Abstract
A 59-year-old Caucasian man with a past history of Parkinson's Disease(PD) status post bilateral Subthalamic Nucleus(STN) deep brain stimulation(DBS), who also had treatment-resistant(TR) obsessive-compulsive disorder(OCD), and treatment-resistant depression(TRD), presented for further evaluation and management of his TR OCD. After an unsuccessful attempt to treat his OCD by reprogramming his existing STN DBS, he was offered bilateral ventral capsule/ventral striatum(VC/VS) DBS surgery. In addition to the expected improvement in OCD symptoms, he experienced significant improvement in both PD-related apathy and depression along with resolution of suicidal ideation. Furthermore, the patient's festinating gait dramatically improved. This case demonstrates that DBS of both the STN and VC/VS appears to have an initial signal of safety and tolerability. This is the first instance where both the STN and the VC/VS DBS targets have been implanted in an individual and the first case where a patient with PD has received additional DBS in mood-regulatory circuitry.
Keywords: Deep Brain Stimulation, Parkinson's Disease, Depression, Apathy, Obsessive-Compulsive Disorder
BACKGROUND
In the treatment of PD, the STN DBS target is quite effective in reducing core symptoms of PD, but has been associated with worsening or de novo neuropsychiatric symptoms in PD patients[1]. For TR OCD, DBS of the limbic (ventromedial) portion of the STN as well as the ventral capsule/ventral striatum (VC/VS) have both shown initial promise[1, 2]. VC/VS DBS has also shown some initial promise in exerting anti-depressant effects in idiopathic TRD[3], although a large formal randomized control trail failed to show efficacy [4].
Depression as a consequence of PD appears to be pathophysiologically related to mesocortical/mesolimbic degeneration, which induces a hypodopaminergic state[5]. Along with the neuropathological evidence, PD depression has been shown to robustly respond to dopamine agonist therapy[6]. Parkinsonian apathy is also a hypodopaminergic state and has been shown to respond to levodopa therapy[7]. The festinating gait has been associated with limbic and cognitive dysfunction in Parkinson's disease[8, 9] and gait disorders have been shown to respond to antidepressant interventions[10].
CASE REPORT
A 59 year-old Caucasian male with a history of PD status post bilateral STN DBS, TR OCD, and TRD presented to the psychiatric brain stimulation clinic requesting evaluation for further management of OCD and TRD. He was diagnosed with tremor predominant PD ten years prior. Five years prior, he was evaluated at an academic medical center for DBS for uncontrolled PD tremor while on levodopa (1600mg daily). Three years prior he underwent bilateral STN DBS with significant motor improvement, and eventually was able to stop levodopa.
The onset of the patient's OCD symptoms began when he was ten years old while his depressive symptoms began in his mid twenties. Additionally, he had been hospitalized five times for suicidal ideations (SI), without any history of attempts. His most recent hospitalizations were six years prior and approximately twenty years prior, when he received electroconvulsive therapy (ECT). He noted no noticeable change in his OCD symptoms following STN DBS implantation. His OCD symptoms included ruminations over cleanliness, fear of contamination, and compulsive bathing rituals (hand washing up to 200 times per day). He also manifested prominent neurovegetative symptoms including poor appetite, low energy, and poor sleep. The patient had failed all standard of care anti-OCD medications and behavioral therapy.
Due to the previously reported success of limbic STN DBS treatment for OCD in a patient with PD[11], the team changed his current STN DBS programming to not only address motor, but to also potentially address his OCD symptoms. Therefore, the contact settings were changed from C+, −2 (monopolar) only to C+, −2/-0 (double negative) bilaterally. During these programming sessions, his motor improvements were maintained with keeping −2 contacts activated, while additionally attempting to stimulate the limbic STN with the deep contact (−0). This deep contact has consistently been shown to elicit behavioral change because it is typically in or close to the ventromedial STN (limbic) and has a greater proximity to the medial forebrain bundle. We then attempted interleaving contacts 0/2 with the same contact configuration and stimulation parameters. This change resulted in only minimal effects on his Yale Brown Obsessive Compulsive Scale (YBOCS), but worsened his mood, leading to new onset suicidal ideation. Therefore, his settings were restored to their previous values, and the team decided to switch strategies.
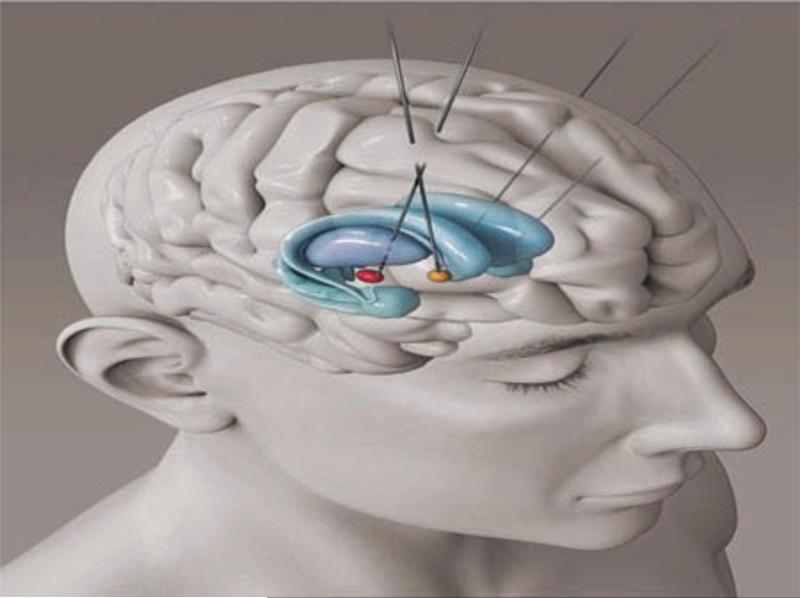
As a result of the recent success of the VC/VS DBS target in managing treatment-resistant OCD[1], the patient elected to receive (through the Food and Drug Administration humanitarian device exemption) VC/VS DBS implantation primarily to treat his TR OCD. For this procedure, Medtronic version 3391 leads were implanted bilaterally in the VC/VS. Affect changes were noticeable starting at 3V; the final setting was 6V, both contact 0 & 1 (−), case (+) 210us, 130Hz bilaterally (Figure 1). No adverse effects occurred during implantation or programming.
Figure 1.
Dual implantations in the STN and VCVS. [To view this figure in color, please see the online version of this Journal.]
Several clinical scales were utilized pre- and post-procedure to assess severity and degree of change in the subject's Major Depressive Disorder (MDD) and OCD symptoms (Table 1).
Table 1.
MDD and OCD Symptom Severity: Clinical Scores over Time
| Time | Baseline | 2 Months | 5 Months | 7 Months | 9 Months |
|---|---|---|---|---|---|
| SSI | 5 | 0 | 0 | 12 | 0 |
| HAMD17 | 30 | 10 | 7 | 29 | 6 |
| AS | 22 | 7 | 9.5 | 37 | 13 |
| Y-BOCS | 32 | 12 | 11 | 32 | 10 |
SSI, Suicide Severity Index; HAMD17, 17-Item Hamilton Rating Scale for Depression; AS, Apathy Scale; Y-BOCS, Yale-Brown Obsessive Compulsive Scale
The Hamilton Depression Rating Scale -17 Item (HAMD-17) was reduced from categorically severe to remission. His YBOCS score dropped from the extreme range to mild. The Apathy Scale (AS) score also decreased to mild. Finally, the Scale for Suicidal Ideation (SSI) indicated complete resolution of his suicidal ideation.
An additional and unpredicted benefit of the VC/VS DBS implantation was a marked improvement in the patient's gait. His festinating gait largely disappeared per his wife. At 7 months post-operation, his DBS battery required replacement and his festinating gait returned and was so much worse that he even required a walker. He was implanted with bilateral Medtronic Activa RC (model 37612) pulse generators and all therapeutic benefit returned (OCD, mood, apathy, and gait). After two weeks of stimulation post battery replacement, he no longer required the walker and returned to his post VC/VS DBS baseline.
DISCUSSION
This case is the first report of VC/VS DBS implantation in a patient with Parkinson's disease. Additionally, this procedure demonstrates that DBS implantation in the VC/VS can be safe alongside bilateral STN DBS.
Depression
Although the VC/VS DBS target has been shown to exert anti-depressant effects in idiopathic TRD [1, 3], a large formal randomized control trial failed to show efficacy. It is worth noting, however, that this trial specifically excluded all patients with comorbid psychiatric or neurodegenerative conditions [4]. In contrast, the results of the current study alongside the other literature on the subject may suggest a potential interaction between comorbid psychiatric or neurodegenerative conditions and TRD that allow VC/VS DBS to have a greater effect in these patients than in the population with only TRD. No DBS target has been shown to significantly improve depression comorbid with PD prior to this report[1, 12]. These findings are particularly notable when considering that depression related to PD is rated as the most disabling symptom[12]. In our patient, TRD pre-dated the development of PD; however, there is no pathophysiologic reason to expect a different response in those whose TRD develops secondary to PD. This is an area that warrants further study. Presently, PD-related depression poses a significant hurdle for clinicians as STN DBS can be associated with stimulation-associated depression and suicidality[1]. Furthermore, DBS of the ventral striatum has been shown to increase ventral striatal dopamine release[13]. Given that reduced dopamine release is speculated to be the pathophysiological cause for PD depression[5], VC/VS DBS may be an intervention for enhancing dopamine transmission in the ventral striatum and mood regulatory circuits of PD patients. The VC/VS DBS target could represent a new and promising option for the management of TRD comorbid or resulting from PD.
OCD
The patient benefitted from VC/VS DBS in a manner that was consistent with the current literature [1, 2]. From the current evidence, this finding most likely directly relates to the patient's lifelong idiopathic OCD. With that said, there is equal reason to expect that this treatment would be beneficial in patients with OCD or Impulse Control Disorders that occurred secondary to PD. This is a valid area for further study.
The implantation of bilateral STN DBS did not provide any noticeable relief or change in OCD symptoms. While this is in clear contrast to the findings of Fontaine et al.(2004), who successfully treated a patient's PD and OCD symptoms simultaneously using bilateral STN DBS[11], the disparity in outcomes is likely due to differences in the relative position of DBS electrodes. While Fontaine et al.(2004) targeted the limbic STN, our patient's electrodes were placed in the sensorimotor (dorsolateral) STN[12]. This is currently the only other study to our knowledge that utilized the STN placement for OCD. Additionally, the DBS stimulator was not set to the deepest contact during initial motor programming. When the deepest contact was activated, the patient experienced a worsening in mood. Short-term changes in mood have been documented in other cases of STN DBS[14], and more research into the mechanism behind this phenomenon is recommended.
Apathy
A novel finding of this report is that DBS of the VC/VS appears to have positive effects on PD-related apathy. Similar to PD-related depression, apathy is a frequent and significant comorbidity of PD and can be exacerbated by STN DBS[15]. These changes have been associated with ventral striatal (specifically nucleus accumbens-NAc) volume, where in severe parkinsonism NAc volume decreases[16] and atrophy of this region is associated with increased apathy[17]. Disease-related atrophy occurs independent of age, possibly multi-factorial with neuronal loss, neuronal shrinkage, and a reduction in synaptic connections[16]. As previously mentioned, VC/VS DBS has been shown to increase striatal dopamine activity[13] and it may be that the positive effect of VC/VS DBS on PD-related apathy is related to restoration of NAc function to normal levels.
Gait
Prior to treatment, the patient exhibited a festinating gait commonly seen in patients with advanced Parkinson's; however, with DBS of the VC/VS his festinating gait resolved, only returning when his battery needed replacement. In a study of nigrostriatal and mesocortical changes in dopamine during movement, significant differences in striatal dopamine activity were found between PD patients and healthy patients[8]. Furthermore, a connection between PD gait and affective state has been established[18]. It should be noted that this study did not utilize an objective measurement of the patient's gait at his pre-operative baseline, as the observed postop improvement was entirely unexpected. This effect was only identified via subjective observation so only limited conclusions should be drawn and more study of this effect is certainly needed. Further exploration of the therapeutic effects of neurostimulation on mood circuits for improvement of gait, as well as the mechanism underlying this effect, is certainly warranted particularly as it relates to VC/VS DBS.
Conclusion
In conclusion, this case presents many new and significant findings in the effects and possible applications of VC/VS DBS to the Parkinson's population. This case represents the first time VC/VS DBS has been used alongside STN DBS, and that it is feasible to perform simultaneous stimulation of both targets in individuals with Parkinson's disease. Importantly, the findings of this case illustrate that this approach is safe in PD patients, and may conceivably be extended to the population of patients with PD-related depression. This is feasible given our success treating a patient with comorbid idiopathic TRD and PD. As expected, the implantation of VC/VS DBS provided significant relief to the patient's OCD behaviors, and lead to improvements in comorbid apathy, depression as well as festinating gait.
Acknowledgments
We would like to express deepest thanks to MUSC medical illustrator Emma Vought for her valuable assistance in providing a figure to illustrate our DBS placements in the STN and VC/VS.
Funding NRW and TRH are both funded through NIDA grant R25 DA020537
Dr. Revuelta reports grants from NIH/NINDS, grants from NIH/NCATS/SCTR, grants and personal fees from CHELSEA, personal fees from LUNDBECK, grants from NIH/NCCAM, grants from BARMORE FUND, grants from BIOTIE, grants from ICON, personal fees from TEVA, grants and personal fees from UCB, outside the submitted work.
Footnotes
Author Contributions Nolan Williams served as both the primary point of contact for the patient and as the research coordinator for this case study. Collaboration between Nolan Williams, Baron Short, and Gonzalo Revuelta lead to the conception and successful implementation of the interventional procedure. Throughout the project, the aforementioned authors worked in conjunction with Thomas Hopkins, Jonathan Snipes, Greg Sahlem Mark George, and Istvan Takacs to review and critique both the interventional procedure as well as the findings of this case study. Thomas Hopkins also assisted Nolan Williams in preparing the final manuscript.
Competing Interests All other authors have no competing interests to disclose.
Ethics Approval This is a single case report and is thus not a study. No IRB approval was needed, or granted. The patient signed all clinical informed consent documents regarding his surgeries.
Provenance and peer review Externally reviewed.
Patient Consent Obtained.
REFERENCES
- 1.Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013;123(11):4546–56. doi: 10.1172/JCI68341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg BD, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bewernick BH, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Dougherty DD, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biological Psychiatry. (0) doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Vriend C, et al. Depression and impulse control disorders in Parkinson's disease: two sides of the same coin? Neurosci Biobehav Rev. 2014;38:60–71. doi: 10.1016/j.neubiorev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Lemke MR, et al. Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in Parkinson's disease. J Neurol Sci. 2006;248(1-2):266–70. doi: 10.1016/j.jns.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Czernecki V, et al. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia. 2002;40(13):2257–67. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi Y, Kanno T, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S, et al. Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson's disease. Brain. 2001;124(4):784–792. doi: 10.1093/brain/124.4.784. [DOI] [PubMed] [Google Scholar]
- 9.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318(14):876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 10.Morgante F, Fasano A. Improvement with duloxetine in primary progressive freezing gait. Neurology. 2010;75(23):2130–2. doi: 10.1212/WNL.0b013e318200d7a3. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine D, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. J Neurosurg. 2004;100(6):1084–6. doi: 10.3171/jns.2004.100.6.1084. [DOI] [PubMed] [Google Scholar]
- 12.Williams NR, Foote KD, Okun MS. STN vs. GPi Deep Brain Stimulation: Translating the Rematch into Clinical Practice. Mov Disord Clin Pract (Hoboken) 2014;1(1):24–35. doi: 10.1002/mdc3.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figee M, et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry. 2014;75(8):647–52. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Mallet L, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121–34. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch-Darrow L, et al. The trajectory of apathy after deep brain stimulation: from pre-surgery to 6 months post-surgery in Parkinson's disease. Parkinsonism Relat Disord. 2011;17(3):182–8. doi: 10.1016/j.parkreldis.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavridis I, Boviatsis E, Anagnostopoulou S. The human nucleus accumbens suffers parkinsonism-related shrinkage: a novel finding. Surg Radiol Anat. 2011;33(7):595–9. doi: 10.1007/s00276-011-0802-1. [DOI] [PubMed] [Google Scholar]
- 17.Carriere N, et al. Apathy in Parkinson's disease is associated with nucleus accumbens atrophy: A magnetic resonance imaging shape analysis. Mov Disord. 2014 doi: 10.1002/mds.25904. [DOI] [PubMed] [Google Scholar]
- 18.Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J Neurol Sci. 2006;248(1-2):173–6. doi: 10.1016/j.jns.2006.05.015. [DOI] [PubMed] [Google Scholar]