Abstract
This meta-analysis of randomized controlled trials (RCTs) examined the efficacy and safety of the combination of electroconvulsive therapy (ECT) and antipsychotic medication (except for clozapine) versus the same antipsychotic monotherapy for treatment-resistant schizophrenia (TRS). Two independent investigators extracted data for a random effects meta-analysis and pre-specified subgroup and meta-regression analyses. Weighted and standard mean difference (WMD/SMD), risk ratio (RR) ±95% confidence intervals (CIs), number needed to treat (NNT), and number needed to harm (NNH) were calculated. Eleven studies (n = 818, duration = 10.2±5.5 weeks) were identified for meta-analysis. Adjunctive ECT was superior to antipsychotic monotherapy regarding (1) symptomatic improvement at last-observation endpoint with an SMD of -0.67 (p<0.00001; I2 = 62%), separating the two groups as early as weeks 1–2 with an SMD of -0.58 (p<0.00001; I2 = 0%); (2) study-defined response (RR = 1.48, p<0.0001) with an NNT of 6 (CI = 4–9) and remission rate (RR = 2.18, p = 0.0002) with an NNT of 8 (CI = 6–16); (3) PANSS positive and general symptom sub-scores at endpoint with a WMD between -3.48 to -1.32 (P = 0.01 to 0.009). Subgroup analyses were conducted comparing double blind/rater-masked vs. open RCTs, those with and without randomization details, and high quality (Jadad≥adadup analyses were Jadad<3) studies. The ECT-antipsychotic combination caused more headache (p = 0.02) with an NNH of 6 (CI = 4–11) and memory impairment (p = 0.001) with an NNH of 3 (CI = 2–5). The use of ECT to augment antipsychotic treatment (clozapine excepted) can be an effective treatment option for TRS, with increased frequency of self-reported memory impairment and headache.
Trial registration
CRD42014006689 (PROSPERO).
Introduction
Treatment-resistant schizophrenia (TRS) remains a great clinical challenge [1, 2]. Clozapine is the most efficacious antipsychotic drug for this population [2, 3], but many patients cannot tolerate clozapine due to its adverse drug reactions (ADRs) [3, 4]. Apart from agranulocytosis, clozapine may cause sedation, constipation, and/or an increased the risk of metabolic syndrome, thereby lowering treatment adherence [5–7]. Augmenting strategies, such as antipsychotic polypharmacy, adjunctive use of antidepressants, mood stabilizers, fatty acid supplements, glutamatergic agents and electroconvulsive therapy (ECT) [8–10], have been considered for TRS.
ECT has shown to be an effective and safe augmentation in TRS including clozapine-resistant schizophrenia [11, 12]. It has been recommended for TRS by the Task Force Report of the American Psychiatric Association on ECT [13]. A number of randomized controlled trials (RCTs) [14–24] compared the efficacy and safety of ECT combined with antipsychotic medication other than clozapine with the same antipsychotic monotherapy. So far, the results have been conflicting.
A systematic review of 22 RCTs concluded that adjunctive ECT added to any type of antipsychotics could produce symptomatic improvement in TRS [25]. In another review of 12 RCTs Tharyan et al. [26] suggested that ECT combined with all types of antipsychotics may be an effective treatment for schizophrenia.
To the best of our knowledge no meta-analyses on ECT added to non-clozapine antipsychotics for TRS have been published. The present meta-analysis of RCTs evaluates the efficacy and safety of ECT added to non-clozapine antipsychotic medications for TRS, comparing this combination to antipsychotic monotherapy. In addition to international literature sources, Chinese databases that are not usually reviewed in the international literature were also searched.
Methods
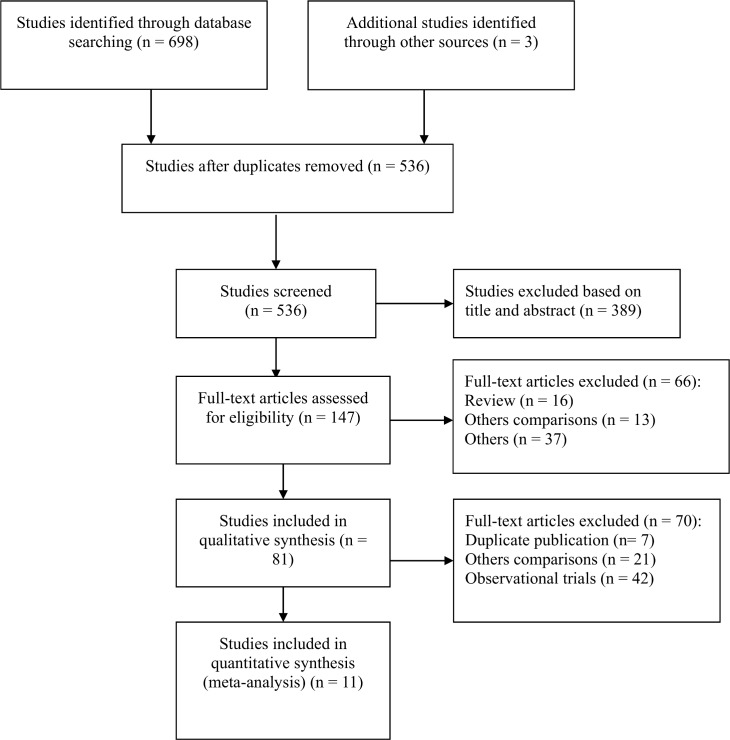
This meta-analysis is based on the methodology recommended by the Cochrane Collaboration [27] and prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [28] (S1 Fig).
Selection criteria
According to the PICOS acronym, the following selection criteria were used: Participants (P): patients with schizophrenia according to any diagnostic criteria. Intervention (I): ECT added to non-clozapine antipsychotic medications. Comparison (C): the same non-clozapine antipsychotic monotherapy or combined with sham ECT. Outcomes (O): efficacy and safety. Study design (S): RCTs reporting the efficacy and safety of adjunctive ECT for schizophrenia. Case series, observational trials, non-randomized trials, and non-original research (reviews and meta-analyses) were excluded.
Outcome parameters
Clinical outcomes were based on intent-to-treat (ITT) analysis, if provided. The primary outcome measure was endpoint symptomatic improvement measured by the change in total psychopathology of the Positive and Negative Syndrome Scale (PANSS) [29], or the Brief Psychiatric Rating Scale (BPRS) [30]. Key secondary outcomes included early (at 1 to 2 weeks) symptomatic improvement, PANSS positive, negative and general psychopathology sub-scores, response and remission defined by individual studies, patient-reported ADRs during the study period, and all-cause discontinuation.
Search methods
Major English (PubMed, PsycINFO, Embase, Cochrane Library databases, the Cochrane Controlled Trials Register) and Chinese databases (WanFang Database, Chinese Biomedical Database and China Journal Net) were searched from their inception until November 10, 2015 for RCTs using the following search terms: Electroconvulsive/Electroconvulsive therapy, schizophrenia, randomized controlled trial, placebo, and trial. The keywords were used in combination with the Boolean operators AND, OR, and NOT. The search was supplemented by using the “related article” function. Reference lists of eligible studies and relevant review articles were hand-searched. Authors were contacted for unpublished data if necessary.
Data extraction
Selection of studies, data extraction and synthesis and assessment of bias were conducted independently by two authors (WZ and XLC). All information was checked by another author (YQX). Inconsistencies were resolved by discussion.
Assessment of risk of bias
The methodological quality of RCTs was assessed by risk of bias [31] rating the method of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other biases. Each domain was rated as “high risk”, “unclear risk”, or “low risk” [31]. In addition, the Jadad scale (range: 0–5) was used to assess study quality in five domains: “randomization,” “double blinding,” “description withdrawals and dropouts,” “generation of random numbers,” and “allocation concealment” [32]. RCTs were classified as high-quality when their Jadad total score was ≥3 and low quality when their Jadad score was <3 [33]. The GRADE system (S1 Table) was used to rate the quality of primary and secondary outcomes of the meta-analysis [34, 35].
Data synthesis and statistical analyses
In order to combine studies, the random effects model [36] was used in all cases with the aid of the Review Manager Version 5.3 software (http://www.cochrane.org). Weighted or standard mean difference (WMD/SMD) and risk ratio (RR) with 95% confidence intervals (CIs) were calculated for continuous and dichotomous data, respectively. When RR was significant (p<0.05), the number needed to treat (NNT) or number needed to harm (NNH) were calculated. All statistical differences were considered significant when p<0.05.
In case of I2≥50% for the effect of primary outcomes, a sensitivity analyses was conducted after removing the three studies [14, 19, 23] that had an outlying effect size of SMD>-1.0. Furthermore, 5 comparative subgroup analyses were also conducted to identify potential moderators or mediators. These subgroup analyses were (1) Chinese vs. non-Chinese studies; (2) double blind/rater-masked vs. non-blinded studies; (3) duration of treatment <12 vs. ≥12 weeks; (4) mean number of ECT sessions <9 ECTs vs. ≥9 (since the number of ECT sessions was reported inconsistently, the median split of the number of recommended ECT sessions according to the ECT guidelines for schizophrenia in China were used, i.e., 6 to 12 sessions [37]; (5) high vs. low quality studies (≥3 vs. <3); and (6) co-starting vs. augmenting with antipsychotic. Since 2 RCTs [21, 24] provided data only for key secondary outcomes, subgroup analyses of endpoint symptomatic improvement were conducted with the remaining 9 RCTs.
Additionally, a meta-regression analysis was performed involving sample size, trial duration, Jadad score, mean age, percentage of male patients and illness duration to identify potential moderators or mediators of the effect on endpoint symptomatic improvement using the STATA Version 12.0 software (http://www.stata.com).
Finally, funnel plots, Egger’s intercept [38], Duval and Tweedie's Trim-and-fill procedure [39], and Rosenthal's fail-safe method [40] were used with the Comprehensive Meta-Analysis Version 2 software (http://www.meta-analysis.com) to assess publication bias of the primary outcomes.
Results
Literature search
A total of 701 potentially relevant articles in the initial database search (698 trials) and other sources (3 trials) were ascertained. Eventually 11 RCTs met the selection criteria for meta-analysis (Table 1, Fig 1 and S2 Fig).
Table 1. ECT RCTs for schizophrenia and sample characteristics.
| Author | Country | Na | Design | schizophrenia patients | ECT | Outcomes | Jadad score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (weeks) | Blinding | APs | Co-treatmentb | Age (years) | Male (%) | Criteria | Refractory criteria | Bilateral/unilateral | Electricity dose | Section (times) | Psychotic scale | Cognition | ||||
| Chanpattana, 1999 | Thailand | 30 | 24 | Open-label | Flu | Augmentation | 34.9 | 43.3 | NR | ≥2 APs | Bilatera | 346mC | 14 | BPRS | MMSE | 3 |
| Goswami, 2003 | India | 25 | 4 | Double-blind | CPZ | Costart | 29.5 | 64.0 | DSM-IV | ≥3 APs | Bilateral | NR | NR | BPRS | N/A | 4 |
| Jiang, 2009 | China | 67 | 12 | Rater-masked | RIS | Augmentation | 39.0 | 55.2 | CCMD-3 | ≥3 APs | Bilateral | NR | 12 | PANSS | WCST | 3 |
| Li, 2015 | China | 160 | 8 | Open-label | ZIP | Augmentation | 34.5 | 62.5 | DSM-IV | ≥3 APs | NR | NR | 12 | BPRS | NR | 2 |
| Liu, 2012 | China | 65 | 12 | Rater-masked | OLA | Costart | 39.0 | 35.9 | ICD-10 | ≥3 APs | Bilateral | 188C | 36 | PANSS | NR | 3 |
| Wang, 2015 | China | 126 | 8 | Open-label | ZIP | Augmentation | 34.1 | 58.7 | CCMD-3 | ≥3 APs | NR | NR | 12 | BPRS | WMSC | 3 |
| Wang, 2013 | China | 72 | 4 | Open-label | OLA | Augmentation | 45.5 | 59.7 | CCMD-3 | ≥3 APs | Bilateral | 20 Hz | 10–12 | PANSS | NR | 2 |
| Zhang, 2014 | China | 42 | 12 | Open-label | OLA | Augmentation | 35.5 | 69.1 | CCMD-3 | ≥3 APs | NR | NR | 8–12 | PANSS | NR | 3 |
| Zhou, 2009 | China | 63 | 12 | Open-label | OLA | Costart | 42.6 | 36.5 | CCMD-3 | ≥3 APs | Bilateral | NR | 9.3 | PANSS | WMS | 2 |
| Zhang, 2012 | China | 84 | 8 | Rater-masked | OLA | Costart | 38.4 | 44.0 | CCMD-3 | ≥3 APs | NR | NR | 16 | PANSS | NR | 3 |
| Zhang, 2012 | China | 84 | 8 | Open-label | QUE | Augmentation | 34.1 | 59.5 | CCMD-3 | ≥4 APs | Bilateral | NR | 7.6 | PANSS | NR | 2 |
aThis number reflects the total sample size recruited, including patients on RCTs.
bCo-treatment with ECT was started at the same time that other antipsychotic or added as an augmentation strategy.
CData not provide for the control group
APs = antipsychotics; BPRS = Brief Psychiatric Rating Scale; CCMD-3 = China's mental disorder classification and diagnosis standard 3th edition; CPZ = chlorpromazine; DSM-IV = Diagnostic and Statistical; ECT = electroconvulsive therapy; Flu = flupenthixol; ICD-10 = International Classification of Diseases, 10th edition; MMSE = Mini-Mental Status Exam; NR = not report; OLA = olanzapine; PANSS = Positive and Negative Syndrome Scale; QUE = quetiapine; RIS = risperidone; ZIP = ziprasidone; WCST = Wisconsin Card Sorting Test; WMS = Wechsler Memory Scale
Fig 1. PRISMA flow diagram.
RCTs and patient characteristics
Eleven RCTs with 818 patients (sample size range = 25–160, median = 67.0) compared add-on ECT (n = 414) to an antipsychotic medication, including chlorpromazine, flupenthixol, olanzapine, quetiapine, risperidone, and ziprasidone with the same antipsychotic monotherapy (n = 404). TRS was defined as failure to respond to ≥2 antipsychotics (1 trial), ≥3 antipsychotics (9 trials), and ≥4 antipsychotics (1 trial). Nine RCTs were conducted in China (n = 763), and 1 each in Thailand (n = 30) and India (n = 25). Only one RCT [15] used sham ECT in the control group.
Patients were 37.0±4.5 years (range = 29.5–45.5 years, median = 35.5 years), 55.4±10.0% were males (range = 36.5%-69.1%, median = 58.7%) and the mean illness duration was 13.0±4.3 years (range = 7.3–20.0 years, median = 12.5 years). The RCTs lasted 10.2±5.5 weeks (range = 4–24 weeks, median = 8.0 weeks). ECT courses comprised 14.2±8.0 sessions (range = 7.6–36.0 sessions, median = 8.0 sessions.
Assessment of risk of bias
While 6 RCTs with a specific description regarding random sequence generation were rated as low risk, 6 RCTs were rated as high risk for allocation concealment. Masked assessors and double blindness were administered in 4 and 1 RCT, respectively. Regarding outcome data, 1 RCT reported loss to follow-up, but failed to use ITT analysis for incomplete outcome data. In addition, none of the studies registered their protocol, preventing formal assessment of reporting bias. Other biases were rated as low risk in all RCTs (S3 Fig).
Quality assessment
The Jadad score was 2.6±0.7 (range = 2–4, median = 3) (Table 1); 7 RCTs were classified as high quality (Jadad score ≥ 3) and the remaining 4 as low quality (Jadad score < 3) (Table 1). The quality of evidence in GRADE analyses for each outcome ranged from ‘‘low” (22.2%) to “moderate” (44.4%) to ‘‘high” (33.3%) (S1 Table).
Improvement of psychiatric symptoms
Primary outcome
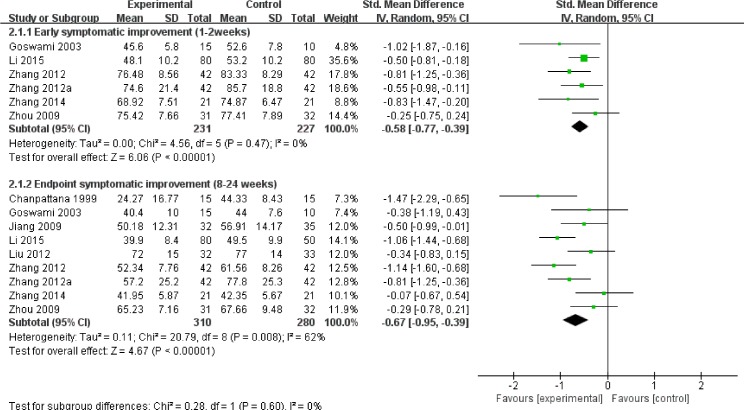
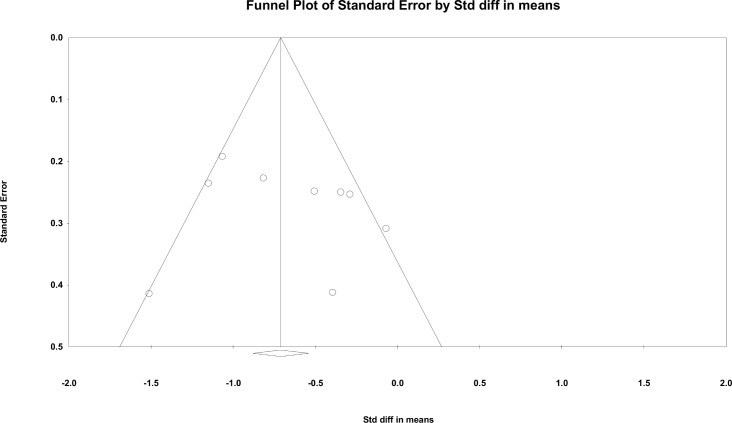
Fig 2 demonstrates that the adjunctive ECT-antipsychotic combination outperformed the comparator on endpoint symptomatic improvement of the total scores of PANSS (6 trials) or BPRS (3 trials) with a SMD of -0.67 [(CI:-0.95,-0.39) (p<0.00001; I2 = 62%)]. The results remained consistent when the three outlier RCTs [14, 19, 23] were removed resulting in a SMD of -0.44 [(95%CI:-0.65, -0.22) (P<0.0001; I2 = 0%)]. Furthermore, added-on ECT was significantly superior in all but one preplanned subgroup analyses regarding endpoint symptomatic status (Table 2). In the exploratory meta-regression analyses, there were non-significant moderating effects on the endpoint symptomatic improvement including sample size (p = 0.25), mean age (p = 0.52), illness duration (p = 0.63), Jadad score (p = 0.28), trial duration (p = 0.56) and proportion of male patients (p = 0.46). The funnel plot was symmetrical (Fig 3), and the Egger’s test did not identify publication bias (p = 0.58). The fail-safe method indicated that an additional 134 studies would result in a negative result.
Fig 2. ECT added to non-clozapine antipsychotic medications for treatment-resistant schizophrenia: improvement in total psychopathology at 1–2 weeks and study endpoint.
Table 2. Subgroup and sensitivity analysis of the effect of mediator variables on the outcome of “endpoint symptomatic improvement”.
| Variables | Subjects (studies) | SMDs (95%CI) | I2 (%) | P |
|---|---|---|---|---|
| 1. Chinese studies | 535 (7) | -0.63 (-0.93, -0.33) | 64 | <0.0001 |
| Non-Chinese studies | 55 (2) | -0.92 (-1.99, 0.14) | 0 | 0.09 |
| 2. Double blind/rater-masked | 271 (5) | -0.74 (-1.16, -0.33) | 61 | 0.0005 |
| Non-blinded | 319 (4) | -0.59 (-1.04, -0.15) | 72 | 0.009 |
| 3. Trial duration < 12 weeks | 323 (4) | -0.95 (-1.20, -0.70) | 8 | <0.00001 |
| Trial duration ≥ 12weeks | 267 (5) | -0.46 (-0.82, -0.10) | 51 | 0.01 |
| 4. the number of ECTa: mean <9 sessions | 84 (1) | -0.81 (-1.25, -0.36) | NA | 0.0004 |
| mean ≥9 sessions | 481 (7) | -0.68 (-1.03, -0.33) | 70 | 0.0002 |
| 5. High quality (Jadad score ≥ 3) | 313 (6) | -0.63 (-1.03, -0.23) | 64 | 0.002 |
| Low quality (Jadad score < 3) | 277 (3) | -0.74 (-1.18, -0.31) | 66 | 0.0009 |
| 6. Co-starting with an antipsychotic | 237 (4) | -0.49 (-0.75, -0.23) | 1 | 0.0003 |
| Augmenting with an antipsychotic | 353 (5) | -0.83 (-1.26, -0.41) | 70 | 0.0001 |
aOnly 8 RCTs reported the number of ECT sessions. Bold values are p<0.05
CI: 95% confidence interval; ECT = electroconvulsive therapy; SMDs = standardized mean differences; NA = not applicable
Fig 3. ECT added to non-clozapine antipsychotic medications for treatment resistant schizophrenia: publication bias.
Secondary outcomes
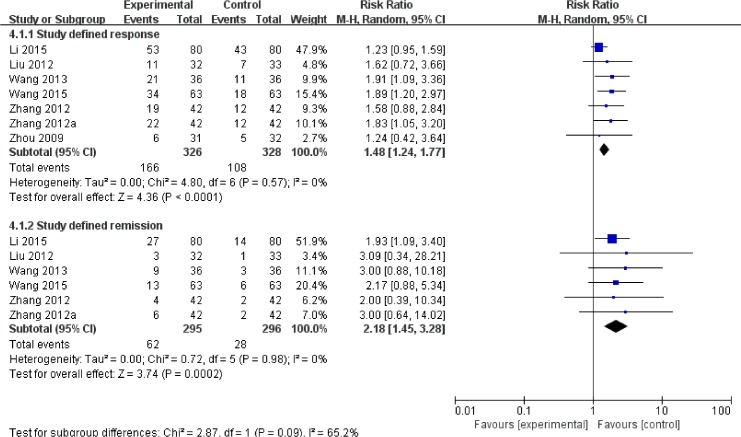
Fig 2 and Fig 4 illustrate that adjunctive ECT outperformed the comparator on early symptomatic improvement in the total scores of PANSS (6 trials) or BPRS (2 trials) at 1 to 2 weeks with a SMD of -0.58 [(CI:-0.77, -0.39) (p<0.00001; I2 = 0%)] and for study-defined response (reduction in PANSS or BPRS total scores ≥50%) (RR = 1.48, p<0.0001) with a NNT of 6 (CI = 4–9) and remission (reduction in PANSS or BPRS total scores h a SM(RR = 2.18, p = 0.0002) with a NNT of 8 (CI = 6–16). Similar results were observed in terms of endpoint of PANSS positive and general symptom sub-scores with a WMD between -3.48 to -1.32 (P = 0.01 to 0.009), but not with respect to the PANSS negative symptom sub-score with a WMD of -1.01 (P = 0.17) (S4 Fig).
Fig 4. ECT added to non-clozapine antipsychotic medications for treatment resistant schizophrenia: study-defined response and remission.
ADRs and discontinuation
Headache (RR = 5.35, p = 0.02) with a NNH of 6 (CI = 4–11) and memory impairment (RR = 14.30, p = 0.001) with a NNH of 3 (CI = 2–5) were significantly more frequent with ECT-antipsychotic co-treatment over antipsychotic monotherapy (S5 Fig). In one study [16] the number of discontinuation due to ADRs was 2 and 0 in the ECT-antipsychotic co-treatment and antipsychotic monotherapy groups, respectively. The remaining RCTs did not report all-cause discontinuation rate.
Discussion
In this meta-analysis of 11 RCTs (n = 818) comparing add-on ECT to non-clozapine antipsychotics with antipsychotic monotherapy for TRS, the combination treatment was superior in terms of the primary and key secondary efficacy outcomes. Importantly, adjunctive ECT was both safe and well tolerated.
In terms of the primary outcome measurement, adjunctive ECT was significantly superior to antipsychotic monotherapy with a medium effect size; according to Cohen [41] criteria, SMD of –0.2, –0.5, and –0.8 were defined as small, medium and large effect size, respectively. While the heterogeneity of the primary outcome result was 62%, the resultant effect size was still -0.44 and heterogeneity decreased to 0% after conducting a sensitivity analyses. The result was the same across all but one a priori defined subgroup analyses, namely non-Chinese studies. Subgroup analyses did not reveal a significant difference between the Chinese (SMD = -0.63, 95%CI:-0.93, -0.33) and non-Chinese studies (SMD = -0.92, 95%CI:-1.99, 0.14). In meta-regression analyses, pre-specified baseline moderators or mediators of primary outcomes could not be identified. Publication bias was not detected by the funnel plot and Egger’s test.
Adjunctive ECT was significantly superior to antipsychotic monotherapy with a moderate effect size of -0.58 after 1 to 2 weeks, which increased to the larger effect size of -0.67 after 8 weeks suggesting an overall reliability of the results. The majority of patients responded better to adjunctive ECT than to antipsychotic monotherapy: 50.9% vs. 32.9%, NNT = 6. The remission rates similarly favored adjunctive ECT: 21.0% vs. 9.5%, NNT = 8. While the optimal number of ECT sessions for schizophrenia remains unclear, 12 to 20 sessions have been shown to be adequate [42, 43]. The number of ECT sessions were 14.2±8.0 (range = 7.6–36.0, median = 8.0) in ten studies. Thus, an ECT course of 14 sessions appears to be reasonable to target the symptoms of schizophrenia. Of note, in seven of the eleven studies, the electrode placement was reported. In all seven of these studies, bilateral placement was utilized.
Adjunctive ECT was generally safe and well-tolerated. Sixteen patients reported headache (18.3% vs. 2.2% on antipsychotic monotherapy, NNH = 6) and 32 experienced memory impairment in the adjunctive ECT group (34.3% vs. 1.5% on antipsychotic monotherapy, NNH = 3), which were consistent with Wang et al's study [25]. These ADRs were mostly mild, transient and tolerable [17, 18].
In a recent meta-analysis on adjunctive ECT combined with any type of antipsychotics for TRS (22 RCTs), 11 RCTs focused on concurrent use of ECT with non-clozapine antipsychotics [25]. However, the authors did not examine the separate effect of this strategy. Another systematic review concluded that ECT may be an effective and safe augmentation strategy to clozapine in TRS, but the effects of ECT combined with non-clozapine were not examined [12].
Several limitations of this meta-analysis need to be mentioned. First, blinding methods for raters were only reported in 4 studies and only 7 of the 11 RCTs were rated as high quality. Second, there was statistical heterogeneity of endpoint symptomatic improvement resulting from methodological and clinical heterogeneity. This limitation was partly offset by one sensitivity analysis, 5 subgroup analyses and 7 meta-regression analyses to identify potential moderators or mediators of the effect on primary outcome. Third, most studies had relatively short duration (median = 8.0 weeks). Fourth, although serious adverse effects, such as confusion, could occur after ECT, apart from the ADRs mentioned above, no serious adverse effects were reported in the studies. Finally, few objective measures evaluated neurocognitive functioning. Only 2 RCTs [17, 24] tested cognitive functions with the Wechsler Memory Scale without data for the control group in one RCT [24]; 1 RCT [14] applied the modified Mini-Mental State Examination; one RCT [16] used the Wisconsin Card Sorting Test.
Conclusions
This systematic review and meta-analysis of 11 RCTs with 818 patients concluded that ECT added to a non-clozapine antipsychotic medication for TRS is more effective than antipsychotic monotherapy. The ECT/non-clozapine antipsychotic combination is safe and tolerable. Future studies should examine clinical factors that predict efficacy and tolerability of ECT for patients with schizophrenia.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was supported by the McLean Hospital—Chinese Psychiatric Society Initiative in Psychiatry (MCIP), the Start-up Research Grant (SRG2014-00019-FHS) and the Multi-Year Research Grant (MYRG2015-00230-FHS) from the University of Macau. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kho KH, Blansjaar BA, de Vries S, Babuskova D, Zwinderman AH, Linszen DH. Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia—an open label study. European archives of psychiatry and clinical neuroscience. 2004;254(6):372–9. Epub 2004/11/13. 10.1007/s00406-004-0517-y . [DOI] [PubMed] [Google Scholar]
- 2.Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443–57. Epub 2008/04/18. 10.1093/schbul/sbn018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanpattana W, Chakrabhand ML, Kongsakon R, Techakasem P, Buppanharun W. Short-term effect of combined ECT and neuroleptic therapy in treatment-resistant schizophrenia. J ECT. 1999;15(2):129–39. Epub 1999/06/23. . [PubMed] [Google Scholar]
- 4.Meltzer HY. Dimensions of outcome with clozapine. Br J Psychiatry Suppl. 1992;(17):46–53. Epub 1992/05/01. . [PubMed] [Google Scholar]
- 5.Iqbal MM, Rahman A, Husain Z, Mahmud SZ, Ryan WG, Feldman JM. Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry. 2003;15(1):33–48. . [DOI] [PubMed] [Google Scholar]
- 6.Young CR, Bowers MB Jr., Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):381–90. . [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Xiang YT, Su YA, Shu L, Yu X, Correll CU, et al. Clozapine in schizophrenia and its association with treatment satisfaction and quality of life: Findings of the three national surveys on use of psychotropic medications in China (2002–2012). Schizophr Res. 2015;168(1–2):523–9. 10.1016/j.schres.2015.07.048 . [DOI] [PubMed] [Google Scholar]
- 8.Englisch S, Zink M. Treatment-resistant Schizophrenia: Evidence-based Strategies. Mens sana monographs. 2012;10(1):20–32. Epub 2012/06/02. 10.4103/0973-1229.91588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porcelli S, Balzarro B, Serretti A. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol. 2012;22(3):165–82. Epub 2011/09/13. 10.1016/j.euroneuro.2011.08.005 . [DOI] [PubMed] [Google Scholar]
- 10.Vayisoglu S, Anil Yagcioglu E. [Augmentation strategies in patients with schizophrenia who show partial response to clozapine treatment]. Turk psikiyatri dergisi = Turkish journal of psychiatry. 2014;25(3):201–11. Epub 2014/09/16. . [PubMed] [Google Scholar]
- 11.Petrides G, Malur C, Braga RJ, Bailine SH, Schooler NR, Malhotra AK, et al. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. A J Psychiatry. 2015;172(1):52–8. Epub 2014/08/27. 10.1176/appi.ajp.2014.13060787 . [DOI] [PubMed] [Google Scholar]
- 12.Lally J, Tully J, Robertson D, Stubbs B, Gaughran F, MacCabe JH. Augmentation of clozapine with electroconvulsive therapy in treatment resistant schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2016;171(1–3):215–24. Epub 2016/02/02. 10.1016/j.schres.2016.01.024 . [DOI] [PubMed] [Google Scholar]
- 13.Weiner RD. The practice of electroconvulsive therapy: Recommendations for treatment, training, and privileging: A Task Force Report of the American Psychiatric Association: American Psychiatric Pub; 2008. [Google Scholar]
- 14.Chanpattana W, Chakrabhand ML, Sackeim HA, Kitaroonchai W, Kongsakon R, Techakasem P, et al. Continuation ECT in treatment-resistant schizophrenia: a controlled study. J ECT. 1999;15(3):178–92. Epub 1999/09/24. . [PubMed] [Google Scholar]
- 15.Goswami U, U. K, B. S. Efficacy of Electroconvulsive Therapy in Treatment Resistant Schizophreinia: A double-blind study. Indian journal of psychiatry. 2003;45(1):: 26–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang XQ, Yang KR, Zhou B, Jing P, Zheng LF, Gao XF, et al. Study on efficacy of modified electroconvulsive therapy(MECT)together with risperidone in treatment treatment-resistant schizophrenia(TRS) [In Chinese]. Chinese Journal of Nervous and Mental Diseases. 2009;35(2):79–83. 10.3969/j.issn.1002-0152.2009.02.005 [DOI] [Google Scholar]
- 17.Zhou HJ, Mao ZR, Chen YJ, Song BF, Shi DQ. A comparative study of modified electroconvulsive therapy combined with olanzapine in the treatment of treatment-refractory schizophrenia [In Chinese]. Journal of Neuroscience and Mental Health. 2009;9(4):328–31. 10.3969/j.issn.1009-6574.2009.04.017 [DOI] [Google Scholar]
- 18.Liu W, Guo XM, Wei BX, Chen JG, Du YT, Zhang R. A randomized controlled trials of olanzapine combined with MECT in the treatment of refractory schizophrenia [In Chinese]. Zhongguo Yaowu Yu Linchuang. 2012;12(1):115–7. 10.3969/j.issn.1671-2560.2012.01.058 [DOI] [Google Scholar]
- 19.Zhang SR, Zhang JL, Li XZ, Yang YY, Lang LS. Clinical efficacy of olanzapine combined with MECT in the treatment of patients with treatment refractory schizophrenia [In Chinese]. Renmin Junyi. 2012;55(02):141–3. [Google Scholar]
- 20.Zhang ZP. A comparative study of quetiapine combined with MECT in the treatment of refractory schizophrenia [In Chinese]. Journal of Clinical Psychosomatic Diseases. 2012;18(1):77–8. 10.3969/j.issn.1672-187X.2012.01.031-0077-02 [DOI] [Google Scholar]
- 21.Wang F, Guo DW. A comparative study of olanzapine combined with MECT in treatment-resistant schizophrenia [In Chinese]. Chinese Journal of Clinical Rational Drug Use. 2013;6(24):99. [Google Scholar]
- 22.Zhang JY. A comparative study of olanzapine combined with MECT in the treatment of refractory schizophrenia [In Chinese]. Yanbian Yixue. 2014;9(27):35–6,7. [Google Scholar]
- 23.Li N, Wan ZY. Clinical efficacy analysis of ziprasidone combined with MECT in the treatment of refractory schizophrenia [In Chinese]. Medical Information. 2015;28(4):194–5. [Google Scholar]
- 24.Wang GK. Efficacy of ziprasidone combined with MECT in the treatment of refractory schizophrenia [In Chinese]. Medical Information. 2015;28(14):43–. [Google Scholar]
- 25.Wang WZ, Pu CC, Jiang JL, Cao QY, Wang JJ, Zhao M, et al. Efficacy and safety of treating patients with refractory schizophrenia with antipsychotic medication and adjunctive electroconvulsive therapy: a systematic review and meta-analysis [In Chinese]. Shanghai Archives of Psychiatry. 2015;27(04):206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia. Cochrane Database Syst Rev. 2005;(2):CD000076 Epub 2005/04/23. 10.1002/14651858.CD000076.pub2 . [DOI] [PubMed] [Google Scholar]
- 27.Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. 2009;34(18):1929–41. Epub 2009/08/15. 10.1097/BRS.0b013e3181b1c99f . [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–9, W64. Epub 2009/07/23. . [DOI] [PubMed] [Google Scholar]
- 29.Kay SR, Fiszbein A,., Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):463–8. [DOI] [PubMed] [Google Scholar]
- 30.Overall JE, Gorham DR. The Brief Psychiatric Rating-Scale. Psychological Reports. 1962;10(3):799–812. [Google Scholar]
- 31.Higgins J, Higgins J, Prof. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2008. [Google Scholar]
- 32.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. Epub 1996/02/01. . [DOI] [PubMed] [Google Scholar]
- 33.Shang A, Huwiler-Muntener K, Nartey L, Juni P, Dorig S, Sterne JA, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet. 2005;366(9487):726–32. Epub 2005/08/30. 10.1016/s0140-6736(05)67177-2 . [DOI] [PubMed] [Google Scholar]
- 34.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. Epub 2011/01/07. 10.1016/j.jclinepi.2010.07.015 . [DOI] [PubMed] [Google Scholar]
- 35.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490 Epub 2004/06/19. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. Epub 1986/09/01. . [DOI] [PubMed] [Google Scholar]
- 37.Shen YF. Electroconvulsive therapy in Psychiatry (In Chinese). Beijing: People's Medical Publishing House; 2009. [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Epub 1997/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duval S,., Tweedie R,. Trim and Fill: A Simple Funnel-Plot–Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics. 2000;56(2):455–63(9). [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–41. [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 42.Tang WK, Ungvari GS. Efficacy of electroconvulsive therapy in treatment-resistant schizophrenia: a prospective open trial. Prog Neuropsychopharmacol B ol Psychiatry. 2003;27(3):373–9. Epub 2003/04/15. 10.1016/s0278-5846(02)00354-8 . [DOI] [PubMed] [Google Scholar]
- 43.Kendell RE. Kendell RE. The present status of electroconvulsive therapy. Br J Psychiatry. 1981;139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.