Abstract
Background
A discordant immune response (DIR) is a failure to satisfactorily increase CD4 counts on ART despite successful virological control. Literature on the clinical effects of DIR has not been systematically evaluated. We aimed to summarise the risk of mortality, AIDS and serious non-AIDS events associated with DIR with a systematic review.
Methods
The protocol is registered with the Centre for Review Dissemination, University of York (registration number CRD42014010821). Included studies investigated the effect of DIR on mortality, AIDS, or serious non-AIDS events in cohort studies or cohorts contained in arms of randomised controlled trials for adults aged 16 years or older. DIR was classified as a suboptimal CD4 count (as defined by the study) despite virological suppression following at least 6 months of ART. We systematically searched PubMed, Embase, and the Cochrane Library to December 2015. Risk of bias was assessed using the Cochrane tool for assessing risk of bias in cohort studies. Two authors applied inclusion criteria and one author extracted data. Risk ratios were calculated for each clinical outcome reported.
Results
Of 20 studies that met the inclusion criteria, 14 different definitions of DIR were used. Risk ratios for mortality in patients with and without DIR ranged between 1.00 (95% CI 0.26 to 3.92) and 4.29 (95% CI 1.96 to 9.38) with the majority of studies reporting a 2 to 3 fold increase in risk.
Conclusions
DIR is associated with a marked increase in mortality in most studies but definitions vary widely. We propose a standardised definition to aid the development of management options for DIR.
Background
Antiretroviral therapy (ART) substantially reduces the incidence of acquired immunodeficiency syndrome (AIDS) and mortality, with increased CD4 cell count significantly and independently associated with improved prognosis [1–4]. Some patients do not achieve CD4 cell count reconstitution with ART, despite achieving suppression of HIV viral load in the blood [5]. This paradoxical response is referred to by various terms in the literature including discordant immune response (DIR), poor or suboptimal immune reconstitution, incomplete immune recovery or restoration and immunological non-response. Here, we use the term discordant immune response as it was the term most frequently used by the included studies [6–10]. There is currently no agreed case definition for DIR.
Over 13 million people worldwide are on ART, with a further 22 million eligible [11]. Understanding limitations to its success will be critical in improving individual responses to treatment and regimen durability. The 2013 World Health Organization (WHO) consolidated guidelines on treatment of HIV now favour use of HIV viral load monitoring for routine identification of ART treatment failure [12], but CD4 cell counts for patients established on ART remain an important clinical and prognostic tool and are essential for identifying DIR [13, 14].
Much research has focused on CD4 reconstitution on ART, but the mechanisms promoting DIR are not well understood. Damage to CD4 T cells begins prior to ART initiation due to direct effects of the HIV virus on thymic tissue and depletion of progenitor cells[15]. Thymic output may be disproportionally affected in patients who start ART at lower CD4 counts leading to under-reconstitution of naïve CD4 T cells[16, 17]. Lymph node fibrosis is also major feature and correlates with duration of HIV infection prior to ART initiation [18, 19]. Untreated HIV infection leads to a significant activation of the immune system [20], resulting in a cycle of systemic inflammation, persistent T cell activation, exhaustion and death [21–23]. The extent of immune activation at the time of ART initiation is associated with the development of DIR [4, 24] and predicts mortality on ART [25]. HIV induced T cell dysfunction and inflammation are closely related to serious non-AIDS events [20, 26]. Persistent immune activation is often detected despite virologically suppressive ART [27] and can be driven by microbial translocation[28], low level persistent HIV viral replication[29], and latent co-infections such as CMV [30, 31] and tuberculosis [32, 33]. Innate immune cells including monocytes, macrophages and NK cells also perpetuate immune activation, but this axis is more specifically driven microbial translocation, LPS antigenaemia and circulating soluble CD14 and does not necessarily correlate with T cell activation [34–37].
Non-systematic reviews have previously been carried out into aetiologies, prevalence and potential management of DIR [38–44]. However, the literature is heterogeneous and in order to better understand the burden of DIR, we sought to systematically characterise the risk of mortality, AIDS and serious non-AIDS events associated with DIR across the published literature.
Methods
The study protocol was registered with the Centre for Review Dissemination, University of York (registration number CRD42014010821). The systematic review has been reported in accordance with the PRISMA guidelines [45] (See S1 Checklist).
Eligibility criteria
Participants
Participants were aged 16 years or older and no restrictions were placed on language or geographical region.
Participants with DIR were defined as patients who had been taking ART for at least 6 months and who were virologically suppressed, but had a suboptimal CD4 count according to study definitions. Studies defined a suboptimal CD4 count in terms of either a failure to achieve a pre-specified rise in CD4 count or a pre-specified absolute CD4 value at a specific time point following ART initiation. Virological suppression was defined as at least one single HIV viral load measurement of below 1000 copies/ml after at least 6 months of ART. Studies that did not report on the virological status of the cohort were not included.
Outcomes
Studies were included if they estimated the risk of mortality, AIDS or serious non-AIDS events associated with DIR. Studies were included if death was verified by clinician review, tracing or verbal autopsy. AIDS was defined as any illness that met criteria for a WHO stage 4 condition [46]. Serious non-AIDS events were defined as illnesses not included in the WHO Clinical Staging System, and which were non-communicable. These include non-communicable cardiovascular, liver, renal and bone diseases as well as non-AIDS related malignancies. Studies reporting AIDS and serious non-AIDS events were deemed to meet our inclusion criteria if the events had been verified at least by clinician review of participant records.
Study design
Studies were eligible for inclusion if they were cohort studies or randomised controlled trials (RCTs). We excluded editorials and comments, case reports and case series, qualitative studies, mathematical modelling studies, and economic analyses.
Information sources and search methods
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL, in the Cochrane Library issue 1, 2016); MEDLINE (PubMed; 1966 to 31st December 2015); EMBASE (OVID; 1980 to 31st Decemeber 2015). Table 1 shows the search strategy used in Medline (PubMed); this was modified for the other electronic databases.
Table 1. Search strategy.
| Search | |
|---|---|
| #16 | Search (#5) AND #15 |
| #15 | Search (((((((((#6) OR #7) OR #8) OR #9) OR #10) OR #11) OR #12) OR #13) OR #14) OR #15 Field: Title/Abstract |
| #14 | Search incomplete CD4* response Field: Title/Abstract |
| #13 | Search discordant* Field:Title/Abstract |
| #12 | Search immunovirological discordance* Field: Title/Abstract |
| #11 | Search low CD4* Field: Title/Abstract |
| #10 | Search insufficient CD4* Field: Title/Abstract |
| #9 | Search suboptimal CD4* Field: Title/Abstract |
| #8 | Search low responder* Field: Title/Abstract |
| #7 | Search suboptimal immune response*[Title/Abstract] |
| #6 | Search suboptimal immune reconstitution [Title/Abstract] |
| #5 | Search (#1) AND #4 |
| #4 | Search (#2) OR #3 |
| #3 | Search (antiretroviral[Title/Abstract]) OR ART[Title/Abstract] |
| #2 | Search antiretroviral therapy [Title/Abstract] |
| #1 | Search "HIV Infections"[Mesh] |
Study Selection and Data Collection
Titles of studies identified from the database search were independently reviewed by two authors (CK and KG) and were excluded if the study was unrelated to the review subject. Remaining studies underwent independent abstract review and then full text review by the same two reviewers. Pre-piloted data extraction forms were independently applied to all studies that underwent full text review. Where disagreement occurred, a consensus was reached by discussion or a third reviewer was consulted (PM). Where outcome data were not reported, the lead study author was contacted.
Risk of bias
Risk of bias assessment was based on the Cochrane Tool for Assessing Risk of Bias in Cohort Studies [47]. The Cochrane Tool for Assessing Risk of Bias in Randomised Control Trials was not used because no RCTs targeting DIR were identified that met the inclusion criteria. Potential sources of bias were assessed in three domains: ‘study design’; ‘comparability’; and ‘assessment of outcomes’.
The study design domain assessed whether participants were selected to be representative of adults on ART, and if there were clear selection criteria for those with and without DIR. Studies with more stringent selection criteria based on, for example, frequency of CD4 and viral load monitoring or attendance at routine clinics prior to enrolment, were deemed to be at a high risk of bias because they might exclude populations at higher risk of DIR and therefore were not representative of the entire population of patients with DIR. The comparability domain assessed if patients with and without DIR were managed according to the same standardised protocol and if outcomes were reported after appropriate adjustment for potential confounding variables. For the assessment of outcomes domain, outcomes had to be measured using clinician review, case note review, verbal autopsy or autopsy. Studies that did not report at least one of these methods were deemed high risk of bias for this category. The minimum acceptable follow-up period was one year as this is the highest risk period for adverse clinical outcomes post ART initiation [48].
An overall risk of bias assessment was made for each individual domain. A domain would be classified as high risk of bias if any one question within it failed the specified criteria. Where insufficient information had been reported in a study to make a judgement on the risk of bias, that question was recorded as unclear. ‘Unclear’ and ‘high risk’ categories were then combined for the purposes of analysis [49].
Summary measures and synthesis of results
For each study, the proportion of participants with and without DIR who died, and/or experienced an AIDS-related, or serious non-AIDS-related event were estimated. Risk ratios and 95% confidence were extracted from the manuscript or calculated. A meta-analysis with pooled effect estimates were planned but could not be carried out due to considerable variation in both DIR definition and length of follow-up.
Results
Study selection
2782 study titles were identified by the search. Twenty studies met inclusion criteria for full-text review (Fig 1). The two most common reasons for exclusion were that the study did not report a clinical outcome (36%) and the cohort was not virally suppressed (23%). Authors from the study by Young et al were contacted to clarify if participants had been on ART for at least 6 months but the data were no longer available and so the study was excluded.
Fig 1. Flow of paper selection from those identified following literature search through to inclusion.
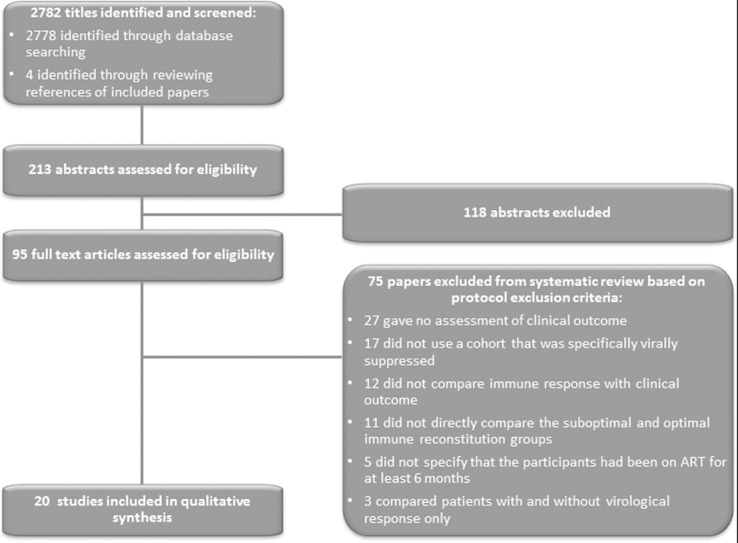
Study characteristics
Twenty studies were included [4, 6–9, 50–64], all of which were cohort studies. For three studies, the cohort was established de novo to investigate the effects of DIR on clinical outcomes [4, 51, 58, 64]. One study analysed DIR and outcomes in the control arm of an RCT assessing ART regimes for individuals initiating ART [50]. The remaining 14 studies conducted secondary analysis of existing datasets comprising of national or international cohorts of HIV infected patients who had data collected prospectively and systematically during routine clinical care [6–9, 52–57, 59–63].
Seventeen studies recruited participants from HIV care clinics. Five studies included participants from resource-limited countries [4, 9, 58, 63, 64]: three from countries with a generalised HIV epidemic (Uganda, South Africa) [4, 58, 64]; one from Senegal [63]; and one from an international collaboration of both low-, and middle-income countries [9]. Participants were ART-naïve in 16 studies [4, 6–9, 50, 51, 53, 55–60, 63, 64] whereas four studies included patients who were ART naïve and experienced [52, 54, 61, 62].
For included studies, the median proportion of male participants ranged from 31%– 100% and median age ranged from range 34 to 43 years (Table 2). Median CD4 cell count at ART initiation was reported for 15 studies and ranged from 80–221 cells/mm3. Median HIV viral load at ART initiation was reported for 10 studies and ranged from 4.5 log10−5.1 log10 copies/ml. The threshold for defining virological suppression ranged from <50 copies/ml to <1000 copies per ml. Participant follow-up ranged from 1 to 7 years.
Table 2. Description of 20 included studies.
| Study author | Study design | Year of publication | Median Duration of follow-up | Country | Setting | Relevant Outcomes examined | ART naïve? | %Male | Median age (years) | Median CD4 (cells/uL) at ART initiation | Median HIV VL (log10 copies/mL) at ART initiation |
| BAKER [50] | Control arm of ART RCT | 2008 | 5 years | USA | Community, 80 sites | Predictors and clinical outcomes of patients with DIR | Yes | 80 | 39 | 221 | 5.0 |
| BATISTA [63] | Established HIV cohort2 | 2015 | 7 years | Senegal | HIV care clinic | Frequency and risk factors for DIR, and incidence of OI and death | Yes | 35 | 40 | Not reported | Not reported |
| DRONDA [51] | Prospective cohort study1 | 2002 | 3 years | Spain | HIV care clinic | Immunologic and clinical outcomes of patients with DIR | Yes | 74 | 36 | 196 | 5.0 |
| ENGSIG [52] | Established HIV cohort2 | 2010 | 4.7 years | Denmark | HIV care clinics, 8 sites | Predictors and mortality of patients with DIR | No | 78 | 435 | Not reported | Not reported |
| FALSTER [53] | Established HIV cohort2 | 2008 | 5.4 years | Australia | HIV care clinics, number of sites not reported | Prevalence of DIR, and and clinical outcomes | Yes | 935 | Not reported | Not reported | Not reported |
| GILSON [6] | Established HIV cohort2 | 2010 | 3 years | UK | HIV care clinics, 10 sites | Predictors and clinical outcomes | Yes | 75 | 37 | 170 | 5 |
| Study author | Study design | Year of publication | Median Duration of follow-up | Country | Setting | Relevant Outcomes examined | ART naïve? | %Male | Median age (years) | Median CD4 (cells/uL) at ART initiation | Median HIV VL (log10 copies/mL) at ART initiation |
| GRABAR [54] | Established HIV cohort2 | 2000 | 18 months | France | HIV care clinics, 68 sites | Clinical outcomes of patients with DIR | No | 79 | 37 | 150 | 4.54 |
| GUTERRIEZ [55] | Established HIV cohort2 | 2008 | 2.3 years | Spain | HIV care clinics, 10 sites | Predictors and clinical outcomes of patients with DIR | Yes | 75 | 37 | 160 | 5.0 |
| HUNT [4] | Prospective cohort study1 | 2011 | 2 years | Uganda | HIV care clinic | Mortality according to CD4 account3 | Yes | 30 | 34 | 135 | 5.1 |
| KAUFMANN [56] | Established HIV cohort2 | 2004 | 5 years | Switzerland | HIV care clinics, number of sites not reported | Predictors and clinical outcomes of patients with DIR | Yes | 74 | 38 | 180 | 4.9 |
| LOUTFY [57] | Established HIV cohort2 | 2010 | 2.7 years | Canada | HIV care clinics, 9 sites | Clinical outcomes of patients with DIR | Yes | 83 | 40 | 180 | 5.0 |
| MOORE [7] | Established HIV cohort2 | 2005 | 3.7 years | Canada | HIV care clinic | Predictors and mortality in patients with DIR | Yes | 775 | 39 | 199 | Not reported |
| NAKANJAKO [58] | Prospective cohort study1 | 2008 | 1.8 years | Uganda | HIV care clinic | Prevalence of DIR and clinical outcomes | Yes | 31 | 38 | 98 | Not reported |
| Study author | Study design | Year of publication | Median Duration of follow-up | Country | Setting | Relevant Outcomes examined | ART naïve? | %Male | Median age (years) | Median CD4 (cells/uL) at ART initiation | Median HIV VL (log10 copies/mL) at ART initiation |
| NICASTRI [61] | Established HIV cohort2 | 2005 | 3.7 years | Italy | Hospital, 63 sites | Immunologic and clinical outcomes | No | 72 | 35 | 185 | 4.78 |
| PACHECO [59] | Established HIV cohort2 | 2009 | 6 years | Spain | Hospital, 10 sites | CD4 count recovery, predictors and mortality in patients with DIR | Yes | 325 | Not reported | Not reported | Not reported |
| TAKUVA [64] | Prospective cohort study1 | 2014 | 2 years | South Africa | HIV care clinic, 1 site | Mortality and AIDS associated with DIR | Yes | 36 | 39 | 80 | Not reported |
| TAN [8] | Established HIV cohort2 | 2008 | 3.2 year | USA | HIV care clinic | Clinical outcomes in patients with DIR | Yes | 76 | 38 | 213 | 5.4 |
| TAIWO [62] | Established HIV cohort2 | 2009 | Not reported | USA | HIV care clinics, 4 sites | Clinical outcomes in patients with DIR | No | 100 | 42 | Not reported | Not reported |
| TUBOI [9] | Established HIV cohort2 | 2010 | 1 year | Multi-centre4 | HIV care clinics, 31 centres | Mortality in patients with DIR | Yes | 39 | 34 | 100 | Not reported |
| ZOUFALY [10] | Established HIV cohort2 | 2010 | 3.8 years | Germany | HIV care clinics, 11 sitess | Predictors and clinical outcomes in patients with DIR | Yes | 77 | 39 | 80 | Not reported |
ART = anti-retroviral therapy, cART = combination anti-retroviral therapy, DIR = discordant immune response, VL = Viral load, PI = Protease inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, DDI = didanosine, TDF = tenofovir, LMIC- = Low and middle income countries.
1 Patients are enrolled specifically for the aims of the current study.
2 Retrospective analysis of prospectively collected data.
3Analysis of clinical outcomes in DIR is a secondary analysis in this study.
4Includes countries from Africa, South America and Asia.
5Not reported for entire cohort therefore median value from optimal immune response group reported.
Risk of bias
Two (10%) studies had a high risk of bias in study design; 6 (30%) in comparability; and 11 (55%) in assessment of outcomes (Table 3).
Table 3. Risk of bias assessment for 20 included studies.
| Study | Study design | Comparability | Assessment of outcomes | Overall risk of bias | ||||||||
| Were participants selected to be representative of the wider population? | Were there clear selection criteria for those with and without DIR? | Risk of bias | Are patients with and without DIR managed to standardised protocol? | Are outcomes reported after adjustment for important confounding variables? | Risk of bias | Were procedures for measuring outcome sufficient? | Was follow-up long enough for outcome detection? | Were incomplete outcome data adequately assessed? | Are outcomes reported in full and not selectively reported? | Risk of bias | ||
| BAKER [50] | Yes | Yes | Low | Yes | Unclear | High | Yes | Yes | Unclear | Yes | High | High |
| BATISTE [63] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | No | Yes | High | High |
| DRONDA [51] | Yes | Yes | Low | Yes | No | High | Yes | Yes | Yes | Yes | Low | High |
| ENGSIG [52] | No | Yes | High | Yes | No | High | Yes | Yes | Unclear | Yes | High | High |
| FALSTER [53] | Yes | Yes | Low | Yes | No | High | Yes | Yes | Unclear | Yes | High | High |
| GILSON [6] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Unclear | Yes | High | High |
| GRABAR [54] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Unclear | Yes | High | High |
| GUTERRIEZ [55] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| HUNT [4] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| KAUFMANN [56] | No | Yes | High | Yes | No | High | Unclear | Yes | No | Yes | High | High |
| LOUTFY [57] | Yes | Yes | Low | Yes | Yes | Low | Unclear | Yes | No | Yes | High | High |
| MOORE [7] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| NAKANJAKO [58] | Yes | Yes | Low | Yes | No | High | Yes | Yes | No | Yes | High | High |
| NICASTRI [61] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| PACHECO [59] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | No | Yes | High | High |
| TAKUVA [64] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| TAN [8] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| TAIWO [62] | Yes | Yes | Low | Yes | Yes | Low | Yes | Unclear | Unclear | Yes | High | High |
| TUBOI [9] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Yes | Yes | Low | Low |
| ZOUFALY [10] | Yes | Yes | Low | Yes | Yes | Low | Yes | Yes | Unclear | Yes | High | High |
For the study design domain, Engsig et al required a viral load of <50 copies /ml for more than three consecutive years before the start of the DIR observation period [52], and Kaufmann et al required a viral load of <1000 copies/mL during the entire 5-year observation period [56]. The frequency of visits these criteria would require may have excluded patients at higher risk of DIR. All studies detailed clear selection criteria for participants with and without DIR.
For the comparability domain, participants with and without DIR were managed according to the same treatment protocols for all studies but 6 studies did not appropriately evaluate the effects of confounders on outcomes [50–53, 56, 58].
For the assessment of outcomes domain, two studies gave no information on how deaths [56, 57] or AIDS events [57] were ascertained. Eleven studies did not describe how missing data were handled [6, 50, 52, 53, 56–60, 62, 63].
Definition of DIR
Definitions of DIR varied significantly and were classified into two categories: a failure to achieve a prespecified absolute CD4 count at a predefined time point; or a failure to achieve a prespecified rise in CD4 count from baseline at a predefined time point (Table 4). Five studies explored several potential definitions of DIR [6, 10, 57, 58, 64].
Table 4. Effect of DIR on rate of clinical outcomes, according to DIR definitions, for 20 studies reporting clinical outcomes.
| Definition of discordant immune response | First Author | HIV viral load cut off | Number virologically suppressed | Number virologically suppressed with DIR | Effect of DIR on risk of Mortality | Effect of DIR on risk of AIDS | Effect of DIR on risk of AIDS or mortality | ||||||
| DIR number of participants (%) | IR number of participants (%) | Risk ratio (min CI–max CI) | DIR number of participants (%) | IR number of participants (%) | Risk ratio (min CI–max CI) | DIR number of participants (%) | IR number of participants (%) | Risk ratio (min CI–max CI) | |||||
| Failure to achieve rise in CD4 count of > = 50 cells/mm3 at 6 months after ART initiation | MOORE [7] | <500 at 6 months | 1084 | 235 | 53 (22.6) | 61 (7.2) | 3.14 (2.24–4.40) | NR | NR | NR | NR | NR | NR |
| TAN [8] | Undetected at 6 months | 320 | 35 | 4 (11.4) | 11 (3.9) | 2.96 (1.00–8.80) | 6 (17.1) | 30 (10.5) | 1.63 (0.73–3.54) | NR | NR | NR | |
| TUBOI [9] | <500 at 6 months | 6234 | 1260 | 23 (4.5) | 51 (1.0) | 1.78 (1.09–2.90) | NR | NR | NR | NR | NR | NR | |
| Failure to achieve rise in CD4 count of > = 50 cells/mm3 at 8 months after ART initiation | BAKER [50] | <400 at 8 months | 850 | 149 | 7 (4.7) | 19 (2.7) | 1.73 (0.74–4.03) | 16 (10.7) | 33 (4.7) | 2.28 (1.29–4.02) | NR | NR | NR |
| Failure to achieve rise in CD4 count of > = 50 cells/mm3 at 12 months after ART initiation | GRABAR [54] | <1000 at 6 months | 1486 | 387 | NR | NR | NR | NR | NR | NR | 37 (9.6) | 51 (4.8) | 1.99 (1.33–2.99) |
| GUTERRIEZ [55] | <500 throughout follow-up | 650 | 108 | 8 (7.4) | 10 (1.8) | 4.01 (1.62–9.94) | 3 (2.8) | 15 (2.8) | 1.00 (0.30 -–3.41) | NR | NR | NR | |
| Failure to achieve rise in CD4 count of > = 100 cells/mm3 at 12 months after ART initiation | DRONDA [51] | <50 quarterly for 2 years | 288 | 76 | NR | NR | NR | NR | NR | NR | 7 (9.2) | 40 (18.9) | 0.86 (0.41–1.80) |
| NAKANJAKO [58] | <400 quarterly for 2 years | 339 | 151 | NR | NR | NR | 14 (9.3) | 9 (4.8) | 1.94 (0.86–4.35) | NR | NR | NR | |
| Failure to achieve rise in CD4 count of > = 100 cells/mm3 at 12 months after ART | NICASTRI [61] | <500 at 12 months | 1117 | 336 | NR | NR | NR | NR | NR | NR | Not reported | Not reported | Odds ratio 2.32 (1.36–3.95) |
| Failure to achieve rise in CD4 count of > = 100 cells/mm3 at 8 months after ART initiation | GILSON [6] | <50 twice over one year | 2584 | 571 | 26 (4.6) | 24 (2.0) | 2.29 (1.33–3.97) | 15 (2.6) | 33 (2.8) | 0.96 (0.53–1.76) | NR | NR | NR |
| Failure to achieve an absolute CD4 count of > = 174cells/mm3 at 6 months after ART initiation1 | HUNT [4] | <1000 throughout follow-up | 451 | 107 | 3 (2.8) | 6 (1.7) | 1.00 (0.26–3.92) | NR | NR | NR | NR | NR | NR |
| Failure to achieve an absolute CD4 count of > = 350 cells/mm3 at 9 months after ART initiation | FALSTER [53] | <400 twice over one year | 292 | 83 | NR | NR | NR | NR | NR | NR | 14 (3.5) | 35 (2.2) | 2.06 (0.89–4.79) |
| Failure to achieve an absolute CD4 count of > = 200 cells/mm3 at 12 months after ART | ZOUFALY [10] | <50 throughout follow-up | 1085 | 248 | NR | NR | NR | 18 (7.3) | 11 (1.3) | 2.70 (1.29–5.66) | NR | NR | NR |
| LOUTFY [57] | <50 throughout | 2028 | 404 | NR | NR | NR | NR | NR | NR | 14 (3.5) | 35 (2.2) | 1.61 (0.87–2.96) | |
| Failure to achieve an absolute CD4 count of > = 250 cells/mm3 at 22 months after ART initiation | PACHECO [59] | <1000 quarterly for 2 years | 147 | 40 | 5 (12.5) | 4 (3.7) | 3.23 (0.89–11.77) | NR | NR | NR | NR | NR | NR |
| Failure to achieve an absolute CD4 count of > = 200 cells/mm3 at 36 months after ART initiation | ENGSIG [52] | <50 over 3 years | 291 | 55 | 11 (20) | 11 (4.7) | 4.29 (1.96–9.38) | NR | NR | NR | NR | NR | NR |
| Failure to achieve an absolute CD4 count of > = 500 cells/mm3 at 60 months after ART | KAUFMANN [56] | <1000 over 5 years | 293 | 105 | 22 (21.0) | 18 (9.6) | 2.19 (1.23–3.89) | NR | NR | NR | NR | NR | NR |
NR not reported. DIR discordant immune response IR concordant immune response. VL viral load.
Eleven studies defined DIR based on a failure to achieve a rise in CD4 from ART initiation [6–9, 50, 51, 54, 55, 58, 61, 63] and used CD4 count thresholds of a failure to achieve a rise of at least 50 cells/ mm3 at 6 months [7–9, 63]; at least 100 cells/ mm3 at 12 months [51, 58]; at least 50 cells/ mm3 at 12 months [54, 55]; at least 50 cells/ mm3 at 8 months [50]; and at least 100 cells/ mm3 at 8 months [6].
Nine studies defined DIR based on a failure to achieve an absolute CD4 count at a predefined time point [4, 10, 52, 53, 56, 57, 59, 62, 64] and used the following CD4 count thresholds: 200 cells/ mm3 at 6 months[64], 200 cells/ mm3 at 12 months [10, 57]; of 350 cells/ mm3 at 9 months [53]; of 250 cells/ mm3 at 22 months [59]; 200 cells/ mm3 at 36 months [52]; and 500cells/ mm3 at 60 months [56]. Hunt and colleagues described mortality according to tertiles of CD4 counts in patients with viral suppression rather than use a single definition for DIR [4] so we compared mortality in the highest tertile (>177 cells/mm3) to the lowest tertile (<95 cells/mm3), using the lowest tertile of CD4 counts as the ‘DIR group’.
HIV VL cut offs used by studies to define virological suppression were as follows: 50 copies/ml (seven studies) [6, 10, 51, 52, 57, 62, 63]; 400 copies/ml (four studies) [50, 53, 58, 64]; 500 copies/ml (four studies) [7, 9, 55, 61]; and 1000 copies/ml (four studies) [4, 54, 56, 59]. One study only reported using an ‘undetectable’ VL [8]. The time period for the definition of virological suppression was as follows: a one off cut off point between 6 months to a year post ART (eight studies) [7–9, 50, 54, 61, 63, 64]; two measurements over one year (two studies) [6, 53]; quarterly measurements for 2 years (two studies) [51, 58, 59], and quarterly measurements throughout the period of follow-up (seven studies) [4, 10, 55–57, 62, 65].
Effect of DIR on risk of mortality
The risk of mortality ranged between 3% to 23% for patients with DIR and 1% to 7% for patients without DIR, over a median follow-up time of 2 years and 3.7 years respectively. Ten studies estimated the effect of DIR on mortality [4, 6–9, 50, 52, 55, 56, 59] (Table 4). Risk ratios ranged between 1.00 (95% CI 0.26–3.92) and 4.29 (95% CI 1.96–9.38). Six of nine studies showed a significantly higher risk of mortality in participants with DIR compared to participants without DIR [6–9, 55, 56] (Fig 2A). Two studies reported on the absolute risk of mortality in participants with DIR in resource-limited settings [4, 9], with Tuboi et al finding DIR to be significantly associated with an increased risk of death.
Fig 2.
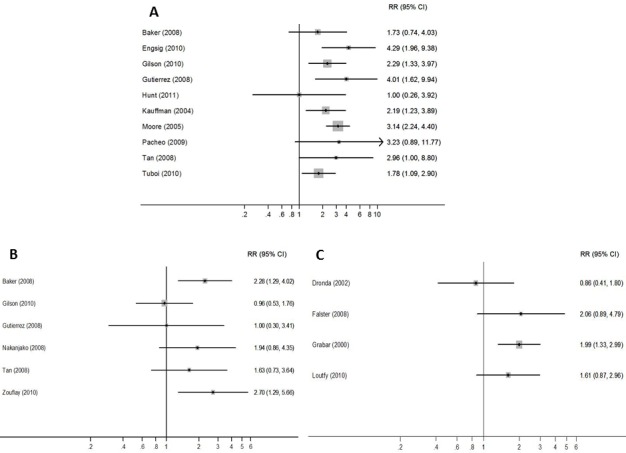
Forest plot showing risk of clinical outcomes for patients with DIR across those studies reporting each outcome: A) Mortality B) AIDS events C) Combined mortality and AIDS events.
Ten studies reported the incidence of mortality in participants with DIR (Table 5). Six found the incidence of mortality to be significantly higher in participants with DIR compared to participants without DIR [6–9, 55, 64]. Incidence rate ratios ranged from 1.78 (95% CI 1.09–2.90) to 4.01 (95% CI 1.62–9.94). One study from a sub-Saharan Africa setting (South Africa) reported rates of mortality and found an IRR of 1.78 (1.09–2.90). No differences in risk of mortality were found according to DIR defined as absolute or rise, CD4 count cut off or time period post ART initiation.
Table 5. Effect of DIR on rate of clinical outcomes, according to DIR definitions, for 10 studies reporting incidence data.
| Definition of discordant immune response (time periods are length of time following ART initiation) | First Author | HIV viral load cut off | Number virologically suppressed | Number virologically suppressed with DIR | Effect of DIR on rate of Mortality | Effect of DIR on rate of AIDS | Effect of DIR on rate of AIDS or mortality | ||||||
| DIR number of participants (per 100py) | IR number of participants (per 100 py) | Incidence rate ratio (min CI–max CI) | DIR number of participants (per 100 py) | IR number of participants (per 100 py) | Incidence rate ratio (min CI–max CI) | DIR number of participants (per 100 py) | IR number of participants (per 100 py) | Incidence rate ratio (min CI–max CI) | |||||
| Failure to achieve rise in CD4 count of > = 50 cells/mm3 after 6 months | BATISTA [63] | <50 at 6 months | 657 | 102 | NR | NR | NR | NR | NR | NR | 47 (9.8) | 202 (7.8) | 1.21 (0.85–1.72) |
| MOORE [7] | <500 at 6 months | 1084 | 235 | 53 (5.7) | 61 (1.8) | 3.2 (3.9–12.7) | NR | NR | NR | NR | NR | NR | |
| Failure to achieve an absolute CD4 count of > = 200 cells/mm3 after 6 months | TAKUVA [64] | <400 at 6 months | 4129 | NR | NR | NR | 2 (1.44–2.79) | NR | NR | 1.67 (1.27–2.21) | NR | NR | NR |
| Failure to achieve rise in CD4 count of > = 100 cells/mm3 after 8 months | GILSON [6] | <50 twice over one year | 2584 | 571 | 26 (3.5) | 24 (0.5) | 7.00 (3.9–12.7) | 15 (2.0) | 33 (0.7) | 2.9 (1.4–5.4) | NR | NR | NR |
| Failure to achieve rise in CD4 count of > = 50 cells/mm3 after 12 months | GRABAR [54] | <1000 at 6 months | 1486 | 387 | NR | NR | NR | NR | NR | NR | 37 (6.6) | 51 (1.8) | 3.7 (2.3–5.7) |
| Failure to achieve an absolute CD4 count of > = 200 cells/mm3 after 12 months | ZOUFALY [10] | <50 throughout | 1085 | 248 | 18 (4.4) | 11 (1.6) | 2.8 (1.2–6.4) | NR | NR | NR | NR | NR | NR |
| LOUTFY [57] | <50 throughout | 2028 | 404 | NR | NR | NR | NR | NR | NR | 14 (1.1) | 35 (0.8) | 1.4 (0.7–2.6) | |
| Failure to achieve an absolute CD4 count of > = 250 cells/mm3 after 22 months | PACHECO [59] | <1000 quarterly for 2 years | 147 | 40 | 5 (2.4) | 4 (0.7) | 3.2 (0.70–16.4) | NR | NR | NR | NR | NR | NR |
| Failure to achieve an absolute CD4 count of > = 200 cells/mm3 after 36 months | ENGSIG [52] | <50 over 3 years | 291 | 55 | 26 (3.5) | 24 (0.5) | 4.4 (1.7–11.3) | NR | NR | NR | NR | NR | NR |
| Failure to achieve an absolute CD4 count of > = 200 cells/mm3 after 6 months | TAIWO [62] | <50 biannually throughout | NR | NR | NR | NR | 5.96 (0.40–87.8) | NR | NR | HR 22.8 (1.89–275) | NR | NR | HR 10.7 (1.65–70) |
NR not reported. DIR discordant immune response IR concordant immune response. VL viral load. py person years HR hazard ratio
Effect of DIR on risk of AIDS and serious non-AIDS events
Six studies reported AIDS events [6, 8, 10, 50, 55, 58]. The risk ratio for associations between DIR and AIDS events ranged from 0.96 (95% CI 0.53–1.76) to 2.70 (95% CI 1.29–5.66) (Fig 2B). One of these reported AIDS events in a low resource setting (Uganda) with a risk ratio of 1.94 (0.86–4.35). Five studies reported combined AIDS events or mortality [51, 53, 54, 57, 61] and risk ratios ranged from 0.86 (95% CI 0.41–1.80) to 2.06 (95% CI 0.89–4.79) (Fig 2C). One study from a low resource setting (Senegal) reported an incidence rate ratio of 1.21 (0.85–1.72).
Four studies detailed AIDS events. The most commonly reported pathologies were oesophageal candidiasis, tuberculosis, AIDS related cancers, pneumocystis jirovecii and bacterial pneumonia [50, 55, 58, 60]. Only Baker et al included serious non-AIDS events [50], reporting events in eight of 143 patients (5.6%) with DIR compared to 31 of 671 patients (4.6%) without.
Discussion
The main finding of this review was that we found definitions used to categorise DIR varied widely, with 14 different definitions used in the 20 included studies. This greatly limits the ability to draw conclusions about clinical burden in this patient cohort. This paper has synthesised existing data and suggested the definition for DIR to be a rise of less than 50 cells/uL at 6 months following ART initiation in those who have achieved virological suppression but with a CD4 count of less than 350 cells/uL. This provides a starting point for the development of consensus within the field. For the majority of included studies, mortality in patients with DIR was two to three times higher than in those with a satisfactory immune response. To our knowledge, this is the first review to systematically examine the fate of adults with DIR. Two further important gaps in the literature were identified: the large majority of current data reports on cohorts from high income countries; and only one study reported on the burden of serious non-AIDS events. Both the clinical burden of DIR in low income settings and the global burden of serious non-AIDS events remain unclear. It is not possible to draw comparisons between the risks associated with DIR in low resource to high resource settings with the current literature.
Although pooled meta-analysis was inhibited by heterogeneity in length of follow-up, mortality rates remain substantially and significantly elevated in patients with DIR in studies that reported rates adjusted for time. The relationship between DIR and AIDS is less clear and may be complicated by challenges in diagnosing or reporting AIDS conditions. Alternatively, other conditions such as serious non-AIDS events may be contributing to mortality and this warrants further investigation.
The scope of this review was also limited by heterogeneity in DIR definitions. In order to address this, we advocate the use of a standard definition. To define DIR based on a failure to achieve a rise in CD4 from baseline is more reflective of the amount of time spent at a lower CD4 count, which is an important predictor of poor outcomes [14]. In contrast, an absolute CD4 count at a given time point may only tell us about that point in time, when other factors such as co-existing infections may be affecting the CD4 count. The expected rate of CD4 reconstitution following ART initiation is 20 to 30 cells per month in the first 6 months and then 5 to 10 cells per month between 6 months and 24 months [66, 67]. Therefore, when choosing a time point to measure DIR, we believe that 6 months after ART initiation is logical.
Many studies included in this review based their definition of DIR on a failure to achieve a rise of 50 cells/uL at 6 months post ART initiation. Whilst this is a relatively strict CD4 cut off, these studies still reported a high proportion of virologically-suppressed patients with DIR. We would therefore recommend defining DIR as a rise of less than 50 cells/uL at 6 months following ART initiation in patients who have achieved virological suppression. This definition has the benefit of identifying a high risk group of patients early on in the course of their ART management to allow for increased benefit of any potential intervention. It is logical that this definition would only apply to those commencing ART with a CD4 <350 cells/uL so as not to over diagnose DIR in a population starting with higher CD4 counts. The heterogeneity in definitions for DIR and outcome measures means that it is not currently possible to compare the utility of definitions to predict clinical outcomes. We recommend further studies to clinically validate a standardised definition.
This review should be interpreted in the light of several limitations. Firstly, the majority of studies were carried out using data collected from ongoing multicentre cohort studies, meaning cohorts are likely to be highly selected in terms of laboratory monitoring and attending follow-up visits. This limits the generalizability of the studies, and may mean that the risk of adverse clinical outcomes in individuals with DIR could be underestimated. Secondly, the HIV viral load limit defining virological suppression varied across studies. However, it remains unclear whether differences in viral load below 1000 copies/ml are biologically significant [68]. Lastly, individual studies did not distinguish between early mortality in patients starting ART with advanced immunosuppression and long term mortality due to poor immune reconstitution. This could be addressed in future studies.
There are currently no effective therapeutic options to reduce the excess mortality associated with DIR and further research is required. One approach under evaluation is to target underlying drivers of immune activation and inflammation. The addition of raltegravir to standard two class regimes at ART initiation has the aim of decreasing viral set point but as yet only two small studies have shown any effect on immune responses [69, 70]. Similarly a recent trial with valganciclovir to tackle ongoing CMV replication failed to show any improvement in CD4 count [71]. Although probiotics can improve the systemic pro-inflammatory profile, there is no evidence that this can improve CD4 counts [72]. To address generalised inflammation, anti-inflammatory agents such as statins and anti-rheumatic agents have been tested [73, 74]. Whilst statins reduced peripheral immune cell activation, there is no evidence that they can improve CD4 T cell count. Studies investigating the role of quinolones in reducing HIV related immune activation have shown only small decreases in inflammatory markers [75]. Immunomodulatoy agents such as IL-2 have shown limited success [76, 77] and current focus is being placed on IL-7 therapy [78, 79] with several ongoing trials in progress. Lastly, agents aimed at stimulating thymic output have also been tested in early studies [80, 81].
Practical management options may be more accessible in the short term. Standardised guidelines could recommend continuation of prophylactic therapies such as co-trimoxazole and isoniazid for patients with DIR, or could prompt investigation for subclinical opportunistic infections such as tuberculosis and CMV. Although prevention of DIR through early diagnosis of HIV infection and prompt treatment with ART is likely the most effective intervention [82–85], a large proportion of patients worldwide continue to present with advanced HIV infection [86, 87].
This systematic review highlights that a wide range of definitions have been used to characterise clinical outcomes in patients with DIR. These patients are at an increased risk of mortality and are in need of special attention, including integration into HIV clinical trials. We have suggested a definition for DIR based on the limited available data in order to help begin the process of arriving at a consensus definition that could be used to guide clinical care and in future research. We recommend that further studies validate this definition for DIR to aid the development of consensus guidelines.
Supporting Information
(DOC)
Acknowledgments
We would like to thank Vittoria Lutje who performed and supported the literature search for this review.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
CK was supported by a Wellcome Trust Training Fellowship, grant number: 099934/Z/12/A. PM was supported by the Wellcome Trust (grant number: WT089673). PG is supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiebaut R, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS (London, England). 2007;21(9):1185–97. Epub 2007/05/16. 10.1097/QAD.0b013e328133f285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. The New England journal of medicine. 2015. Epub 2015/07/21. 10.1056/NEJMoa1506816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. The New England journal of medicine. 2015. Epub 2015/07/21. 10.1056/NEJMoa1507198 . [DOI] [PubMed] [Google Scholar]
- 4.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, et al. Impact of CD8+ T Cell Activation on CD4+ T Cell Recovery and Mortality in HIV-infected Ugandans Initiating Antiretroviral Therapy. AIDS (London, England). 2011;25(17):2123–31. 10.1097/QAD.0b013e32834c4ac1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pakker NG, Kroon ED, Roos MT, Otto SA, Hall D, Wit FW, et al. Immune restoration does not invariably occur following long-term HIV-1 suppression during antiretroviral therapy. INCAS Study Group. AIDS (London, England). 1999;13(2):203–12. Epub 1999/04/15. . [DOI] [PubMed] [Google Scholar]
- 6.Gilson RJ, Man SL, Copas A, Rider A, Forsyth S, Hill T, et al. Discordant responses on starting highly active antiretroviral therapy: suboptimal CD4 increases despite early viral suppression in the UK Collaborative HIV Cohort (UK CHIC) Study. HIV medicine. 2010;11(2):152–60. Epub 2009/09/08. 10.1111/j.1468-1293.2009.00755.x . [DOI] [PubMed] [Google Scholar]
- 7.Moore DM, Hogg RS, Yip B, Wood E, Tyndall M, Braitstein P, et al. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. Journal of acquired immune deficiency syndromes (1999). 2005;40(3):288–93. Epub 2005/10/27. . [DOI] [PubMed] [Google Scholar]
- 8.Tan R, Westfall AO, Willig JH, Mugavero MJ, Saag MS, Kaslow RA, et al. Clinical outcome of HIV-infected antiretroviral-naive patients with discordant immunologic and virologic responses to highly active antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999). 2008;47(5):553–8. Epub 2008/02/21. 10.1097/QAI.0b013e31816856c5 . [DOI] [PubMed] [Google Scholar]
- 9.Tuboi SH, Pacheco AG, Harrison LH, Stone RA, May M, Brinkhof MW, et al. Mortality associated with discordant responses to antiretroviral therapy in resource-constrained settings. Journal of acquired immune deficiency syndromes (1999). 2010;53(1):70–7. Epub 2009/12/26. 10.1097/QAI.0b013e3181c22d19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoufaly A, an der Heiden M, Kollan C, Bogner JR, Fatkenheuer G, Wasmuth JC, et al. Clinical outcome of HIV-infected patients with discordant virological and immunological response to antiretroviral therapy. J Infect Dis. 2011;203(3):364–71. Epub 2011/01/07. 10.1093/jinfdis/jiq055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.http://www.unaids.org/sites/default/files/documents/20141118_FS_WADreport_en.pdf. UNAIDS Global Statistics Fact Sheet 2014 2014 [cited 2015 23rd March].
- 12.http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [cited 2014 15th December].
- 13.Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, Kirk O, et al. The incidence of AIDS-defining illnesses at a current CD4 count >/ = 200 cells/muL in the post-combination antiretroviral therapy era. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(7):1038–47. Epub 2013/08/08. 10.1093/cid/cit423 . [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS (London, England). 2009;23(3):335–42. Epub 2008/12/31. 10.1097/QAD.0b013e328321823f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandera A, Ferrario G, Saresella M, Marventano I, Soria A, Zanini F, et al. CD4+ T cell depletion, immune activation and increased production of regulatory T cells in the thymus of HIV-infected individuals. PLoS One. 2010;5(5):e10788 Epub 2010/06/04. 10.1371/journal.pone.0010788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vrisekoop N, van Gent R, de Boer AB, Otto SA, Borleffs JC, Steingrover R, et al. Restoration of the CD4 T cell compartment after long-term highly active antiretroviral therapy without phenotypical signs of accelerated immunological aging. J Immunol. 2008;181(2):1573–81. Epub 2008/07/09. . [DOI] [PubMed] [Google Scholar]
- 17.Li T, Wu N, Dai Y, Qiu Z, Han Y, Xie J, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(9):944–51. Epub 2011/10/01. 10.1093/cid/cir552 . [DOI] [PubMed] [Google Scholar]
- 18.Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, Schacker TW, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8(1):e1002437 Epub 2012/01/14. 10.1371/journal.ppat.1002437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz A, Alos L, Leon A, Mozos A, Caballero M, Martinez A, et al. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS (London, England). 2010;24 (13):2029–39. . [DOI] [PubMed] [Google Scholar]
- 20.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078 Epub 2014/05/17. 10.1371/journal.ppat.1004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delobel P, Nugeyre MT, Cazabat M, Sandres-Saune K, Pasquier C, Cuzin L, et al. Naive T-cell depletion related to infection by X4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy. Journal of Virology. 2006;80(20):10229–36. Epub 2006/09/29. 10.1128/jvi.00965-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funderburg NT, Andrade A, Chan ES, Rosenkranz SL, Lu D, Clagett B, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One. 2013;8(12):e83514 Epub 2013/12/25. 10.1371/journal.pone.0083514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, et al. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis. 2014;210(4):619–29. Epub 2014/03/04. 10.1093/infdis/jiu125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi RT, Spritzler J, Chan E, Asmuth DM, Rodriguez B, Merigan TC, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. Journal of acquired immune deficiency syndromes (1999). 2006;42(4):426–34. Epub 2006/07/01. 10.1097/01.qai.0000226789.51992.3f . [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Medicine. 2008;5(10):e203 Epub 2008/10/24. 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapadula G, Chatenoud L, Gori A, Castelli F, Di Giambenedetto S, Fabbiani M, et al. Risk of Severe Non AIDS Events Is Increased among Patients Unable to Increase their CD4+ T-Cell Counts >200+/mul Despite Effective HAART. PLoS One. 2015;10(5):e0124741 Epub 2015/05/29. 10.1371/journal.pone.0124741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Current opinion in HIV and AIDS. 2014;9(1):80–6. Epub 2013/11/28. 10.1097/coh.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallet MA, Rodriguez CA, Yin L, Saporta S, Chinratanapisit S, Hou W, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS (London, England). 2010;24(9):1281–90. Epub 2010/06/19. 10.1097/QAD.0b013e328339e228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavigner M, Delobel P, Cazabat M, Dubois M, L'Faqihi-Olive FE, Raymond S, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4(10):e7658 Epub 2009/10/31. 10.1371/journal.pone.0007658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett L, Stapleton SN, Fudge NJ, Grant MD. Immune resilience in HIV-infected individuals seronegative for cytomegalovirus. AIDS (London, England). 2014;28(14):2045–9. Epub 2014/09/30. 10.1097/qad.0000000000000405 . [DOI] [PubMed] [Google Scholar]
- 31.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203(10):1474–83. Epub 2011/04/20. 10.1093/infdis/jir060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5(5):e10527 Epub 2010/05/19. 10.1371/journal.pone.0010527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermans SM, van Leth F, Kiragga AN, Hoepelman AI, Lange JM, Manabe YC. Unrecognised tuberculosis at antiretroviral therapy initiation is associated with lower CD4+ T cell recovery. Tropical medicine & international health: TM & IH. 2012;17(12):1527–33. Epub 2012/11/08. 10.1111/tmi.12001 . [DOI] [PubMed] [Google Scholar]
- 34.Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS (London, England). 2015;29(1):43–51. Epub 2014/11/12. 10.1097/qad.0000000000000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–90. Epub 2011/01/22. 10.1093/infdis/jiq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189(3):1491–9. Epub 2012/06/30. 10.4049/jimmunol.1200458 . [DOI] [PubMed] [Google Scholar]
- 37.BenMarzouk-Hidalgo OJ, Torres-Cornejo A, Gutierrez-Valencia A, Ruiz-Valderas R, Viciana P, Lopez-Cortes LF. Differential effects of viremia and microbial translocation on immune activation in HIV-infected patients throughout ritonavir-boosted darunavir monotherapy. Medicine (Baltimore). 2015;94(17):e781 Epub 2015/05/02. 10.1097/md.0000000000000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunological reviews. 2013;254(1):343–54. Epub 2013/06/19. 10.1111/imr.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48(3):328–37. Epub 2009/01/07. 10.1086/595851 . [DOI] [PubMed] [Google Scholar]
- 40.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clinical and Developmental Immunology. 2012;2012:670957 Epub 2012/04/05. 10.1155/2012/670957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117(21):5582–90. Epub 2011/03/16. 10.1182/blood-2010-12-322453 . [DOI] [PubMed] [Google Scholar]
- 42.Battegay M, Nuesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6(5):280–7. Epub 2006/04/25. 10.1016/s1473-3099(06)70463-7 . [DOI] [PubMed] [Google Scholar]
- 43.Cenderello G, De Maria A. Discordant responses to cART in HIV-1 patients in the era of high potency antiretroviral drugs: clinical evaluation, classification, management prospects. Expert Rev Anti Infect Ther. 2015:1–12. Epub 2015/10/30. 10.1586/14787210.2016.1106937 . [DOI] [PubMed] [Google Scholar]
- 44.Schechter M, Tuboi SH. Discordant immunological and virological responses to antiretroviral therapy. The Journal of antimicrobial chemotherapy. 2006;58(3):506–10. Epub 2006/07/21. 10.1093/jac/dkl263 . [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097 Epub 2009/07/22. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. WHO; [cited 2015 31st July].
- 47.https://bmg.cochrane.org/sites/bmg.cochrane.org/files/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf. Tool to Assess Risk of Bias in Cohort Studies [cited 2014 12th December].
- 48.Mugyenyi P, Walker AS, Hakim J, Munderi P, Gibb DM, Kityo C, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375(9709):123–31. Epub 2009/12/17. 10.1016/s0140-6736(09)62067-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.http://handbook.cochrane.org/chapter_8/8_8_2_2_studies_assessed_as_at_unclear_risk_of_bias.htm. [cited 2015 5th August].
- 50.Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. Journal of acquired immune deficiency syndromes (1999). 2008;48(5):541–6. Epub 2008/07/23. 10.1097/QAI.0b013e31817bebb3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dronda F, Moreno S, Moreno A, Casado JL, Perez-Elias MJ, Antela A. Long-term outcomes among antiretroviral-naive human immunodeficiency virus-infected patients with small increases in CD4+ cell counts after successful virologic suppression. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002;35(8):1005–9. Epub 2002/10/02. 10.1086/342695 . [DOI] [PubMed] [Google Scholar]
- 52.Engsig FN, Gerstoft J, Kronborg G, Larsen CS, Pedersen G, Roge B, et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect Dis. 2010;10:318 Epub 2010/11/04. 10.1186/1471-2334-10-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falster K, Petoumenos K, Chuah J, Mijch A, Mulhall B, Kelly M, et al. Poor baseline immune function predicts an incomplete immune response to combination antiretroviral treatment despite sustained viral suppression. Journal of acquired immune deficiency syndromes (1999). 2009;50(3):307–13. 10.1097/QAI.0b013e3181945ed4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grabar S, Le Moing V, Goujard C, Leport C, Kazatchkine MD, Costagliola D, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Annals of internal medicine. 2000;133(6):401–10. Epub 2000/09/23. . [DOI] [PubMed] [Google Scholar]
- 55.Gutierrez F, Padilla S, Masia M, Iribarren JA, Moreno S, Viciana P, et al. Patients' characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Current HIV research. 2008;6(2):100–7. Epub 2008/03/14. . [DOI] [PubMed] [Google Scholar]
- 56.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;41(3):361–72. Epub 2005/07/12. 10.1086/431484 . [DOI] [PubMed] [Google Scholar]
- 57.Loutfy MR, Genebat M, Moore D, Raboud J, Chan K, Antoniou T, et al. A CD4+ cell count <200 cells per cubic millimeter at 2 years after initiation of combination antiretroviral therapy is associated with increased mortality in HIV-infected individuals with viral suppression. Journal of acquired immune deficiency syndromes (1999). 2010;55(4):451–9. Epub 2010/11/26. . [DOI] [PubMed] [Google Scholar]
- 58.Nakanjako D, Kiragga A, Ibrahim F, Castelnuovo B, Kamya MR, Easterbrook PJ. Sub-optimal CD4 reconstitution despite viral suppression in an urban cohort on Antiretroviral Therapy (ART) in sub-Saharan Africa: Frequency and clinical significance. AIDS research and therapy. 2008;5:23 10.1186/1742-6405-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pacheco YM, Jarrin I, Del Amo J, Moreno S, Iribarren JA, Viciana P, et al. Risk factors, CD4 long-term evolution and mortality of HIV-infected patients who persistently maintain low CD4 counts, despite virological response to HAART. Current HIV research. 2009;7(6):612–9. Epub 2009/11/26. . [DOI] [PubMed] [Google Scholar]
- 60.Zoufaly A, an der Heiden M, Kollan C, Bogner JR, Fätkenheuer G, Wasmuth JC, et al. Clinical Outcome of HIV-Infected Patients with Discordant Virological and Immunological Response to Antiretroviral Therapy. J Infect Dis. 2011;203(3):364–71. 10.1093/jinfdis/jiq055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicastri E, Chiesi A, Angeletti C, Sarmati L, Palmisano L, Geraci A, et al. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76(2):153–60. Epub 2005/04/19. 10.1002/jmv.20352 . [DOI] [PubMed] [Google Scholar]
- 62.Taiwo BO, Li X, Palella F, Jacobson LP, Margolick JB, Detels R, et al. Higher risk of AIDS or death in patients with lower CD4 cell counts after virally suppressive HAART. HIV medicine. 2009;10(10):657–60. Epub 2009/07/16. 10.1111/j.1468-1293.2009.00739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batista G, Buve A, Ngom Gueye NF, Manga NM, Diop MN, Ndiaye K, et al. Initial suboptimal CD4 reconstitution with antiretroviral therapy despite full viral suppression in a cohort of HIV-infected patients in Senegal. Med Mal Infect. 2015;45(6):199–206. Epub 2015/04/25. 10.1016/j.medmal.2015.03.009 . [DOI] [PubMed] [Google Scholar]
- 64.Takuva S, Maskew M, Brennan AT, Long L, Sanne I, Fox MP. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. Journal of the International AIDS Society. 2014;17:18651 Epub 2014/03/07. 10.7448/IAS.17.1.18651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Long-term mortality in HIV positive individuals virally suppressed for more than three years with incomplete CD4 recovery. Clinical Infectious Diseases. 2014. 10.1093/cid/ciu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith CJ, Sabin CA, Lampe FC, Kinloch-de-Loes S, Gumley H, Carroll A, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS (London, England). 2003;17(7):963–9. Epub 2003/04/18. 10.1097/01.aids.0000060352.78202.79 . [DOI] [PubMed] [Google Scholar]
- 67.Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. Epub 2004/07/06. 10.1016/s0140-6736(04)16589-6 . [DOI] [PubMed] [Google Scholar]
- 68.Castro P, Plana M, Gonzalez R, Lopez A, Vilella A, Nicolas JM, et al. Influence of episodes of intermittent viremia ("blips") on immune responses and viral load rebound in successfully treated HIV-infected patients. AIDS research and human retroviruses. 2013;29(1):68–76. Epub 2012/11/06. 10.1089/aid.2012.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negredo E, Massanella M, Puertas MC, Buzon MJ, Puig J, Perez-Alvarez N, et al. Early but limited effects of raltegravir intensification on CD4 T cell reconstitution in HIV-infected patients with an immunodiscordant response to antiretroviral therapy. The Journal of antimicrobial chemotherapy. 2013;68(10):2358–62. Epub 2013/05/17. 10.1093/jac/dkt183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pallikkuth S, Fischl MA, Pahwa S. Combination antiretroviral therapy with raltegravir leads to rapid immunologic reconstitution in treatment-naive patients with chronic HIV infection. J Infect Dis. 2013;208(10):1613–23. Epub 2013/08/08. 10.1093/infdis/jit387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi TJ, Walmsley S, Szadkowski L, Raboud J, Rajwans N, Shannon B, et al. A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(9):1331–8. Epub 2013/08/16. 10.1093/cid/cit539 . [DOI] [PubMed] [Google Scholar]
- 72.Stiksrud B, Nowak P, Nwosu FC, Kvale D, Thalme A, Sonnerborg A, et al. Reduced Levels of D-dimer and Changes in Gut Microbiota Composition After Probiotic Intervention in HIV-Infected Individuals on Stable ART. Journal of acquired immune deficiency syndromes (1999). 2015;70(4):329–37. Epub 2015/08/11. 10.1097/qai.0000000000000784 . [DOI] [PubMed] [Google Scholar]
- 73.Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203(6):756–64. Epub 2011/02/18. 10.1093/infdis/jiq115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakanjako D, Ssinabulya I, Nabatanzi R, Bayigga L, Kiragga A, Joloba M, et al. Atorvastatin reduces T-cell activation and exhaustion among HIV-infected cART-treated suboptimal immune responders in Uganda: a randomised crossover placebo-controlled trial. Tropical medicine & international health: TM & IH. 2015;20(3):380–90. Epub 2014/12/03. 10.1111/tmi.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savarino A, Shytaj IL. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12:51 Epub 2015/06/19. 10.1186/s12977-015-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanussi S, Simonelli C, Bortolin MT, D'Andrea M, Crepaldi C, Vaccher E, et al. Immunological changes in peripheral blood and in lymphoid tissue after treatment of HIV-infected subjects with highly active anti-retroviral therapy (HAART) or HAART + IL-2. Clin Exp Immunol. 1999;116(3):486–92. Epub 1999/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogler MA, Teppler H, Gelman R, Valentine F, Lederman MM, Pomerantz RJ, et al. Daily low-dose subcutaneous interleukin-2 added to single- or dual-nucleoside therapy in HIV infection does not protect against CD4+ T-cell decline or improve other indices of immune function: results of a randomized controlled clinical trial (ACTG 248). Journal of acquired immune deficiency syndromes (1999). 2004;36(1):576–87. Epub 2004/04/21. . [DOI] [PubMed] [Google Scholar]
- 78.Imamichi H, Degray G, Asmuth DM, Fischl MA, Landay AL, Lederman MM, et al. HIV-1 viruses detected during episodic blips following interleukin-7 administration are similar to the viruses present before and after interleukin-7 therapy. AIDS (London, England). 2011;25(2):159–64. Epub 2010/12/03. 10.1097/QAD.0b013e328340a270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beq S, Delfraissy JF, Theze J. Interleukin-7 (IL-7): immune function, involvement in the pathogenesis of HIV infection and therapeutic potential. Eur Cytokine Netw. 2004;15(4):279–89. Epub 2005/01/04. 15627636. [PubMed] [Google Scholar]
- 80.Plana M, Garcia F, Darwich L, Romeu J, Lopez A, Cabrera C, et al. The reconstitution of the thymus in immunosuppressed individuals restores CD4-specific cellular and humoral immune responses. Immunology. 2011;133(3):318–28. Epub 2011/04/20. 10.1111/j.1365-2567.2011.03442.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacobson JM, Wang H, Bordi R, Zheng L, Gross BH, Landay AL, et al. A randomized controlled trial of palifermin (recombinant human keratinocyte growth factor) for the treatment of inadequate CD4+ T-lymphocyte recovery in patients with HIV-1 infection on antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999). 2014;66(4):399–406. Epub 2014/05/13. 10.1097/qai.0000000000000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12):e1004543 Epub 2014/12/17. 10.1371/journal.ppat.1004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208(8):1202–11. Epub 2013/07/16. 10.1093/infdis/jit311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. The Journal of antimicrobial chemotherapy. 2013;68(5):1169–78. Epub 2013/01/22. 10.1093/jac/dks533 . [DOI] [PubMed] [Google Scholar]
- 85.Okulicz JF, Le TD, Agan BK, Camargo JF, Landrum ML, Wright E, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA internal medicine. 2015;175(1):88–99. Epub 2014/11/25. 10.1001/jamainternmed.2014.4010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Late presentation for HIV care across Europe: update from the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study, 2010 to 2013. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2015;20(47). Epub 2015/12/02. 10.2807/1560-7917.es.2015.20.47.30070 . [DOI] [PubMed] [Google Scholar]
- 87.Dickson N, McAllister S, Sharples K, Paul C. Late presentation of HIV infection among adults in New Zealand: 2005–2010. HIV medicine. 2012;13(3):182–9. Epub 2011/11/19. 10.1111/j.1468-1293.2011.00959.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.


