Abstract
Background
Anterior cingulate cortex (ACC) and striatum are part of the emotional neural circuitry implicated in major depressive disorder (MDD). Music is often used for emotion regulation, and pleasurable music listening activates the dopaminergic system in the brain, including the ACC. The present study uses functional MRI (fMRI) and an emotional nonmusical and musical stimuli paradigm to examine how neural processing of emotionally provocative auditory stimuli is altered within the ACC and striatum in depression.
Method
Nineteen MDD and 20 never-depressed (ND) control participants listened to standardized positive and negative emotional musical and nonmusical stimuli during fMRI scanning and gave subjective ratings of valence and arousal following scanning.
Results
ND participants exhibited greater activation to positive versus negative stimuli in ventral ACC. When compared with ND participants, MDD participants showed a different pattern of activation in ACC. In the rostral part of the ACC, ND participants showed greater activation for positive information, while MDD participants showed greater activation to negative information. In dorsal ACC, the pattern of activation distinguished between the types of stimuli, with ND participants showing greater activation to music compared to nonmusical stimuli, while MDD participants showed greater activation to nonmusical stimuli, with the greatest response to negative nonmusical stimuli. No group differences were found in striatum.
Conclusions
These results suggest that people with depression may process emotional auditory stimuli differently based on both the type of stimulation and the emotional content of that stimulation. This raises the possibility that music may be useful in retraining ACC function, potentially leading to more effective and targeted treatments.
Introduction
Emotion regulation is a critical skill for emotional health and well-being [1]. Music is a powerful inducer of emotion, and people often report using music as a tool for regulating their emotional state [2–4]. Music is used for mood manipulations in clinical and laboratory settings [5–10]. Music is also used for mood change in the population generally, and as a coping strategy specifically in depression. College students diagnosed with depression report using music to reduce stress and anxiety [3]. Additionally, a European survey of public advice for the best self-help measures for dealing with depression placed listening to music near the top of the list, with 69% of respondents agreeing that they would recommend this as a useful self-help method. In this survey, 82% of respondents with depression who were already in treatment agreed that music was helpful in this regard [4]. Studies examining pleasurable musical experiences have associated enjoyment of music and activation in ventral striatal and ventral tegmental brain areas, specifically the nucleus accumbens (NAc) [11, 12]. Music listening may be rewarding, Menon and Levitin argue, because it mediates dopamine release, a neurotransmitter of reward, via the ventral tegmental-NAc network. Direct evidence of dopamine release to music has recently been found using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) [13].
Emotion regulation capabilities are determined in part by emotional biases in cognition. Individuals who have never had depression typically show a positivity bias in their cognitive processes [14]; they allocate greater attentional resources and show greater memory for positive versus negative information. While positive bias is typical, individuals with depression show an overall bias toward negative information. Depression is characterized by difficulties with emotion regulation [1], leading to prolonged negative affect and reduced responsiveness to previously enjoyed activities [15]. Negative affect may be the result of an increased susceptibility to negative emotional bias, including increased negative cognitions (attentional biases [16], outlook [17], rumination [15], and negative self-talk [18]), stemming from disordered brain activity [19]. Emotional responsiveness in depression is often altered for both positive and negative information. Individuals with MDD show reduced brain responses compared to control participants to emotionally positive words in reward centers of the brain, including ventral striatal and dorsomedial prefrontal regions [20]. Overall negativity biases in depression may comprise both negative hypersensitivity and positive hyposensitivity. Negative words are more readily processed by individuals with depression, as evidenced by shorter reaction times and larger evoked brain potentials, and responses to positive words are muted compared to control participants [21–23]. The negativity bias seen for emotional words may also occur in response to non-linguistic auditory information, such as music. If individuals with depression show a bias for negative musical stimuli—that is, increased brain responses or more negative ratings compared to control participants—it would be evidence that depression involves general hypersensitivity to negative information not limited to verbal rumination. Imaging studies have extended this negativity bias to music. Participants with MDD show reduced responses compared to control participants in ventromedial PFC (vmPFC) to their favorite music, despite similar enjoyment ratings between groups [24].While this dysregulation has been shown as an anhedonic response to favorite music [24], it has yet to be confirmed for potentially unfamiliar, emotionally evocative musical stimuli. By using standardized stimuli, familiarity effects and potential effects of participants’ self-awareness of loss of pleasure for familiar music are minimized.
The current research probes neural responsiveness to musical and nonmusical emotional stimuli that individuals are likely to encounter in their everyday lives. Emotional responses to music and other nonmusical stimuli have been studied separately in control participants, but though the patterns of responsiveness seem similar, the magnitude of these responses has not been directly compared for musical and nonmusical stimulus types. Individuals use music for emotion regulation [2]. Additionally, according to Thaut’s Rational Scientific Mediating Model of Music Therapy [25], the nonmusical goals of music therapy are achieved through the action of music on brain regions associated with nonmusical domains of functioning. Therefore, it is important to understand how underlying neural mechanisms may differ for these two stimulus types.
A large body of research has identified the anterior cingulate cortex (ACC) as a primary region implicated in major depressive disorder (MDD) [26]. [27]. The ACC is involved in both positive and negative emotional systems. The ACC receives projections from dopaminergic neurons in the ventral tegmental area and from top-down cognitive input in the prefrontal cortex [28]. It serves as a mediator between sensory inputs via the thalamus and appraisal via the prefrontal cortex, and is involved in monitoring for highly salient information [29–32]. Because of this, the ACC serves a critical function in task-switching, cognitive control, and emotional amplification and suppression [33]. While there is some disagreement on the number of discrete functional regions of the ACC, as well as the labeling of those regions [34–37], the ACC is often divided into two main areas: ventral (vACC) and dorsal (dACC). The vACC encompasses the perigenual, or rostral (rACC), and subgenual (sgACC) portions of the ACC. It is often described as the emotional ACC, because activation in this region is typically found to emotional stimuli [34]. Additionally, projections from the dopaminergic system reach this area of the ACC first. The dACC is defined posterior to the crossing of the corpus callosum to the motor cortex. It is described as the cognitive ACC, because it is more often activated in cognitive tasks that probe executive function and control, such as the Stroop task, which requires inhibition of prepotent responses [34, 35]. Volumetric studies repeatedly show decreased ACC size in participants with depression compared to control participants [38], including sufficient sensitivity and specificity to provide secondary means of diagnosis [39]. Unlike studies showing ongoing atrophy of affected brain regions with psychiatric disorders—for example, reduced hippocampal volume following PTSD [40]—the ACC in depression does not change size during the course of the disorder, nor with treatment [39]. This suggests that ACC volume may be a marker of depression vulnerability, in addition to its value in diagnosis. Functional responsiveness of the ACC has been linked to treatment success [27, 41]. Different treatment regimens target different subregions of the ACC; successful cognitive therapies are associated with increased activation in dorsal ACC, whereas medication based therapies often target ventral and rostral portions of the ACC [42]. Glutamate cycling, indicating general neural activity, has also been shown to be reduced in the vACC in depression [36], which suggests that tonic levels of activity in the vACC are lower in depression. For these reasons, we have focused on the ACC as the main region of interest in this study.
The current study uses fMRI and musical and nonmusical auditory-processing probes to determine whether activation within the ACC and striatum elicited by emotionally evocative auditory stimuli differ between the two stimulus types in never-depressed (ND) control participants. Further, this study measures differences in ACC and striatal activation to positive and negative emotional probes between participants with MDD and ND control participants, to confirm whether participants with depression also show negative bias for emotionally evocative non-linguistic auditory stimuli. We hypothesize that individuals with depression will show greater responsiveness to negative stimuli and reduced responsiveness to positive stimuli in ACC and striatum when compared with ND control participants.
Methods and Materials
Participants
ND participant population
This study was approved by the Human Subjects Committee of the University of Kansas Medical Center. Participants were recruited through email and flyer advertisements requesting volunteers with or without depression. All participants gave written consent prior to participation, according to the principles expressed in the Declaration of Helsinki. ND control participants (n = 22; 9 males; MAGE = 28.50; SDAGE = 11.14; RangeAGE = 18–59) were recruited with no history of depression or other psychiatric disorder, determined by administration of the Structured Clinical Interview for DSM Disorders, non-patient version (SCID-I/NP) [43]. Depression was assessed on the day of testing with the Beck Depression Inventory—Second Edition (BDI-II) [44], with a score greater than 18 indicative of high levels of depression. Based on this criterion, two participants scored greater than 18 on this measure on the day of testing and were excluded from further analyses (final group: n = 20).
MDD participant population
Twenty individuals with MDD (n = 20; 9 males; MAGE = 34.15; SDAGE = 13.64; RangeAGE = 18–56) were enrolled. Participants were all experiencing a current depressive episode at the time of scanning, determined by screening for research purposes using the SCID-I/NP [43]. Participants had no current or past manic episodes, no comorbid anxiety disorders, and no current alcohol abuse or dependence. One participant was taking medication for depression (Sertraline) at the time of the study, and was excluded from analyses; the final 19 participants were unmedicated. Five participants were currently undergoing counseling for depression, and 13 participants had received treatment in the past (Counseling: n = 7, Medication: n = 6). Behavioral treatments for depression, including counseling, have been shown to impact brain functioning [45, 46]; however, participants in this study were all experiencing a current depressive episode at the time of testing. Therefore, we believe measurements taken from this sample to be representative of the experience of clinically significant depressive symptoms that have not been ameliorated by the participants’ current or previous treatment. Four participants had a history of alcohol or drug dependence, fully remitted a minimum of one year prior to participation. One participant had a history of post-traumatic stress disorder (PTSD) in full remission. BDI-II scores were collected, but not used as criteria for inclusion/exclusion for the MDD group.
Participants in both groups were right-handed, had no contraindications for MRI (metal implanted in body, pregnancy), conditions and medications affecting blood flow (hypertension, diabetes), brain function (other psychiatric illness or medications), or neurological conditions (e.g., head injury, stroke). All participants had at least a high school education (MED = 15.22 years; SDED = 2.74 years), and were within normal or above average range of IQ (MIQ = 118.95; SDIQ = 11.65) as assessed by the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) [47]. The final groups (ND: n = 20, MDD: n = 19) did not significantly differ on age (t(37) = -1.02, p = .32, MDIFF = -4.08, SEMDIFF = 4.01), sex (χ2(1) = 0.03, p = .86), years of education (t(37) = 1.51, p = .14, MDIFF = 1.32, SEMDIFF = 0.87), IQ (t(37) = 1.16, p = .25, MDIFF = 4.37, SEMDIFF = 3.76), or years of musical training (χ2(4) = 1.80, p = .77).
Materials
Stimuli
Emotionally evocative positive and negative musical examples from Western art music, and positive and negative nonmusical stimuli selected from the International Affective Digital Sound set (IADS) [48] were identified and validated through a separate rating study [49]. More than 400 10-second-duration audio clips from 12 pieces of Western art music were rated for emotional valence (positive-negative) and arousal (high-low) using the circumplex model of affect [50–52]. A final set of 36 musical (18 positive, 18 negative), 24 nonmusical (12 positive, 12 negative), and 9 neutral (pure tone) stimuli were used in this imaging study. These stimuli are fully described in a separate publication [49], and are available from the corresponding author (RL). In the previous rating study, positive and negative stimuli were rated significantly differently on valence. Additionally, musical and nonmusical stimuli were given comparable emotional valence and arousal ratings. Emotionally neutral pure tones were also identified and validated as intermediate between positive and negative stimuli. The three categories did not differ on arousal rating.
Questionnaires
Anxiety was assessed with the Beck Anxiety Inventory (BAI) [53]. This twenty-one item questionnaire assesses severity of anxiety symptoms over the previous week. The Affect Intensity Measure (AIM), a brief, 40-item validated self-report tool for measuring strength of positive and negative emotions, was collected to assess affect intensity [54]. Each item is rated on a six-point scale (Never—Almost Always). The AIM returns a total score from all items (Range = 40–240) and three subscale scores: Positive Affectivity (AIMPA; 15 items), Negative Intensity (AIMNI; 6 items), and Negative Reactivity (AIMNR; 6 items) [54, 55]. All participants underwent both SCID administration and BDI-II testing as part of this study. The SCID was used prior to enrolment to determine eligibility. The BDI-II, BAI, and AIM were collected on the day of fMRI testing as a measure of current mood and depressive symptoms, and those ND participants who scored high on the BDI-II were excluded as noted above.
Procedures
fMRI methods
Participants underwent a single fMRI scanning session with anatomical scanning and five functional scanning runs. Scanning was conducted on a 3 Tesla Siemens Skyra scanner (Siemens, Erlangen, Germany). Participants’ heads were immobilized with cushions. Following automated scout image acquisition and shimming procedures to optimize field homogeneity, a structural scan was completed. High-resolution T1-weighted anatomic images were acquired with a 3D MPRAGE sequence (TR/TE = 2300/2.01 msec, flip angle = 9°, FOV = 256 mm, matrix = 256x192, slice thickness = 1 mm), used for slice localization for the functional scans, Talairach transformation, and coregistration with fMRI data. Participants were given the option to have their de-identified structural images included in a database accessible to researchers at the institution, reducing the cost of future studies. Following structural scans, five gradient echo blood oxygen level dependent (BOLD) sequences were acquired in 50 interleaved oblique axial slices at a 40° angle (repetition time/echo time [TR/TE] = 3000/25 msec, flip angle = 90°, field of view [FOV] = 220 mm, matrix = 64x64, slice thickness = 3 mm, 0 mm skip, in-plane resolution = 2.9x2.9 mm, 105 data points, 5 min: 24 sec).
To minimize susceptibility artifact and optimize signal in ventromedial prefrontal regions, participants were positioned in the scanner with the angle of the AC-PC plane between 17° and 22° in scanner coordinate space, verified with a localization scan. This careful positioning ensured that the 40° slice acquisition angle was applied the same way for all subjects. Head positioning and slice orientation parameters were verified in pilot tests and are now applied routinely at the imaging center in all fMRI studies targeting ventromedial regions of the brain.
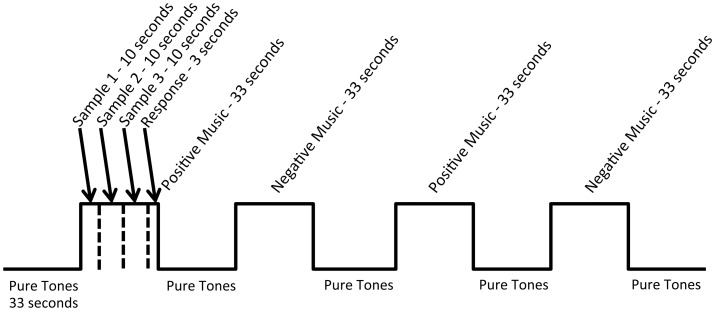
During the functional runs, auditory stimuli were presented in blocks of three clips from the same experimental condition (Fig 1) using E-Prime 2.0 software (Psychology Software Tools, Inc., Sharpsburg, PA), with six stimuli from each experimental condition presented during each run. To ensure that participants were attending to the emotional content of the stimuli, they were instructed to think about whether each clip was emotionally positive or negative while listening, and after each block of clips, were asked to rate whether the preceding block as a whole consisted of positive or negative stimuli. During three functional runs, participants listened to alternating groups of positive music, negative music, and pure tones. During the remaining two functional runs, participants listened to alternating groups of positive and negative nonmusical stimuli (IADS) [48] and pure tones. Pure tones were used as the baseline to control for general auditory stimulation and pitch, while neither being musical nor nonmusical. This stimulus condition was validated as emotionally neutral compared to the musical and nonmusical stimuli in the previously published rating study [49]. Stimuli were presented through MR compatible earbuds (Sensimetrics Corporation, Malden, MA) at 70dB, or as loud as comfortably possible to ensure the stimuli were heard over the noise of the scanner. Volume levels were adjusted for each subject with an audio test in the scanner prior to the task. Participants heard music not included in the task [56] concurrent with the noise of a functional BOLD sequence and provided visual feedback (thumbs up or down) to indicate whether the volume should be raised or lowered. In addition, noise-canceling headphones were placed over the earbuds to block scanner noise. This system was designed to present audio stimuli against the noise of the MR environment and has been used successfully during fMRI scanning.
Fig 1. fMRI Paradigm.
An example functional run from the blocked emotional stimulus paradigm.
Emotion rating methods
Using methods developed in our previous ratings studies [49], participants gave valence and arousal ratings for the stimuli following fMRI scanning. Ratings were collected after scanning to ensure that the stimuli were novel during scanning. Auditory stimuli were presented through computer speakers, using E-Prime 2.0 software running on a PC computer outside the scanning environment. Participants were allowed to adjust the volume to a comfortable level during a practice session. After each stimulus, participants were presented with a biaxial diagram, with valence rating coded on the x axis, and arousal coded on the y axis [49]. To encourage participants to rate their experience of emotion, rather than emotions that they simply recognized, participants were explicitly instructed to rate how each stimulus made them feel. Responses were collected via mouse click, and the mouse position in pixels was recorded for both x and y (Origin (x = 314, y = 240); RangeX = 76 (left)–542 (right); RangeY = 16 (upper)–464 (lower), resulting in a single valence and arousal rating per 10-second stimulus per subject.). The procedure lasted approximately twenty minutes. Following the rating procedure, participants completed questionnaires and were debriefed.
Analysis
Questionnaires
Group differences (MDD, ND) on each of the self-report measures were assessed using two-sample t-tests.
Emotion ratings
Average valence and arousal ratings for each condition (Valence—Positive, Negative: Type—Musical, Nonmusical) given by participants in the two diagnosis Groups (MDD, ND), were compared using separate 2x2x2 mixed model analysis of covariance (ANCOVA) tests to determine whether the groups were responding to the two stimulus types differently. Gender, age, and years of musical training were included as covariates in each analysis. Planned analyses using one-tailed t-tests directly tested whether MDD participants rated the negative stimuli as more negative and the positive stimuli as less positive compared to ND participants by comparing average ratings of valence across the diagnostic groups. Demographics, summary questionnaire scores, and emotion ratings for each participant are provided in Supporting Information S1 File.
fMRI preprocessing
fMRI data were analyzed using the Analysis of Functional NeuroImages (AFNI) statistical package [57]. Preprocessing steps included trilinear 3D motion correction, 3D spatial smoothing to 4 mm with a Gaussian filter, and high pass filter temporal smoothing. Images were resampled to a voxel-wise resolution of 2.5 mm3. Each participant’s structural image was realigned to the first functional image obtained within the participant’s scanning session, and normalized to the space defined by Talairach and Tournoux’s stereotaxic atlas [58] with the AFNI <@auto-tlrc> algorithm. Normalization to atlas space was confirmed by visual inspection for all participants. Anatomic data from one participant (MDD group) could not be successfully normalized using the <@auto-tlrc> algorithm, and was transformed manually in AFNI by defining key anatomic points (anterior commissure, posterior commissure, anterior point, posterior point, superior point, inferior point, right point, left point, and two points on the mid-sagittal plane). Volumes with excessive signal artifact (>50% voxels considered outliers were censored from each dataset prior to statistical analyses. Additionally, motion of greater than 1 mm between successive TRs resulted in the censoring of that TR and the two adjacent TRs. No functional runs were discarded for excessive motion (i.e.>30%).
fMRI statistical analyses
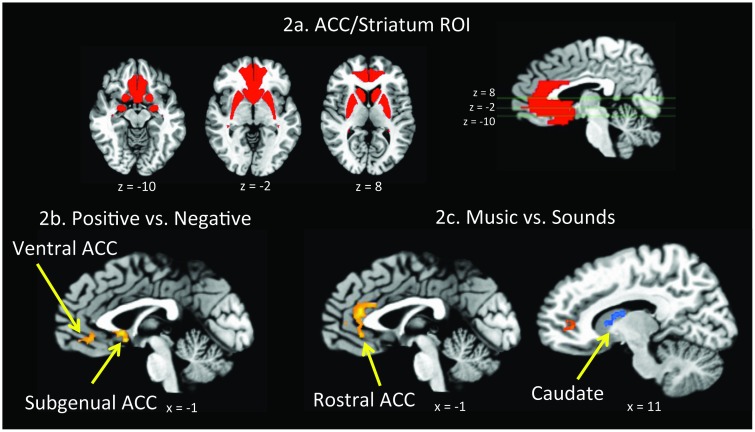
Activation maps were analyzed using statistical parametric methods [59] contained within the AFNI software [57]. Statistical contrasts were conducted using multiple regression analysis with the general linear model (GLM). Regressors representing the experimental conditions of interest were modeled with a hemodynamic response filter and entered into multiple regression analysis using a two-stage mixed-effects model. Motion estimates were also entered into the model as nuisance regressors. Contrasts between conditions of interest were assessed with t statistics using the <3dttest++> command. We conducted whole brain exploratory analyses and a region of interest (ROI) analysis focused on regions implicated in emotion processing in depression, including ACC (Brodmann areas (BA) 32 and 33, sgACC (BA25), and striatum (amygdala, caudate, nucleus accumbens, and putamen), defined anatomically from Talairach template masks within AFNI and combined into a single ACC/striatum mask (Fig 2A). Main effects of Valence (Positive, Negative) and Stimulus Type (Musical, Nonmusical), and the interaction of Valence and Stimulus Type were assessed over the whole brain in the ND group, and also in the ACC/striatum ROI mask to confirm activation to the task within the ACC and striatum. Whole brain and ROI comparisons were also conducted between ND and MDD groups to determine whether depression status interacted with Valence or Stimulus Type within these critical regions. Statistical parametric maps were overlaid on three-dimensional renderings of the Talairach template brain (TT_N27). Activations were considered significant if they survived a statistical threshold of pcorrected <.01 in the whole brain analyses, or pcorrected <.05 within the ACC/striatum ROI mask (small volume corrected for multiple comparisons determined by Monte Carlo simulations using AFNI’s <3dClustSim> command).
Fig 2. Region of Interest and fMRI Results—ND Participants.
2a. Anterior cingulate cortex and striatum region of interest (ACC/striatum ROI). 2b. Among ND participants, Ventral and Subgenual regions of the ACC exhibited significantly greater activation to positive versus negative stimuli. 2c. Among ND participants, Rostral ACC showed significantly greater activation to musical versus nonmusical stimuli, while right Caudate showed significantly greater activation to nonmusical versus musical stimuli.
Results and Discussion
Questionnaires
Descriptive statistics and results of the two-sample t-tests are provided in Table 1. Participants with depression reported higher total BDI-II scores and total BAI scores, confirming that this group was experiencing greater depressive symptoms at the time of testing, as well as higher anxiety. Participants with depression also had significantly higher scores on the negative intensity (AIMNI) subscale of the AIM, as well as somewhat higher scores, though not significant, on the negative reactivity (AIMNR) subscale, indicating that they generally experience negative situations with greater intensity, and may have greater reactivity to negative situations, than do ND participants.
Table 1. Comparisons of Questionnaire Scores between MDD* and ND† participants.
| Score | t | Df | p | M(SD) MDD | M(SD) ND | MDIFF | SEMDIFF |
|---|---|---|---|---|---|---|---|
| BDI-II‡ | -10.78** | 37 | <.001 | 30.16 (10.63) | 3.00 (3.67) | -27.16 | 2.52 |
| BAI§ | -7.46** | 37 | <.001 | 10.09 (10.09) | 2.65 (3.36) | -17.77 | 2.38 |
| AIM¶ Total | -1.35 | 37 | .19 | 143.11 (23.08) | 134.25 (17.73) | -8.86 | 6.57 |
| AIM Positive Affectivity | 0.43 | 37 | .67 | 52.00 (13.76) | 53.55 (8.48) | 1.55 | 3.64 |
| AIM Negative Intensity | -4.25** | 37 | <.001 | 22.63 (6.12) | 15.50 (4.24) | -7.13 | 1.68 |
| AIM Negative Reactivity | -1.65 | 37 | .11 | 24.47 (4.10) | 22.45 (3.56) | -2.02 | 1.23 |
*Major depressive disorder,
†Never depressed,
‡Beck Depression Inventory, 2nd Edition,
** significant a p <.01,
§Beck Anxiety Inventory,
¶Affect Intensity Measure.
Emotion ratings
The results of the ANCOVA for Valence rating revealed a significant main effect for Valence (F(1, 34) = 9.45, p = .004, η2 = .22), with positive stimuli being rated as more positive than negative stimuli (MPOS = 357.96, SEMPOS = 3.87; MNEG = 269.51, SEMNEG = 4.49). No other effects or interactions passed significance criteria.
There was a trend toward a significant interaction of Valence by Type (F(1, 34) = 3.10, p = .09, η2 = .08), with positive music being rated somewhat more positive than positive nonmusical stimuli (MDIFF = -42.81, MPOS_MUS = 379.36, SEMPOS_MUS = 4.84; MPOS_NONMUS = 336.55, SEMPOS_NONMUS = 6.38), and negative music being significantly more positive as negative nonmusical stimuli but with a smaller mean difference (MDIFF = -20.79, MNEG_MUS = 279.91, SEMNEG_MUS = 6.62; MNEG_NONMUS = 259.12, SEMNEG_NONMUS = 5.93). Finally, there was a trend toward a significant interaction of Valence by Type by Age (F(1, 34) = 3.21, p = .08, η2 = .09). Though not significant, this three-way interaction was characterized by older participants (> = 27 years) rating negative nonmusical stimuli as marginally more negative than did younger participants (t(37) = -1.87, p = .07, MDIFF = -21.19, SEMDIFF = 11.36), but no difference was found for positive nonmusical stimuli (t(37) = -1.40, p = .17, MDIFF = -18.12, SEMDIFF = 12.91), positive music (t(37) = 1.05, p = .30, MDIFF = 10.54, SEMDIFF = 10.02) or negative music (t(37) = -0.50, p = .62, MDIFF = -6.64, SEMDIFF = 13.18).
There was not a significant interaction of Valence by Group (F(1, 34) = 0.80, p = .38, η2 = .02), which means that diagnostic group was not a factor in how the participants were rating the valence of these stimuli. The planned t-tests comparing Valence ratings of positive (t(37) = 0.32, p = .75, MDIFF = 2.46, SEMDIFF = 7.79) and negative stimuli (t(37) = 1.16, p = .25, MDIFF = 10.50, SEMDIFF = 9.03) between the two groups were non-significant, confirming this result. All other effects and interaction terms in the ANCOVA were non-significant (All F’s < 2.5).
The results of the ANCOVA for Arousal rating revealed no significant effects. However, there was a trend toward a significant interaction of Valence by Group (F(1, 34) = 3.51, p = .07, η2 = .09), with ND participants rating positive stimuli as slightly more arousing than MDD participants (MDIFF = 17.21, SEMDIFF = 10.60, MND_POS = 224.67, SEMND_POS = 8.12, MMDD_POS = 207.46, SEMMDD_POS = 6.72), and no difference for negative stimuli (MDIFF = -3.35, SEMDIFF = 9.77, MND_NEG = 223.70, SEMND_NEG = 7.44, MMDD_NEG = 227.05, SEMMDD_NEG = 6.26). There was also a trend toward a significant interaction of Type by Age (F(1, 34) = 3.57, p = .07, η2 = .10), with younger participants (< 27 years) rating nonmusical stimuli as more arousing than did older participants (t(37) = -2.66, p = .01, MDIFF = -31.45, SEMDIFF = 11.83), but no difference was found for musical stimuli (t(37) = -0.29, p = .77, MDIFF = -3.42, SEMDIFF = 11.75). There was also a trend for the main effect of Age (F(1, 34) = 3.00, p = .09, η2 = .08). Though not significant, the pattern of arousal ratings indicated that younger participants rated all stimuli as slightly more arousing than did older participants (M<27 = 230.20, SEM<27 = 6.36; M> = 27 = 212.76, SEM> = 27 = 5.48). All other effects and interaction terms were non-significant (All F’s < 2.5).
As there were no significant main effects or interactions of diagnostic group for valence or arousal rating, these null findings suggest the two groups did not differ in their subjective responses to the stimuli. Therefore, differences in subjective labeling of the stimuli cannot be the explanation for the fMRI results that follow.
ND Participants: whole brain fMRI results
Main effect of Valence
When all positive stimuli were compared to all negative stimuli, the right auditory cortex showed significantly greater activation to positive stimuli, while bilateral occipital gyri showed significantly greater activation to negative stimuli. There was significantly greater activation to positive stimuli in ventral anterior cingulate and right hippocampus. Activation focused in the hippocampus also spread into the right dorsal amygdala (Table 2).
Table 2. Whole-brain activations to the emotional auditory functional MRI task, Main effect of Valence—ND Participants.
| Contrast and Region | X | Y | Z | Peak t statistic | Cluster size (mm3) |
|---|---|---|---|---|---|
| ND‡: Main effect of Valence; Positive > Negative | |||||
| Right Auditory cortex | 54 | -4 | 1 | 6.20 | 2891 |
| vACC§ | -4 | 36 | -9 | 5.10 | 2172 |
| Right Hippocampus/Amygdala | 26 | -11 | -9 | 4.69 | 1641 |
| Right Superior Temporal cortex | 59 | -19 | 9 | 4.38 | 1313 |
| ND: Main effect of Valence; Negative > Positive | |||||
| Right Occipital cortex | 24 | -86 | 9 | -4.88 | 2766 |
| Left Occipital cortex | -31 | -81 | 6 | -5.36 | 1797 |
Coordinates for the maximally activated voxel are provided in Talairach space. Correction for multiple comparisons, whole-brain corrected p < .01; t > 2.09, cluster >1125 mm3).
‡Never depressed,
§Ventral anterior cingulate cortex.
Main effect of Stimulus Type
In stark contrast to the focal Valence results, the main effect of stimulus type revealed broad differences in activation across the brain. Activation foci are listed in Table 3; however, clusters were large and encompassed several regions. Activation to musical stimuli was stronger in bilateral middle frontal gyrus, anterior and dorsal cingulate gyrus, bilateral precuneus, bilateral inferior parietal lobule, bilateral occipital gyrus, and bilateral fusiform gyrus. Activation to nonmusical stimuli was greater in thalamus, striatum, dorsomedial prefrontal cortex (DMPFC), bilateral dorsolateral prefrontal cortex (DLPFC), bilateral ventrolateral PFC (VLPFC), auditory cortex bilaterally (middle temporal gyrus, superior temporal gyrus), and bilateral cerebellum.
Table 3. Whole-brain activations to the emotional auditory functional MRI task, Main effect of Stimulus Type—ND participants.
| Contrast and Region | X | Y | Z | Peak t statistic | Cluster size (mm3) |
|---|---|---|---|---|---|
| Main effect of Stimulus Type; Musical > Nonmusical | |||||
| Left (and Right) Precuneus/Parietal/dACC§ | -26 | -56 | 51 | 6.27 | 63266 |
| Right Inferior Temporal/Occipital/Fusiform Gyri | 49 | -64 | -1 | 6.09 | 13641 |
| Left Middle Occipital/Fusiform Gyri | -49 | -76 | 6 | 5.27 | 9766 |
| Right Middle Frontal Gyrus | 26 | 26 | 34 | 4.39 | 1609 |
| Left Middle Frontal Gyrus | -21 | 34 | 34 | 5.73 | 1516 |
| Left Insula | -39 | 9 | 9 | 5.15 | 1469 |
| Right Cerebellum | 19 | -49 | -51 | 5.32 | 1234 |
| Main effect of Sound Type; Nonmusical > Musical | |||||
| Right (and Left) Cerebellum | 21 | -64 | -29 | -8.55 | 23344 |
| Right Auditory cortex | 46 | -16 | 6 | -7.23 | 18328 |
| Left Auditory cortex | -56 | -46 | 16 | -6.93 | 14500 |
| Left DLPFC¶/VLPFC** | -39 | 14 | 29 | -6.76 | 14156 |
| Right DLPFC/VLPFC | 36 | 24 | 21 | -6.12 | 13797 |
| Left (and Right) Thalamus/Striatum | -6 | -11 | 11 | -6.23 | 7813 |
| Right DMPFC†† | 1 | 11 | 59 | -6.74 | 4859 |
| Left Striatum | -21 | -11 | -4 | -6.43 | 4594 |
| Right Parahippocampus/Amygdala | 26 | -6 | -9 | -6.01 | 2734 |
| Right Cerebellum | 9 | -46 | -29 | -5.25 | 2438 |
| Left DMPFC | -4 | 41 | 36 | -4.17 | 1438 |
Coordinates for the maximally activated voxel are provided in Talairach space. Correction for multiple comparisons, whole-brain corrected p < .01; t > 2.09, cluster >1125 mm3).
§Dorsal anterior cingulate cortex,
¶Dorsolateral prefrontal cortex,
**Ventrolateral prefrontal cortex,
††Dorsomedial prefrontal cortex.
Interaction of Valence by Stimulus Type
In the interaction of Valence by Stimulus Type, bilateral auditory cortex and bilateral precentral gyrus showed significant activation characterized by greater activation to positive music versus negative music, with no difference in activation to positive versus negative nonmusical stimuli. Right inferior frontal cortex had greater activation to negative versus positive music, with no difference for positive versus negative nonmusical stimuli. Right parietal cortex had significantly greater activation for negative versus positive music, and greater activation for positive versus negative nonmusical stimuli (Table 4).
Table 4. Whole-brain activations to the emotional auditory functional MRI task, Interaction of Valence by Stimulus Type—ND Participants.
| Contrast and Region | X | Y | Z | Peak t statistic | Cluster size (mm3) |
|---|---|---|---|---|---|
| ND‡: Positive > Negative; Musical > Nonmusical | |||||
| Left Auditory cortex | -59 | -16 | 11 | 7.48 | 13172 |
| Right Auditory cortex | 49 | -6 | 4 | 5.65 | 12891 |
| Left Precentral Gyrus | -49 | -11 | 41 | 4.39 | 1406 |
| Right Precentral Gyrus | 44 | -9 | 44 | 4.53 | 1344 |
| ND: Negative > Positive; Musical > Nonmusical | |||||
| Right Parietal cortex | 36 | -59 | 41 | -3.61 | 1734 |
| Right Inferior Frontal Gyrus | 51 | 19 | 14 | -4.04 | 1391 |
Coordinates for the maximally activated voxel are provided in Talairach space. Correction for multiple comparisons, whole-brain corrected p < .01; t > 2.09, cluster >1125 mm3).
‡Never depressed.
ND ACC ROI fMRI results
Within the ACC/striatum mask, there was a significant main effect of Valence, with greater activation to positive versus negative stimuli in vACC (pcorrected <.01), and sgACC (pcorrected <.04) (Fig 2B). There was also a main effect of Stimulus Type in rACC, with greater activation for musical compared to nonmusical stimuli (pcorrected <.001), and in right caudate with greater activation for nonmusical stimuli compared to music (pcorrected <.02; Fig 2C; Table 5).
Table 5. Significant activations within the ACC*/Striatum ROI† –ND participants.
| Contrast and Region | X | Y | Z | Peak t statistic | Cluster size (mm3) |
|---|---|---|---|---|---|
| ND‡: Main effect of Valence; Positive > Negative | |||||
| vACC§ | -4 | 36 | -9 | 5.10 | 1391 |
| sgACC¶ | 1 | 6 | -6 | 5.41 | 656 |
| ND: Main effect of Valence; Negative > Positive | |||||
| NS** | |||||
| ND: Main effect of Sound Type; Musical > Nonmusical | |||||
| rACC†† | -1 | 34 | 19 | 5.44 | 3641 |
| ND: Main effect of Sound Type; Nonmusical > Musical | |||||
| Right Caudate | 11 | 11 | 6 | -4.41 | 500 |
Coordinates for the maximally activated voxel are provided in Talairach space. Correction for multiple comparisons, small volume corrected p < .05; t > 2.09, cluster > 375 mm3).
*Anterior cingulate cortex,
†Region of Interest,
‡Never depressed,
§Ventral anterior cingulate cortex,
¶Subgenual anterior cingulate cortex,
**No significant clusters,
††Rostral anterior cingulate cortex.
Comparison of MDD and ND groups: whole brain fMRI results
No areas of the cortex were significantly activated in the Group by Valence interaction (all pcorrected > .05).
Comparing group responses to musical versus nonmusical stimuli (Interaction of Group by Stimulus Type), significant activations were found in the anterior cingulate and the dorsolateral prefrontal cortex (Table 6).
Table 6. Activations to the emotional auditory functional MRI task, Group Interactions.
| Contrast and Region | X | Y | Z | Peak t statistic | Cluster size (mm3) |
|---|---|---|---|---|---|
| Group by Valence Interaction; ND‡ > MDD‡‡: Positive > Negative | |||||
| NS** | |||||
| Group by Valence Interaction; MDD > ND: Positive > Negative | |||||
| NS** | |||||
| Group by Stimulus Type Interaction; ND > MDD: Musical > Nonmusical | |||||
| dACC§§ | -1 | 31 | 14 | 4.22 | 1672 |
| Left DLPFC¶ | -21 | 46 | 26 | 3.92 | 1531 |
| Group by Stimulus Type Interaction; MDD > ND: Musical > Nonmusical | |||||
| NS | |||||
| Group by Valence by Stimulus Type Interaction | |||||
| NS |
Coordinates for the maximally activated voxel are provided in Talairach space. Correction for multiple comparisons, whole-brain corrected p < .01; t > 2.03, cluster > 1125 mm3)
‡Never depressed,
‡‡Major depressive disorder,
*No significant clusters,
§§Dorsal anterior cingulate cortex,
¶Dorsolateral prefrontal cortex.
No areas of the cortex were significantly activated in the three-way Group by Valence by Stimulus Type interaction (all pcorrected > .05). One small cluster in the left caudate tail showed a trend toward a significant interaction (pcorrected > .06; [TAL XYZ = -26–46 4] 906 mm3). In this region, ND participants showed greater activation for all positive versus all negative stimuli, whereas MDD participants showed greater activation for positive versus negative music, and greater activation for negative versus positive nonmusical stimuli.
Comparison of MDD and ND groups: ACC ROI fMRI results
Interaction of Group by Valence
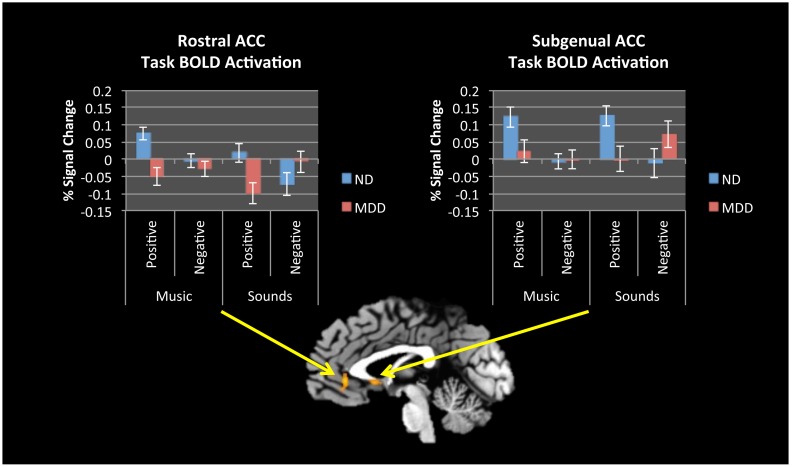
Comparing all positive to all negative stimuli, significant differences were found between MDD and ND groups in rACC (pcorrected <.01), with ND participants increasing activation from baseline to positive stimuli and decreasing activation from baseline to negative stimuli, whereas participants with depression showed no difference from baseline to negative stimuli and a significant decrease to positive stimuli (Fig 3; Table 7). There was also a significant group difference in sgACC (pcorrected <.03), with ND participants increasing activation from baseline for positive stimuli with no difference from baseline to negative stimuli, whereas participants with depression exhibited the opposite pattern. No significant group differences were found in striatum.
Fig 3. fMRI Results—Group by Valence.
Rostral and Subgenual ACC showed differential task activation between ND and MDD groups to positive versus negative stimuli. Graphs show mean activation over the entire cluster. Error bars denote standard error.
Table 7. Significant activations within the ACC*/Striatum ROI† –Group comparison.
| Contrast and Region | X | Y | Z | Peak t statistic | Cluster size (mm3) |
|---|---|---|---|---|---|
| Group by Valence Interaction; ND‡ > MDD‡‡: Positive > Negative | |||||
| rACC†† | -1 | 34 | 1 | 3.92 | 625 |
| sgACC¶ | 1 | 11 | -1 | 4.00 | 484 |
| Group by Valence Interaction; MDD > ND: Positive > Negative | |||||
| NS** | |||||
| Group by Stimulus Type Interaction; ND > MDD: Musical > Nonmusical | |||||
| dACC§§ | -1 | 31 | 14 | 4.22 | 656 |
| Group by Stimulus Type Interaction; MDD > ND: Musical > Nonmusical | |||||
| NS | |||||
| Group by Valence by Stimulus Type Interaction | |||||
| vACC§ (p <.09) | -9 | 36 | -6 | -4.64 | 359 |
Coordinates for the maximally activated voxel are provided in Talairach space. Correction for multiple comparisons, small volume corrected p < .05; t > 2.03, cluster > 375 mm3).
*Anterior cingulate cortex,
†Region of Interest,
‡Never depressed,
‡‡Major depressive disorder,
††Rostral anterior cingulate cortex,
¶Subgenual anterior cingulate cortex,
**No significant clusters,
§§Dorsal anterior cingulate cortex,
§Ventral anterior cingulate cortex.
Interaction of Group by Stimulus Type
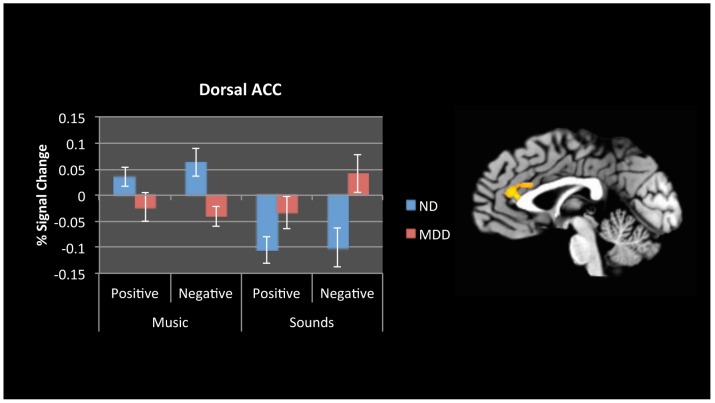
Comparing group responses to musical versus nonmusical stimuli, only one region survived thresholding (pcorrected <.01). ND participants had greater activation in dACC to musical versus nonmusical stimuli, whereas participants with depression had greater activation to nonmusical stimuli compared to music, with relatively greater activation to negative nonmusical stimuli, less activation to positive nonmusical stimuli, then positive music, and finally, negative music (Fig 4; Table 7). The area of activation covers both vACC and dACC; however, the maximally activated voxel was in dACC.
Fig 4. fMRI Results—Group by Stimulus Type.
Dorsal ACC showed differential task activation between ND and MDD groups to musical versus nonmusical stimuli. Graphs show mean activation over the entire cluster. Error bars denote standard error.
Three-way interaction
There was a trend toward a significant cluster in vACC in the Group by Valence by Stimulus Type interaction (pcorrected <.09; Table 7). In this region, both ND and MDD participants had greater activation to positive versus negative music; however, ND participants had greater activation to positive versus negative nonmusical stimuli, whereas MDD participants had greater activation to negative versus positive nonmusical stimuli.
Discussion
This experiment was designed as a probe for investigating neural circuitry of emotion and reward in depression. First, the paradigm was tested in ND control participants to determine whether musical and nonmusical stimuli activated these circuits. After confirming the paradigm activated a priori defined ACC and striatal regions, activation was directly compared within the anatomic mask with an unmedicated group of participants experiencing a current depressive episode. Both groups reported similar emotional experiences from the stimuli; however, the ACC showed differences in activation. No group differences were observed in striatum.
ND control participants
Positive versus Negative stimuli
Positive stimuli activated vACC to a greater extent than negative stimuli for both groups. Activation in this region was characterized by a pure valence effect—no differences were seen based on stimulus type. The vACC receives dopamine projections from the ventral tegmental area, and sends projections dorsally and laterally to executive control areas of the cortex [28]. Blunted activation in this region has been associated both with transient sadness [35] and with depression [26], suggesting that this region is critical for the experience of positive emotions. These findings corroborate previous research [11, 13] and suggest that the dopaminergic system is active during music listening. While familiarity has been shown to impact both liking [60] as well as neural responsiveness for music [61], we have no reason to believe that familiarity would differ for the positive and negative stimuli presented in this study. We chose to use Western art music examples, rather than popular or film music, to minimize familiarity effects. The dopaminergic system was also activated by nonmusical stimuli in this study, as evidenced by greater activation in caudate to nonmusical stimuli versus music. The musical and nonmusical stimuli used in this study were equally emotional [49]. However, the nonmusical stimuli were concrete, nameable items or experiences, whereas the music was abstract. It is possible that another feature, such as self-referential memory, may have driven activation in the caudate, but this is speculative. Further work is needed to elucidate the differential roles of ACC and striatum in processing emotional stimuli of various types.
Musical versus Nonmusical stimuli
The responses to musical versus nonmusical stimuli were described by very different patterns of activation. Greater activation to nonmusical compared to musical stimuli in thalamus, amygdala, cerebellum and auditory cortex—regions associated with early emotion processing—suggests that nonmusical stimuli activate primary emotion networks more than music. However, greater activation to nonmusical stimuli was also found in lateral and dorsomedial prefrontal cortex, areas associated with top-down executive control, object recognition, language processing, and reappraisal. Music, on the other hand, activated rostral anterior cingulate cortex, precuneus, and bilateral parietal and occipital cortices more than nonmusical stimuli. These regions, collectively, are associated with the default mode network (DMN), a network of regions that tend to be more active when a person is focused more on their own internal state, rather than engaged in an external task [62, 63]. These two systems have been described in models of voluntary and automatic reappraisal strategies [64]. The DMN has been implicated in autobiographical processing [65, 66]. The fact that music activates the DMN, while nonmusical stimuli show greater activation in the network generally associated with tasks of executive function, is neural evidence that supports Myer’s [67] theory that the ambiguity in music is what allows it to be what he called a “metaphorizing medium,” a scaffold that provides structure, but not content, that allows individual listeners the freedom to impose personal meaning onto this structure.
These stimuli were matched for valence and arousal [49]; therefore, the differences seen here cannot be attributed to differences in the emotional qualities of the stimuli, and must, therefore, be interpreted in terms of cognitive identification and appraisal strategies. Together, these findings show that even when carefully matched for emotional content, separable brain networks process music compared with other emotional sounds. Emotional nonmusical stimuli activate early emotion monitoring systems (thalamus, amygdala, and cerebellum). Executive control areas, such as DLPFC, VLPFC, and DMPFC are activated as well, suggesting that object identification and voluntary reappraisal may be taking place. Music activates DMN and reward processing areas, such as ACC, suggesting that emotional processing in music relies more on autobiographical memory, idiosyncratic meaning assignment, and automatic appraisal, than does emotional processing in everyday sounds, which are more concrete and activate linguistic processing areas to a greater degree.
Comparison of MDD and ND groups
Positive versus Negative stimuli
When all stimuli were compared based on emotional valence, vACC (rACC and sgACC) showed relatively more activation to positive stimuli in ND participants. In rACC, this relative difference was driven by ND participants showing increased activation to positive stimuli, with a significant decrease from baseline to negative stimuli. Participants with depression showed the opposite pattern: no difference from baseline for negative stimuli, but a significant decrease from baseline to positive stimuli. In sgACC, ND participants had increased activation for positive stimuli and no difference from baseline to negative stimuli, whereas participants with depression had increased activation for negative stimuli and no difference from baseline to positive stimuli. This represents both a hypoactivation to positive and a hyperactivation to negative stimuli among depressed participants. The sgACC has been shown to be the most effective stimulation site for deep brain stimulation in treatment-resistant depression [68]. Although the activation found in this study extends beyond the sgACC, the entire ACC receives projections from the midbrain dopaminergic neurons (ventral tegmental areas) and is implicated in emotional functioning in depression [69–71]. Again, familiarity could have an impact on these results [61]; however, while we did not measure familiarity directly in this study, we did measure musical training—which did not differ between groups. For these reasons, we feel confident that our results reflect differential emotional processing between the groups, rather than familiarity.
The current findings using standardized emotional auditory stimuli replicate those found by Osuch and colleagues [24], who showed that participants with depression had reduced activation to their favorite music in this region. Here, we show that decreased reactivity to positive stimuli in depression can generalize to evocative emotional stimuli, including music and positive nonmusical stimuli, and that this effect extends to other subregions of ACC. The vACC inhibits amygdala response [33], and has been shown to deactivate during cognitive tasks [34]. Activation patterns observed in this region could indicate monitoring for a change from one’s current mood state, as ND participants showed a change in activation only to negative stimuli and participants with depression showed a change only to positive stimuli.
Musical versus Nonmusical stimuli
By matching for arousal, we were also able to directly compare responses to music and nonmusical stimuli, further extending the work from Osuch and colleagues [24]. Comparing musical to nonmusical stimuli between the groups, activation was found in perigenual and dorsal ACC. In this region, ND participants showed greater activation to all music compared to all nonmusical stimuli. Participants with depression showed greater activation to nonmusical stimuli in this region, with the biggest response for negative nonmusical stimuli, smaller response for positive music, then positive nonmusical stimuli, and negative music showing the smallest response. Even sad music can be enjoyable and aid in emotion regulation [72–77]. When emotional content is mild—as in this study—and stimuli are not dissonant or designed to be unpleasant yet evoke negative emotions—as in the negative music condition—emotional classification for the music requires a decision between competing streams of information.
Emotion ratings
Self-reported emotion ratings for Valence and Arousal indicate that depression status did not systematically influence how participants rated the stimuli. In the current study, enjoyment and pleasure were not directly measured, however, the lack of group differences in subjective experience is comparable to the findings of Osuch and colleagues [24]. In that study, participants gave equivalent enjoyment ratings for their favorite music, yet showed reduced activation in reward centers of the brain, suggesting a potential neural marker of anhedonia.
Implications
The current study sought to determine whether emotional musical and nonmusical stimuli were processed similarly by healthy participants. Specifically, we had hypothesized that negative music and nonmusical stimuli might be processed differently, given the growing body of literature focused on the enjoyment of sad music [75–77]. In addition, the study compared the evoked brain responses to those stimuli in people with and without MDD to determine if the pattern of response was affected by MDD. By comparing musical and nonmusical stimuli, the current study provides a broader understanding of how individuals with MDD process different types of auditory stimuli. Music is currently used for mood manipulations in clinical and laboratory settings [5–10]; therefore, the results may ultimately have significant clinical implications for treating depression, or for the use of music as an affective probe for determining risk of developing the disorder. Additionally, music therapists have been using music to impact mood and depression in terminal illness [78] and Alzheimer’s disease [79], and are now extending this to mood disorders that are not related to a physical illness, with promising results [80–83]. The transitory nature of music might make it a useful tool for mood modification; however, the mechanisms by which this may occur are not fully defined. Koelsch and colleagues [81] argue that music therapy may be useful in treating depression, PTSD, and other mood disorders by acting on both the NAc-VTA reward processing loop, and by potentially reactivating the anterior hippocampal formation, which has been shown to have a reduced volume in these disorders [84]. The current results suggest that, similar to other forms of treatment, the mechanisms by which music and other forms of emotional auditory stimulation may function in depression could be by reactivating the ACC. As the link between ACC and emotion regulation has also been established in other psychiatric conditions such as borderline personality disorder [85–87], the present results may also have implications for psychiatric conditions beyond depression.
Limitations
Although the stimuli used in this study were carefully matched for both valence and arousal, the measure used to assess emotional ratings was based on self-report. While participants were explicitly instructed to rate how the stimuli made them feel, this self-report style measure did not allow for examination of whether the emotion was truly experienced by participants or simply recognized. Also, the fMRI employed a block-design, which did not allow us to probe responses to individual stimuli, or exclude trials to which participants may have been responding differently than expected. Future studies should include psychophysiological measurements, such as heart rate variability, respiration, and skin conductance, and further fMRI studies could use event-related designs that would allow individual variability in response to be measured. Additionally, a limited number of examples from Western art music are used in the current study. An increase in the number and variety of musical examples would be beneficial. Though these examples were selected empirically to control for familiarity and linguistic confounds, other genres of music, such as popular songs or opera, might elicit stronger emotions. Additionally, although there is evidence to suggest that many of the emotional cues in music, such as harmonic expectancies, are learned through enculturation rather than explicit musical training [88], and that emotional responses to music are influenced by familiarity [61], there is a possibility that brain responses could differ based on musical preference or training. While the lack of a familiarity measure is a limitation of this study, we did measure musical training. In this sample of participants musical training ranged from none (n = 8) to more than ten years (n = 7); however, the two groups were matched for years of musical training, thereby limiting the confounding effect of training across group.
Conclusions
In conclusion, the present project revealed that in healthy participants, positive auditory stimuli activated reward-processing areas of the brain that are implicated in depression. This set of studies focused on the ACC, which showed differential responsiveness to these mild emotional stimuli in participants with depression, and striatum. By using fMRI and a standardized set of musical and nonmusical emotion-processing probes, the current study provides insight into finer distinctions of stimulus type and may have implications for therapeutic interventions or risk assessment. The pattern of responsiveness in the ACC among participants with depression in this study raises the question of whether music, and specifically positive music, may be useful in retraining the ACC and improving functioning. A longitudinal study with a music-listening intervention would be critical to determine whether activation in ACC is malleable in this population. It is also possible that both the initial emotional response to a stimulus and the inability to sustain activation of positive emotional neural circuitry may lead to persistent depression, as reported by Heller and colleagues [89]. Results from this and other studies of affective responsivity in MDD may lead to more effective and targeted treatments.
Supporting Information
Key: study_id = unique subject identifier; male (1 = male, 0 = female); age (years), ed = education in years; mus_train (1 = None, 2 = 1–3 years, 3 = 4–6 years, 4 = 7–10 years, 5 = more than 10 years); MDD = Major Depressive Disorder classification (1 = MDD group, 0 = Never Depressed group); AIMtot = Affect Intensity Measure total score; AIM_PA = AIM positive affectivity subscore; AIM_NI = AIM negative intensity subsore; AIM_NR = AIM negative reactivity subscore; BDI_Tot = Beck Depression Inventory total score; BAI_Tot = Beck Anxiety Inventory total score; WASI_iq = estimated IQ from two subtests of Wechsler Abbreviated Scale of Intelligence; NegNonMus_X = average valence rating for negative nonmusical stimuli; NegNonMus_Y = average arousal rating for negative nonmusical stimuli; NegMus_X = average valence rating for negative musical stimuli; NegMus_Y = average arousal rating for negative musical stimuli; PosNonMus_X = average valence rating for positive nonmusical stimuli; PosNonMus_Y = average arousal rating for positive nonmusical stimuli; PosMus_X = average valence rating for positive musical stimuli; PosMus_Y = average arousal rating for positive musical stimuli.
(CSV)
Acknowledgments
These data have been presented in part as posters at scientific conferences.[90–93] All authors were involved in writing the paper and had final approval of the submitted and published versions.
Data Availability
Imaging data are now available at OpenfMRI.org. The link to access the data files is https://openfmri.org/dataset/ds000171/. Behavioral data are in the Supporting Information files.
Funding Statement
This work was supported by funding to RL from The Society for Education, Music and Psychology Research (SEMPRE: Arnold Bentley New Initiatives Fund), and the University of Kansas Doctoral Student Research Fund. MR imaging was provided by pilot funding from the Hoglund Brain Imaging Center. The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund and funding from the National Institutes of Health (UL1 TR000001). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or any individual institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gross JJ, Munoz RF. Emotion Regulation and Mental-Health. Clinical Psychology-Science and Practice. 1995;2(2):151–64. [Google Scholar]
- 2.Juslin PN, Sloboda JA. Music and emotion: theory and research. Oxford; New York: Oxford University Press; 2001. viii, 487 p. p. [Google Scholar]
- 3.Aselton P. Sources of stress and coping in American college students who have been diagnosed with depression. J Child Adolesc Psychiatr Nurs. 2012;25(3):119–23. 10.1111/j.1744-6171.2012.00341.x [DOI] [PubMed] [Google Scholar]
- 4.Holzinger A, Matschinger H, Angermeyer M. What to do about depression? Self-help recommendations of the public. Int J Soc Psychiatry. 2012;58(4):343–9. 10.1177/0020764010397262 [DOI] [PubMed] [Google Scholar]
- 5.Pignatiello MF, Camp CJ, Rasar LA. Musical mood induction: an alternative to the Velten technique. J Abnorm Psychol. 1986;95(3):295–7. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland G, Newman B, Rachman S. Experimental investigations of the relations between mood and intrusive unwanted cognitions. Br J Med Psychol. 1982;55(Pt 2):127–38. [DOI] [PubMed] [Google Scholar]
- 7.Clark DM. On the Induction of Depressed Mood in the Laboratory—Evaluation and Comparison of the Velten and Musical Procedures. Advances in Behaviour Research and Therapy. 1983;5(1):27–49. 10.1016/0146-6402(83)90014-0 [DOI] [Google Scholar]
- 8.Clark DM, Teasdale JD. Constraints on the Effects of Mood on Memory. Journal of Personality and Social Psychology. 1985;48(6):1595–608. 10.1037//0022-3514.48.6.1595 [DOI] [Google Scholar]
- 9.Clark DM, Teasdale JD, Broadbent DE, Martin M. Effect of Mood on Lexical Decisions. Bulletin of the Psychonomic Society. 1983;21(3):175–8. [Google Scholar]
- 10.Vastfjall D. Emotion induction through music: a review of the musical mood induction procedure. Musicae Scientiae. 2001;5(1):173–211. [Google Scholar]
- 11.Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28(1):175–84. 10.1016/j.neuroimage.2005.05.053 [DOI] [PubMed] [Google Scholar]
- 12.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98(20):11818–23. 10.1073/pnas.191355898 98/20/11818 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257–62. 10.1038/nn.2726 [DOI] [PubMed] [Google Scholar]
- 14.Kakolewski KE, Crowson JJ Jr., Sewell KW, Cromwell RL. Laterality, word valence, and visual attention: a comparison of depressed and non-depressed individuals. Int J Psychophysiol. 1999;34(3):283–92. [DOI] [PubMed] [Google Scholar]
- 15.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Arlington, VA: American Psychiatric Publishing, Inc.; 1994. [Google Scholar]
- 16.Abramson LY, Seligman ME, Teasdale JD. Learned helplessness in humans: critique and reformulation. J Abnorm Psychol. 1978;87(1):49–74. [PubMed] [Google Scholar]
- 17.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165(8):969–77. 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- 18.Ingram RE, Smith TW. Depression and Internal Versus External Focus of Attention. Cognitive Therapy and Research. 1984;8(2):139–51. 10.1007/Bf01173040 [DOI] [Google Scholar]
- 19.Heller W, Nitschke JB. Regional brain activity in emotion: A framework for understanding cognition in depression. Cognition & Emotion. 1997;11(5–6):637–61. [Google Scholar]
- 20.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163(10):1784–90. 10.1176/appi.ajp.163.10.1784 [DOI] [PubMed] [Google Scholar]
- 21.Atchley RA, Ilardi SS, Enloe A. Hemispheric asymmetry in the processing of emotional content in word meanings: the effect of current and past depression. Brain Lang. 2003;84(1):105–19. [DOI] [PubMed] [Google Scholar]
- 22.Enloe AA, Ilardi SS, Atchley RA, Cromwell RL, Sewell KW. Word valence, attention, and hemispheric activity in depressed, remitted, and nondepressed controls. Brain and Cognition. 2001;46(1–2):129–33. 10.1016/S0278-2626(01)80049-X [DOI] [PubMed] [Google Scholar]
- 23.Ilardi SS, Atchley RA, Enloe A, Kwasny K, Garratt G. Disentangling attentional biases and attentional deficits in depression: An event-related potential P300 analysis. Cognitive Therapy and Research. 2007;31(2):175–87. 10.1007/S10608-006-9113-Y [DOI] [Google Scholar]
- 24.Osuch EA, Bluhm RL, Williamson PC, Theberge J, Densmore M, Neufeld RW. Brain activation to favorite music in healthy controls and depressed patients. Neuroreport. 2009;20(13):1204–8. 10.1097/WNR.0b013e32832f4da3 [DOI] [PubMed] [Google Scholar]
- 25.Thaut M. Rhythm, music, and the brain: scientific foundations and clinical applications. New York: Routledge; 2005. x, 247 p. p. [Google Scholar]
- 26.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–74. 10.1146/annurev.psych.53.100901.135148 [DOI] [PubMed] [Google Scholar]
- 27.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. 10.1038/npp.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeve J. The motivated and emotional brain In: Reeve J, editor. Understanding Motivation and Emotion. 5th ed New York: John Wiley & Sons; 2009. [Google Scholar]
- 29.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703. 10.1176/appi.ajp.2012.11071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duval ER, Hale LR, Liberzon I, Lepping R, J NP, Filion DL, et al. Anterior cingulate cortex involvement in subclinical social anxiety. Psychiatry Res. 2013;214(3):459–61. 10.1016/j.pscychresns.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 31.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci U S A. 2010;107(47):20582–6. 10.1073/pnas.1009076107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21(2):676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–22. [DOI] [PubMed] [Google Scholar]
- 35.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306. [DOI] [PubMed] [Google Scholar]
- 36.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4 10.3389/fnsys.2010.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211(1):37–46. 10.1016/j.pscychresns.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 39.Niida A, Niida R, Matsuda H, Inada T, Motomura M, Uechi A. Identification of atrophy of the subgenual anterior cingulate cortex, in particular the subcallosal area, as an effective auxiliary means of diagnosis for major depressive disorder. Int J Gen Med. 2012;5:667–74. 10.2147/IJGM.S34093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, et al. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34(11):2808–16. 10.1002/hbm.22100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: A potential predictor of treatment response. Neuroreport. 1997;8(4):1057–61. [DOI] [PubMed] [Google Scholar]
- 42.Quide Y, Witteveen AB, El-Hage W, Veltman DJ, Olff M. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neurosci Biobehav Rev. 2012;36(1):626–44. 10.1016/j.neubiorev.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 43.First MB, Spitzer RL, Gibbon M, W WJB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version (SCID-I/NP). Non-patient Edition ed New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 44.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 45.Buchheim A, Viviani R, Kessler H, Kachele H, Cierpka M, Roth G, et al. Changes in prefrontal-limbic function in major depression after 15 months of long-term psychotherapy. PLoS One. 2012;7(3):e33745 10.1371/journal.pone.0033745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66(9):886–97. 10.1016/j.biopsych.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 48.Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37(2):204–15. [PubMed] [Google Scholar]
- 49.Lepping RJ, Atchley RA, Savage CR. Development of a Validated Emotionally Provocative Musical Stimulus Set for Research. Psychology of Music. 2015;online before print. 10.1177/0305735615604509 [DOI] [Google Scholar]
- 50.Russell JA. A Circumplex Model of Affect. Journal of Personality and Social Psychology. 1980;39(6):1161–78. [Google Scholar]
- 51.Hevner K. Experimental studies of the elements of expression in music. American Journal of Psychology. 1936;48:246–68. 10.2307/1415746 [DOI] [Google Scholar]
- 52.Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology. 2005;17(3):715–34. 10.1017/S0954579405050340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck AT, Steer RA. BAI, Beck Anxiety Inventory: Manual. San Antonio, TX: The Psychological Corporation: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- 54.Diener E, Larsen RJ, Levine S, Emmons RA. Intensity and frequency: dimensions underlying positive and negative affect. J Pers Soc Psychol. 1985;48(5):1253–65. [DOI] [PubMed] [Google Scholar]
- 55.Bryant FB, Yarnold PR, Grimm LG. Toward a measurement model of the affect intensity measure: A three-factor structure. Journal of Research in Personality. 1996;30(2):223–47. 10.1006/Jrpe.1996.0015 [DOI] [Google Scholar]
- 56.Mussorgsky MP. Pictures at an Exhibition: Great Gates of Kiev Mussorgsky/Gorchakov—Pictures at an Exhibition Prokofiev—Romeo & Juliet (selections G Nowak). Bordeaux, France: Sonoris; 1995. [Google Scholar]
- 57.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 58.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers Inc.; 1988. [Google Scholar]
- 59.Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2(2):166–72. [DOI] [PubMed] [Google Scholar]
- 60.Witvliet CVO, Vrana SR. Play it again Sam: Repeated exposure to emotionally evocative music polarises liking and smiling responses, and influences other affective reports, facial EMG, and heart rate. Cognition & Emotion. 2007;21(1):3–25. [Google Scholar]
- 61.Pereira CS, Teixeira J, Figueiredo P, Xavier J, Castro SL, Brattico E. Music and emotions in the brain: familiarity matters. PLoS One. 2011;6(11):e27241 10.1371/journal.pone.0027241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17(4):766–77. 10.1093/cercor/bhk030 [DOI] [PubMed] [Google Scholar]
- 63.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–8. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829, 33–57. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. 10.1016/j.tics.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 66.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. 10.1162/jocn.2008.21029 [DOI] [PubMed] [Google Scholar]
- 67.Meyer LB. Emotion and meaning in music. Chicago: University of Chicago Press; 1956. 307 p. p. [Google Scholar]
- 68.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69(4):301–8. 10.1016/j.biopsych.2010.09.034 [DOI] [PubMed] [Google Scholar]
- 69.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–31. 10.1016/S0079-6123(00)26027-5 [DOI] [PubMed] [Google Scholar]
- 70.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–9. [DOI] [PubMed] [Google Scholar]
- 71.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. 10.1007/s00429-008-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McFerran KS, Saarikallio S. Depending on music to feel better: Being conscious of responsibility when appropriating the power of music. Arts in Psychotherapy. 2014;41(1):89–97. 10.1016/j.aip.2013.11.007 [DOI] [Google Scholar]
- 73.Carlson E, Saarikallio S, Toiviainen P, Bogert B, Kliuchko M, Brattico E. Maladaptive and adaptive emotion regulation through music: a behavioral and neuroimaging study of males and females. Frontiers in Human Neuroscience. 2015;9 Artn 466 10.3389/Fnhum.2015.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van den Tol AJM, Edwards J. Exploring a rationale for choosing to listen to sad music when feeling sad. Psychology of Music. 2013;41(4):440–65. 10.1177/0305735611430433 [DOI] [Google Scholar]
- 75.Brattico E, Bogert B, Alluri V, Tervaniemi M, Eerola T, Jacobsen T. It's Sad but I Like It: The Neural Dissociation Between Musical Emotions and Liking in Experts and Laypersons. Front Hum Neurosci. 2015;9:676 10.3389/fnhum.2015.00676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vuoskoski JK, Thompson WF, McIlwain D, Eerola T. Who Enjoys Listening to Sad Music and Why? Music Perception. 2012;29(3):311–7. 10.1525/MP.2012.29.3.311 [DOI] [Google Scholar]
- 77.Peltola H, Eerola T. Fifty shades of blue: Classification of music-evoked sadness. Musicae Scientiae. 2015. [Google Scholar]
- 78.Hilliard RE. The use of music therapy in meeting the multidimensional needs of hospice patients and families. J Palliat Care. 2001;17(3):161–6. [PubMed] [Google Scholar]
- 79.Guetin S, Portet F, Picot MC, Pommie C, Messaoudi M, Djabelkir L, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer's type dementia: randomised, controlled study. Dement Geriatr Cogn Disord. 2009;28(1):36–46. 10.1159/000229024 [DOI] [PubMed] [Google Scholar]
- 80.Koelsch S. Towards a neural basis of music-evoked emotions. Trends in Cognitive Sciences. 2010;14(3):131–7. 10.1016/J.Tics.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 81.Koelsch S, Offermanns K, Franzke P. Music in the Treatment of Affective Disorders: An Exploratory Investigation of a New Method for Music-Therapeutic Research. Music Perception. 2010;27(4):307–16. 10.1525/Mp.2010.27.4.307 [DOI] [Google Scholar]
- 82.Erkkila J, Punkanen M, Fachner J, Ala-Ruona E, Pontio I, Tervaniemi M, et al. Individual music therapy for depression: randomised controlled trial. Br J Psychiatry. 2011;199(2):132–9. 10.1192/bjp.bp.110.085431 [DOI] [PubMed] [Google Scholar]
- 83.Maratos A, Crawford MJ, Procter S. Music therapy for depression: it seems to work, but how? Br J Psychiatry. 2011;199(2):92–3. 10.1192/bjp.bp.110.087494 [DOI] [PubMed] [Google Scholar]
- 84.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–49. 10.1002/hipo.20156 [DOI] [PubMed] [Google Scholar]
- 85.Niedtfeld I, Schulze L, Kirsch P, Herpertz SC, Bohus M, Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol Psychiatry. 2010;68(4):383–91. 10.1016/j.biopsych.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 86.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic structural changes in borderline personality disorder. J Psychiatr Res. 2008;42(9):727–33. 10.1016/j.jpsychires.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155(3):231–43. 10.1016/j.pscychresns.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leino S, Brattico E, Tervaniemi M, Vuust P. Representation of harmony rules in the human brain: further evidence from event-related potentials. Brain Res. 2007;1142:169–77. 10.1016/j.brainres.2007.01.049 [DOI] [PubMed] [Google Scholar]
- 89.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106(52):22445–50. 10.1073/pnas.0910651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lepping RJ, Atchley RA, Martin LE, Patrician TM, Stroupe N, Brooks WM, et al. Limbic responses to positive and negative emotionally evocative music: An fMRI study. Society for Neuroscience annual meeting 2012 Oct 13–18; New Orleans, LA 2012.
- 91.Lepping RJ, Atchley RA, Martin LE, Brooks WM, Clair AA, Ingram RE, et al. Musical and nonmusical sounds evoke different patterns of neural activity: An fMRI study. Psychonomic Society annual meeting; 2012 Nov 14–18; Minneapolis, MN 2012.
- 92.Lepping RJ, Atchley RA, Martin LE, Patrician TM, Ingram RE, Clair AA, et al. The effect of musical experiences and musical training on neural responses to emotionally evocative music and non-musical sounds. Social and Affective Neuroscience Society annual conference 2013 Apr 12–13; San Francisco, CA 2013
- 93.Lepping RJ, Atchley RA, Patrician TM, Stroupe NN, Martin LE, Ingram RE, et al. Music to my ears: Neural responsiveness to emotional music and sounds in depression. Society of Biological Psychiatry annual scientific convention 2013 May 16–18; San Francisco, CA 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Key: study_id = unique subject identifier; male (1 = male, 0 = female); age (years), ed = education in years; mus_train (1 = None, 2 = 1–3 years, 3 = 4–6 years, 4 = 7–10 years, 5 = more than 10 years); MDD = Major Depressive Disorder classification (1 = MDD group, 0 = Never Depressed group); AIMtot = Affect Intensity Measure total score; AIM_PA = AIM positive affectivity subscore; AIM_NI = AIM negative intensity subsore; AIM_NR = AIM negative reactivity subscore; BDI_Tot = Beck Depression Inventory total score; BAI_Tot = Beck Anxiety Inventory total score; WASI_iq = estimated IQ from two subtests of Wechsler Abbreviated Scale of Intelligence; NegNonMus_X = average valence rating for negative nonmusical stimuli; NegNonMus_Y = average arousal rating for negative nonmusical stimuli; NegMus_X = average valence rating for negative musical stimuli; NegMus_Y = average arousal rating for negative musical stimuli; PosNonMus_X = average valence rating for positive nonmusical stimuli; PosNonMus_Y = average arousal rating for positive nonmusical stimuli; PosMus_X = average valence rating for positive musical stimuli; PosMus_Y = average arousal rating for positive musical stimuli.
(CSV)
Data Availability Statement
Imaging data are now available at OpenfMRI.org. The link to access the data files is https://openfmri.org/dataset/ds000171/. Behavioral data are in the Supporting Information files.