Abstract
Background
Cardiac involvement in systemic sclerosis (SSc) adversely affects long-term prognosis, often remaining undetectable despite close clinical examination and 2D echocardiographic monitoring. Speckle derived strain of the right ventricle (RV) was utilized to detect occult abnormalities in regional and global contractility in SSc patients.
Methods and Results
138 SSc patients with technically adequate echocardiograms were studied, and compared with 40 age- and gender-matched healthy non-SSc controls. Standard assessment of RV chamber function included tricuspid annular plane systolic excursion (TAPSE) and fractional area change (FAC). RV longitudinal systolic speckle-derived strain (RVLSS) was assessed in the basal, mid and apical free wall. TAPSE, was not different between groups (p=0.307). While FAC was lower in SSc patients compared to controls (mean 48.9 vs. 55, p=0.002), the mean FAC was still within the normal range (>35). In contrast, RVLSS measures were significantly different between groups, both globally (-20.4 vs -17.7%, p=0.005), and regionally: decreased in the apex (-8.5 vs -17.1%, p<0.0001) and mid segments (-12.4 vs -20.9%, p<0.0001) and increased in the base (-32.2 vs -23.3%, p=0.0001)for SSc group. The regional difference in the base compared to the apex was significantly greater for SSc than controls (p<0.0001 for interaction). The differences observed in regional strain between SSc and control were unchanged after adjusting for RVSP.
Conclusions
Speckle-derived strain reveals a heterogenous pattern of regional heart strain in SSc that is not detected by conventional measures of function, suggestive of occult RV myocardial disease.
Keywords: systemic sclerosis, longitudinal strain, right ventricle, pulmonary hypertension
Systemic sclerosis (scleroderma, SSc) is a complex disease that is characterized by a prominent vasculopathy, dysregulation of the immune system, and fibrosis of multiple organ systems including the skin, heart, lungs, kidneys, gastrointestinal tract, and blood vessels.1 Cardiac involvement is common in SSc and includes conduction abnormalities, arrhythmias, myocardial and pericardial involvement. In addition to intrinsic heart disease, there is unique vascular insult of the systemic and pulmonary vascular beds, each of which adversely affects long-term prognosis.1-3 Development of pulmonary arterial hypertension (PAH) in particular has a strong correlation with reduced mortality, with 50% 3-year survival rates in patients with NYHA Class II at the time of diagnosis.4-7
Despite frequent echocardiographic monitoring, significant right ventricular (RV) dysfunction and PAH are commonly undetected until the patient is symptomatic late in the course of the disease process. The lack of detection of right heart dysfunction by standard 2D echocardiographic analysis is in part due to the complex geometric configuration of the RV chamber, and misalignment of the Doppler beam for accurate noninvasive assessment of cardiac hemodynamics.8
Speckle tracking echocardiography (STE) is a novel imaging modality used in conjunction with conventional 2D echocardiogram that involves a fairly precise software-based algorithm, and is not limited by Doppler beam angulation.8 Pixels, or speckles, are generated within the myocardium from random reflection, refraction, and scattering of the ultrasound beam. Tracking of the speckles within a particular myocardial segment over time allows for specific quantification of how each segment “deforms”, or shortens during systole and lengthens during diastole. This results in an estimate of the relative velocity of motion of a particular segment within the myocardium in space as a function of time, defined as strain-derived deformation parameter.8 Strain is defined as the magnitude of myocardial contraction and relaxation, while strain rate is the change in strain over unit time, and is a measure of myocardial contractility.8,9 STE provides a precise estimate of regional and global systolic function, that is not user-dependent, or angle-dependent unlike other Doppler-derived techniques.
In the present study, we used STE to determine whether there is a distinct pattern of myocardial dysfunction of the RV in SSc patients, suggestive of intrinsic microvascular disease, patchy myocardial fibrosis, or emerging PAH.
METHODS
Study Population
SSc patients were identified through the IRB-approved Johns Hopkins Scleroderma Center database. Clinical features of the disease are recorded prospectively at baseline and at return visits, typically occurring at 6-month intervals, in all consenting participants. All participants meet 1980 American College of Rheumatology criteria for systemic sclerosis,10 or at least 3 of 5 CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) criteria, or have definite Raynaud’s phenomenon, abnormal nailfold capillaries and SSc specific autoantibodies. Center standard practice includes annual echocardiograms to screen for the development of PAH, regardless of clinical symptoms. All patients with SSc who had a clinically indicated echocardiogram performed at the Johns Hopkins Bayview Medical Center between January and December 2012 were eligible for inclusion in this cross sectional analysis. If multiple echocardiograms were performed on the same patient within this specified time frame, the first study performed was analyzed. Of the 162 SSc patients who met these inclusion criteria, 138 (85%) patients had adequate 2D image quality to allow for complete visualization and strain mapping of the RV free wall.
A cohort of age-and gender-matched non-SSc controls, who underwent clinically indicated echocardiograms during the study period, was also evaluated. Control patients were included for this analysis if LV ejection fraction was normal (≥55%). Exclusion criteria included: history of hospitalization for heart failure, hemodynamically significant valvular disease (any stenosis and/or regurgitation greater than mild in severity), coronary artery disease (segmental wall motion abnormality, history of myocardial infarction), ischemic, dilated or hypertrophic cardiomyopathy, primary pulmonary disease, and/or systemic disease associated with secondary pulmonary disease (e.g. sarcoidosis, SSc, connective tissue disease), no evidence of intracardiac shunting, and no evidence of congenital heart disease. Control patients underwent extensive chart review to exclude any cardiovascular and/or pulmonary disease. Exclusion criteria included: hypertension, diabetes mellitus, atherosclerotic cardiovascular disease, atrial fibrillation and any known history of arrhythmia, stroke, peripheral vascular disease, known history of chronic obstructive pulmonary disease, or sleep apnea. Control patients had to have echocardiographic study quality that was technically adequate to allow for off-line strain analysis.
A Cardiologist (MM), board certified in Echocardiography, was blinded to disease status and analyzed each study including 2D measures and speckle-based strain. To assess for intraobserver and interobserver variability, 20 studies with adequate study quality were randomized for reanalysis six months following initial analysis by two independent Cardiologists (MM, VKT), again blinded to disease status.
Clinical Parameters
Demographic data, disease characteristics, smoking history, medication exposures, history of cardiovascular and pulmonary comorbidities, pulmonary function testing and clinically obtained autoantibody tests results were abstracted for SSc patients from the Scleroderma Center’s database. Hypertension was defined as an average systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥ 90 mmHg at two or more visits, a documented history of hypertension by the patient’s primary care provider, or use of an anti-hypertensive medication for an indication other than Raynaud’s phenomenon. Atherosclerotic cardiovascular disease was defined by the presence of peripheral arterial disease (history of ankle-brachial index < 0.9, history of claudication, history of amputation or ulceration due to macrovascular disease without other clear indication) or coronary artery disease (history of angina, abnormal stress/pharmacologic stress test, abnormal coronary angiogram, history of myocardial infarction, or history of coronary revascularization). SSc cutaneous subtype was defined by established criteria,11 and SSc disease duration was calculated as the time interval between the first SSc symptom (either Raynaud’s or first non-Raynaud’s symptom) and the echocardiogram date. Measurements of forced vital capacity and diffusing capacity were standardized by age and gender.12,13
For the non-SSc control patients that met our echocardiographic inclusion criteria, extensive chart review was performed to ensure that controls met the exclusion criteria of no known cardiopulmonary disease as outlined above.
Echocardiographic Acquisition and Measurements
Echocardiograms were performed at a single clinical site using Phillips ie33 ultrasound machine (Phillips Healthcare, Andover, MA) with subjects in the left lateral decubitus position. 2D-directed methods were used to obtain linear measurements of RV chamber size (basal diameter, midventricular diameter, and longitudinal dimension) from the apical 4-chamber view in end-systole in accordance with American Society of Echocardiography (ASE) guidelines.14,15 RV internal dimension at end-diastole (RVIDD) was measured from the parasternal long axis view. Distal measures of the RV outflow tract (RVOT) diameter were measured from the left parasternal short axis view at the level of the pulmonary valve annulus respectively. Right atrial size (RA) was estimated using volumetric area from the apical 4-chamber view. RV function was assessed using TAPSE and 2-dimensional FAC, with abnormal function defined as < 16mm and < 35% respectively. In the absence of RVOT obstruction and tricuspid or pulmonic stenosis, tricuspid regurgitant (TR) velocity was used to estimate right ventricular systolic pressure (RVSP) using the modified Bernoulli equation and adding estimated RA pressure based on inferior vena cava dimension and collapsibility with sniff.15,16 RVSP of > 36 mmHg was identified as abnormal in accordance with ASE guidelines. Pulmonary vascular resistance (PVR) was noninvasively estimated utilizing the Abbas equation: [TR velocity / time velocity integral of the RVOT × 10 + 0.16] with an abnormal value defined as greater than 2.0 Wood Units.17 Left ventricular diastolic parameters including mitral inflow with early diastolic (E) and late diastolic (A) velocities, and tissue Doppler medial e’ velocities were obtained by convention.18
Following acquisition of standard 2D echocardiographic images, additional images were acquired at 70 – 90 frames per second at end-expiration, and subsequently analyzed using commercially available strain software (Epsilon, Milwaukee, WI).9 From the 4-chamber apical view, peak systolic longitudinal strain of the RV free wall segments was obtained by tracing the RV chamber endocardial borders in end-systolic still frames.8,9,19,20 In post-processing, automated tracking was visually verified and manually adjusted to ensure adequate border delineation. Longitudinal strain is traditionally defined as the percentage shortening of a regional area of interest (ROI) relative to its original length, and by convention is expressed as a negative percentage.19,20 The extent of myocardial deformation, defined as the peak longitudinal systolic strain, was expressed as percentage of longitudinal shortening in systole compared with diastole for each RV segment of interest.8,19 Worsening strain refers to a less negative number (i.e. a lower absolute value) than expected for a ROI, or an area of hypokinesis or diminished deformation along the longitudinal axis. Improved strain, on the other hand, refers to a more negative number (i.e. a higher absolute value) than expected for a ROI, or an area of hyperkinesis or enhanced deformation along the longitudinal axis. RV longitudinal systolic strain (RVLSS) was calculated as the average of regional strain from the basal, mid, and apical RV free wall segments, and compared to published standard reference values.21,22
STATISTICAL ANALYSIS
Baseline characteristics and echocardiography parameters were compared between patients with SSc and controls. Differences in continuous variables and dichotomous/categorical variables were examined using a Student’s t test or a chi square test, respectively. Generalized estimating equations (GEE) analysis was performed to account for clustering of RV strain values across the 3 RV segments within a given individual. First, we examined whether global RVLSS, defined as a repeated strain measure in the basal, midventricular, and apical segments, differed between patients with SSc and controls. We then modeled RVLSS as a function of segment and disease status (SSc versus controls). Segmental differences were examined utilizing RVLSS in the apex as the reference group; therefore for all multivariable analyses, differences in RVLSS were examined in the midventricular and basal segments compared to the apex, respectively. As we detected regional differences in strain, we performed further analyses to determine whether these regional differences differed by SSc or control status by 1) modeling the interaction between region and disease status and 2) performing stratified analysis by disease status.
Our models demonstrated significant differences in RVLSS in basal segments compared to apical segments in patients with SSc compared to controls. In light of this, we examined the base-apex difference in RVLSS further. We defined “abnormal strain” as being present if the base-apex RVLSS difference was greater than 2 standard deviations from the mean base-apex RVLSS difference for controls. We then examined whether any SSc phenotypic characteristics were different between SSc patients who had abnormal strain versus those who had normal strain using a Student’s t test or a chi square test where appropriate.
Statistical analyses were performed using Stata version 13.0 (College Station, TX, USA) and SAS version (Cary, NC, USA). Statistical significance was defined by a 2-sided p-value ≤0.05. Intrareader reliability was assessed by interclass correlation coefficient. Interreader reliability was assessed using Spearman correlation.
RESULTS
Baseline Patient Characteristics
The final study population consisted of 138 patients with SSc, and 40 age- and gender-matched non-SSc control patients without known cardiovascular and/or pulmonary disease. SSc patients were predominantly female (87.7%), white (75.9% Caucasian, 19.7% Black, 4.4% other), with a mean age of 54.3 ± 12.6 years. Antibody positivity within the SSc group consisted of 29.2% anticentromere, 27.9% Scl-70, and 16.5% RNA polymerase 3. 39.9% of SSc patients had diffuse disease, while 60.1% had limited disease. Control patients were intentionally frequency matched by age and gender, and by inclusion criteria, did not have any known cardiovascular and/or pulmonary disease. Additional characteristics of participants are detailed in Table 1.
Table 1.
Baseline patient demographics, medications, co-morbidites, and SSc-specific characteristics for systemic sclerosis patients and non-SSc controls.
| Scleroderma N=138 | Controls N=40 | p-value | |
|---|---|---|---|
| Age (years), mean ± SD | 54.3 ± 12.6 | 53.5 ± 14 | 0.704 |
| Women, no. (%) | 121 (87.7) | 35 (87.7) | 0.976 |
| Race, no. (%), N=137 for scleroderma | NA | ||
| White | 104 (75.9) | ||
| Black | 27 (19.7) | ||
| Other | 6 (4.4) | ||
| Hypertension, no. (%) | 50 (36.2) | NA | |
| Diabetes Mellitus, no. (%) | 9 (6.5) | NA | |
| Atherosclerotic cardiovascular disease* | 9 (6.5) | NA | |
| Atrial Fibrillation, no. (%) | 3 (2.2) | NA | |
| Ever Smoker, no. (%) | 55 (39.9) | 15 (37.5) | 0.788 |
| Vasodilator Medications, no. (%) | NA | ||
| Calcium Channel Blockers | 61 (44.2) | ||
| Phosphodiesterase 5 inhibitors | 15 (10.9) | ||
| Endothelin Receptor Antagonists | 3 (2.2) | ||
| Prostacyclin analogs | 0(0) | ||
| Scleroderma disease duration (years), median (interquartile range) | 13.5 (6.7, 22.4) | NA | |
| Scleroderma subtype, no. (%) | NA | ||
| Limited | 83 (60.1) | ||
| Diffuse | 55 (39.9) | ||
| Autoantibody status, no. (%) | NA | ||
| ANA, n=138 | 135 (98.7) | ||
| Centromere, n=137 | 40 (29.2) | ||
| Topoisomerase 1, n=136 | 38 (27.9) | ||
| RNA polymerase III, n=115 | 19 (16.5) | ||
| Pulmonary function data, mean ± SD | NA | ||
| Forced vital capacity (% predicted), N=134 SSc | 82.5 (18.5) ± 1.6 | ||
| Diffusing capacity (% predicted), N=132 SSc | 81.4 (25.4) ± 2.21 |
Defined by presence of peripheral arterial disease and/or coronary artery disease. NA= Not applicable or not available
There were no clinically significant differences in conventional 2D echocardiographic parameters of the right ventricular chamber size and function between systemic sclerosis patients and controls
Conventional 2D measures of RV function were assessed in the 2 groups (Table 2). Mean RV FAC (%), while statistically different between the two groups (p=0.002), was within normal clinical limits for both SSc and controls (48.9 ± 10.9 versus 55 ± 10.7). The fairly similar distributions of FAC between the two groups are illustrated in Supplemental Figure 1. Similarly, TAPSE was within normal clinical limits between SSc and controls, and did not differ between groups (2.16 ± 0.47 versus 2.25 cm ± 0.40, P=0.307, Supplemental Figure 2). There were no clinically significant differences in LV ejection fraction when comparing patients with SSc to controls. While there were statistically significant differences in linear dimensions of RV base, length, RVIDD, distal RVOT dimension at the level of PV annulus, and RA size between SSc and controls, these values were not clinically significant and did not reach pathological values (Supplemental Table 1). PVR, as estimated by the Abbas technique,16 was elevated in SSc patients compared to controls (1.48 ± 0.45 versus 1.24 ± 0.26 Wood Units, p = 0.002), as was RVSP (31.4 ± 13.3 mmHg versus 22.6 ± 4.4mm Hg, p = 0.0001), although values did not reach pathologic values.
Table 2.
Conventional 2D echocardiographic parameters and speckle-based strain parameters for systemic sclerosis patients and non-SSc controls.
| Parameter | Scleroderma N=138 | Controls N=40 | p-value | Normal Values |
|---|---|---|---|---|
| Conventional 2D Measures | ||||
| LV Ejection Fraction (%) | 58.5 ± 6.3 | 62.9 ± 5.9 | 0.0001 | ≥ 55 14 |
| Mitral E/A | 1.1 ± 0.38 | 1.4 ± 0.22 | 0.96 | 1.28 ± 0.25 18 |
| LV Septal E/e’ | 11 ± 4.3 | 9.4 ± 2.8 | 0.01 | ≥ 15 18 |
| RV FAC (%) | 48.9 ± 10.9 | 55 ± 10.7 | 0.002 | > 35 15 |
| TAPSE (cm) | 2.16 ± 0.47, N=107 | 2.25 ± 0.40, N=38 | 0.307 | > 1.6 15 |
| PVR (Wood Units) | 1.48 ± 0.45 | 1.24 ± 0.26 | 0.002 | > 3 15 |
| RVSP (mm Hg) | 31.4 ± 13.3 | 22.6 ± 4.4 | 0.0001 | ≥ 35 15 |
| Strain Parameters | ||||
| Basal RVLSS (%) | -32.2 ± 13.5 | -23.3 ± 4.5 | 0.0001 | -25 ± 6 22 |
| Midventricular RVLSS (%) | -12.4 ±7.6 | -20.9 ± 3.2 | <0.0001 | -27 ± 5 22 |
| Apical RVLSS (%) | -8.5 ± 6.7 | -17.1 ± 4.1 | <0.0001 | -24 ± 6 22 |
| Global RVLSS (%) | -17.7 ± 5.9 | -20.4± 2.4 | 0.005 | -26 ± 4 22 |
Regional abnormalities in RVLSS in systemic sclerosis are primarily due to hyperkinesis of the base and hypokinesis of the midventricular and apical segments
Our age- and gender-matched control patients had global RV free wall strain of -20.4 ± 2.4% that was less than published reference standards of -26 ± 4 in normal adults without cardiopulmonary disease.22 Patients with SSc had diminished global RVLSS (expected mean difference 2.71%) compared to controls (p<.0001) consistent with RV dysfunction. The distribution of strain in each RV segment is illustrated in Figure 1 for SSc patients and controls.
Figure 1.
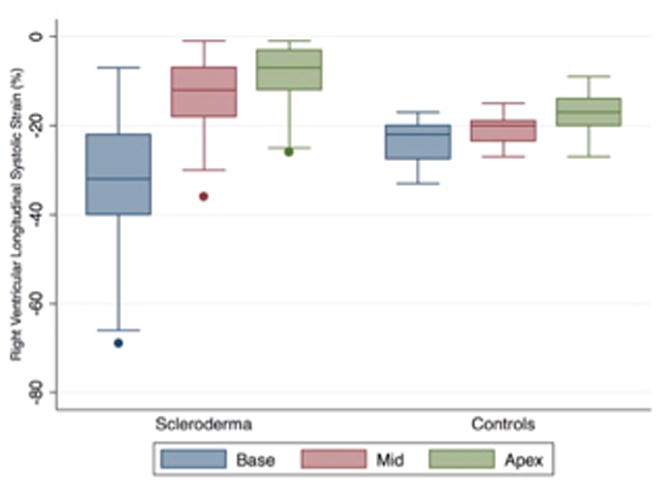
Distribution of strain by right ventricular free wall segment in systemic sclerosis.
We sought to determine whether these differences were secondary to a global reduction in strain or a regional pattern of RV dysfunction unique to SSc. While the pattern of strain was similar across RV free wall segments in controls, a heterogeneous pattern was observed across these same segments in SSc. RVLSS was decreased in the apex (SSc -8.5% versus control -17.1%, p<0.0001) and mid (SSc -12.4% versus control -20.9%, p<0.0001) segments in patients with SSc suggestive of diminished systolic contractility in these regions (Table 2). In contrast, patients with SSc had increased RVLSS in the base (SSc -32.2% versus control -23%, p=0.0001). The base-apex and mid-apex difference was significantly greater in SSc patients compared to controls (p<0.0001 and p<0.0001, respectively, for interaction). Among controls, regional differences in RVLSS were detected in the basal and mid segment relative to the apex (-3.8 and -6.2 respectively; p<0.0001) but not in the mid-apex comparison (Table 3). In contrast, SSc patients had significant regional differences throughout (base-apex difference -23.6, mid-apex difference -3.9, both p<0.0001), especially when comparing the basal to apical segments.
Table 3.
Regional differences in right ventricular longitudinal systolic strain by disease status between SSc patients and controls without cardiovascular and/or pulmonary disease.
| Parameter | β estimate | 95% CI | p-value |
|---|---|---|---|
| Scleroderma stratum | |||
| Intercept | -8.5 | -9.6, -7.4 | <0.0001 |
| Mid vs apex | -3.9 | -5.1, -2.7 | <0.0001 |
| Base vs apex | -23.6 | -26.3, -20.9 | <0.0001 |
| Control stratum | |||
| Intercept | -17.1 | -18.3, -15.8 | <0.0001 |
| Mid vs apex | -3.8 | -4.9, -2.8 | <0.0001 |
| Base vs apex | -6.2 | -8.2, -4.2 | <0.0001 |
Figure2 demonstrates regional strain curves from the 6 segments of the RV (basal, mid, and apical free wall and apical, mid, and basal interventricular septum) from a representative control (Figure 2A), and a patient with SSc (Figure 2B). In the control patient, there is evidence of synchronous systolic shortening and diastolic lengthening of each of the six RV segments throughout the cardiac cycle. In the SSc patient, there is hyperkinesis of the basal segment (red strain curve) while the mid and apical segments (cyan and yellow strain curves respectively) are hypokinetic in systole. While included in the graphical representation, interventricular strain was not assessed in this study.
Figure 2.
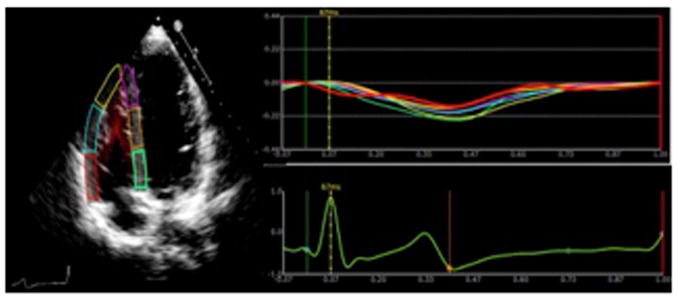
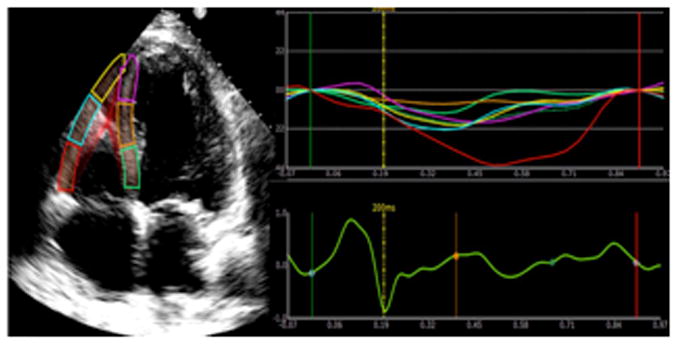
Panel A demonstrates normal right ventricular longitudinal systolic strain in a non-SSc control. Panel B shows abnormal right ventricular longitudinal systolic strain in a patient with systemic sclerosis.
SSc patients had higher mean RVSP than controls (SSc 31.4 versus control 22.6 mmHg, p=0.0001). There were 38 (27.5%) SSc patients with RVSP > 36 mmHg. When restricting our analysis to those with a RVSP<35 mmHg, however, we found that the differences observed in regional strain between SSc patients and controls were unchanged (data not shown).
Examination of SSc-specific characteristics that may associate with abnormal regional right ventricular strain
We were interested in identifying SSc phenotypic characteristics that may associate with abnormal regional strain. We defined “abnormal strain” as being present if the base-apex RVLSS difference was greater than 2 standard deviations from the mean base-apex RVLSS difference for controls. Based on this definition, 81 SSc patients (58.7%) had evidence of abnormal strain. When comparing SSc patients with abnormal strain to those without, there were no statistically significant differences in age, race, smoking status, cutaneous subtype, SSc disease duration, autoantibody status, history of myopathy, vasodilator medication use, extent of dyspnea (examined by MRC, Borg and UCSD dyspnea scales), FVC, DLCO, RVSP, FAC or PVR.
Inter- and intra-observer variability of right ventricular longitudinal strain parameters
Intraobserver agreement was excellent (ICC 0.981, 0.984, and 0.970 for RVLSS of the base, midventricular, and apical RV segments respectively). Interobserver agreement of RVLSS for the same segments was 0.96, 0.90, 0.78 for RVLSS of the base, midventricular, and apical RV segments respectively based on Spearman correlation.
DISCUSSION
In the present study, we utilized novel echocardiographic techniques to detect global and regional RV systolic dysfunction in a large, well-characterized cohort of SSc patients. Our study demonstrated that standard linear measurements of RV chamber size were not clinically significantly different between SSc patients and controls, however speckle-derived strain revealed a heterogenous pattern of regional heart strain in SSc. In this study, we defined hyperkinesis as a more negative number than expected for a ROI (higher absolute value), representative of enhanced deformation and contractility along the longitudinal axis. Hypokinesis, on the other hand, was defined as a less negative number than expected for a ROI (lower absolute value) or diminished deformation along the longitudinal axis. The pattern of basal hyperkinesis and hypokinesis of the apical and mid segments in SSc patients suggests occult myocardial dysfunction segmental changes in strain that is not appreciable by standard echocardiographic imaging, and was observed in patients with SSc even after restricting our analysis to patients with an RVSP < 35 mmHg. Our study demonstrates a diminishing gradation in systolic shortening of the RV free wall from base to apex in a large cohort of SSc patients that was observed independent of pulmonary pressures.
Despite well-described limitations, 2D echocardiography is the mainstay of serial monitoring of SSc patients given its high specificity and high positive predictive value in the evaluation for PAH.23,24 While standard 2D measures of RV size and function are able to identify grossly apparent chamber dilatation, depressed systolic function, and regional wall motion abnormalities, there may be subtle changes in global and regional systolic function not detectable by conventional measures. Our study demonstrates RV dysfunction that is readily detected with a method of strain analysis used in conjunction with conventional 2D imaging that has the potential to provide additional insight into the course of scleroderma heart disease.
There has been extensive investigation correlating standard echocardiographic measures with morbidity and mortality in patients with SSc. Recent studies in small selected cohorts have suggested that global speckle-based strain of both the right and left ventricles is reduced in patients with SSc, and is associated with morbidity, especially in patients with concomitant elevation in pulmonary pressures.5,24-29 Our study complements and significantly adds to the body of data that occult changes in RV myocardial contractility occurs in SSc regardless of pulmonary pressures, and that these changes occur in a specific regional pattern.
RA and RV chamber enlargement in SSc is directly related to onset of heart failure symptoms as well as mortality.1,6,23 Measures of RV systolic function such as TAPSE, tissue Doppler of the tricuspid annulus S’, RV fractional area change (RV FAC), and Tei Index all correlate with decreased survival.5,24,26-29 Noninvasive measures of PVR have been also been shown to predict PAH in SSc, and correlate with diminished 6-minute walk test (6-MWT) and DLCO.28
In fact, volumetric measures of RV chamber size and function may be abnormal in SSc patients even prior to the elevation in RVSP.30,31 Early studies utilizing tissue Doppler index (TDI) have demonstrated global reduction of RV strain abnormalities in small cohorts of SSc patients with interstitial lung disease, and similar to our study, occur independent of RVSP.32-35 Studies utilizing TDI based methods have also demonstrated that abnormalities in RV and LV systolic and diastolic function can be followed prospectively, and correlate with disease duration and with elevation in RVSP.35
Given the known association of longitudinal strain and increase in afterload, we also considered the possibility that increases in RV afterload may contribute to our findings of heterogenous RV free wall contractility patterns in SSc patients. It has been well described that the normal ventricular response to increasing RV afterload is a concomitant increase in RV contractility, either by heterometric or homometric adaptation.36 In a normal RV, these adaptive responses will occur until the afterload increases beyond a certain pathologic threshold, after which, the RV can no longer match its load, and the RV-PA unit becomes uncoupled resulting in decline in systolic function.36 Given this hemodynamic rationale, measures of RV contractility such as FAC, TAPSE, and RVLSS should be unchanged or arguably even greater in situations of modestly increased afterload. In our SSc population, there was no pathologic increase in afterload, with average PVR 1.48 ± 0.45 WU by noninvasive estimation (Table 2). To further analyze the effect of small increases in RV afterload on our findings, we performed additional analysis in those patients who had PVR >1.5 WU, and found that there were no significant differences of conventional 2D measures of RV systolic function (FAC or TAPSE) between groups. Segmental and global RVLSS differences between SSc patients and controls were also unchanged when restricting our analysis to PVR > 1.5 WU.
There have been no studies to date that demonstrate whether there is a distinct echocardiographic pattern defining the segmental nature of RV dysfunction that accompanies SSc. While patchy myocardial fibrosis can be seen by cardiac magnetic resonance imaging and is related to disease duration as well as overt RV dysfunction, current recommended echocardiographic measures may not detect these subtle changes. RV FAC and TAPSE provide global assessment of systolic function, however may not detail whether there are regional differences in contractility.4,28 Furthermore, image and parameter acquisition by 2D assessment is frequently limited by appropriate beam alignment and tend to be sonographer-dependent, with great variability in interobserver interpretation.15
Our study demonstrates RV dysfunction that is readily detected with a method of strain analysis used in conjunction with conventional 2D imaging has the potential to provide additional insight into the course of scleroderma heart disease. Based on our findings, we hypothesize that prior to the development of overt RV failure and PAH, there are subclinical changes in regional RV strain that are a precursor to impending myocardial dysfunction. Hypokinesis of the RV apex may not be appreciable by 2D visualization, and therefore may also not be captured by TAPSE, which measures the systolic excursion of the tricuspid annulus and basal RV segments towards the apex. Our study detected differences in RV apical function that may have important implications in predicting disease course.
Prior findings from our group utilizing multi-beat pressure volume analysis have demonstrated RV contractile dysfunction and RV-pulmonary vascular uncoupling in SSc patients with PAH compared to patients with idiopathic PAH.37 Importantly, these differences were not detected by MRI or standard 2D echocardiographic measures. The techniques employed in this study however are invasive, require sophisticated equipment, and not practical for routine clinical use. STE, on the other hand, can be utilized in conjunction with conventional 2D echocardiogram and provide reproducible and reliable information in regards to global and regional RV contractility.
Furthermore, there has been limited information in regards to global and regional abnormalities of RV systolic function in regards to phenotypic subsets of SSc (i.e. diffuse versus limited cutaneous disease or in unique autoantibody groups) as most studies to date have been performed in small cohorts of all-comers with SSc. Our study, performed in a large well characterized SSc cohort, suggests that regional abnormalities are present in all subtypes without clear distinction. Prospective studies are required to define whether abnormalities in RVLSS predict development of PAH and mortality, and whether early intervention with vasodilator or immunosuppressive therapies can improve outcomes in patients with abnormal strain.
Our study had several limitations. First, the ability to perform speckle-based strain is largely dependent on 2D image quality. As this was a retrospective study, frame rates that were used during original clinically indicated acquisition of 2D images, tended to result in artifactual strain rates when off-line strain analysis was performed. It is well recognized that while STE adds incremental clinical value over conventional methods alone, standardization of post-processing is needed, especially given vendor-specific variability in strain measures. These inherent differences may therefore limit the reproducibility of our study results.38,39 Since 2D tissue Doppler and tricuspid inflow patterns are not routinely performed as part of our protocol for clinically indicated echocardiograms, S’ as a surrogate of RV systolic function was not available for analysis. Similarly, pulmonary acceleration time, a noninvasive surrogate of PAH, was also not available for analysis. Diastolic function of the RV was not assessed on the present study. Another potential limitation of our study was that RVLSS was not followed in serial observations to determine whether this observed pattern of RV myocardial dysfunction was of clinical consequence and impacts morbidity and mortality, which we propose as a future study.
In summary, speckle-based strain revealed global and regional differences in RV function in patients with SSc that was not fully detected by conventional 2D measures. We identified a specific heterogenous pattern of RV dysfunction in SSc patients where there is hyperkinesis of the base and hypokinesis of the apical and mid segments, which remained significant after restricting our analysis to patients with RVSP<35 mmHg. These findings suggest occult RV myocardial disease that may precede the development of PAH, and may serve as an important target for vasodilator-guided therapies.
Supplementary Material
CLINICAL PERSPECTIVE.
In this study we utilized novel speckle tracking echocardiographic techniques for the noninvasive detection of global and regional systolic right ventricular (RV) dysfunction prior to the development of overt chamber dilatation, dysfunction, and clinical symptoms of right heart failure and pulmonary hypertension (PAH) in a large cohort of systemic sclerosis patients. We found that standard linear measurements of RV chamber size and function were not clinically different between SSc patients and controls, however novel speckle-derived strain revealed a heterogenous pattern of regional heart strain in SSc in which there was hyperkinesis of the RV base and hypokinesis of the apical and mid segments. This pattern suggested the presence of occult myocardial dysfunction with segmental changes in RV strain that is not appreciable by standard echocardiographic imaging. Despite frequent echocardiographic monitoring, significant RV dysfunction and PAH are commonly undetected until the patient is symptomatic late in the course of the disease process. Early detection of subclinical RV dysfunction utilizing these techniques prior to the onset of PAH may serve as a future therapeutic goal for earlier initiation of vasodilator and immunomodulating therapies, and therefore has numerous clinical implications in the morbidity and mortality of systemic sclerosis patients.
Acknowledgments
The authors would like to thank Adrianne Woods for assistance with database management and quality control, and the sonographers at Johns Hopkins Bayview Medical Center for study acquisition.
SOURCES OF FUNDING
This investigator-initiated study was supported by the Donald B. and Dorothy L. Stabler Foundation, the Scleroderma Research Foundation, the Catherine Keilty Memorial Fund for Scleroderma Research, the Martha McCrory Professorship, and the National Institutes of Health (NIH/NIAMS K23 AR061439).
Footnotes
DISCLOSURES
None.
References
- 1.Desai CS, Lee DC, Shah SJ. Systemic sclerosis and the heart: current diagnosis and management. Current Opinion in Rheumatology. 2011;23:545–54. doi: 10.1097/BOR.0b013e32834b8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parks JL, Taylor MH, Parks LP. Systemic sclerosis and the heart. Rheumatic Disease Clinics of North America. 2014;40:87–102. doi: 10.1016/j.rdc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Piccone MC, Zito C, Bagnato G, Oreto G, Di Bella G, Bagnato G, Carerj S. Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovascular Ultrasound. 2013;11:6–14. doi: 10.1186/1476-7120-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hachulla E, Launay D, Yaici A, Berezne A, de Groote P, Sitbon O, Mouthon L, Guillevin L, Hatron PY, Simonneau G, Clerson P, Humbert P, French PAH-SSc Network Primary arterial hypertension associated with systemic sclerosis in patients with functional class II dyspnoea: Mild symptoms but severe outcome. Rheumatology. 2010;49:940–4. doi: 10.1093/rheumatology/kep449. [DOI] [PubMed] [Google Scholar]
- 5.Frea S, Capriolo M, GrossoMarra W, Cannillo M, Fusaro E, Libertucci D, Morello M, Gaita F. Echo Doppler predictors of pulmonary arterial hypertension in patients with systemic sclerosis. Echocardiography. 2011;28:860–9. doi: 10.1111/j.1540-8175.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 6.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2012;51:1017–26. doi: 10.1093/rheumatology/ker269. [DOI] [PubMed] [Google Scholar]
- 7.Fisher MR, Mathai SC, Champion HC, Girgis RE, Houston-Harris T, Hummers L, Krishnan JA, Wigley F, HAssoun PM. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis and Rheumatism. 2006;54:3043–50. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 8.Blessberger H, Binder T. Two-dimensional speckle tracking echocardiography: Basic principles. Heart. 2010;96:716–722. doi: 10.1136/hrt.2007.141002. [DOI] [PubMed] [Google Scholar]
- 9.Leitman M, Lysyansky P, Sidenok S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain: a novel software for real-time echocardiographic assessment of myocardial function. Journal of the American Society of Echocardiography. 2004;17:1021–9. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for Scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis and Rheumatism. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 11.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. Journal of Rheumatology. 1988;15:202–5. [PubMed] [Google Scholar]
- 12.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American Journal of Respiratory and Critical Care Medicine. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit. American Review of Respiratory Disease. 1987;135:805–11. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MK, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. Journal of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2010;23:686–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. Journal of the American College Cardiology. 1985;6:359–65. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 17.Abbas AE, Fourtuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. Journal of the American College Cardiology. 2003;41:1021–7. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. Journal of the American Society of Echocardiography. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Marwick TH, Leano RL, Brown J, Sun J, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with two-dimensional speckle-tracking echocardiography: definition of normal range. Journal of the American College of Cardiology Cardiovascular Imaging. 2009;2:80–4. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria BJ, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. Journal of the American Society of Echocardiography. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: A novel index of left ventricular systolic function. Journal American Society of Echocardiography. 2004;17:630–3. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Reference values for right ventricular strain in patients without cardiopulmonary disease: A prospective evaluation and meta-analysis. Echocardiography. 2014;32:787–96. doi: 10.1111/echo.12806. [DOI] [PubMed] [Google Scholar]
- 23.Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, Desai A, Seibold JR. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. Journal of Rheumatology. 2008;35:458–65. [PubMed] [Google Scholar]
- 24.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill HS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. Journal of the American College Cardiology. 2002;39:1214–9. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 25.Brierre G, Blot-Souletie N, Degano B, Tetu L, Bongard V, Carrie D. New echocardiographic prognostic factors for mortality in pulmonary arterial hypertension. European Journal of Echocardiography. 2010;11:516–22. doi: 10.1093/ejechocard/jeq011. [DOI] [PubMed] [Google Scholar]
- 26.Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, Vliegen HW, Delgado V. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circulation Cardiovascular Imaging. 2012;5:628–36. doi: 10.1161/CIRCIMAGING.111.971465. [DOI] [PubMed] [Google Scholar]
- 27.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Coretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PE. Tricuspid annular displacement predicts survival in pulmonary hypertension. American Journal of Respiratory Critical Care Medicine. 2006;174:1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 28.Mathai SC, Sibley CT, Forfia PR, Mudd JO, Fisher MR, Tedford RJ, Lechtzin N, Boyce D, Hummers LK, Housten T, Zaiman AL, Girgis RE, Hassoun PM. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis and pulmonary arterial hypertension. Journal of Rheumatology. 2011;38:2410–8. doi: 10.3899/jrheum.110512. [DOI] [PubMed] [Google Scholar]
- 29.Matias C, Perez de Isla L, Vasconcelo M, Almeria C, Rodrigo JL, Serra V, Zamorano J. Speckle-tracking-derived strain and strain rate: a technique for the evaluation of early alterations in right ventricular systolic function in patients with systemic sclerosis and normal pulmonary artery pressures. Journal of Cardiovascular Medicine. 2009;10:129–134. doi: 10.2459/JCM.0b013e32831af028. [DOI] [PubMed] [Google Scholar]
- 30.Pigatto E, Peluso D, Zanatta E, Polito P, Miatton P, Bourji K, Badano LP, Punzi L, Cozzi F. Evaluation of right ventricular function performed by 3D-echocardiography in scleroderma patients. Reumatismo. 2015;66:259–63. doi: 10.4081/reumatismo.2014.773. [DOI] [PubMed] [Google Scholar]
- 31.Ciurzyński M1, Bienias P, Irzyk K, Kostrubiec M, Bartoszewicz Z, Siwicka M, Stelmaszczyk-Emmel A, Górska E, Demkow U, Pruszczyk P. Serum endothelin-1 and NT-proBNP, but not ADMA, endoglin and TIMP-1 levels, reflect impaired right ventricular function in patients with systemic sclerosis. Clinical Rheumatology. 2014;33:83–9. doi: 10.1007/s10067-013-2354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Andrea A, Stisi S, Bellissimo S, Vigorito F, Scotto di Uccio F, Tozzi N, Moscato F, Pezzullo E, Calabrò R, Scherillo M. Early impairment of myocardial function in systemic sclerosis: non-invasive assessment by Doppler myocardial and strain rate imaging. European Journal of Echocardiography. 2005;6:407–18. doi: 10.1016/j.euje.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Durmus E, Sunbul M, Tigen K, Kivrak T, Ozen G, Sari I, Direskeneli H, Basaran Y. Right ventricular and atrial functions in systemic sclerosis patients without pulmonary hypertension. Speckle-tracking echocardiographic study Herz. 2015;40:709–15. doi: 10.1007/s00059-014-4113-2. [DOI] [PubMed] [Google Scholar]
- 34.Mele D, Censi S, La Corte R, Merli E, Lo Monaco A, Locaputa A, Ceconi C, Trotta F, Ferrari R. Abnormalities of left ventricular function in asymptomatic patients with systemic sclerosis using Doppler measures of myocardial strain. Journal of the American Society Echocardiography. 2008;21:1257–64. doi: 10.1016/j.echo.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.D’Alto M, Cuomo G, Romeo E, Argiento P, Iudici M, Vettori S, Giovanna Russo M, Calabrò R, Valentini G. Tissue Doppler imaging in systemic sclerosis: a 3-year longitudinal study. Seminars in Arthritis and Rheumatism. 2014;43:673–80. doi: 10.1016/j.semarthrit.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right Ventricular Function in Cardiovascular Disease, Part II. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 37.Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, Boyce D, Kelemen BW, Bacher AC, Shah AA, Hummers LK, Wigley FM, Russell SD, Saggar R, Saggar R, Maughan WL, Hassoun PM, Kass DA. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circulation Heart Failure. 2013;6:953–63. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson MR, Hurst RT, Raslan SF, Cha S, Wilansky S, Lester SJ. Echocardiographic measures of myocardial deformation by speckle-tracking technologies: the need for standardization? Journal of the American Society Echocardiography. 2012;25:1189–94. doi: 10.1016/j.echo.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voight JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. Journal of the American Society of Echocardiography. 2015;28:1171–81. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


