weak (38). Given the high degree of sequence conservation amongst the different Sestrins, these are rather surprising findings. It is possible that Sestrin3 and other Sestrins may have another ligand that is similar to leucine, such as other hydrophobic amino acids or metabolites. Furthermore, although yeast TOR is leucine-responsive, no Sestrin family members are encoded by the yeast genome (3), suggesting an alternative mode of leucine sensing. As described above, leucyl-tRNA synthetase could be that alternative leucine sensor because its role is conserved from mammals to yeast (31, 33). Therefore, if Sestrin2 is indeed a direct leucine sensor, its role seems to be largely mammalian specific and/or restricted to certain experimental conditions.
Fig 2. Structure comparison of human Sestrin2 in the absence (green, PDB ID: 5CUF) or presence (blue, PDB ID: 5DJ4) of leucine.
The leucine binding pocket is marked by the red circle. The calculated R.M.S. difference between the two structures is 0.70 Å based on the FATCAT server (http://fatcat.burnham.org/fatcat/). The illustration of protein structures was generated with PYMOL (Delano Scientific, LLC).
Several additional findings pose further obstacles on the road for Sestrin2 to win the title of a leucine sensor. First, genetic analysis showed that the single Sestrin protein encoded by the Drosophila genome is as capable of regulating AMPK and TORC1 activities in vivo and in vitro, as the mammalian Sestrins (7). Second, all of the original cell culture studies conducted by at least two different laboratories on Sestrin1 and 2 (4) or Sestrin3 (40), which demonstrated their ability to activate AMPK and inhibit TORC1, were done under normal culture conditions, namely in the presence of leucine and other amino acids. Therefore, there is little evidence that leucine affects Sestrin function in cells other than amino acid-starved triple Sestrin-null HEK293 cells and mouse fibroblasts. Furthermore the genetic analysis of Sestrin function in Drosophila or mice revealed that Sestrins are fully capable of inhibiting TORC1 activity in vivo under amino acid-replete conditions (7–9, 13). Although it needs to be determined whether direct binding of Sestrin to leucine has a physiological role in vivo, all Sestrins can activate AMPK and inhibit TORC1 in intact animals in the presence of physiological amounts of leucine. Even if one postulates that AMPK activation does not contribute to the TORC1 inhibitory activity of Sestrins, it should be acknowledged that Sestrin-mediated TORC1 inhibition can take place in the presence of amino acids.
Another important aspect of Sestrin biology is the requirement of stress to induce Sestrin expression in both cells (4, 40) and tissues (4, 8, 9). Leucine starvation results in the transcriptional induction of Sestrin2 due to activation of the protein kinase GCN2 and its target transcription factor ATF4 (41). GCN2 is a physiological sensor for most amino acids, including leucine and glutamine, whose function is conserved from yeast to mammals (42, 43). Therefore, glutamine starvation, which does not affect physiological leucine concentrations, inhibits TORC1 through GCN2-mediated Sestrin2 induction (41), as seen in other studies in which Sestrin abundance has been increased by exposure to genotoxic or oxidative stress (1–3). Thus, it remains to be clarified whether direct leucine binding indeed impairs the mTORC1-inhibiting function of Sestrin2 under these stresses.
Finally, Wolfson et al. suggested that Sestrin2 inhibition could be used to increase muscle protein synthesis by activating mTORC1 (38). This proposal is contradicted by numerous genetic studies performed using various model organisms, including worms, flies and mice. Such studies show that loss of Sestrin in muscle results in or facilitates various pathologies, including autophagy defects, accumulation of damaged mitochondria, oxidative muscle damage and myofiber degeneration (6, 7, 44). Furthermore, Sestrin knockout mice are predisposed to various systemic metabolic defects, including fat accumulation and insulin resistance (8, 9, 13). Therefore, the use of systemic Sestrin2 inhibitors proposed by these authors would be expected to accelerate age-associated muscle degeneration and other metabolic derangements, rather than alleviate them. The assessment of the therapeutic value of Sestrin inhibitors requires proper genetic analysis of Sestrin-deficient animal models, all of which are readily available. So far, the genetic studies mentioned above indicate that Sestrin induction is needed for stress reduction, a function that does not seem to be opposed by physiological leucine concentrations.
Gloss.
Environmental and metabolic stresses, such as DNA damage and nutrient deprivation, induce the expression of evolutionarily conserved proteins called Sestrins. In both flies and mammals, Sestrin inhibits the nutrient-responsive protein kinase complex Target of Rapamycin Complex 1 (TORC1), which regulates the biosynthesis of macromolecules including proteins, lipids, and nucleic acids. The mechanisms through which Sestrins inhibit TORC1 activity are both indirect, depending on activation of the adenosine monophosphate (AMP)-activated protein kinase (AMPK), and direct, mediated through interaction with the TORC1 regulating protein complex GATOR. New findings suggest that the ability of Sestrins to interact with GATOR is regulated by the amino acid leucine. Here we discuss whether and how this finding fits what has already been learned about the physiological functions of Sestrin in mammals and insects.
Acknowledgments
Funding: Research was supported by the NIH (1R21AG050903 to U.-S.C. and J.H.L., 1R01DK102850 to J.H.L. and 2P42ES010337 to M.K.).
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
References and Notes
- 1.Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- 2.Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, Skaliter R, Gudkov AV, Chumakov PM, Feinstein E. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanli T, Linher-Melville K, Tsakiridis T, Singh G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS One. 2012;7:e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison A, Chen L, Wang J, Zhang M, Yang H, Ma Y, Budanov A, Lee JH, Karin M, Li J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J. 2015;29:408–417. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Budanov AV, Talukdar S, Park EJ, Park H, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, Wolfe AM, Perkins GA, Ellisman MH, Bier E, Scadeng M, Foretz M, Viollet B, Olefsky J, Karin M. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HW, Park H, Ro SH, Jang I, Semple IA, Kim DN, Kim M, Nam M, Zhang D, Yin L, Lee JH. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun. 2014;5:4233. doi: 10.1038/ncomms5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee SG, Bae SH. Antioxidant function of Sestrins mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic Biol Med. 2015;88:205–211. doi: 10.1016/j.freeradbiomed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, An S, Ro SH, Teixeira F, Park GJ, Kim C, Cho CS, Kim JS, Jakob U, Lee JH, Cho US. Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat Commun. 2015;6:10025. doi: 10.1038/ncomms10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao R, Xiong X, Liangpunsakul S, Dong XC. Sestrin 3 Protein Enhances Hepatic Insulin Sensitivity by Direct Activation of the mTORC2-Akt Signaling. Diabetes. 2014;64:1211–1223. doi: 10.2337/db14-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding B, Parmigiani A, Yang C, Budanov AV. Sestrin2 facilitates death receptor-induced apoptosis in lung adenocarcinoma cells through regulation of XIAP degradation. Cell Cycle. 2015;14:3231–3241. doi: 10.1080/15384101.2015.1084447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng M, Yin N, Li MO. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell. 2014;159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, Budanov AV. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014;9:1281–1291. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JS, Ro SH, Kim M, Park HW, Semple IA, Park HL, Cho US, Wang W, Guan KL, Karin M, Lee JH. Sestrin2 regulates mTOR complex 1 (mTORC1) through modulation of GATOR complexes. Sci Rep. 2015;5:9502. doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Guan KL. Amino acid signaling in TOR activation. Annual review of biochemistry. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- 20.Jewell JL, Guan KL. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci. 2013;38:233–242. doi: 10.1016/j.tibs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chantranupong L, Wolfson RL, Sabatini DM. Nutrient-sensing mechanisms across evolution. Cell. 2015;161:67–83. doi: 10.1016/j.cell.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas JD, Zhang YJ, Wei YH, Cho JH, Morris LE, Wang HY, Zheng XF. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell. 2014;26:754–769. doi: 10.1016/j.ccell.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efeyan A, Schweitzer LD, Bilate AM, Chang S, Kirak O, Lamming DW, Sabatini DM. RagA, but Not RagB, Is Essential for Embryonic Development and Adult Mice. Dev Cell. 2014;29:321–329. doi: 10.1016/j.devcel.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YC, Park HW, Sciarretta S, Mo JS, Jewell JL, Russell RC, Wu X, Sadoshima J, Guan KL. Rag GTPases are cardioprotective by regulating lysosomal function. Nat Commun. 2014;5:4241. doi: 10.1038/ncomms5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshiro N, Rapley J, Avruch J. Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging. J Biol Chem. 2014;289:2658–2674. doi: 10.1074/jbc.M113.528505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen K, Sidik H, Talbot W. The Rag-Ragulator Complex Regulates Lysosome Function and Phagocytic Flux in Microglia. Cell Reports. 2016;14:547–559. doi: 10.1016/j.celrep.2015.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung J, Genau HM, Behrends C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol Cell Biol. 2015;35:2479–2494. doi: 10.1128/MCB.00125-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477–481. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL, Sabatini DM. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52:495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Dokudovskaya S, Rout MP. SEA you later alli-GATOR--a dynamic regulator of the TORC1 stress response pathway. J Cell Sci. 2015;128:2219–2228. doi: 10.1242/jcs.168922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014;9:1–8. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016;351:53–58. doi: 10.1126/science.aad2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Ma JJ, Tan M, Yao P, Hu QH, Eriani G, Wang ED. Modular pathways for editing non-cognate amino acids by human cytoplasmic leucyl-tRNA synthetase. Nucleic Acids Res. 2011;39:235–247. doi: 10.1093/nar/gkq763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29:2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Yang YL, Loh KS, Liou BY, Chu IH, Kuo CJ, Chen HD, Chen CS. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Exp Gerontol. 2013;48:371–379. doi: 10.1016/j.exger.2012.12.011. [DOI] [PubMed] [Google Scholar]