Abstract
Large conductance Ca2+- and voltage-activated K+ (BK) channels are widely distributed in the postnatal central nervous system (CNS). BK channels play a pleiotropic role in regulating the activity of brain and spinal cord neural circuits by providing a negative feedback mechanism for local increases in intracellular Ca2+ concentrations. In neurons, they regulate the timing and duration of K+ influx such that they can either increase or decrease firing depending on the cellular context, and they can suppress neurotransmitter release from presynaptic terminals. In addition, BK channels located in astrocytes and arterial myocytes modulate cerebral blood flow. Not surprisingly, both loss and gain of BK channel function have been associated with CNS disorders such as epilepsy, ataxia, mental retardation, and chronic pain. On the other hand, the neuroprotective role played by BK channels in a number of pathological situations could potentially be leveraged to correct neurological dysfunction.
1. OVERVIEW
Large conductance Ca2+-, and voltage-activated K+ channels (also known as BK [for Big Potassium], Maxi-K, or Slo1) are expressed in various tissues. As their name indicates, opening of BK channels leads to a massive efflux of K+ ions that hyperpolarizes cellular membrane potential. This property, combined with their ability to act as a coincidence detector for concomitant elevation in intracellular Ca2+ concentration ([Ca2+]i) and membrane depolarization, puts BK channels in a unique position to control the activity of excitable cells via negative feedback regulation of Ca2+ influx through voltage-activated Ca2+ (CaV) channels. In the central nervous system (CNS), BK channels located in the plasma membrane of neurons influence the shape, frequency, and propagation of action potentials (APs), as well as neurotransmitter release from presynaptic terminals. BK channels located in the nuclear envelope of neurons can also directly influence gene transcription and neuronal morphology (a topic addressed in Chapter “Functional Role of Mitochondrial and Nuclear BK Channels” by Gao and Li of the present volume). Furthermore, BK channels expressed in non-neuronal cell populations, such as astrocytes or vascular smooth muscle cells, can regulate cerebral blood flow, thereby influencing brain activity. In the present chapter, we review the expression patterns of BK channel subunits in the CNS, the mechanisms by which BK channels shape neuronal excitability, neurotransmission, and neurovascular coupling, as well as the neurological functions and pathologies in which BK channels are implicated. Aspects relevant to BK channels expressed in the pituitary gland, inner ear, and cerebral blood vessels are addressed in Chapters “BK Channels in the Pituitary and Hypothalamus” by Duncan and Shipston, “BK Channels in the Inner Ear” by Pyott and Duncan, and “BK Channels in the Vascular System” by Krishnamoorthy-Natarajan and Koide, respectively.
2. EXPRESSION PATTERNS OF BK CHANNEL SUBUNITS IN THE CNS
2.1 Pore-Forming Subunit (α)
2.1.1 Localization
In the mature mammalian CNS, the pore-forming α subunit of BK channels (encoded by the KCNMA1 gene in humans) displays a widespread distribution, as assessed by Northern blot, in situ hybridization, autoradiography, or immunohistochemistry. Prominent levels of expression are observed in the olfactory bulb, cortex, basal ganglia, hippocampus, thalamus, habenulo-interpeduncular tract, cerebellum, vestibular nuclei, and spinal cord (Chang, Dworetzky, Wang, & Goldstein, 1997; Grunnet & Kaufmann, 2004; Hu et al., 2001; Knaus et al., 1996; Misonou et al., 2006; Sailer et al., 2006; Sausbier et al., 2006; Tseng-Crank et al., 1994; Wanner et al., 1999). Although BK α immunoreactivity is macroscopically enriched in fiber tracts and terminal fields, it is also present in the somatodendritic compartment of neurons (Knaus et al., 1996; Misonou et al., 2006; Sausbier et al., 2006).
The distribution of BK α subunits at the ultrastructural level varies between neuronal types, as revealed by electron microscopy. In hippocampal pyramidal cells, BK α immunoreactivity is present in the presynaptic membrane facing the synaptic cleft, as well as in the head of dendritic spines, in close proximity to the postsynaptic specialization of glutamatergic synapses (Hu et al., 2001; Sailer et al., 2006). In contrast, in cerebellar Purkinje cells, BK α subunits are found in the soma and at extrasynaptic sites in dendritic shafts and spines, but they are absent from the vicinity of postsynaptic densities (Kaufmann et al., 2009).
Interestingly, Purkinje cell BK α subunits are segregated into two distinct pools: some are clustered in patches of plasma membrane overlying subsurface cisterns (see below) while others are scattered; the clustered pool is restricted to the soma and proximal dendrites while the scattered pool is present throughout the somatodendritic compartment (Kaufmann et al., 2009). This nonhomogenous distribution of somatic BK α subunits is also observed in other populations of projections neurons, both inhibitory (central amygdala medium spiny neurons) and excitatory (layer V pyramidal neurons of the somatosensory cortex, hippocampal CA3 pyramidal neurons, dentate gyrus granule cells, and basolateral amygdala principal neurons), albeit at lower densities than in Purkinje cells (Kaufmann, Kasugai, Ferraguti, & Storm, 2010). In dentate gyrus granule cells, approximately twice as many BK α subunits belong to the scattered pool as to the clustered pool. It is currently unknown whether the scattered and clustered pools of BK α subunits are independent from each other, whether they are molecularly distinct, or whether individual BK channels can be exchanged between each pool, as was previously described for neurotransmitter receptors that are rapidly trafficked between synaptic and extrasynaptic plasma membrane domains (Triller & Choquet, 2005).
Subsurface cisterns are present underneath BK α clusters in both Purkinje cells and dentate gyrus granule cells (Kaufmann et al., 2009, 2010). These cisterns are apical extensions of smooth (ie, ribosome free) endoplasmic reticulum closely apposed against the plasma membrane that may serve as a source of local Ca2+ sparks for coordinated opening of clustered BK channels (Petersen, Tepikin, & Park, 2001). BK channels located in these domains may become activated during AP-stimulated release of Ca2+ from internal stores and thereby exert negative feedback on Ca2+ entry through CaV channels, in a manner reminiscent of their role in smooth muscle cells in response to Ca2+ sparks elicited from sarcoplasmic reticulum stores (Brenner, Perez, et al., 2000; Burdyga & Wray, 2005; Ledoux, Werner, Brayden, & Nelson, 2006; see Chapter “BK Channels in the Vascular System” by Krishnamoorthy-Natarajan and Koide).
2.1.2 Ontogeny
Some insight into the role BK channels play in the CNS can be gained by considering when they are expressed. BK α expression is upregulated during late embryonic and early postnatal development throughout the CNS (Hafidi, Beurg, & Dulon, 2005; Higgins et al., 2010; MacDonald, Ruth, Knaus, & Shipston, 2006). Consistent with this observation, BK currents undergo an abrupt increase during the first 2 weeks after birth in neocortical pyramidal neurons, substantia nigra dopamine neurons, and cochlear inner hair cells (Kang, Huguenard, & Prince, 1996; Kros, Ruppersberg, & Rusch, 1998; Langer, Grunder, & Rusch, 2003; Marcotti, Johnson, Holley, & Kros, 2003; Ramirez-Latorre, 2012). Interestingly, the emergence of BK currents in these cells coincides with their functional maturation, as characterized by increased excitability in the neocortex, high-frequency firing in response to salient stimuli in the substantia nigra, and auditory signal transduction in the cochlea. This developmental pattern suggests that BK channels shape the properties of mature neuronal firing and contribute to experience-dependent plasticity. Interestingly, BK currents are transiently reduced in medial prefrontal cortex pyramidal neurons during adolescence, which may participate in the strong plasticity of prefrontal circuits during this phase of development (Benhassine & Berger, 2009; Ksiazek, Ladno, Szulczyk, Grzelka, & Szulczyk, 2013; Selemon, 2013).
BK α precursor mRNA undergoes extensive alternative splicing and the resulting isoforms exhibit different biophysical and trafficking properties (see Chapter “Posttranscriptional and Posttranslational Regulation of BK Channels” by Shipston and Tian). In particular, BK α isoforms containing the stress-regulated exon (STREX) diversify BK channel function during development. These isoforms possess a C-terminal insert that speeds up activation, slows down deactivation, and increases open probabilities at negative-voltage potentials (Xie & McCobb, 1998). STREX-containing isoforms predominate in the embryonic mouse CNS but are drastically repressed after birth, so that the vast majority of BK α transcripts lack STREX in adulthood (Higgins et al., 2010; MacDonald et al., 2006). Accordingly, fast-gating BK currents consistent with STREX inclusion disappear during the first postnatal week, as observed in neocortical pyramidal neurons (Kang et al., 1996). STREX-containing isoforms remain detectable in a limited number of adult neurons, including dentate gyrus granule cells, CA3 pyramidal neurons, and cerebellar Purkinje cells (Petrik & Brenner, 2007).
The regulation of STREX splicing during development has been proposed as a mechanism whereby BK channels facilitate the generation of spontaneous activity necessary for neural circuit formation during embryogenesis, and then later protect against hyperexcitability in the postnatal CNS (MacDonald et al., 2006). Exclusion of the STREX exon can be triggered by cellular depolarization via a mechanism involving a Ca2+/calmodulin-dependent protein kinase IV-responsive RNA element within the STREX 3′ splice site (Xie & Black, 2001; Xie, Jan, Stoilov, Park, & Black, 2005). Whether this mechanism drives the switch in STREX splicing following birth remains to be determined. Hippocampal neurons also contain a partially processed intron-containing BK α mRNA that promotes the distribution of BK channels to dendritic spines and alters membrane excitability (Bell et al., 2008).
2.2 Auxiliary Subunits (β and γ)
2.2.1 Localization
The transcripts encoding BK channel auxiliary subunits are heterogeneously represented in the CNS. BK β4 (encoded by the KCNMB4 gene) is commonly referred as the “neuronal auxiliary subunit” since it is the most abundant of the four β subunits in the brain and spinal cord, and is expressed at low levels in other tissues (Behrens et al., 2000; Brenner, Jegla, Wickenden, Liu, & Aldrich, 2000; Weiger et al., 2000). The distribution of BK β4 in the brain mostly overlaps with that of BK α, with prominent expression in the olfactory bulb mitral and granule layers, neocortical pyramidal cells, paraventricular nucleus of the hypothalamus, supraoptic nucleus, CA3 pyramidal layer and dentate gyrus granule cell layer, cerebellar Purkinje layer, and facial and trigeminal motor nuclei (Brenner et al., 2005; http://mouse.brain-map.org, Lein et al., 2007; Petrik & Brenner, 2007; Shruti et al., 2012; Weiger et al., 2000).
BK β2 (KCNMB2) is also expressed throughout the brain, although at lower levels than BK β4 (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Weiger et al., 2000). In the mouse forebrain, BK β4 and β2 transcripts are enriched almost fourfold in neurons compared to both oligodendrocytes and astrocytes (Cahoy et al., 2008).
BK β1 (KCNMB1) is the canonical “smooth muscle auxiliary subunit.” Accordingly, it is highly abundant in cerebral artery myocytes but is otherwise undetectable in the brain, with the exception of several hypothalamic subregions, cerebellar Purkinje cells, and several brainstem nuclei (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Chang et al., 1997; Jiang, Wallner, Meera, & Toro, 1999; Salzmann et al., 2010; Tseng-Crank et al., 1994; Weiger et al., 2000). BK β1 is prominently expressed in ependymal cells lining the ventricles and is particularly enriched in tanycytes of the median eminence (Salzmann et al., 2010). Tanycytes are a subtype of ependymal cells with long processes that extend into the hypothalamic parenchyma, thereby coming in close contact with neurons and reaching the fenestrated capillary network, while their tight junctions prevent the diffusion of blood-borne molecules into the adjacent cerebrospinal fluid (Bolborea & Dale, 2013; Langlet, Mullier, Bouret, Prevot, & Dehouck, 2013). It remains to be determined whether functional BK channels are present in tanycytes, and, if so, what role they might play in the physiology of these cells. It is also possible that BK β1 subunits expressed in tanycytes associate with the pore-forming subunit of another (non-BK) ion channel. Such nonconventional functional interactions between ion channel subunits was previously described between the β1 subunit of L-type CaV channels and BK α, between BK β4 and the pore-forming subunit of pH-regulated Slo3 K+ channels, as well as between BK β4 and the Nav1.6 pore-forming subunit of voltage-gated Na+ channels (Seidel et al., 2011; Yang, Zeng, Xia, & Lingle, 2009; Zou, Jha, Kim, & Dryer, 2008b).
BK β3 (KCNMB3) shows particularly weak expression in the CNS (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Poulsen et al., 2009; Uebele et al., 2000).
Another family of BK auxiliary subunits, named γ1–γ4 subunits, was recently identified. Association of these leucine-rich repeat-containing proteins (encoded by LRRC26, LRRC52, LRRC55, and LRRC38, respectively) with BK α tetramers shifts the voltage dependence of BK channel activation toward hyperpolarizing voltages (see Chapter “Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits” by Yan and Li). Comparative analysis of the distribution of BK γ subunits indicated that γ3 is selectively enriched in the brain, while γ1 and γ4 are expressed at lower levels, and γ2 is essentially absent from this tissue (Yan & Aldrich, 2012). BK γ1 subunits located in cerebral arterial myocytes elevate the voltage and Ca2+ sensitivity of BK channels, which contributes to lowering myogenic tone and promoting vasodilation (Evanson, Bannister, Leo, & Jaggar, 2014). The role of γ3 in modulating neuronal physiology still remains to be determined.
Unlike the BK α subunit for which a monoclonal antibody has been thoroughly validated in knockout tissue (Misonou et al., 2006), the specificity of commercially available antibodies raised against BK auxiliary subunits has been questionable (see Bhattarai et al., 2014 for anti-β1 antibodies; Martinez-Espinosa, Yang, Gonzalez-Perez, Xia, & Lingle, 2014 for anti-β2 antibodies; comment in Wang, Jaffe, & Brenner, 2014 for anti-β4 antibodies; and our personal experience with a number of commercial anti-β1 and anti-β4 antibodies). Immunohistochemical characterization of these subunits should therefore be interpreted with caution when used as the sole analytical method (eg, Piwonska, Wilczek, Szewczyk, & Wilczynski, 2008 reporting BK β4 immunoreactivity in neuronal mitochondria and BK β2 immunoreactivity in astrocytes; and Seidel et al., 2011 reporting BK β1 and β4 immunoreactivity in astrocytes).
2.2.2 Functional Signatures
Auxiliary subunits alter BK channel activation and inactivation, subcellular trafficking, and sensitivity to channel activators or blockers (see Chapter “Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits” by Yan and Li). These features have been exploited to identify the subunits that contribute to BK currents in different neuronal populations.
Early studies conducted in brain synaptosomal membranes identified a subtype of BK channels (called “type II”) that shows slow gating kinetics and resistance to the BK channel blocker iberiotoxin (Reinhart, Chung, & Levitan, 1989). These properties were later found to be conferred by the association of auxiliary β4 subunits (see Wang et al., 2014 for review). In the presence of β4, peak currents are not observed until >25 ms after depolarization, which is in contrast to currents from BK α (or BK α with other β subunits) that reach steady state in 2–10 ms, and tail currents are significantly prolonged (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Ha, Heo, & Park, 2004). This is particularly relevant for neurons, where the participation of β4-containing BK channels may broaden APs and increase the afterhyperpolarization (AHP) (Brenner et al., 2005). In addition, β4-containing BK channels exhibit unique pharmacological properties, as they are resistant to blockade by charybdotoxin and iberiotoxin (Meera, Wallner, & Toro, 2000).
BK channels showing the characteristic properties of β4-containing channels have been observed in a variety of neuronal populations, including neurohypophysial terminals, dentate gyrus granule cells, cerebellar Purkinje cells, and trigeminal caudal nuclei (TCN) terminals (Benton, Lewis, Bant, & Raman, 2013; Bielefeldt, Rotter, & Jackson, 1992; Brenner et al., 2005; Deng et al., 2013; Dopico, Widmer, Wang, Lemos, & Treistman, 1999; Samengo, Curro, Barrese, Taglialatela, & Martire, 2014; Shruti et al., 2012). It is somewhat puzzling that a large fraction of CNS BK channels is sensitive to iberiotoxin despite the prominent expression of β4 in this tissue. One possible explanation advanced by Wang and colleagues relates to the stoichiometry of β4 subunits assembling with α tetramers, with a full complement of β4 subunits (ie, 1:1 stoichiometry) being possibly required to confer iberiotoxin resistance (Wang et al., 2014). Alternatively, it is possible that β4 subunits control BK channel function through their ability to restrict plasma membrane localization of BK α tetramers (see Section 2.3); in this scenario, BK β4 may be highly expressed but retained within the endoplasmic reticulum and not present in plasma membrane BK channels (Shruti et al., 2012). Martentoxin and conopeptide Vt3.1 were reported to preferentially inhibit β4-containing BK channels and could help provide novel insights into this question, but, as for iberiotoxin, the ratio of β4 subunits required to drive increased sensitivity to these inhibitors remains to be determined (Li, Chang, et al., 2014; Shi et al., 2008).
Another subtype of BK channels, which was first reported in chromaffin cells of the adrenal gland, exhibits rapid inactivation (Solaro & Lingle, 1992). This fast inactivation was later shown to be conferred by the N-terminal residues of BK β2 subunit (Wallner, Meera, & Toro, 1999; Xia, Ding, & Lingle, 1999). Using this unique inactivating property, β2-containing BK channels have been identified in hippocampus, neocortex, and lateral amygdala pyramidal neurons, as well as in cerebellar Purkinje cells and dorsal root ganglion (DRG) neurons (Faber & Sah, 2003; Haghdoost-Yazdi, Janahmadi, & Behzadi, 2008; Hicks & Marrion, 1998; Li et al., 2007; McLarnon, 1995; Sun, Gu, & Haddad, 2003). There is, to the best of our knowledge, no ligand that selectively discriminates β2-containing BK channels.
Alcohol can increase BK currents in neurons, but the association of β1 renders BK channels insensitive to alcohol-induced potentiation (Feinberg-Zadek & Treistman, 2007; Martin et al., 2004; see Chapter “Modulation of BK Channels by Ethanol” by Dopico). This pharmacological signature has been used to identify β1-containing BK channels in the soma and dendrites of nucleus accumbens medium spiny neurons and magnocellular neurons of the supraoptic nucleus (Dopico et al., 1999; Martin et al., 2004; Wynne, Puig, Martin, & Treistman, 2009).
Finally, the association of γ1 confers resistance to mallotoxin (Almassy & Begenisich, 2012), but this feature has not been exploited yet to identify γ1-containing BK channels in the CNS.
2.3 Subcellular Trafficking
A number of factors regulate the subcellular trafficking of BK channels, including pre-mRNA splicing, posttranslational modifications, and interaction with BK auxiliary subunits and other proteins (reviewed in Chapters “Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits” by Yan and Li, “Posttranscriptional and Posttranslational Regulation of BK Channels” by Shipston and Tian, and “Protein Network Interacting with BK Channels” by Kim and Oh). Examples of parameters promoting the cell surface localization of BK α subunits include incorporation of the alternatively spliced C-terminus ending with the “QEERL” amino acid sequence, palmitoylation of cysteine residues within the S0–S1 linker or the alternatively spliced STREX insert, and interaction with actin (Jeffries et al., 2010; Kim, Ridgway, Zou, Chiu, & Dryer, 2007; Ma et al., 2007; Tian et al., 2008; Zou, Jha, Kim, & Dryer, 2008a). Conversely, inclusion of the SV1 insert or VEDEC C-terminus, myristoylation, ubiquitination, phosphorylation by cyclin-dependent kinase 5 (CDK5), adenylyl cyclase activation, and association with caveolin-1 or cereblon promote BK α endocytosis or retention in the endoplasmic reticulum, thereby reducing surface expression of functional BK channels (Alioua, Li, Wu, Stefani, & Toro, 2011; Alioua et al., 2008; Bai, Surguchev, Joshi, Gross, & Navaratnam, 2012; Jo, Lee, Song, Jung, & Park, 2005; Kim, Ridgway, et al., 2007; Liu et al., 2014; Ma et al., 2007; Pratt, He, Wang, Barth, & Bruchez, 2015; Tian et al., 2008; Zarei et al., 2004).
A large number of proteins physically interacting with BK α subunits are known to play a role in trafficking/scaffolding and therefore likely dictate the subcellular localization of BK channels in neurons (Gorini et al., 2010; Kathiresan, Harvey, Orchard, Sakai, & Sokolowski, 2009; Kim, Jo, Song, Park, & Park, 2007; Singh et al., 2016; Sokolowski, Orchard, Harvey, Sridhar, & Sakai, 2011; Williams et al., 2004). To add another layer of complexity, there is an intricate cross talk between all parameters known to influence the subcellular trafficking of BK channel subunits (see Chapter “Posttranscriptional and Posttranslational Regulation of BK Channels” by Shipston and Tian). For instance, the influence of BK β1 and β4 subunits depends on the C-terminal variant of BK α: both β1 and β4 enhance the cell surface expression of the VEDEC isoform, but β1 targets the QEERL isoform to endosomes, while β4 promotes the retention of the EMVYR isoform in the endoplasmic reticulum (Chen et al., 2013; Kim, Zou, Ridgway, & Dryer, 2007; Shruti et al., 2012; Toro et al., 2006).
Importantly, most studies examining the regulation of BK channel trafficking have been conducted in transfected cells. Findings obtained in these heterologous expression systems may not necessarily reflect what happens in the CNS, where the expression and posttranslational modifications of BK α isoforms and BK auxiliary subunits are cell type-dependent and dynamically regulated. We therefore highlight the few studies that have examined endogenous mechanisms of BK channel subcellular trafficking in neurons.
In mouse hippocampus CA3 pyramidal cells, despite prominent expression of the β4 subunit, β4-containing BK channels may not participate in whole-cell BK currents, as suggested by an equivalent effect of paxilline and iberiotoxin (Shruti et al., 2012). However, genetic deletion of β4 strongly increases whole-cell BK currents, which suggests, in accordance with observations made in transfected cells, that β4 represses the forward trafficking of BK α in these neurons (Shruti et al., 2012).
Notably, some studies have identified proteins implicated in the addressing of BK α to specific neuronal compartments. In Caenorhabditis elegans cholinergic motor neurons, the localization of BK channels to pre-synaptic terminals is dependent upon α-catulin and dystrobrevin, two proteins that interact with cortical cytoskeletal proteins (Oh et al., 2015). In mouse and rat myelinated cerebellar Purkinje cell axons, the cell adhesion molecule Caspr promotes the localization of BK channels to paranodal junctions, where they support axonal AP conduction during high-frequency firing and ensure synaptic output in the deep cerebellar nuclei (Hirono et al., 2015).
Finally, a recent study provided evidence for activation-induced endocytosis of neuronal BK channels. In primary cultures of hippocampal neurons, alcohol acutely potentiates macroscopic BK currents, but prolonged (3–6 h) exposure to alcohol decreases cell surface expression of functional BK channels without altering total levels of BK α expression, indicating their trafficking to intracellular compartments (Palacio et al., 2015).
3. ROLE OF BK CHANNELS IN CNS CELLULAR PHYSIOLOGY
3.1 Neurons
The synergistic effect of depolarization and Ca2+ binding on BK channel opening enables BK channels to act as a coincidence detector (see Chapter “Biophysics of BK Channel Gating” by Pantazis and Olcese), providing a hyperpolarizing drive that contributes to AP repolarization, mediates the fast phase of AHP, and regulates neurotransmitter release and dendritic excitability.
3.1.1 Calcium Nanodomains
Neurons regulate [Ca2+]i using specialized Ca2+-binding proteins, which ensure that increases in neuronal [Ca2+]i are tightly restricted in time and space (Augustine, Santamaria, & Tanaka, 2003; Neher, 1998). At resting Ca2+ concentrations and within the physiological voltage range found in neurons, BK channel open probability is close to null. Furthermore, the relatively low affinity of BK channels for Ca2+ (as compared to other Ca2+-activated channels, such as SK channels) implies that only BK channels located in the immediate vicinity of active Ca2+ influx sources can become activated. The [Ca2+]i required for BK channel activation (~10 μM) is largely restricted to local Ca2+-signaling nanodomains centered around CaV channels (Augustine et al., 2003; Brenner, Jegla, et al., 2000; Fakler & Adelman, 2008). Accordingly, the experimental findings reviewed below have revealed the spatial proximity and even direct physical interaction between BK and CaV channels.
Early functional evidence for the tight spatial coupling between BK and CaV channels in neurons came from the ability of BAPTA, a Ca2+-chelator with fast-binding kinetics, to disrupt BK–CaV interactions and suppress BK currents, unlike the slow buffer EGTA (Edgerton & Reinhart, 2003; Lancaster & Nicoll, 1987; Muller, Kukley, Uebachs, Beck, & Dietrich, 2007; Robitaille, Garcia, Kaczorowski, & Charlton, 1993; Storm, 1987b; Sun et al., 2003). In some instances, the coupling between BK and CaV channels is even completely resistant to buffering by BAPTA (Loane, Lima, & Marrion, 2007). Altogether, these experimental data suggest a ~10–50 nm distance between the two channel pores.
A more detailed understanding of the functional coupling between CaV and BK channels was obtained using subtype-specific CaV channel blockers. In sum, virtually all subtypes of CaV channels have been shown to influence BK channel opening (except R-type), with the specific subtype involved depending upon the subcellular location of the BK channel. For instance, Ca2+ entry via fast-activating P/Q- and N-type channels in presynaptic terminals can indirectly, via a BK channel-dependent shortening of AP duration, reduce neurotransmitter release (Castillo, Weisskopf, & Nicoll, 1994; Kulik et al., 2004; Pelkey, Topolnik, Lacaille, & McBain, 2006; Wu, Westenbroek, Borst, Catterall, & Sakmann, 1999). Alternatively, the more slowly activating L- and T-type channels located primarily in the dendrites and cell body have been shown to gate BK channels and thereby regulate their role in synaptic summation and AP propagation (Dobremez et al., 2005; Engbers, Anderson, Zamponi, & Turner, 2013; Obermair, Szabo, Bourinet, & Flucher, 2004; Rehak et al., 2013).
The coupling of BK channels with specific CaV subtypes may also vary across different brain regions. Near-coincident activation of N-type CaV and BK channels in hippocampal CA1 pyramidal neurons suggests a very close spatial relationship between these channels, which is not seen for L-type channels in this cell type (Marrion & Tavalin, 1998). In cortical pyramidal neurons, both N- and L-type channels provide Ca2+ to gate BK channel opening (Sun et al., 2003), while in chromaffin cells, L- and P/Q-type channels play a greater role than N-type channels in gating BK channels (Prakriya & Lingle, 1999). Additionally, low-voltage-activated T-type channels in cerebellar neurons complex with BK channels to reduce synaptically evoked neural firing (Engbers et al., 2013). By associating with a diverse set of CaV partners, BK channels can adapt to display diverse temporal responses that may enable them to serve different roles in regulating neural activity (Berkefeld & Fakler, 2008).
Although early electrophysiological studies provided important evidence for a functional coupling between BK and CaV channels, direct physical association between channel subunits was not demonstrated until fairly recently (Bao, de Jong, Alevra, & Schild, 2015; Berkefeld et al., 2006; Grunnet & Kaufmann, 2004; Indriati et al., 2013; Kaufmann et al., 2009; Loane et al., 2007; Samaranayake, Saunders, Greene, & Navaratnam, 2004). A first coimmunoprecipitation study using a rat brain membrane preparation found that BK channels predominantly complex with L-type CaV channels, and to a limited extent with P/Q-type, but not with N-type channels (Grunnet & Kaufmann, 2004). A subsequent coimmuno-precipitation study however showed that BK channels assemble with N-type channels in the rat whole brain, and in the hippocampus in particular, to a greater extent than with L-type channels (Loane et al., 2007). The discrepancy between these two studies possibly results from the use of different antibodies against CaV channels. When mass spectrometry was used to identify proteins interacting with BK α subunit affinity-purified from rat brain plasma membranes, it was found that BK channels complex with L-, P/Q-, and N-type channels, with P/Q-type associations being most abundant (Berkefeld et al., 2006). Consistent with pharmacological observations, R-type CaV channels were not specifically copurified with BK α in the latter study.
High-resolution imaging further corroborated the physical colocalization of BK and CaV channels in neurons. Specifically, SDS-digested, freeze-fracture replica, immunogold double-labeling experiments showed that BK channel clusters on cerebellar Purkinje cell soma and primary dendrites virtually always colocalize with P/Q-type CaV channels (Indriati et al., 2013; Kaufmann et al., 2009).
In contrast to BK channels tightly associated with CaV channels, BK channels located further away from Ca2+ sources may play a role in sensing global [Ca2+]i and provide an “emergency brake” under excitotoxic conditions where [Ca2+]i rises above the physiological range (Hu et al., 2001; Runden-Pran, Haug, Storm, & Ottersen, 2002).
3.1.2 Regulation of Neural Firing
BK channels can shape the AP waveform and influence firing frequency in complex ways. In modeling studies, BK channel currents can slow down or speed up firing, depending on the relative magnitude of other ion channels. For example, when hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are present, slowly activating BK channels (a feature of β4-containing channels; see Section 2.2.2) increase firing rates in cells that are normally relatively silent. This effect is reversed in neurons with high basal firing, where the same slow-activating BK channels reduce overall firing rates (Ly, Melman, Barth, & Ermentrout, 2011). This mixed effect suggests that BK channels, together with HCN channels, act as a gain control mechanism to flatten F–I curves (Ly et al., 2011).
In CNS neurons, pharmacological or genetic inhibition of BK channels can increase or decrease evoked or spontaneous firing activity (Bielefeldt & Jackson, 1993; Brenner et al., 2005; Du et al., 2005; Gu, Vervaeke, & Storm, 2007; Jin, Sugaya, Tsuda, Ohguchi, & Sugaya, 2000; Li et al., 2007; Matthews, Weible, Shah, & Disterhoft, 2008; Meredith et al., 2006; Nelson, Gittis, & du Lac, 2005; Nelson, Krispel, Sekirnjak, & du Lac, 2003; Pitts, Ohta, & McMahon, 2006). These findings are part of a consistent pattern of experimental evidence supporting the notion that BK channels should not be thought of as strictly excitatory or inhibitory, but rather, similar to dynamic range compressors used in sound recording that reduce the volume of loud sounds and amplify quiet ones. In other words, BK channels can turn down the firing of neurons that are too active and turn up the firing of quiet ones.
What is an explanation for the diversity of BK channel effects on neural activity? It may seem counterintuitive that K+ conductance (ie, efflux) can increase firing output, since its net effect should be membrane hyperpolarization. However, the timing of BK channel activation (during the falling phase of the AP, when Ca2+ from CaV channels has had a chance to accumulate, or in later spikes during a high-frequency train) and the presence of other channels in the membrane contribute to its net effect on firing output. Understanding the timing of BK channel activation during isolated or sustained firing may prove helpful in explaining these bidirectional effects.
The three major phases of the AP are: (1) a depolarization phase mediated by voltage-gated Na+ and Ca2+ channel currents, (2) a repolarization phase involving fast-activating voltage-gated K+ currents, and (3) an AHP phase driven by slowly activating/deactivating voltage-gated K+ currents that sets the spike refractory period. In isolation, BK α homotetramers show fast-activation kinetics, and thus, can contribute to the repolarization phase and the AHP (Faber & Sah, 2002; Gu et al., 2007; Montgomery & Meredith, 2012; Shao, Halvorsrud, Borg-Graham, & Storm, 1999; Shruti, Clem, & Barth, 2008; Storm, 1987a). In neurons, other currents and cell-specific factors regulate whether BK channels contribute to the repolarization or AHP phases of the AP and, as a result, BK currents can either slow down or speed up spontaneous firing depending on the context.
These nuances have been investigated in detail in the vestibular nucleus, where firing properties and long-term changes in intrinsic excitability are dependent on BK currents (Gittis & du Lac, 2007; Nelson et al., 2003). This brain region contains fast-firing projection neurons and slower-firing GABAergic interneurons, and BK channels are differentially implicated in the regulation of firing in these two populations (Gittis & du Lac, 2007; Gittis, Moghadam, & du Lac, 2010; Smith, Nelson, & Du Lac, 2002). In projection neurons, Kv3 currents activate faster than BK currents and dominate the repolarization phase, while in GABA interneurons that lack Kv3, BK currents dominate the repolarization phase (Gittis et al., 2010). In cells with higher basal firing, pharmacological inhibition of fast-activating BK channels using iberiotoxin increases firing rates and reduces the AHP, whereas for a subset of cells with lower firing rates, iberiotoxin slightly decreases firing rates and has less pronounced effects on AHP (Smith et al., 2002). These data support the notion that BK channels contribute preferentially either to the repolarization or to the AHP phases of the AP, depending on the coexpression of other voltage-activated currents, and will thereby either increase or decrease firing rates.
Notably, the contribution of BK channels to firing response gain was dependent on the relative activity levels of the cells examined (Gittis et al., 2010; Smith et al., 2002). For instance, BK channel contribution to repolarization is greatest in low-frequency firing neurons in an excited state (Gittis et al., 2010). The interplay between neural activity levels and the role of BK channels in firing might be related to activity-dependent second messengers, which can have bidirectional effects on BK channel gating. In cell-excised patches from vestibular neurons, application of activated Ca2+/calmodulin-dependent kinase II or a peptide inhibitor of protein kinase C increases BK open probability, raising the possibility that BK contribution to neural excitability can be dynamically regulated depending upon activation of different signaling cascades (van Welie & du Lac, 2011).
The state-dependent effects of BK channels on neural excitability have also been demonstrated in the suprachiasmatic nucleus (SCN) of the hypothalamus, a GABAergic circuit controlling circadian rhythms where [Ca2+]i, cAMP-dependent second messengers, fast-activating K+ currents, and BK currents display circadian oscillations (Doi et al., 2011; Granados-Fuentes, Norris, Carrasquillo, Nerbonne, & Herzog, 2012; Hong, Jeong, Min, & Lee, 2012; Ikeda & Ikeda, 2014; Ikeda et al., 2003; Kudo, Loh, Kuljis, Constance, & Colwell, 2011; Meredith et al., 2006; O’Neill, Maywood, Chesham, Takahashi, & Hastings, 2008; Panda et al., 2002; Pitts et al., 2006). Paradoxically, total BK currents are inversely correlated with neuronal Ca2+ levels, which positively regulate BK currents (Meredith et al., 2006); however, they are also inversely correlated with fast-activating K+ currents that mediate fast-firing rates (Granados-Fuentes et al., 2012; Kudo et al., 2011). These findings make it increasingly apparent that disambiguating BK channel contribution to the regulation of neural excitability will likely also require cell-by-cell characterization of other voltage-gated K+ channels.
Examination of BK channel contribution to neural excitability in cerebellar Purkinje cells raises additional problems in understanding how BK channels contribute to the regulation of neural excitability. Pharmacological experiments indicate that BK currents generally suppress activity in Purkinje cells by increasing the magnitude of the AHP, which supports an inhibitory role of BK channels in these cells (Edgerton & Reinhart, 2003; Swensen & Bean, 2003; Womack & Khodakhah, 2002). Yet, when BK channels are genetically ablated, a substantial decrease in Purkinje cell tonic firing rates is observed, which supports an excitatory role of BK channels in these cells (Sausbier et al., 2004). Reconciling these two experimental observations is difficult, but might be accounted for if one considers that acute pharmacological inhibition of BK currents and constitutive loss of BK channels are fundamentally different interventions. Unlike acute pharmacological inhibition, constitutive knockout of BK α may elicit homeostatic/compensatory changes in other voltage-gated currents to protect against hyperexcitability. Investigating this possibility will require systematic examination of other currents regulating neural excitability and AP generation in the cerebellar circuit of BK α knockout mice.
Experimental studies focused on other neuronal populations have provided evidence for a proexcitatory role of BK channels. For example, genetic ablation of BK β4 subunit causes hyperexcitability of dentate gyrus granule cells in conjunction with faster gating and higher density of BK channels (Brenner et al., 2005). Furthermore, in neocortical pyramidal neurons primed by seizure activity, increased BK current amplitude is linked to both single-cell and network hyperexcitability, and the elevated firing output of postseizure neurons can be normalized by application of the BK channel blocker paxilline (Shruti et al., 2008). In snail serotonergic neurons, silencing synapsin expression also increases intrinsic excitability while concomitantly increasing the magnitude of BK currents (Brenes, Vandael, Carbone, Montarolo, & Ghirardi, 2015). Paxilline reduces firing frequency down to control levels in this system as well, further buttressing the idea that excessive BK channel activity can generate neuronal hyperexcitability.
Some of the differential effects BK channels have on neural excitability may depend on the specific β subunit that is coexpressed with the pore-forming α subunit. For example, when assembled with the fast-inactivating β2 subunit found in adrenal medullary chromaffin cells, the role of the BK channel is limited to the repolarization phase, which acts to increase the spontaneous firing of these cells (Martinez-Espinosa et al., 2014). In contrast, when assembled with the β4 subunit, the role of the BK channel is more critical during the AHP phase, where it lengthens spike refractory periods to increase interspike intervals and decrease overall firing rates (Brenner et al., 2005; Cloues & Sather, 2003; Petrik, Wang, & Brenner, 2011). This effect may be due to the relatively slow activation kinetics of β4-containing channels that prevent BK channels from opening during the AP (Brenner, Jegla, et al., 2000), or from the role of β4 in reducing BK channel current density via intra-cellular sequestration (Shruti et al., 2012). Overall, it is clear that the different biophysical characteristics and cellular localization properties conferred by the β subunits can profoundly influence firing output in neurons.
3.1.3 Regulation of Neurotransmitter Release
The role of BK channels in regulating neural activity does not end with their role in shaping AP waveform and firing rate. The pattern of BK α expression within terminal areas of prominent fiber tracts is consistent with its presynaptic targeting and involvement in the regulation of neurotransmitter release (Knaus et al., 1996; Misonou et al., 2006; Raffaelli, Saviane, Mohajerani, Pedarzani, & Cherubini, 2004). Determining whether presynaptic BK channels inhibit or facilitate neurotransmitter release has not been inconsequential, since the small size of most synapses makes isolating the contribution of synaptic vs somatic BK currents methodologically challenging. Nevertheless, multiple lines of evidence including neurotransmitter release from synaptosome preparations, direct examination of the brain largest synapses, indirect measures of synaptic function such as neurotransmitter release probability, and the manipulation of synapse-specific proteins have provided important insights into the functional role of presynaptic BK channels. Additionally, recent technological advancements in superresolution scanning and genetically encoded voltage sensors have facilitated glimpses into even small synapses (≤1 μm), where the hallmark properties of BK channel conductances can be visualized (Novak et al., 2013). Based on these findings and the literature discussed below, BK channels appear to be an important regulator of neurotransmitter release at diverse synapses in the brain.
Once an AP propagates to the presynaptic terminal, it stimulates Ca2+ entry through CaV channels that serves both as a signal to initiate exocytosis of neurotransmitter-containing vesicles (reviewed in Kaeser & Regehr, 2014), and as a requisite cofactor for activating local BK channels. In general, presynaptic BK channels are thought to act as negative regulators of neurotransmitter release by hyperpolarizing the plasma membrane in response to AP-stimulated depolarization and Ca2+ entry through CaV channels (Wang, 2008). In addition, Ca2+ influx in the terminal is modulated by the width of the presynaptic AP, which is in part determined by BK channel activity (see Section 3.1.2). Experimental evidence for an inhibitory role of BK channels on neurotransmitter release was first obtained at neurosecretory terminals and neuromuscular junctions (Obaid, Flores, & Salzberg, 1989; Robitaille & Charlton, 1992; Robitaille et al., 1993; Dopico et al., 1999), and later at central synapses. In organotypic hippocampal slice cultures, BK channel blockade by iberiotoxin or paxilline increases the probability of glutamate release at synapses between CA3 pyramidal neurons, as reflected by increased frequency of spontaneous excitatory postsynaptic currents (EPSCs), increased amplitude of evoked EPSCs, and reduced paired-pulse ratio (Raffaelli et al., 2004). Likewise, iberiotoxin increases the frequency of spontaneous EPSCs in acute spinal cord slices (Furukawa, Takasusuki, Fukushima, & Hori, 2008).
The inhibitory influence of BK channels on excitatory synaptic transmission was also observed in synaptosomes subjected to depolarization-induced release of radioactively labeled neurotransmitter. Using this approach, pharmacological blockade of BK channels enhances neurotransmitter release from glutamatergic terminals in cortex, hippocampus, and TCN, but not in cerebellum (Martire et al., 2010; Samengo et al., 2014). In contrast, BK channel inhibition does not affect GABA release from cortical, hippocampal, cerebellar, and TCN synaptosomes; noradrenaline release from cortical, hippocampal, and hypothalamic synaptosomes; or dopamine release from striatal synaptosomes (Martire et al., 2010; Samengo et al., 2014). Experiments performed on synaptosomes therefore suggested that BK channels selectively regulate excitatory transmission. However, electrophysiological recordings have provided more refined insights into the influence of BK channels on inhibitory transmission at individual synapses. In primary hippocampal cultures, iberiotoxin can indeed enhance the amplitude of evoked inhibitory postsynaptic currents (IPSCs) in a subset of neurons receiving a monosynaptic GABAergic innervation (Martire et al., 2010). Similarly, the probability of GABA release onto neurons of the central amygdala is enhanced by iberiotoxin and paxilline, as reflected by increased amplitude of evoked IPSCs and increased frequency of miniature IPSCs (Li, Madison, & Moore, 2014). Iberiotoxin also augments glycine release in the spinal cord (Shoudai et al., 2007). In addition, BK channel activity mediates the inhibitory influence of presynaptic α1-adrenoreceptors on GABA release onto ventral tegmental area dopaminergic neurons (Velasquez-Martinez, Vazquez-Torres, Rojas, Sanabria, & Jimenez-Rivera, 2015).
The functional implication of BK channels in inhibiting neurotransmitter release plays an important role in preventing overexcitation. In monosynaptically connected neonatal rat hippocampal CA3 pyramidal neurons, BK currents decrease the transmitter release probability and increase the synaptic failure rate, even at low-stimulation frequencies (Raffaelli et al., 2004). In adult rat CA3 terminals and mossy fiber boutons, similar Ca2+-dependent negative feedback control of transmitter release is also observed, but only under “excited” conditions, when 4-aminopyridine treatment is used to pharmacologically broaden APs (Alle, Kubota, & Geiger, 2011; Hu et al., 2001). Notably, this is in contrast to the strong contribution of CA3 somatic BK channels to AP repolarization even under baseline conditions (Hu et al., 2001). Thus, the ability of BK channels to brake network activity is expressed differently at the soma and at the synapse.
Presynaptic BK channels have also been characterized in invertebrates. Although genetic ablation of BK channels is not thought to alter neurotransmitter release at the Drosophila neuromuscular junction (Gho & Ganetzky, 1992; Lee, Ueda, & Wu, 2008), a recent study using archaerhodopsin to image synaptic voltage changes found that during periods of repetitive firing, BK channels inhibit recurrent electrical activity (Ford & Davis, 2014). Taken together, physiological data suggest that BK channels at and around synaptic terminals support high-fidelity neurotransmitter release during periods of repetitive firing by inhibiting neurotransmitter release from overly excited synapses.
BK channel trafficking to the synapse is an important determinant of neurotransmitter release regulation. Experiments manipulating cytoskeletal proteins to affect synaptic BK channel clustering alter the ability of BK channels to act as a brake on neurotransmitter release. In neonatal rat hippocampal neuron cultures, stimulating cytoskeletal rearrangement with leptin and insulin signaling promotes presynaptic BK clustering, which is necessary for BK channels to block recurrent electrical activity (O’Malley & Harvey, 2007; O’Malley, Irving, & Harvey, 2005). Additionally, BK clustering at the synapse appears to be an important mechanism for normal synapse maintenance. Mutations in the presynaptic protein cysteine string protein α (CSPα), which enhance synaptic BK expression, are associated with overall synapse loss and neurodegeneration (Kyle, Ahrendt, Braun, & Braun, 2013). Taken together, these findings suggest that while synaptic BK currents must be large enough to inhibit excessive neurotransmitter release during an excited state, they must also be not so large as to limit normal neurotransmission.
In some cases, presynaptic BK channel activity increases the amplitude of postsynaptic responses by facilitating Ca2+ entry in the nerve terminal (Pattillo et al., 2001; Xu & Slaughter, 2005). This is observed in embryonic Xenopus nerve-muscle co-cultures, where BK channel blockers (iberiotoxin and charybdotoxin) reduce transmitter release by approximately a third (Pattillo et al., 2001). This observation was hypothesized to result from a precisely timed broadening of the presynaptic AP, which reduces Ca2+ currents in varicosities (Pattillo et al., 2001). Importantly, opposite observations were made at adult frog neuromuscular junctions, where the same blockers produce a twofold increase in transmitter release, suggesting that the timing of BK channel contribution to AP repolarization is developmentally regulated (Robitaille & Charlton, 1992; Robitaille et al., 1993). In nonspiking rod photoreceptors of the salamander retina, BK currents can also facilitate neurotransmitter release through a Ca2+-dependent positive feedback loop (Xu & Slaughter, 2005). Specifically, BK channel activity produces K+ accumulation in the synaptic cleft, which in turn increases Ca2+ channel currents and hence synaptic transmission (Xu & Slaughter, 2005). Taken together, these data indicate that BK channels located at the presynaptic terminal generally inhibit neurotransmitter release; however, under rare conditions, they may enhance synaptic transmission.
3.1.4 Regulation of Dendritic Excitability
In addition to their effects on regulating neuronal firing rates and neurotransmitter release, BK channels exert an additional level of control on neurotransmission by modulating dendritic excitability. Dendritic electrogenesis stems from the backpropagation of axonal APs into the dendritic tree, as well as from locally generated Na+ and Ca2+ spikes (Hausser, Spruston, & Stuart, 2000). Backpropagating APs can amplify dendritic APs and facilitate the induction of long-term potentiation (Magee & Johnston, 1997). Likewise, although dendritic spikes do not necessarily trigger axonal AP generation, they contribute to synaptic plasticity (Golding, Staff, & Spruston, 2002; Holthoff, Kovalchuk, Yuste, & Konnerth, 2004; Rancz & Hausser, 2006). In layer 5 pyramidal neurons, BK channels suppress AP backpropagation during high-frequency firing and increase the threshold for dendritic spike generation (Benhassine & Berger, 2009; Grewe, Bonnan, & Frick, 2010). BK channels also restrict dendritic Ca2+ spikes both temporally and spatially. In CA1 pyramidal cells, BK channels shorten dendritic Ca2+-dependent spikes, although dendritic BK channels are not activated by backpropagating APs in this neuronal population (Golding, Jung, Mickus, & Spruston, 1999; Poolos & Johnston, 1999). BK channel inhibition was also shown to improve the propagation of dendritic Ca2+ spikes in cerebellar Purkinje neurons, thereby facilitating a form of short-term synaptic plasticity at distant spines (Rancz & Hausser, 2006).
The pathophysiological implications of BK-mediated reduction in dendritic excitability were recently highlighted in mice lacking the Fragile X Mental Retardation Protein (FMRP). These mice exhibit reduced BK channel activity (further discussed in Section 4.7) and, consistent with the findings reported above, recordings performed at distal apical dendrites of layer 5 neocortical neurons revealed an increased amplitude of dendritic Ca2+ spikes, as well as an increased amplitude of Ca2+ transients associated with bursts of backpropagating APs, in the absence of FMRP. Most strikingly, local application of a BK channel activator (BMS-191011) onto distal dendrites was able to normalize these phenotypes (Zhang et al., 2014).
3.2 Astrocytes
BK channels also play an important role for CNS function in nonneuronal cell types. In astrocytes, BK channels are targeted to endfoot processes that wrap parenchymal blood vessels where they play a critical role in the rapid dilation of intracerebral arterioles produced by adjacent neuronal activity (Filosa et al., 2006; Price, Ludwig, Mi, Schwarz, & Ellisman, 2002). Specifically, following neuronal stimulation, astrocytic BK channels are activated by the elevation of endfoot Ca2+ concentration and produce a local K+ efflux in the perivascular space that activates inward rectifier K+ (Kir) channels located on arteriolar smooth muscle cells (Filosa et al., 2006). Accordingly, the coupling between neuronal activity and vasodilation is suppressed by iberiotoxin and by genetic inactivation of BK α (Filosa et al., 2006). Similarly, BK channels located in the glia limitans, the barrier of astrocytic endfoot processes that surrounds the brain and spinal cord, participate in the dilation of pial arterioles in response to somatosensory stimulation (Paisansathan, Xu, Vetri, Hernandez, & Pelligrino, 2010). However, at higher astrocytic endfoot Ca2+ concentration, BK channels mediate the opposite response (ie, vasoconstriction), by raising perivascular K+ concentration above a crossover threshold that depolarizes, instead of hyperpolarizing, arteriolar smooth muscle cells (Girouard et al., 2010). This situation occurs following aneurysmal subarachnoid hemorrhage, where astrocytic Ca2+ concentrations are sufficiently elevated to invert neurovascular coupling, potentially contributing to subsequent ischemia and neurological deficits (Koide, Bonev, Nelson, & Wellman, 2012).
BK channels and other markers of astrocytic endfeet are downregulated in whole brain samples from mouse models of vascular amyloid deposition representing different stages of cerebral amyloid angiopathy, consistent with a general disruption of the neurovascular unit in this disorder associated with the development of Alzheimer’s disease (AD) (Wilcock, Vitek, & Colton, 2009). In addition, pharmacological blockade of BK channels by paxilline prevents the blood–brain barrier disruption induced by HIV-infected astrocytes, which suggests that BK channels may be a target for reducing neuroAIDS pathogenesis (Eugenin, Clements, Zink, & Berman, 2011).
3.3 Microglia
BK currents have been identified in microglial cells of the hippocampus, striatum, neocortex, and entorhinal cortex, as well as in the spinal cord following nerve injury (Bordey & Spencer, 2003; Hayashi et al., 2011; Schilling & Eder, 2007, 2015). Intrathecal administration of a BK channel opener produces microglial activation in the dorsal horn of the spinal cord, as reflected by cell body enlargement and positive staining for the Ca2+-binding protein Iba1 (Hayashi et al., 2011). Spinal nerve injury increases BK currents in spinal microglia and S-ketamine suppresses this effect, which led the authors to propose that the blockade of microglial BK currents by S-ketamine inhibits microglial hyperactivation and mediates the antiallodynic properties of this drug in neuropathic pain (Hayashi et al., 2011). However, the pharmacological approach used in this study does not rule out a role of neuronal BK channels in this phenomenon.
Finally, the density of microglial BK current is not different between brain regions, between juvenile and aged brains, or between ramified and dystrophic microglial cells, thereby discounting a role for BK channels in microglia senescence (Schilling & Eder, 2015). K+ efflux through BK channels has been hypothesized to play a role in the process motility that enables microglial cells to survey their microenvironment, but experimental evidence supporting this premise still needs to be generated (Schilling & Eder, 2015).
3.4 Other Cell Types
There is, to the best of our knowledge, only one report mentioning the presence of BK channels in oligodendrocytes. Specifically, BK currents were found to regulate Ca2+ influx in oligodendroglial precursor cells (Buttigieg, Eftekharpour, Karimi-Abdolrezaee, & Fehlings, 2011). BK channel expression and function gradually diminish during the maturation of precursors into oligodendrocytes, which suggests that BK currents could play a role in the differentiation of these cells (Buttigieg et al., 2011).
Furthermore, BK channels are present in cerebral blood vessels and meninges (Poulsen et al., 2009; Wulf et al., 2008). As in other blood vessels, BK channels play a critical role in promoting cerebral artery vasodilation in response to Ca2+ sparks from the sarcoplasmic reticulum (reviewed in Chapter “BK Channels in the Vascular System” by Krishnamoorthy-Natarajan and Koide).
4. ROLE OF BK CHANNELS IN CNS FUNCTION AND PATHOLOGIES
Owing to their multifaceted role in shaping neuronal activity, BK channels help maintain a physiological range of circuit output in the brain and spinal cord. Accordingly, both insufficient and excessive BK channel activity can have detrimental effects on the CNS, as illustrated in Fig. 1. The following section reviews neurological functions and pathologies, in which BK channels have been implicated.
Fig. 1.
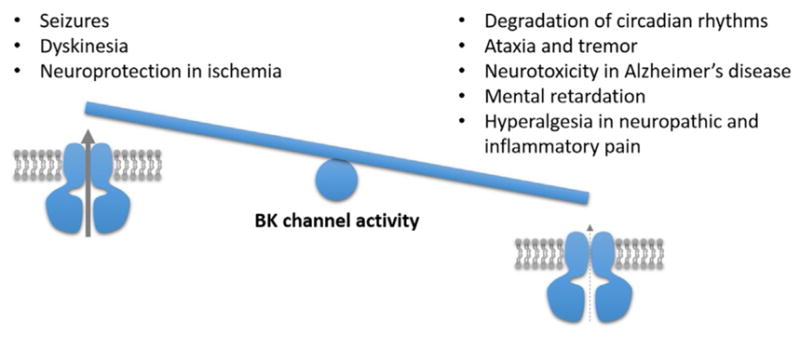
Summary of the physiological and behavioral outcomes resulting from excessive (left) and insufficient (right) BK channel activity in the CNS. In some instances, this balance can be tipped by systemic pharmacological manipulation, such that BK channel blockade can normalize abnormalities elicited by BK channel hyperfunction (eg, anticonvulsant effect of paxilline) and BK channel activation can correct abnormalities generated by BK channel hypofunction (eg, analgesic effect of NS-1619). Mechanistic explanations and bibliographic references are provided in the text.
4.1 Control of the Circadian Rhythm
The SCN of the hypothalamus acts as a circadian pacemaker coordinating physiological and behavioral rhythms in mammals (Moore, 2013). Accordingly, SCN neurons exhibit spontaneous rhythms in electrical activity, with high firing rates during daytime and low firing rates or silence at night. This oscillating pattern persists in constant darkness and is independent from synaptic inputs (de Jeu, Hermes, & Pennartz, 1998; Kuhlman & McMahon, 2004; Welsh, Logothetis, Meister, & Reppert, 1995). BK channels contribute to the ionic mechanisms controlling the circadian rhythm of SCN neuronal activity by regulating the magnitude and duration of the AHP (Cloues & Sather, 2003; Montgomery, Whitt, Wright, Lai, & Meredith, 2013). Kcnma1 was identified as a cycling transcript in the mouse SCN under both entrained (light–dark cycles) and free-running (constant darkness) conditions, with mRNA levels peaking during the dark phase (Meredith et al., 2006; Panda et al., 2002; Pitts et al., 2006). Accordingly, BK current amplitude and density increase during the dark phase in SCN neurons, alongside a decrease in their firing (Montgomery et al., 2013; Pitts et al., 2006).
Consistent with a role of BK channels in silencing SCN neuron activity at night, genetic ablation of BK α selectively increases nighttime firing frequency without affecting daytime frequency (Meredith et al., 2006; Montgomery et al., 2013). In contrast, pharmacological experiments have produced paradoxical results, as iberiotoxin significantly reduces daytime firing in loose patch but not whole-cell recordings, and minimally elevates nighttime firing (Cloues & Sather, 2003; Pitts et al., 2006). These discrepant findings possibly reflect the implication of β4-containing BK channels, which are removed in BK α knockout mice but unaffected by iberiotoxin. The strongly reduced AHP observed in BK α knockout SCN neurons during nighttime is consistent with the hypothesis that slowly deactivating β4-containing BK channels contribute to the suppression of nighttime SCN neuron firing (Montgomery et al., 2013).
At the behavioral level, BK α deletion reduces the amplitude of wheel-running activity, home-cage activity, and body temperature circadian rhythms, but it does not alter the period and phase of these rhythms (Meredith et al., 2006). BK α knockout mice are able to entrain to photic zeitgebers, indicating that BK channels are not involved in retinal signaling of ambient light information to the SCN (Meredith et al., 2006). At the molecular level, the absence of BK α does not affect the transcription of core “clock genes”—the set of transcription factors that form autoregulatory feedback loops driving the rhythmic expression of downstream proteins (Meredith et al., 2006). These data imply that the degradation of behavioral and physiological rhythms in BK α knockout mice does not result from a disruption of the transcriptional clockwork.
The role of BK channels in the control of circadian patterning extends beyond mammals. The expression of Drosophila KCNMA1 orthologue, slowpoke, also displays daily oscillations: head mRNA levels peak at the beginning of the dark phase, and protein levels accumulate during the dark phase and drop during the light phase (Ceriani et al., 2002). At the behavioral level, slowpoke null mutations weaken the rhythmic behavior of flies by disrupting the typical bursts of activity occurring around the circadian phase transitions (Ceriani et al., 2002). In contrast to the mammalian timekeeping system, fly brain BK channels do not control the activity of core pacemaker cells (small ventral lateral neurons) but rather mediate the rhythmicity of downstream output pathways (dorsal clusters) (Fernandez et al., 2007).
A decay in circadian rhythms is also observed with aging (Monk, 2005). The circadian modulation of BK channel activity in SCN neurons was recently shown to be age-dependent, with BK currents no longer increasing during nighttime in aged SCN neurons (Farajnia, Meijer, & Michel, 2015). The loss of BK current oscillation in aged SCN neurons alters AP waveform and increases [Ca2+]i at night, changes that could mediate the degradation of behavioral rhythms and other Ca2+-induced disorders in older organisms (Farajnia et al., 2015).
4.2 Epilepsy
Epilepsy is a chronic disorder in which neuronal hyperexcitability and excessive synchronization generate abnormal brain electrical activity (seizures), which can in turn produce absences, loss of consciousness, limb stiffening and/or jerking (convulsions), or atonia. The first evidence for a genetic link between BK channels and epilepsy came from the association between the duplication of a chromosomal segment encompassing the human gene encoding BK β3 (KCNMB3) and seizures exhibited by some dup(3q) syndrome patients (Riazi et al., 1999). Gene duplication is hypothesized to increase the expression of β3, but the functional impact of β3 upregulation on BK channel activity is difficult to predict, in particular given the very low abundance of this subunit in the brain (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Poulsen et al., 2009; Uebele et al., 2000).
A truncation mutation in KCNMB3 (delA750) was later found to confer susceptibility to idiopathic generalized epilepsy (Lorenz, Heils, Kasper, & Sander, 2007). This mutation selectively alters the properties of one of the four β3 splice variants, β3b, by lowering voltage sensitivity and causing rapid inactivation of BK channels containing this isoform (Hu et al., 2003). Whether BK β3 truncation alters AP waveform and neuronal excitability is unknown. Notably, genetic ablation of BK α subunits causes movement disorders (see Section 4.3.), but does not produce an epileptic phenotype in mice and even elevates seizure thresholds in flies, which does not support a causative role for BK channel hypoactivity in epileptogenesis (Kuebler, Zhang, Ren, & Tanouye, 2001; Sausbier et al., 2004).
In contrast, a number of studies indicate that BK channel hyperactivity increases neuronal excitability. In some instances, BK currents can facilitate high-frequency firing by accelerating spike repolarization and enhancing the AHP amplitude, thereby accelerating the recovery of Na+ channels from inactivation (see Section 3.1.2). Accordingly, a gain-of-function mutation in the human gene encoding BK α (KCNMA1, D434G) was identified in a large family of patients exhibiting generalized epilepsy, paroxysmal non-kinesigenic dyskinesia (ie, involuntary movements of the body), or both (Du et al., 2005). The D434G mutation speeds up BK channel activation and enhances Ca2+ sensitivity via an increase in intrinsic gating, Ca2+ affinity, and allosteric coupling between Ca2+ binding and channel opening (Diez-Sampedro, Silverman, Bautista, & Richerson, 2006; Du et al., 2005; Lee & Cui, 2009; Wang, Rothberg, & Brenner, 2009; Yang et al., 2010). While these effects are preserved when β1, β2, or β4 subunits assemble with BK α, the D434G mutation slows down activation kinetics, decreases voltage dependence, and does not alter Ca2+ sensitivity in the presence of β3b (Lee & Cui, 2009). The relevance of this distinction for neuronal physiology remains unclear given the low levels of β3 expression in the CNS.
Further evidence for a causal link between increased BK channel function and seizure activity comes from the manipulation of BK β4. Genetic deletion of BK β4 reduces AP half-width, shortens the AHP, and increases firing rates in dentate gyrus granule cells, which exhibit particularly high levels of β4 expression in wild-type mice (Brenner et al., 2005). At the systemic level, BK β4 knockout mice exhibit spontaneous nonconvulsive seizures that appear to originate in the hippocampus and spread into the whole neocortex (Brenner et al., 2005). Interestingly, a single-nucleotide polymorphism (SNP) located 3′ of the human gene encoding BK β4 (KCNMB4, rs398702) confers strong predisposition to mesial temporal lobe epilepsy associated with hippocampal sclerosis in an Irish population (Cavalleri et al., 2007). The impact of this allelic variation on BK β4 expression is currently unknown. In mouse dentate granule cells, the effects of BK β4 deletion on AP waveform are reversed by paxilline, consistent with the idea that the β4 subunit reduces BK currents in wild-type neurons (Brenner et al., 2005). One can therefore speculate that paxilline might suppress temporal lobe seizures in BK β4 knockout mice, and possibly in human carriers of the rs398702 allele. Indeed, the anticonvulsant properties of paxilline have been demonstrated in other models, as reviewed below.
Seizure activity potentiates BK currents in neocortical neurons, enhancing their intrinsic excitability and recurrent activity in the local network. In layer 2/3 pyramidal neurons from the mouse somatosensory cortex, whole-cell BK currents are increased fivefold 24 h after picrotoxin-induced generalized seizures in mice (Shruti et al., 2008). This enhancement of BK channel activity is associated with increased evoked firing rate, which is normalized by pharmacological blockade of BK channels, both in isolated neurons and in acute brain slices (Shruti et al., 2008). At the behavioral level, systemic administration of paxilline 24 h after an initial seizure episode elicited by picrotoxin or pentylenetetrazole virtually abolishes seizures upon second exposure to these chemoconvulsants (Sheehan, Benedetti, & Barth, 2009). Consistent with a direct suppression of neural activity, paxilline pretreatment also blocks the induction of Fos immunoreactivity in the neocortex and hippocampus (Sheehan et al., 2009). Similar findings were obtained in rats injected with pilocarpine. Twenty-four hours after seizure induction, dentate gyrus granule cells exhibit an increased firing rate, with a reduced duration of APs and a larger amplitude of fast AHP, which are consistent with enhanced BK channel activity (Mehranfard, Gholamipour-Badie, Motamedi, Janahmadi, & Naderi, 2014). Again, these changes are normalized by paxilline (Mehranfard et al., 2014). Importantly, BK channel blockade does not exert an anticonvulsant effect in animals that had no previous seizure experience, and even reduces the protective effect of cannabidiol in a first seizure episode (Sheehan et al., 2009; Shirazi-zand, Ahmad-Molaei, Motamedi, & Naderi, 2013).
What is the mechanism mediating the seizure-induced upregulation of BK channel activity? Postseizure BK channel hyperactivity could result from increased transcription and/or translation of BK α. Accordingly, in a rat model of genetic predisposition to audiogenic seizures (Krushinskii–Molodkina rats), exposure to audiogenic stimuli transiently upregulates BK α protein levels in the dentate gyrus, with a significant increase detected 3 days but not 14 days postseizure (Savinaa, Levina, Poletaevab, Fedotovab, & Shchipakinaa, 2014). Alternatively, the increase in neuronal BK currents might result from post-translational modification of existing channels. For example, ubiquitination of BK α subunits by the DNA damage-binding protein 1 (DDB1)/Cullin-4A/RING-box protein 1/Cereblon ubiquitin ligase complex prevents their trafficking from the endoplasmic reticulum to the cell surface and inhibition of this process increases BK currents (Jo et al., 2005; Liu et al., 2014). In accordance with a proepileptogenic effect of BK channel hyperactivity, mice with genetic inactivation of DDB1 in forebrain excitatory neurons develop spontaneous seizures in adulthood, sometimes leading to convulsive death (Liu et al., 2014). In addition, they have an enhanced sensitivity to pentylenetetrazol-induced seizures, which is ameliorated by paxilline pre-treatment (Liu et al., 2014).
BK channel activity has also been implicated in the pathogenesis of alcohol withdrawal seizures. Ablation of neuronal BK channels in D. melanogaster completely prevents alcohol-induced enhancement of seizure susceptibility (Ghezzi, Krishnan, & Atkinson, 2014). Conversely, deletion of BK β4 prolongs handling-induced convulsions in mice withdrawn from chronic intermittent alcohol inhalation (Kreifeldt, Cates-Gatto, Roberts, & Contet, 2015). These findings corroborate the notion that enhanced BK channel activity promotes abnormal hyperexcitability and seizures.
In some instances, seizure activity or susceptibility have been correlated with a downregulation of BK channels. In the pilocarpine model of mesial temporal lobe epilepsy, in which pilocarpine precipitates status epilepticus followed by a latent period and later by the appearance of spontaneous recurrent seizures, BK α mRNA and protein expression progressively diminishes in the cortex and hippocampus (Ermolinsky, Arshadmansab, Pacheco Otalora, Zarei, & Garrido-Sanabria, 2008; Pacheco Otalora et al., 2008). In this model of chronic seizure activity, BK channel downregulation may represent a compensatory adaptation to offset sustained neuronal hyper-excitability. Alternatively, if this downregulation affects the presynaptic compartment, as suggested by the prominent decrease in BK α immunoreactivity in the terminal fields of hippocampal granule cells and entorhinal inputs to the hippocampus, it could directly contribute to increased glutamate release and drive hyperactivity of downstream neurons (Pacheco Otalora et al., 2008). Interestingly, BK α downregulation in the dentate gyrus of pilocarpine rats is accompanied by a relative increase in the proportion of STREX-containing BK α isoforms, whose gating kinetics promotes repetitive firing (Ermolinsky et al., 2011; Xie & McCobb, 1998). Altogether, it is possible that a decreased expression of presynaptic BK channels and a preferential expression of STREX-containing variants in the soma of glutamatergic neurons concomitantly drive neuronal hyperexcitability in these chronically epileptic rats.
BK current density was also decreased in inferior colliculus neurons 24 h into withdrawal from subchronic alcohol gavage, a time point at which rats exhibit increased audiogenic seizure susceptibility, and partially returned to baseline 24 h later, when seizure susceptibility is normalized, suggesting that this decrease might play a role in seizure induction, either directly or through a downstream area (N’Gouemo & Morad, 2014). Importantly, recordings were obtained from rats that had not been exposed to audiogenic stimulation, ruling out that BK channel hypoactivity was elicited by seizures. Additional studies are needed to determine whether this decrease in BK currents is indeed mediating neuronal hyperexcitability in the inferior colliculus during alcohol withdrawal (Evans, Li, & Faingold, 2000).
Overall, there is abundant evidence that altered BK channel activity is linked to changes in firing output that can be associated with seizures. Findings that show up- or downregulation of BK currents in different seizure models are consistent with the fact that BK currents can speed up or slow down firing depending on their cellular context (see Section 3.1.2). Critical issues to be resolved are how abnormal activity occurring during seizures drives changes in BK currents, and how changes in BK currents either remedy or exacerbate abnormal excitability.
4.3 Movement Disorders
BK channels have been repeatedly implicated in movement disorders, a phenomenon consistent with their abundant expression in the cerebellum. Ablating BK channel activity leads to tremor and cerebellar ataxia. Early evidence for a tremorgenic effect of BK channel inhibition came from the demonstration that the indole diterpene mycotoxins causing ryegrass staggers in ruminants grazing on fungus-infected pastures (eg, penitrems, lolitrems, paxilline) are selective blockers of BK currents (Knaus et al., 1994). Accordingly, BK α knockout mice exhibit muscular tremor and are insensitive to the tremorgenic effects of lolitrem B and paxilline (Imlach et al., 2008; Typlt et al., 2013). On the other hand, genetic deletion of BK β1 or β4 does not produce tremors and does not affect the tremorgenic effect of these mycotoxins (Imlach et al., 2008).
In contrast, a gain-of-function mutation in KCNMA1 has been associated with paroxysmal nonkinesigenic dyskinesia (D434G; Du et al., 2005). Two additional de novo heterozygous point mutations in KCNMA1 were later identified in children affected by early-onset paroxysmal non-kinesigenic dyskinesia and developmental delay (E884K and N1053S; Zhang, Tian, Gao, Jiang, & Wu, 2015). The functional impact of these two mutations on BK channel activity is however unknown.
Constitutive deletion of BK α in mice also produces abnormal gait and deficient sensorimotor coordination (Sausbier et al., 2004). These deficits are recapitulated in mice lacking BK α exclusively in cerebellar Purkinje neurons, indicating the critical role of BK channel activity in these cells for normal motor coordination (Chen et al., 2010). Genetic deletion of BK β4, but not BK β1, reduces the ataxic effect of BK channel inhibitors (Imlach et al., 2008). The circuitry mediating the influence of BK channel activity on Purkinje cell output has been elegantly worked out. The absence of BK α depolarizes Purkinje neurons resting membrane potential and decreases their spontaneous activity (Sausbier et al., 2004). In addition, the reduced firing of Purkinje cells perturbs the downstream neuronal circuitry, ultimately resulting in reduced excitatory feedback from the inferior olive (Chen et al., 2010). This disruption of the olivocerebellar feedback loop further contributes to silencing Purkinje neurons, thereby disabling the sole output of the cerebellar cortex.
The ataxic behavior produced by genetic inactivation of BK α is associated with the emergence of a beta oscillatory field potential in the cerebellar cortex, along with a highly rhythmic bursting pattern of Purkinje cells (Cheron et al., 2009). Interestingly, spike rhythmicity of Purkinje cells is increased in other rodent models of ataxia, which suggests that it may represent a general pathophysiological mechanism of cerebellar ataxia (Bearzatto et al., 2006; Cheron et al., 2004; Cheron, Servais, Wagstaff, & Dan, 2005; LeDoux & Lorden, 2002; Servais et al., 2005, 2007).
In a mouse model of spinocerebellar ataxia type 1 (SCA1, ATXN-1 [82Q] mice overexpressing mutant human ataxin-1 in Purkinje neurons), a reduction in BK currents contributes to the depolarization block that abolishes Purkinje cell repetitive firing in juvenile mice and is associated with motor impairment at this age (Dell’Orco et al., 2015). Importantly, these phenotypes can be rescued by virally mediated overexpression of BK α in the cerebellum of ATXN-1[82Q] mice, which normalizes the AHP amplitude and firing frequency of 6-week-old Purkinje neurons and ameliorates motor function in young adult mice (Dell’Orco et al., 2015).
4.4 Cerebral Ischemia
BK channels are well suited to provide an “emergency brake” to limit glutamate release in instances of excessive Ca2+ influx, such as during cerebral ischemic stroke (Hu et al., 2001). Accordingly, intravenous administration of a BK channel opener (BMS-204352) 1–2 h after middle cerebral artery occlusion reduces cortical infarct volume in rats (Gribkoff et al., 2001). Consistent with a protective role of BK channels in ischemic stroke, BK α knockout mice display larger infarct volume, more severe neurological deficits, and higher mortality than their wild-type littermates (Liao et al., 2010). Interestingly, these pharmacological and genetic manipulations do not impact cerebral blood flow, ruling out a role of vascular BK channels in their effects (Gribkoff et al., 2001; Liao et al., 2010). Pharmacological blockade or genetic inactivation of BK α also exacerbates NMDA-induced neurotoxicity in vivo and neuronal death in hippocampal or cerebrocortical slice cultures exposed to ischemia-like conditions, which further suggests that neuronal BK channels exert their neuroprotective effect by dampening excitotoxicity (Katsuki, Shinohara, Fujimoto, Kume, & Akaike, 2005; Liao et al., 2010; Runden-Pran et al., 2002). It is also possible that BK channels protect against neuronal death via their interactions with the proapoptotic proteins p53 and fas-associated protein with death domain (Sakai & Sokolowski, 2015). An additional mechanism may involve mitochondrial BK channels, which are known to play a critical cardioprotective role in ischemia–reperfusion injury of the heart (Singh et al., 2013; Xu et al., 2002; see Chapter “Functional Role of Mitochondrial and Nuclear BK Channels” by Gao and Li).
Increased BK channel activity also mediates the neuroprotective effect of the traditional Chinese medicine Baifuzi and the antipsychotic chlorpromazine in rodent models of cerebral ischemia (Chi et al., 2010; Li, Zhang, et al., 2014), as well as the neuroprotective effect of resveratrol and estrogen receptor activators in hippocampal slices deprived from oxygen and glucose (Zhang et al., 2008, 2009). Interestingly, Baifuzi promotes BK channel activation via a direct interaction with the STREX insert of BK α, a domain that also mediates BK channel sensitivity to hypoxia (Chi et al., 2010; McCartney et al., 2005). BK channel activation also contributes to the neuroprotective effect of mitochondrial division inhibitor mdivi-1 in a model of spinal cord ischemia–reperfusion injury (Liu et al., 2015).
Despite these promising preclinical findings, the therapeutic efficacy of BMS-204352 failed to be demonstrated in a phase III clinical trial involving acute stroke patients, possibly due to the intrinsic limitations of that particular compound (Jensen, 2002; see Chapter “Developing the Molecular Pharmacology of BK Channels for Therapeutic Benefit” by Kaczorowski and Garcia). The abundance of evidence pointing to a neuroprotective role of BK channels in cerebral ischemic stroke calls for this therapeutic direction to be further explored, although side effects associated with CNS-wide BK channel activation will be a major hurdle to overcome.
4.5 Alzheimer’s Disease
A genome-wide association study found that a SNP in the gene encoding BK α (KCNMA1, rs16934131) is nominally associated with late-onset AD risk in humans (Beecham et al., 2009). Although a separate study was unable to replicate this association, the same SNP was found to influence age-at-onset and disease duration between different late-onset AD genotypes (Burns et al., 2011). A more recent genome-wide association study, coupled with an analysis of known genetic risk loci for AD and related dementias, found that a SNP in the gene encoding BK β2 (KCNMB2, rs9637454) was strongly associated with hippocampal sclerosis, a comorbid neuropathological feature of AD (Beecham et al., 2014).
While the functional consequence of these point mutations are unknown, rodent models of AD have shed some light on the mechanism through which BK channels may contribute to AD pathogenesis. Intracellular injection of amyloid-β1–42 (Aβ1–42) peptides into neocortical pyramidal cells suppresses BK channel activity, which broadens evoked AP spike width and indirectly enhances Ca2+ influx (Yamamoto et al., 2011). This effect is reversed by prior exposure of the animal to electroconvulsive shock (ECS), a treatment known to facilitate BK channel opening (Sakagami et al., 2005; Yamamoto et al., 2011). Accordingly, in a triple-transgenic mouse model of AD that displays intraneuronal Aβ accumulation prior to the appearance of extracellular Aβ plaques and neurofibrillary tangles (4 months old 3xTg mice; Oddo et al., 2003), spike broadening is observed in neocortical neurons and BK currents cannot be detected unless the mice are first subjected to ECS (Yamamoto et al., 2011). A similar effect of Aβ accumulation on BK channels is also observed in brain mitochondrial inner membranes, as ventricular injection of Aβ1–42 in rats decreases the abundance of BK α and the open probability of BK channels in this intracellular compartment (Jafari et al., 2015).
Consistent with these findings, both ventricular injection of isopimaric acid, a BK channel opener, and the use of transcranial magnetic stimulation ameliorate cognitive deficits and cortical hyperexcitability in 3xTg mice (Wang, Kang et al., 2015; Wang, Zhang, et al., 2015). Interestingly, these treatments not only restore BK channel activity but also reduce brain Aβ content, which reveals a two-way relationship between BK currents and Aβ accumulation (Wang, Kang, et al., 2015; Wang, Zhang, et al., 2015).
BK channels seem to play an intricate role in AD pathogenesis. In contrast to the therapeutic effect of BK channel activation discussed above, increased activity of presynaptic BK channels was shown to account for reduced basal excitatory transmission in the hippocampus of another mouse model of preplaque AD (6–9 weeks old TgCRND8 mice; Chishti et al., 2001; Ye, Jalini, Mylvaganam, & Carlen, 2010). As a result, treatments enhancing BK channel activity could potentially aggravate synaptic dysfunction while being beneficial to counteract neurotoxicity in AD.
4.6 Adult-Onset Neuronal Ceroid Lipofuscinosis
BK channel dysfunction has been associated with adult-onset neuronal ceroid lipofuscinosis (ANCL) cases caused by mutations in the gene encoding CSPα. This dominantly inherited neurodegenerative disorder is characterized by the accumulation of an autofluorescent lipopigment (lipofuscin) in neuronal lysosomes and causes seizures, ataxia, psychiatric manifestations, and progressive dementia (Noskova et al., 2011; Velinov et al., 2012). CSPα acts as a presynaptic chaperone for proteins involved in neurotransmitter vesicle trafficking (Chamberlain & Burgoyne, 2000). CSPα knockout mice suffer from a progressive neurodegenerative disorder similar to ANCL (Fernandez-Chacon et al., 2004). Interestingly, CSPα genetic ablation dramatically increases whole brain synaptosomal BK α protein levels without altering BK α mRNA levels (Ahrendt, Kyle, Braun, & Braun, 2014; Kyle et al., 2013). This finding is consistent with the idea that CSPα suppresses BK α trafficking to the presynaptic terminals, and likely enhances neurotransmitter release (see Section 3.1.3). Synaptosomal BK α protein abundance was also significantly elevated in the postmortem cortex of an ANCL patient (Donnelier et al., 2015), but it remains to be determined whether BK channel upregulation actually contributes to the etiology of ANCL.
4.7 Mental Retardation
De novo mutations in KNCMA1 have been identified in several cases of mental retardation (Laumonnier et al., 2006; Zhang et al., 2015). In a patient affected by severe mental deficiency and autism (but not epilepsy), physical disruption of the KCNMA1 gene by chromosomal translocation led to haploinsufficiency and decreased BK currents in lymphoblastoid cell lines (Laumonnier et al., 2006). Dysregulation of BK α expression is also hypothesized to contribute to intellectual disability in humans carrying a nonsense mutation (R419X) in CRBN, the gene encoding cereblon (Higgins, Hao, Kosofsky, & Rajadhyaksha, 2008). Cereblon is a cytosolic protein that promotes the ubiquitination of BK α and suppresses the surface expression of BK channels in neurons (Jo et al., 2005; Liu et al., 2014). Taken together, these findings suggest that both decreased and increased BK channel activity may lead to mental retardation.
Two SNPs in the gene encoding BK β4 (KCNMB4) were also found to be among the strongest predictors of an autism spectrum disorder diagnosis, with a first one (rs968122) conferring vulnerability and the other one (rs12317962) being protective (Skafidas et al., 2014). The impact of these SNPs on BK β4 expression and function is currently unknown.
Mechanistic insights into the link between BK channels and mental retardation have recently been provided by the study of FMRP knockout mice, a mouse model of Fragile X Syndrome (FXS). In contrast with its conventional role in the control of dendritic mRNA translation (Darnell & Klann, 2013), FMRP was shown to regulate glutamate release at CA3-CA1 synapses by interacting with BK β4 in the presynaptic terminals of CA3 pyramidal cells (Deng et al., 2013). Specifically, the loss of FMRP broadens APs, reduces the fast AHP, increases presynaptic Ca2+ influx, and facilitates neurotransmitter release during repetitive activity (Deng et al., 2013). These effects result from a reduction in the Ca2+ sensitivity of BK channels and are corrected by genetic deletion of BK β4. These findings suggest that in wild-type animals FMRP enhances BK channel activity by sequestering β4 subunits, thereby reducing their influence on the gating kinetics and/or trafficking of BK α tetramers (Deng & Klyachko, 2016; Deng et al., 2013). Furthermore, BK β4 deletion normalizes epileptiform neuronal activity in hippocampal slices from FMRP knockout mice even though it promotes neuronal hyperexcitability in the presence of FMRP (Deng & Klyachko, 2016).
A missense mutation in the gene encoding FMRP (R138Q), which impairs the presynaptic function of FMRP and disrupts the interaction between FMRP and BK β4 without altering its canonical postsynaptic function, was recently identified in an FXS patient, thereby providing a unique opportunity to interrogate the role of presynaptic FMRP at the systemic level (Myrick et al., 2015). This case revealed that presynaptic FMRP–BK channel interaction indeed contributes to some of the neuropathological features of FXS, as this patient exhibits intellectual disability and seizures (Myrick et al., 2015). The behavioral relevance of presynaptic FMRP was further confirmed by the striking ability of a BK channel opener (BMS-204352) to normalize the deficits in social recognition and interaction, nonsocial anxiety, and spatial memory of FMRP knockout mice after a single intraperitoneal injection (Hebert et al., 2014).
BK channels are also implicated in the sensory hypersensitivity associated with the loss of FMRP. In FMRP knockout mice, primary somatosensory cortex pyramidal neurons exhibit an increase in the intrinsic excitability of their dendrites, which results from a reduction in Ih and BK currents in this cellular compartment (Zhang et al., 2014). Specifically, the downregulation of dendritic BK channels in these mice enhances AP backpropagation and facilitates the generation of dendritic spikes (Zhang et al., 2014). Accordingly, pharmacological activation of BK channels rescues the hyperexcitable phenotype of FMRP knockout neurons in slices and corrects hypersensitivity to a moderate acoustic stimulus in vivo (Zhang et al., 2014).
Given the bulk of evidence pointing to a causative role of BK channel hypofunction in mental retardation in humans, the cognitive phenotype of BK α knockout mice appears surprisingly mild. Genetic inactivation of BK α abolishes the experience-dependent increase in prepulse inhibition and slows down spatial learning in the Morris water maze, but leaves working memory and spatial reference memory intact (Typlt et al., 2013). It is possible that constitutive deletion of BK α produces compensatory adaptations, particularly in other ion channels, that may mask the more severe phenotypes resulting from BK channel hypoactivity. Alternatively, the behavioral testing that has been conducted in BK α knockout mice may not have tapped into the cognitive domains most impacted in intellectually disabled humans, or BK channels may fulfill a species-specific function with regard to intellect.
4.8 Alcohol Use Disorders
Alcohol use disorders (AUD), formerly known as alcohol abuse and alcohol dependence, represent a spectrum of problematic patterns of alcoholic beverage consumption. Diagnostic criteria of AUD include loss ofcontrol over drinking, craving, and withdrawal symptoms (American Psychiatric Association, 2013). Low level of response to alcohol is a strong predictor of future AUD (Schuckit, 1994; Schuckit, Smith, Anderson, & Brown, 2004). KCNMA1 has been repeatedly detected by genome-wide linkage and association studies of the level of response to alcohol and alcohol dependence, although the signals were statistically weak (Edenberg et al., 2010; Kendler et al., 2011; Schuckit et al., 2005). In addition, both KCNMA1 and KCNMB1 were identified as part of a 39-gene functional network associated with a higher risk of developing alcohol dependence (Han et al., 2013).
BK channels are one of the well-established molecular targets of alcohol (Dopico, Lemos, & Treistman, 1996; see Chapter “Modulation of BK Channels by Ethanol” by Dopico). Residues implicated in alcohol sensing are located between the channel Ca2+-sensing sites and gate (Bukiya et al., 2014; Davis, Scott, Hu, & Pierce-Shimomura, 2014). Clinically relevant concentrations of alcohol (20–25 mM) can lead to either BK current potentiation, refractoriness, or reduction depending on a number of parameters. In particular, the association of BK β1 shifts the activation-to-inhibition crossover to a lower Ca2+ concentration, yielding alcohol-resistant channels in neurons and alcohol-inhibited channels in cerebral artery myocytes (Bukiya, Liu, & Dopico, 2009; Martin et al., 2004; Wynne et al., 2009). Lowering the concentration of Mg2+ also switches the effect of alcohol from activating to inhibitory, which is relevant to the pathophysiology of AUD as chronic alcohol consumption leads to Mg2+ depletion (Marrero, Treistman, & Lemos, 2015; Romani, 2008).
In striatal medium spiny neurons, alcohol-induced potentiation of BK currents rapidly desensitizes, unless auxiliary β4 subunits are present (Martin et al., 2008). An miRNA-mediated BK α isoform switch contributes to this rapid desensitization in supraoptic nucleus and striatal neurons, with miR-9 targeting ALCOREX-containing splice variants to degradation, thereby favoring the expression of alcohol-insensitive STREX-containing isoforms (Pietrzykowski et al., 2008; Velazquez-Marrero et al., 2011). Whether miR-9-driven regulation of BK α splice variants also occurs following in vivo alcohol exposure, possibly in a brain region- or cell type-specific manner, awaits further investigation. Changes in the phosphorylation state of BK α also contribute to rapid desensitization (Velazquez-Marrero, Seale, Treistman, & Martin, 2014). Finally, prolonged exposure of neurons to alcohol (6 h) reduces BK channel cell surface expression (Palacio et al., 2015; Pietrzykowski et al., 2004; Velazquez-Marrero et al., 2011).
The contribution of BK channels to the behavioral effects of alcohol has been investigated by genetically manipulating the pore-forming α subunit in worms and flies, and the auxiliary β subunits in mice. Studies in invertebrates have yielded conflicting results, showing that BK channels mediate the intoxicating effects of alcohol in Caenorhabditis elegans (Davies et al., 2003; Davis et al., 2014) while they mediate rapid tolerance to alcohol-induced sedation and increased seizure susceptibility in Drosophila melanogaster (Cowmeadow, Krishnan, & Atkinson, 2005; Cowmeadow et al., 2006; Ghezzi, Al-Hasan, Larios, Bohm, & Atkinson, 2004; Ghezzi et al., 2014; Ghezzi, Pohl, Wang, & Atkinson, 2010). In mice, deletion of BK β4 promotes rapid tolerance to the hypolocomotor effect of alcohol (Martin et al., 2008) and attenuates alcohol drinking escalation in alcohol-dependent mice (Kreifeldt, Le, Treistman, Koob, & Contet, 2013). Conversely, deletion of BK β1 accelerates drinking escalation in dependent mice (Kreifeldt et al., 2013), while reducing chronic tolerance to alcohol-induced sedation and hypothermia (Kreifeldt et al., 2015). BK channel auxiliary subunits therefore seem to play a critical role in the adaptive response to chronic alcohol exposure in mammals. Whether the interaction of alcohol with BK channels directly contributes to the intoxicating effects of alcohol and/or to the adaptive mechanisms generating chronic tolerance or drinking escalation in mammals is still an open question and a hot topic of investigation.
4.9 Pain
In the spinal cord, BK channels are expressed in subsets of small- and medium-diameter DRG neurons, in which they shorten AP duration, drive the fast AHP, reduce firing frequency, and prolong the refractory period (Li et al., 2007; Lu et al., 2014; Sarantopoulos et al., 2007; Scholz, Gruss, & Vogel, 1998; Shieh et al., 2007; Zhang, Gopalakrishnan, & Shieh, 2003; Zhang, Mok, Katz, & Gold, 2010). BK channels are particularly enriched in isolectin-B4 (IB4) positive small-diameter DRG neurons, a subset of nociceptive primary afferents (Gruss et al., 2001; Zhang et al., 2010). In these neurons, clinically relevant concentrations of ethanol and 2,2,2-trichloroethanol, the active metabolite of chloral hydrate, potentiate BK currents and may contribute to the analgesic effects of these compounds (Gruss, Hempelmann, & Scholz, 2002; Gruss et al., 2001). Consistent with this hypothesis, BK channels located in calcitonin gene-related peptide terminals were recently shown to mediate the analgesic effect of a peripherally acting μ opioid receptor agonist in a model of trigeminal nociception (Baillie, Schmidhammer, & Mulligan, 2015). BK channels are also present on presynaptic terminals in the superficial dorsal horn, where they inhibit transmitter release from primary afferents and inhibitory interneurons (Furukawa et al., 2008; Song & Marvizon, 2005).
At the behavioral level, pharmacological blockade of spinal BK channels with iberiotoxin produces mechanical hyperalgesia (Chen, Cai, & Pan, 2009). The effect of spinal BK channel activation in naïve animals may be biphasic and/or modality dependent, as intrathecal administration of a low dose of the BK channel opener NS-1619 (0.072 μg) produces tactile allodynia, but a higher dose (20 μg) does not affect thermal and mechanical nociceptive thresholds (Chen et al., 2009; Hayashi et al., 2011).
Peripheral nerve injury, a preclinical model of neuropathic pain, reduces the expression and activity of BK channels in small and medium DRG neurons, resulting in increased excitatory transmission in the superficial dorsal horn (Cao, Chen, Li, & Pan, 2012; Chen et al., 2009; Furukawa et al., 2008; Sarantopoulos et al., 2007). This downregulation of BK α is mediated by brain-derived neurotrophic factor activation of TrkB receptors (Cao et al., 2012). Glial cell-derived neurotrophic factor (GDNF) also reduces BK currents in IB4-positive nociceptors, an effect hypothesized to mediate GDNF-induced mechanical hyperalgesia (Hendrich, Alvarez, Chen, & Levine, 2012). Consistent with BK channel activity attenuating nociceptor signaling, intrathecal administration of a BK channel opener (NS-1619) reduces tactile allodynia, as well as mechanical and thermal hyperalgesia, in nerve-ligated rats (Chen et al., 2009). Spinal BK channel activation also contributes to the antiallodynic effect of resveratrol (a plant stilbenoid) in neuropathic pain, and to the analgesic effect of MV8612 (a plant pregnane compound) in inflammatory pain (Bermudez-Ocana, Ambriz-Tututi, Perez-Severiano, & Granados-Soto, 2006; Santos, Trentin, Ferreira, Yunes, & Calixto, 2003). In models of chronic inflammatory pain, genetic ablation of BK α in DRG sensory neurons aggravates nociceptive behavior, while systemic administration of a BK channel opener (NS-1619) exerts analgesic properties (Lu et al., 2014).
Surprisingly, DRG-restricted BK α conditional knockout mice display unaltered nociceptive thresholds in models of acute or neuropathic pain (Lu et al., 2014). This lack of phenotype suggests that the effects of intrathecally administered BK channel modulators may be mediated by other spinal cord neuronal populations, such as interneurons (see below). Another paradoxical finding was that charybdotoxin, a BK channel blocker, suppresses nerve injury-induced tactile allodynia (Hayashi et al., 2011). This discrepancy may stem from the promiscuous action of charybdotoxin on intermediate conductance Ca2+-activated K+ (IK) channels, as well as on voltage-activated K+ channels (Garcia et al., 1991; Garcia, Knaus, Munujos, Slaughter, & Kaczorowski, 1995).
BK channels inhibit the release of inhibitory neurotransmitters from spinal cord interneurons. In particular, they reduce spontaneous glycinergic transmission in the sacral dorsal commissural nucleus and mediate the inhibitory effect of NMDA on opioid release in the dorsal horn (Shoudai et al., 2007; Song & Marvizon, 2005). Interestingly, while nerve ligation reduces BK channel expression in DRG neurons (see above), it increases BK α immunoreactivity in dorsal horn interneurons (Chen et al., 2009). The functional impact of this redistribution awaits further investigation, but suppression of inhibitory signaling within the dorsal horn may potentiate nociception in neuropathic pain.
BK channels are also located at juxtaparanodal sites in spinal cord white matter axons, beneath the myelin sheath (Ye et al., 2012). Under normal conditions, juxtaparanodal BK channels do not modulate axonal conduction, but in a model of spinal cord compression injury, resulting demyelination exposes juxtaparanodal BK channels, whose activity then impairs axonal conduction (Ye et al., 2012). In summary, BK channels are implicated in multiple aspects of sensory information flow in the spinal cord, and changes in BK channel activity upon nerve injury or following treatment can have either detrimental or beneficial consequences.
4.10 In the Eye
BK currents can be detected in a large number of retinal cell types, including retinal pigment epithelial cells (Tao & Kelly, 1996; Wimmers, Halsband, Seyler, Milenkovic, & Strauss, 2008), rod photoreceptors (Pelucchi, Grimaldi, & Moriondo, 2008; Xu & Slaughter, 2005), cone photoreceptors (Barnes & Hille, 1989), retinal bipolar cells (Burrone & Lagnado, 1997; Sakaba, Ishikane, & Tachibana, 1997), amacrine cells (Grimes, Li, Chavez, & Diamond, 2009; Mitra & Slaughter, 2002b), and retinal ganglion cells (Henne & Jeserich, 2004; Wang, Robinson, & Chalupa, 1998). They are also present in retinal arterioles (Mori, Suzuki, Sakamoto, Nakahara, & Ishii, 2011) and retinal pericytes (Quignard, Harley, Duhault, Vanhoutte, & Feletou, 2003), which are both involved in the regulation of retinal blood flow, as well as in proliferating Müller (glial) cells (see Bringmann et al., 2000 for review) and in trabecular meshwork, a smooth muscle-like tissue involved in the regulation of aqueous humor outflow (Soto et al., 2004; Stumpff, Strauss, Boxberger, & Wiederholt, 1997).
In a subset of amacrine cells, BK channels mediate spontaneous miniature outward currents, which are hypothesized to serve as synaptic noise suppressors at quiescent glutamatergic synapses (Mitra & Slaughter, 2002a, 2002b). In A17 amacrine cells, BK channels also suppress activation of CaV channels to limit GABA release onto rod bipolar cells (Grimes et al., 2009). Accordingly, genetic ablation of BK channels reduces rod bipolar cell activity in vivo, although this modulatory role is restricted to intermediate lighting conditions (Tanimoto et al., 2012). Conversely, at the ribbon synapse of rod photoreceptors, BK channel activity enhances neurotransmitter release (Xu & Slaughter, 2005; see Section 3.1.3). Notably, this facilitatory role is only observed within a physiological range of depolarization, beyond which BK currents hyperpolarize the rod and brake neurotransmitter release. Finally, in the retinal pigment epithelium, BK channels regulate the rhythmic phagocytosis of shed photoreceptor outer segments, a process whose dysfunction leads to blindness (Muller, Mas Gomez, Ruth, & Strauss, 2014; Strauss, 2005).
Although there are no journal publications on the topic, multiple patents cover the use of BK channel inhibitors to lower intraocular pressure for the treatment of open-angle ocular hypertension and the prevention of glaucoma (Adorante, WoldeMussie, Ruiz, Kopper, & Moore, 1999; Brnardic et al., 2009; Chen et al., 2004; Gao & Shen, 2006; Goetz, Kaczorowski, Monaghan, Strohl, & Tkacz, 2003). Surprisingly, the drug Rescula (unoprostone isopropyl ophthalmic solution), the only BK channel modulator that has been approved by the U.S. Food and Drug Administration for this indication, is a potent BK channel activator (Cuppoletti, Malinowska, Tewari, Chakrabarti, & Ueno, 2007; Yu et al., 2015). It is hypothesized to exert its therapeutic action by increasing the aqueous humor outflow through the trabecular meshwork (Thieme et al., 2001), while inhibitors claimed in the patent literature are hypothesized to act on BK channels of the nonpigmented ciliary epithelium to block humor inflow (see Chapter “Developing the Molecular Pharmacology of BK Channels for Therapeutic Benefit” by Kaczorowski and Garcia).
5. CONCLUSIONS
BK channels have myriad (and sometimes opposite) effects on neural activity, as underscored not only by experimental data but also by modeling studies. Their ability to regulate firing output and synaptic transmission depends upon their coexpression and intracellular association with other ion channels, particularly Ca2+ channels, but also HCN and Na+ channels among others. Overall, it is clear that early studies investigating how specific conditions change total levels of BK α transcript or protein wereinsufficient to understand how BK channels influence normal neuronal function and confer disease phenotypes. Specific future challenges for experimentalists will be in separating the physiological contribution of intracellular vs plasma membrane BK channels, as well as isolating the contribution of somatic vs presynaptic BK channels. Electrophysiological measures, or biochemical methods that can focus on specific cellular compartments, will be required to fully comprehend how BK channels contribute to firing and synaptic communication.
The regulation of BK α pre-mRNA alternative splicing and isoform-specific mechanisms of posttranscriptional control can have dramatic functional consequences on BK channel activity in vitro. Analyzing the abundance of specific splice variants, their subcellular localization, and interactions with other proteins in different CNS regions and/or cell types will be essential for fully understanding the role BK channels play in diverse pathologies in vivo. Such knowledge may then enable the development of therapeutic approaches targeting critically involved subpopulations of BK channels, in an attempt to circumvent the detrimental effects associated with CNS-wide BK channel activation or inhibition (summarized in Fig. 1).
Acknowledgments
We are grateful for the support of NIH Grants U01 AA-020913 and R21 AA-024198 to C.C., and R21 MH-100612 to A.L.B.
References
- Adorante JS, WoldeMussie E, Ruiz G, Kopper K, Moore AM. Patent No. US5925342 A Method for reducing intraocular pressure in the mammalian eye by administration of potassium channel blockers. 1999
- Ahrendt E, Kyle B, Braun AP, Braun JE. Cysteine string protein limits expression of the large conductance, calcium-activated K(+) (BK) channel. PloS One. 2014;9(1):e86586. doi: 10.1371/journal.pone.0086586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua A, Li M, Wu Y, Stefani E, Toro L. Unconventional myristoylation of large-conductance Ca(2)(+)-activated K(+) channel (Slo1) via serine/threonine residues regulates channel surface expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10744–10749. doi: 10.1073/pnas.1008863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. The Journal of Biological Chemistry. 2008;283(8):4808–4817. doi: 10.1074/jbc.M709802200. [DOI] [PubMed] [Google Scholar]
- Alle H, Kubota H, Geiger JR. Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons. The Journal of Neuroscience. 2011;31(22):8001–8012. doi: 10.1523/JNEUROSCI.0972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almassy J, Begenisich T. The LRRC26 protein selectively alters the efficacy of BK channel activators. Molecular Pharmacology. 2012;81(1):21–30. doi: 10.1124/mol.111.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40(2):331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Bai JP, Surguchev A, Joshi P, Gross L, Navaratnam D. CDK5 interacts with Slo and affects its surface expression and kinetics through direct phosphorylation. American Journal of Physiology. Cell Physiology. 2012;302(5):C766–C780. doi: 10.1152/ajpcell.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie LD, Schmidhammer H, Mulligan SJ. Peripheral mu-opioid receptor mediated inhibition of calcium signaling and action potential-evoked calcium fluorescent transients in primary afferent CGRP nociceptive terminals. Neuropharmacology. 2015;93:267–273. doi: 10.1016/j.neuropharm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. The Journal of General Physiology. 1989;94(4):719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao G, de Jong D, Alevra M, Schild D. Ca(2+)-BK channel clusters in olfactory receptor neurons and their role in odour coding. The European Journal of Neuroscience. 2015;42(11):2985–2995. doi: 10.1111/ejn.13095. [DOI] [PubMed] [Google Scholar]
- Bearzatto B, Servais L, Roussel C, Gall D, Baba-Aissa F, Schurmans S, … Schiffmann SN. Targeted calretinin expression in granule cells of calretinin-null mice restores normal cerebellar functions. FASEB Journal. 2006;20(2):380–382. doi: 10.1096/fj.05-3785fje. [DOI] [PubMed] [Google Scholar]
- Beecham GW, Hamilton K, Naj AC, Martin ER, Huentelman M, Myers AJ, …Montine TJ. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genetics. 2014;10(9):e1004606. doi: 10.1371/journal.pgen.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham GW, Martin ER, Li YJ, Slifer MA, Gilbert JR, Haines JL, Pericak-Vance MA. Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. American Journal of Human Genetics. 2009;84(1):35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Letters. 2000;474(1):99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- Bell TJ, Miyashiro KY, Sul JY, McCullough R, Buckley PT, Jochems J, …Eberwine J. Cytoplasmic BK(Ca) channel intron-containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):1901–1906. doi: 10.1073/pnas.0711796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhassine N, Berger T. Large-conductance calcium-dependent potassium channels prevent dendritic excitability in neocortical pyramidal neurons. Pflügers Archiv. 2009;457(5):1133–1145. doi: 10.1007/s00424-008-0569-3. [DOI] [PubMed] [Google Scholar]
- Benton MD, Lewis AH, Bant JS, Raman IM. Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. Journal of Neurophysiology. 2013;109(10):2528–2541. doi: 10.1152/jn.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Fakler B. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. The Journal of Neuroscience. 2008;28(33):8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, …Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314(5799):615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- Bermudez-Ocana DY, Ambriz-Tututi M, Perez-Severiano F, Granados-Soto V. Pharmacological evidence for the participation of NO-cyclic GMP-PKG-K+ channel pathway in the antiallodynic action of resveratrol. Pharmacology, Biochemistry, and Behavior. 2006;84(3):535–542. doi: 10.1016/j.pbb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Bhattarai Y, Fernandes R, Kadrofske MM, Lockwood LR, Galligan JJ, Xu H. Western blot analysis of BK channel beta1-subunit expression should be interpreted cautiously when using commercially available antibodies. Physiological Reports. 2014;2(10):e12189. doi: 10.14814/phy2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Jackson MB. A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. Journal of Neurophysiology. 1993;70(1):284–298. doi: 10.1152/jn.1993.70.1.284. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Rotter JL, Jackson MB. Three potassium channels in rat posterior pituitary nerve terminals. The Journal of Physiology. 1992;458:41–67. doi: 10.1113/jphysiol.1992.sp019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolborea M, Dale N. Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends in Neurosciences. 2013;36(2):91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A, Spencer DD. Chemokine modulation of high-conductance Ca(2+)-sensitive K(+) currents in microglia from human hippocampi. The European Journal of Neuroscience. 2003;18(10):2893–2898. doi: 10.1111/j.1460-9568.2003.03021.x. [DOI] [PubMed] [Google Scholar]
- Brenes O, Vandael DH, Carbone E, Montarolo PG, Ghirardi M. Knockdown of synapsin alters cell excitability and action potential waveform by potentiating BK and voltage-gated Ca(2+) currents in Helix serotonergic neurons. Neuroscience. 2015;311:430–443. doi: 10.1016/j.neuroscience.2015.10.046. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nature Neuroscience. 2005;8(12):1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. The Journal of Biological Chemistry. 2000;275(9):6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, … Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Francke M, Pannicke T, Biedermann B, Kodal H, Faude F, … Reichenbach A. Role of glial K(+) channels in ontogeny and gliosis: A hypothesis based upon studies on Muller cells. Glia. 2000;29(1):35–44. doi: 10.1002/(sici)1098-1136(20000101)29:1<35::aid-glia4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brnardic E, Doherty JB, Dorsey J, Ellwood C, Fillmore M, Malaska M, … Soukri M. Patent No. WO2009048559 A1 Maxi-k channel blockers and methods of use. 2009
- Bukiya AN, Kuntamallappanavar G, Edwards J, Singh AK, Shivakumar B, Dopico AM. An alcohol-sensing site in the calcium- and voltage-gated, large conductance potassium (BK) channel. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(25):9313–9318. doi: 10.1073/pnas.1317363111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Letters. 2009;583(17):2779–2784. doi: 10.1016/j.febslet.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga T, Wray S. Action potential refractory period in ureter smooth muscle is set by Ca sparks and BK channels. Nature. 2005;436(7050):559–562. doi: 10.1038/nature03834. [DOI] [PubMed] [Google Scholar]
- Burns LC, Minster RL, Demirci FY, Barmada MM, Ganguli M, Lopez OL, …Kamboh MI. Replication study of genome-wide associated SNPs with late-onset Alzheimer’s disease. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2011;156(4):507–512. doi: 10.1002/ajmg.b.31194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Electrical resonance and Ca2+ influx in the synaptic terminal of depolarizing bipolar cells from the goldfish retina. The Journal of Physiology. 1997;505(Pt. 3):571–584. doi: 10.1111/j.1469-7793.1997.571ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg J, Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Molecular and electrophysiological evidence for the expression of BK channels in oligodendroglial precursor cells. The European Journal of Neuroscience. 2011;34(4):538–547. doi: 10.1111/j.1460-9568.2011.07789.x. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, … Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. The Journal of Neuroscience. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XH, Chen SR, Li L, Pan HL. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. Journal of Neurochemistry. 2012;121(6):944–953. doi: 10.1111/j.1471-4159.2012.07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12(2):261–269. doi: 10.1016/0896-6273(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Weale ME, Shianna KV, Singh R, Lynch JM, Grinton B, …Goldstein DB. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: A case-control study. Lancet Neurology. 2007;6(11):970–980. doi: 10.1016/S1474-4422(07)70247-8. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. The Journal of Neuroscience. 2002;22(21):9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD. Cysteine-string protein: The chaperone at the synapse. Journal of Neurochemistry. 2000;74(5):1781–1789. doi: 10.1046/j.1471-4159.2000.0741781.x. [DOI] [PubMed] [Google Scholar]
- Chang CP, Dworetzky SI, Wang J, Goldstein ME. Differential expression of the alpha and beta subunits of the large-conductance calcium-activated potassium channel: Implication for channel diversity. Brain Research. Molecular Brain Research. 1997;45(1):33–40. doi: 10.1016/s0169-328x(96)00230-6. [DOI] [PubMed] [Google Scholar]
- Chen L, Bi D, Tian L, McClafferty H, Steeb F, Ruth P, …Shipston MJ. Palmitoylation of the beta4-subunit regulates surface expression of large conductance calcium-activated potassium channel splice variants. The Journal of Biological Chemistry. 2013;288(18):13136–13144. doi: 10.1074/jbc.M113.461830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Cai YQ, Pan HL. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. Journal of Neurochemistry. 2009;110(1):352–362. doi: 10.1111/j.1471-4159.2009.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Doherty JB, Liu L, Natarajan SR, Shen DM, Tynebor RM. Patent No. WO2004043354 A2 Ophthalmic compositions for treating ocular hypertension. 2004
- Chen X, Kovalchuk Y, Adelsberger H, Henning HA, Sausbier M, Wietzorrek G, …Konnerth A. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(27):12323–12328. doi: 10.1073/pnas.1001745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Gall D, Servais L, Dan B, Maex R, Schiffmann SN. Inactivation of calcium-binding protein genes induces 160 Hz oscillations in the cerebellar cortex of alert mice. The Journal of Neuroscience. 2004;24(2):434–441. doi: 10.1523/JNEUROSCI.3197-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Sausbier M, Sausbier U, Neuhuber W, Ruth P, Dan B, Servais L. BK channels control cerebellar Purkinje and Golgi cell rhythmicity in vivo. PloS One. 2009;4(11):e7991. doi: 10.1371/journal.pone.0007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Servais L, Wagstaff J, Dan B. Fast cerebellar oscillation associated with ataxia in a mouse model of Angelman syndrome. Neuroscience. 2005;130(3):631–637. doi: 10.1016/j.neuroscience.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Chi S, Cai W, Liu P, Zhang Z, Chen X, Gao L, …Qi Z. Baifuzi reduces transient ischemic brain damage through an interaction with the STREX domain of BKCa channels. Cell Death & Disease. 2010;1:e13. doi: 10.1038/cddis.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, … Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. The Journal of Biological Chemistry. 2001;276(24):21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. The Journal of Neuroscience. 2003;23(5):1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcoholism, Clinical and Experimental Research. 2005;29(10):1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Ghezzi A, Al’Hasan YM, Wang YZ, Atkinson NS. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcoholism, Clinical and Experimental Research. 2006;30(5):745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Malinowska DH, Tewari KP, Chakrabarti J, Ueno R. Cellular and molecular effects of unoprostone as a BK channel activator. Biochimica et Biophysica Acta. 2007;1768(5):1083–1092. doi: 10.1016/j.bbamem.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The translation of translational control by FMRP: Therapeutic targets for FXS. Nature Neuroscience. 2013;16(11):1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, …McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115(6):655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Scott LL, Hu K, Pierce-Shimomura JT. Conserved single residue in the BK potassium channel required for activation by alcohol and intoxication in C. elegans. The Journal of Neuroscience. 2014;34(29):9562–9573. doi: 10.1523/JNEUROSCI.0838-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9(16):3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- Dell’Orco JM, Wasserman AH, Chopra R, Ingram MA, Hu YS, Singh V, … Shakkottai VG. Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. The Journal of Neuroscience. 2015;35(32):11292–11307. doi: 10.1523/JNEUROSCI.1357-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Klyachko VA. Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. The Journal of Physiology. 2016;594(1):83–97. doi: 10.1113/JP271031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, … Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77(4):696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Sampedro A, Silverman WR, Bautista JF, Richerson GB. Mechanism of increased open probability by a mutation of the BK channel. Journal of Neurophysiology. 2006;96(3):1507–1516. doi: 10.1152/jn.00461.2006. [DOI] [PubMed] [Google Scholar]
- Dobremez E, Bouali-Benazzouz R, Fossat P, Monteils L, Dulluc J, Nagy F, Landry M. Distribution and regulation of L-type calcium channels in deep dorsal horn neurons after sciatic nerve injury in rats. The European Journal of Neuroscience. 2005;21(12):3321–3333. doi: 10.1111/j.1460-9568.2005.04177.x. [DOI] [PubMed] [Google Scholar]
- Doi M, Ishida A, Miyake A, Sato M, Komatsu R, Yamazaki F, …Okamura H. Circadian regulation of intracellular G-protein signalling mediates inter-cellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nature Communications. 2011;2:327. doi: 10.1038/ncomms1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelier J, Braun ST, Dolzhanskaya N, Ahrendt E, Braun AP, Velinov M, Braun JE. Increased expression of the large conductance, calcium-activated K+ (BK) channel in adult-onset neuronal ceroid lipofuscinosis. PloS One. 2015;10(4):e0125205. doi: 10.1371/journal.pone.0125205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca(2+)-activated K+ channels in isolated neurohypophysial terminals. Molecular Pharmacology. 1996;49(1):40–48. [PubMed] [Google Scholar]
- Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN. Rat supra-optic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. The Journal of Physiology. 1999;519(Pt. 1):101–114. doi: 10.1111/j.1469-7793.1999.0101o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, …Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nature Genetics. 2005;37(7):733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, … Foroud T. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcoholism, Clinical and Experimental Research. 2010;34(5):840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. The Journal of Physiology. 2003;548(Pt. 1):53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbers JD, Anderson D, Zamponi GW, Turner RW. Signal processing by T-type calcium channel interactions in the cerebellum. Frontiers in Cellular Neuroscience. 2013;7:230. doi: 10.3389/fncel.2013.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolinsky B, Arshadmansab MF, Pacheco Otalora LF, Zarei MM, Garrido-Sanabria ER. Deficit of Kcnma1 mRNA expression in the dentate gyrus of epileptic rats. Neuroreport. 2008;19(13):1291–1294. doi: 10.1097/WNR.0b013e3283094bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolinsky BS, Skinner F, Garcia I, Arshadmansab MF, Otalora LF, Zarei MM, Garrido-Sanabria ER. Upregulation of STREX splice variant of the large conductance Ca2+-activated potassium (BK) channel in a rat model of mesial temporal lobe epilepsy. Neuroscience Research. 2011;69(1):73–80. doi: 10.1016/j.neures.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. The Journal of Neuroscience. 2011;31(26):9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MS, Li Y, Faingold C. Inferior colliculus intracellular response abnormalities in vitro associated with susceptibility to ethanol withdrawal seizures. Alcoholism, Clinical and Experimental Research. 2000;24(8):1180–1186. [PubMed] [Google Scholar]
- Evanson KW, Bannister JP, Leo MD, Jaggar JH. LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circulation Research. 2014;115(4):423–431. doi: 10.1161/CIRCRESAHA.115.303407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. The Journal of Neuroscience. 2002;22(5):1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. The Journal of Physiology. 2003;552(Pt. 2):483–497. doi: 10.1113/jphysiol.2003.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/micro-domains. Neuron. 2008;59(6):873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Meijer JH, Michel S. Age-related changes in large-conductance calcium-activated potassium channels in mammalian circadian clock neurons. Neurobiology of Aging. 2015;36(6):2176–2183. doi: 10.1016/j.neurobiolaging.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Feinberg-Zadek PL, Treistman SN. Beta-subunits are important modulators of the acute response to alcohol in human BK channels. Alcoholism, Clinical and Experimental Research. 2007;31(5):737–744. doi: 10.1111/j.1530-0277.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- Fernandez MP, Chu J, Villella A, Atkinson N, Kay SA, Ceriani MF. Impaired clock output by altered connectivity in the circadian network. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5650–5655. doi: 10.1073/pnas.0608260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, …Sudhof TC. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron. 2004;42(2):237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature Neuroscience. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Ford KJ, Davis GW. Archaerhodopsin voltage imaging: Synaptic calcium and BK channels stabilize action potential repolarization at the Drosophila neuromuscular junction. The Journal of Neuroscience. 2014;34(44):14517–14525. doi: 10.1523/JNEUROSCI.2203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa N, Takasusuki T, Fukushima T, Hori Y. Presynaptic large-conductance calcium-activated potassium channels control synaptic transmission in the superficial dorsal horn of the mouse. Neuroscience Letters. 2008;444(1):79–82. doi: 10.1016/j.neulet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Gao YD, Shen DM. Patent No. WO2006044232 A1 Compositions ophtalmiques servant a traiter l’hypertension oculaire. 2006
- Garcia ML, Galvez A, Garcia-Calvo M, King VF, Vazquez J, Kaczorowski GJ. Use of toxins to study potassium channels. Journal of Bioenergetics and Biomembranes. 1991;23(4):615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Knaus HG, Munujos P, Slaughter RS, Kaczorowski GJ. Charybdotoxin and its effects on potassium channels. The American Journal of Physiology. 1995;269(1 Pt 1):C1–C10. doi: 10.1152/ajpcell.1995.269.1.C1. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Al-Hasan YM, Larios LE, Bohm RA, Atkinson NS. slo K(+) channel gene regulation mediates rapid drug tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17276–17281. doi: 10.1073/pnas.0405584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Krishnan HR, Atkinson NS. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addiction Biology. 2014;19(3):332–337. doi: 10.1111/j.1369-1600.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Pohl JB, Wang Y, Atkinson NS. BK channels play a counter-adaptive role in drug tolerance and dependence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(37):16360–16365. doi: 10.1073/pnas.1005439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho M, Ganetzky B. Analysis of repolarization of presynaptic motor terminals in Drosophila larvae using potassium-channel-blocking drugs and mutations. The Journal of Experimental Biology. 1992;170:93–111. doi: 10.1242/jeb.170.1.93. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Firing properties of GABAergic versus non-GABAergic vestibular nucleus neurons conferred by a differential balance of potassium currents. Journal of Neurophysiology. 2007;97(6):3986–3996. doi: 10.1152/jn.00141.2007. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Moghadam SH, du Lac S. Mechanisms of sustained high firing rates in two classes of vestibular nucleus neurons: Differential contributions of resurgent Na, Kv3, and BK currents. Journal of Neurophysiology. 2010;104(3):1625–1634. doi: 10.1152/jn.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MA, Kaczorowski GJ, Monaghan RL, Strohl WR, Tkacz JS. Patent No. WO2003105868 A1 Novel maxi-k channel blockers, methods of use and process for making the same. 2003
- Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. The Journal of Neuroscience. 1999;19(20):8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418(6895):326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gorini G, Ponomareva O, Shores KS, Person MD, Harris RA, Mayfield RD. Dynamin-1 co-associates with native mouse brain BKCa channels: Proteomics analysis of synaptic protein complexes. FEBS Letters. 2010;584(5):845–851. doi: 10.1016/j.febslet.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Norris AJ, Carrasquillo Y, Nerbonne JM, Herzog ED. I(A) channels encoded by Kv1.4 and Kv4.2 regulate neuronal firing in the suprachiasmatic nucleus and circadian rhythms in locomotor activity. The Journal of Neuroscience. 2012;32(29):10045–10052. doi: 10.1523/JNEUROSCI.0174-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Bonnan A, Frick A. Back-propagation of physiological action potential output in dendrites of slender-tufted L5A pyramidal neurons. Frontiers in Cellular Neuroscience. 2010;4:13. doi: 10.3389/fncel.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Jr, Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, …Yeola SW. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nature Medicine. 2001;7(4):471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE, Diamond JS. BK channels modulate pre-and postsynaptic signaling at reciprocal synapses in retina. Nature Neuroscience. 2009;12(5):585–592. doi: 10.1038/nn.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Kaufmann WA. Coassembly of big conductance Ca2+-activated K+ channels and L-type voltage-gated Ca2+ channels in rat brain. The Journal of Biological Chemistry. 2004;279(35):36445–36453. doi: 10.1074/jbc.M402254200. [DOI] [PubMed] [Google Scholar]
- Gruss M, Hempelmann G, Scholz A. Trichloroethanol alters action potentials in a subgroup of primary sensory neurones. Neuroreport. 2002;13(6):853–856. doi: 10.1097/00001756-200205070-00023. [DOI] [PubMed] [Google Scholar]
- Gruss M, Henrich M, Konig P, Hempelmann G, Vogel W, Scholz A. Ethanol reduces excitability in a subgroup of primary sensory neurons by activation of BK(Ca) channels. The European Journal of Neuroscience. 2001;14(8):1246–1256. doi: 10.1046/j.0953-816x.2001.01754.x. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. The Journal of Physiology. 2007;580(Pt. 3):859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Heo MS, Park CS. Functional effects of auxiliary beta4-subunit on rat large-conductance Ca(2+)-activated K(+) channel. Biophysical Journal. 2004;86(5):2871–2882. doi: 10.1016/S0006-3495(04)74339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidi A, Beurg M, Dulon D. Localization and developmental expression of BK channels in mammalian cochlear hair cells. Neuroscience. 2005;130(2):475–484. doi: 10.1016/j.neuroscience.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Haghdoost-Yazdi H, Janahmadi M, Behzadi G. Iberiotoxin-sensitive large conductance Ca2+-dependent K+ (BK) channels regulate the spike configuration in the burst firing of cerebellar Purkinje neurons. Brain Research. 2008;1212:1–8. doi: 10.1016/j.brainres.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Han S, Yang BZ, Kranzler HR, Liu X, Zhao H, Farrer LA, … Gelernter J. Integrating GWASs and human protein interaction networks identifies a gene subnetwork underlying alcohol dependence. American Journal of Human Genetics. 2013;93(6):1027–1034. doi: 10.1016/j.ajhg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290(5492):739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kawaji K, Sun L, Zhang X, Koyano K, Yokoyama T, …Nakanishi H. Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. The Journal of Neuroscience. 2011;31(48):17370–17382. doi: 10.1523/JNEUROSCI.4152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert B, Pietropaolo S, Meme S, Laudier B, Laugeray A, Doisne N, …Briault S. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet Journal of Rare Diseases. 2014;9:124. doi: 10.1186/s13023-014-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Chen X, Levine JD. GDNF induces mechanical hyperalgesia in muscle by reducing I(BK) in isolectin B4-positive nociceptors. Neuroscience. 2012;219:204–213. doi: 10.1016/j.neuroscience.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne J, Jeserich G. Maturation of spiking activity in trout retinal ganglion cells coincides with upregulation of Kv3.1- and BK-related potassium channels. Journal of Neuroscience Research. 2004;75(1):44–54. doi: 10.1002/jnr.10830. [DOI] [PubMed] [Google Scholar]
- Hicks GA, Marrion NV. Ca2+-dependent inactivation of large conductance Ca2+-activated K+ (BK) channels in rat hippocampal neurones produced by pore block from an associated particle. The Journal of Physiology. 1998;508(Pt. 3):721–734. doi: 10.1111/j.1469-7793.1998.721bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JJ, Hao J, Kosofsky BE, Rajadhyaksha AM. Dysregulation of large-conductance Ca2+-activated K+ channel expression in nonsyndromal mental retardation due to a cereblon p.R419X mutation. Neurogenetics. 2008;9(3):219–223. doi: 10.1007/s10048-008-0128-2. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Tal AL, Sun X, Hauck SC, Hao J, Kosofosky BE, Rajadhyaksha AM. Temporal and spatial mouse brain expression of cereblon, an ionic channel regulator involved in human intelligence. Journal of Neurogenetics. 2010;24(1):18–26. doi: 10.3109/01677060903567849. [DOI] [PubMed] [Google Scholar]
- Hirono M, Ogawa Y, Misono K, Zollinger DR, Trimmer JS, Rasband MN, Misonou H. BK channels localize to the paranodal junction and regulate action potentials in myelinated axons of cerebellar Purkinje cells. The Journal of Neuroscience. 2015;35(18):7082–7094. doi: 10.1523/JNEUROSCI.3778-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff K, Kovalchuk Y, Yuste R, Konnerth A. Single-shock LTD by local dendritic spikes in pyramidal neurons of mouse visual cortex. The Journal of Physiology. 2004;560(Pt. 1):27–36. doi: 10.1113/jphysiol.2004.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Jeong B, Min CH, Lee KJ. Circadian waves of cytosolic calcium concentration and long-range network connections in rat suprachiasmatic nucleus. The European Journal of Neuroscience. 2012;35(9):1417–1425. doi: 10.1111/j.1460-9568.2012.08069.x. [DOI] [PubMed] [Google Scholar]
- Hu S, Labuda MZ, Pandolfo M, Goss GG, McDermid HE, Ali DW. Variants of the KCNMB3 regulatory subunit of maxi BK channels affect channel inactivation. Physiological Genomics. 2003;15(3):191–198. doi: 10.1152/physiolgenomics.00110.2003. [DOI] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, …Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. The Journal of Neuroscience. 2001;21(24):9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Ikeda M. Bmal1 is an essential regulator for circadian cytosolic Ca(2)(+) rhythms in suprachiasmatic nucleus neurons. The Journal of Neuroscience. 2014;34(36):12029–12038. doi: 10.1523/JNEUROSCI.5158-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38(2):253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE. The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. The Journal of Pharmacology and Experimental Therapeutics. 2008;327(3):657–664. doi: 10.1124/jpet.108.143933. [DOI] [PubMed] [Google Scholar]
- Indriati DW, Kamasawa N, Matsui K, Meredith AL, Watanabe M, Shigemoto R. Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: Somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. The Journal of Neuroscience. 2013;33(8):3668–3678. doi: 10.1523/JNEUROSCI.2921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari A, Noursadeghi E, Khodagholi F, Saghiri R, Sauve R, Aliaghaei A, Eliassi A. Brain mitochondrial ATP-insensitive large conductance Ca(+)(2)-activated K (+) channel properties are altered in a rat model of amyloid-beta neurotoxicity. Experimental Neurology. 2015;269:8–16. doi: 10.1016/j.expneurol.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Jeffries O, Geiger N, Rowe IC, Tian L, McClafferty H, Chen L, …Shipston MJ. Palmitoylation of the S0–S1 linker regulates cell surface expression of voltage-and calcium-activated potassium (BK) channels. The Journal of Biological Chemistry. 2010;285(43):33307–33314. doi: 10.1074/jbc.M110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS. BMS-204352: A potassium channel opener developed for the treatment of stroke. CNS Drug Reviews. 2002;8(4):353–360. doi: 10.1111/j.1527-3458.2002.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel beta-subunit genes: Cloning and characterization. Genomics. 1999;55(1):57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- Jin W, Sugaya A, Tsuda T, Ohguchi H, Sugaya E. Relationship between large conductance calcium-activated potassium channel and bursting activity. Brain Research. 2000;860(1–2):21–28. doi: 10.1016/s0006-8993(00)01943-0. [DOI] [PubMed] [Google Scholar]
- Jo S, Lee KH, Song S, Jung YK, Park CS. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. Journal of Neurochemistry. 2005;94(5):1212–1224. doi: 10.1111/j.1471-4159.2005.03344.x. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annual Review of Physiology. 2014;76:333–363. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Development of BK channels in neocortical pyramidal neurons. Journal of Neurophysiology. 1996;76(1):188–198. doi: 10.1152/jn.1996.76.1.188. [DOI] [PubMed] [Google Scholar]
- Kathiresan T, Harvey M, Orchard S, Sakai Y, Sokolowski B. A protein interaction network for the large conductance Ca(2+)-activated K(+) channel in the mouse cochlea. Molecular & Cellular Proteomics. 2009;8(8):1972–1987. doi: 10.1074/mcp.M800495-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Shinohara A, Fujimoto S, Kume T, Akaike A. Tetra-ethylammonium exacerbates ischemic neuronal injury in rat cerebrocortical slice cultures. European Journal of Pharmacology. 2005;508(1–3):85–91. doi: 10.1016/j.ejphar.2004.11.058. [DOI] [PubMed] [Google Scholar]
- Kaufmann WA, Ferraguti F, Fukazawa Y, Kasugai Y, Shigemoto R, Laake P, … Ottersen OP. Large-conductance calcium-activated potassium channels in Purkinje cell plasma membranes are clustered at sites of hypolemmal microdomains. The Journal of Comparative Neurology. 2009;515(2):215–230. doi: 10.1002/cne.22066. [DOI] [PubMed] [Google Scholar]
- Kaufmann WA, Kasugai Y, Ferraguti F, Storm JF. Two distinct pools of large-conductance calcium-activated potassium channels in the somatic plasma membrane of central principal neurons. Neuroscience. 2010;169(3):974–986. doi: 10.1016/j.neuroscience.2010.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kalsi G, Holmans PA, Sanders AR, Aggen SH, Dick DM, … Gejman PV. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcoholism, Clinical and Experimental Research. 2011;35(5):963–975. doi: 10.1111/j.1530-0277.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Jo S, Song HJ, Park ZY, Park CS. Myelin basic protein as a binding partner and calmodulin adaptor for the BKCa channel. Proteomics. 2007;7(15):2591–2602. doi: 10.1002/pmic.200700185. [DOI] [PubMed] [Google Scholar]
- Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience. 2007;146(4):1652–1661. doi: 10.1016/j.neuroscience.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. Journal of Neurophysiology. 2007;97(5):3508–3516. doi: 10.1152/jn.00009.2007. [DOI] [PubMed] [Google Scholar]
- Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, … Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33(19):5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, …Sperk G. Distribution of high-conductance Ca(2+)-activated K+ channels in rat brain: Targeting to axons and nerve terminals. The Journal of Neuroscience. 1996;16(3):955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(21):E1387–E1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifeldt M, Cates-Gatto C, Roberts AJ, Contet C. BK channel beta1 subunit contributes to behavioral adaptations elicited by chronic intermittent ethanol exposure. Alcoholism, Clinical and Experimental Research. 2015;39(12):2394–2402. doi: 10.1111/acer.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifeldt M, Le D, Treistman SN, Koob GF, Contet C. BK channel beta1 and beta4 auxiliary subunits exert opposite influences on escalated ethanol drinking in dependent mice. Frontiers in Integrative Neuroscience. 2013;7:105. doi: 10.3389/fnint.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394(6690):281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Ksiazek A, Ladno W, Szulczyk B, Grzelka K, Szulczyk P. Properties of BK-type Ca(+) (+)-dependent K(+) channel currents in medial prefrontal cortex pyramidal neurons in rats of different ages. Frontiers in Cellular Neuroscience. 2013;7:185. doi: 10.3389/fncel.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: Critical for input and output of the circadian system. The Journal of Neuroscience. 2011;31(8):2746–2755. doi: 10.1523/JNEUROSCI.5792-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler D, Zhang H, Ren X, Tanouye MA. Genetic suppression of seizure susceptibility in Drosophila. Journal of Neurophysiology. 2001;86(3):1211–1225. doi: 10.1152/jn.2001.86.3.1211. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. The European Journal of Neuroscience. 2004;20(4):1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Hagiwara A, Fukazawa Y, Lujan R, Saito H, …Shigemoto R. Immunocytochemical localization of the alpha 1A subunit of the P/Q-type calcium channel in the rat cerebellum. The European Journal of Neuroscience. 2004;19(8):2169–2178. doi: 10.1111/j.0953-816X.2004.03319.x. [DOI] [PubMed] [Google Scholar]
- Kyle BD, Ahrendt E, Braun AP, Braun JE. The large conductance, calcium-activated K+ (BK) channel is regulated by cysteine string protein. Scientific Reports. 2013;3:2447. doi: 10.1038/srep02447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. The Journal of Physiology. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P, Grunder S, Rusch A. Expression of Ca2+-activated BK channel mRNA and its splice variants in the rat cochlea. The Journal of Comparative Neurology. 2003;455(2):198–209. doi: 10.1002/cne.10471. [DOI] [PubMed] [Google Scholar]
- Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. The Journal of Comparative Neurology. 2013;521(15):3389–3405. doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Roger S, Guerin P, Molinari F, M’Rad R, Cahard D, …Briault S. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. The American Journal of Psychiatry. 2006;163(9):1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Experimental Brain Research. 2002;145(4):457–467. doi: 10.1007/s00221-002-1127-4. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Lee US, Cui J. {beta} Subunit-specific modulations of BK channel function by a mutation associated with epilepsy and dyskinesia. The Journal of Physiology. 2009;587(Pt. 7):1481–1498. doi: 10.1113/jphysiol.2009.169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ueda A, Wu CF. Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and shaker K+ channel mutations in Drosophila. Neuroscience. 2008;154(4):1283–1296. doi: 10.1016/j.neuroscience.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, …Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li M, Chang S, Yang L, Shi J, McFarland K, Yang X, …Cui J. Conopeptide Vt3.1 preferentially inhibits BK potassium channels containing beta4 subunits via electrostatic interactions. The Journal of Biological Chemistry. 2014;289(8):4735–4742. doi: 10.1074/jbc.M113.535898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gao SB, Lv CX, Wu Y, Guo ZH, Ding JP, Xu T. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. Journal of Cellular Physiology. 2007;212(2):348–357. doi: 10.1002/jcp.21007. [DOI] [PubMed] [Google Scholar]
- Li Q, Madison R, Moore SD. Presynaptic BK channels modulate ethanol-induced enhancement of GABAergic transmission in the rat central amygdala nucleus. The Journal of Neuroscience. 2014;34(41):13714–13724. doi: 10.1523/JNEUROSCI.5284-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Zhang YJ, Zhou L, Han F, Wang MY, Xue MQ, Qi Z. Chlorpromazine confers neuroprotection against brain ischemia by activating BKCa channel. European Journal of Pharmacology. 2014;735:38–43. doi: 10.1016/j.ejphar.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Liao Y, Kristiansen AM, Oksvold CP, Tuvnes FA, Gu N, Runden-Pran E, … Storm JF. Neuronal Ca2+-activated K+ channels limit brain infarction and promote survival. PloS One. 2010;5(12):e15601. doi: 10.1371/journal.pone.0015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ye J, Zou X, Xu Z, Feng Y, Zou X, …Cang Y. CRL4A(CRBN) E3 ubiquitin ligase restricts BK channel activity and prevents epileptogenesis. Nature Communications. 2014;5:3924. doi: 10.1038/ncomms4924. [DOI] [PubMed] [Google Scholar]
- Liu JM, Yi Z, Liu SZ, Chang JH, Dang XB, Li QY, Zhang YL. The mitochondrial division inhibitor mdivi-1 attenuates spinal cord ischemia-reperfusion injury both in vitro and in vivo: Involvement of BK channels. Brain Research. 2015;1619:155–165. doi: 10.1016/j.brainres.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Lima PA, Marrion NV. Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. Journal of Cell Science. 2007;120(Pt. 6):985–995. doi: 10.1242/jcs.03399. [DOI] [PubMed] [Google Scholar]
- Lorenz S, Heils A, Kasper JM, Sander T. Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144(1):10–13. doi: 10.1002/ajmg.b.30369. [DOI] [PubMed] [Google Scholar]
- Lu R, Lukowski R, Sausbier M, Zhang DD, Sisignano M, Schuh CD, … Schmidtko A. BKCa channels expressed in sensory neurons modulate inflammatory pain in mice. Pain. 2014;155(3):556–565. doi: 10.1016/j.pain.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Ly C, Melman T, Barth AL, Ermentrout GB. Phase-resetting curve determines how BK currents affect neuronal firing. Journal of Computational Neuroscience. 2011;30(2):211–223. doi: 10.1007/s10827-010-0246-3. [DOI] [PubMed] [Google Scholar]
- Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Letters. 2007;581(5):1000–1008. doi: 10.1016/j.febslet.2007.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SH, Ruth P, Knaus HG, Shipston MJ. Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS. BMC Developmental Biology. 2006;6:37. doi: 10.1186/1471-213X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275(5297):209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. The Journal of Physiology. 2003;548(Pt. 2):383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero HG, Treistman SN, Lemos JR. Ethanol effect on BK channels is modulated by magnesium. Alcoholism, Clinical and Experimental Research. 2015;39(9):1671–1679. doi: 10.1111/acer.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395(6705):900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Martin GE, Hendrickson LM, Penta KL, Friesen RM, Pietrzykowski AZ, Tapper AR, Treistman SN. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17543–17548. doi: 10.1073/pnas.0801068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, Treistman S. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. The Journal of Neuroscience. 2004;24(29):6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Espinosa PL, Yang C, Gonzalez-Perez V, Xia XM, Lingle CJ. Knockout of the BK beta2 subunit abolishes inactivation of BK currents in mouse adrenal chromaffin cells and results in slow-wave burst activity. The Journal of General Physiology. 2014;144(4):275–295. doi: 10.1085/jgp.201411253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire M, Barrese V, D’Amico M, Iannotti FA, Pizzarelli R, Samengo I, … Taglialatela M. Pre-synaptic BK channels selectively control glutamate versus GABA release from cortical and hippocampal nerve terminals. Journal of Neurochemistry. 2010;115(2):411–422. doi: 10.1111/j.1471-4159.2010.06938.x. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(39):15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CE, McClafferty H, Huibant JM, Rowan EG, Shipston MJ, Rowe IC. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel alpha-subunits. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17870–17876. doi: 10.1073/pnas.0505270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon JG. Inactivation of a high conductance calcium dependent potassium current in rat hippocampal neurons. Neuroscience Letters. 1995;193(1):5–8. doi: 10.1016/0304-3940(95)11651-c. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(10):5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehranfard N, Gholamipour-Badie H, Motamedi F, Janahmadi M, Naderi N. The effect of paxilline on early alterations of electrophysiological properties of dentate gyrus granule cells in pilocarpine-treated rats. Iranian Journal of Pharmaceutical Research. 2014;13(Suppl):125–132. [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nature Neuroscience. 2006;9(8):1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, … Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. The Journal of Comparative Neurology. 2006;496(3):289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Slaughter MM. Calcium-induced transitions between the spontaneous miniature outward and the transient outward currents in retinal amacrine cells. The Journal of General Physiology. 2002a;119(4):373–388. doi: 10.1085/jgp.20028479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Slaughter MM. Mechanism of generation of spontaneous miniature outward currents (SMOCs) in retinal amacrine cells. The Journal of General Physiology. 2002b;119(4):355–372. doi: 10.1085/jgp.20028478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. Aging human circadian rhythms: Conventional wisdom may not always be right. Journal of Biological Rhythms. 2005;20(4):366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- Montgomery JR, Meredith AL. Genetic activation of BK currents in vivo generates bidirectional effects on neuronal excitability. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):18997–19002. doi: 10.1073/pnas.1205573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JR, Whitt JP, Wright BN, Lai MH, Meredith AL. Mis-expression of the BK K(+) channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. American Journal of Physiology. Cell Physiology. 2013;304(4):C299–C311. doi: 10.1152/ajpcell.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. The suprachiasmatic nucleus and the circadian timing system. Progress in Molecular Biology and Translational Science. 2013;119:1–28. doi: 10.1016/B978-0-12-396971-2.00001-4. [DOI] [PubMed] [Google Scholar]
- Mori A, Suzuki S, Sakamoto K, Nakahara T, Ishii K. BMS-191011, an opener of large-conductance Ca2+-activated potassium channels, dilates rat retinal arterioles in vivo. Biological & Pharmaceutical Bulletin. 2011;34(1):150–152. doi: 10.1248/bpb.34.150. [DOI] [PubMed] [Google Scholar]
- Muller A, Kukley M, Uebachs M, Beck H, Dietrich D. Nanodomains of single Ca2+ channels contribute to action potential repolarization in cortical neurons. The Journal of Neuroscience. 2007;27(3):483–495. doi: 10.1523/JNEUROSCI.3816-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Mas Gomez N, Ruth P, Strauss O. CaV1.3 L-type channels, maxiK Ca(2+)-dependent K(+) channels and bestrophin-1 regulate rhythmic photoreceptor outer segment phagocytosis by retinal pigment epithelial cells. Cellular Signalling. 2014;26(5):968–978. doi: 10.1016/j.cellsig.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, … Klyachko VA. Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(4):949–956. doi: 10.1073/pnas.1423094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron. 1998;20(3):389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46(4):623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40(3):609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Morad M. Alcohol withdrawal is associated with a down-regulation of large-conductance Ca(2)(+)-activated K(+) channels in rat inferior colliculus neurons. Psychopharmacology. 2014;231(9):2009–2018. doi: 10.1007/s00213-013-3346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskova L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, …Kmoch S. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. American Journal of Human Genetics. 2011;89(2):241–252. doi: 10.1016/j.ajhg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P, Gorelik J, Vivekananda U, Shevchuk AI, Ermolyuk YS, Bailey RJ, … Korchev YE. Nanoscale-targeted patch-clamp recordings of functional presynaptic ion channels. Neuron. 2013;79(6):1067–1077. doi: 10.1016/j.neuron.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Flores R, Salzberg BM. Calcium channels that are required for secretion from intact nerve terminals of vertebrates are sensitive to omega-conotoxin and relatively insensitive to dihydropyridines. Optical studies with and without voltage-sensitive dyes. The Journal of General Physiology. 1989;93(4):715–729. doi: 10.1085/jgp.93.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Szabo Z, Bourinet E, Flucher BE. Differential targeting of the L-type Ca2+ channel alpha 1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. The European Journal of Neuroscience. 2004;19(8):2109–2122. doi: 10.1111/j.0953-816X.2004.03272.x. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, … LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oh KH, Abraham LS, Gegg C, Silvestri C, Huang YC, Alkema MJ, …Kim H. Presynaptic BK channel localization is dependent on the hierarchical organization of alpha-catulin and dystrobrevin and fine-tuned by CaV2 calcium channels. BMC Neuroscience. 2015;16:26. doi: 10.1186/s12868-015-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley D, Harvey J. MAPK-dependent actin cytoskeletal reorganization underlies BK channel activation by insulin. The European Journal of Neuroscience. 2007;25(3):673–682. doi: 10.1111/j.1460-9568.2007.05347.x. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Irving AJ, Harvey J. Leptin-induced dynamic alterations in the actin cytoskeleton mediate the activation and synaptic clustering of BK channels. FASEB Journal. 2005;19(13):1917–1919. doi: 10.1096/fj.05-4166fje. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320(5878):949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, …Garrido-Sanabria ER. Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Research. 2008;1200:116–131. doi: 10.1016/j.brainres.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. American Journal of Physiology. Heart and Circulatory Physiology. 2010;299(6):H2009–H2017. doi: 10.1152/ajpheart.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio S, Velazquez-Marrero C, Marrero HG, Seale GE, Yudowski GA, Treistman SN. Time-dependent effects of ethanol on BK channel expression and trafficking in hippocampal neurons. Alcoholism, Clinical and Experimental Research. 2015;39(9):1619–1631. doi: 10.1111/acer.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, … Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Pattillo JM, Yazejian B, DiGregorio DA, Vergara JL, Grinnell AD, Meriney SD. Contribution of presynaptic calcium-activated potassium currents to transmitter release regulation in cultured Xenopus nerve-muscle synapses. Neuroscience. 2001;102(1):229–240. doi: 10.1016/s0306-4522(00)00453-x. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron. 2006;52(3):497–510. doi: 10.1016/j.neuron.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Pelucchi B, Grimaldi A, Moriondo A. Vertebrate rod photoreceptors express both BK and IK calcium-activated potassium channels, but only BK channels are involved in receptor potential regulation. Journal of Neuroscience Research. 2008;86(1):194–201. doi: 10.1002/jnr.21467. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Tepikin A, Park MK. The endoplasmic reticulum: One continuous or several separate Ca(2+) stores? Trends in Neurosciences. 2001;24(5):271–276. doi: 10.1016/s0166-2236(00)01787-2. [DOI] [PubMed] [Google Scholar]
- Petrik D, Brenner R. Regulation of STREX exon large conductance, calcium-activated potassium channels by the beta4 accessory subunit. Neuroscience. 2007;149(4):789–803. doi: 10.1016/j.neuroscience.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D, Wang B, Brenner R. Modulation by the BK accessory beta4 subunit of phosphorylation-dependent changes in excitability of dentate gyrus granule neurons. The European Journal of Neuroscience. 2011;34(5):695–704. doi: 10.1111/j.1460-9568.2011.07799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, …Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: Decreased ethanol potentiation and decreased channel density. The Journal of Neuroscience. 2004;24(38):8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Research. 2006;1071(1):54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Piwonska M, Wilczek E, Szewczyk A, Wilczynski GM. Differential distribution of Ca2+-activated potassium channel beta4 subunit in rat brain: Immunolocalization in neuronal mitochondria. Neuroscience. 2008;153(2):446–460. doi: 10.1016/j.neuroscience.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. The Journal of Neuroscience. 1999;19(13):5205–5212. doi: 10.1523/JNEUROSCI.19-13-05205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, Klaerke DA. Differential expression of BK channel isoforms and beta-subunits in rat neurovascular tissues. Biochimica et Biophysica Acta. 2009;1788(2):380–389. doi: 10.1016/j.bbamem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lingle CJ. BK channel activation by brief depolarizations requires Ca2+ influx through L- and Q-type Ca2+ channels in rat chromaffin cells. Journal of Neurophysiology. 1999;81(5):2267–2278. doi: 10.1152/jn.1999.81.5.2267. [DOI] [PubMed] [Google Scholar]
- Pratt CP, He J, Wang Y, Barth AL, Bruchez MP. Fluorogenic green-inside red-outside (GIRO) labeling approach reveals adenylyl cyclase-dependent control of BKalpha surface expression. Bioconjugate Chemistry. 2015;26(9):1963–1971. doi: 10.1021/acs.bioconjchem.5b00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Research. 2002;956(2):183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Harley EA, Duhault J, Vanhoutte PM, Feletou M. K+ channels in cultured bovine retinal pericytes: Effects of beta-adrenergic stimulation. Journal of Cardiovascular Pharmacology. 2003;42(3):379–388. doi: 10.1097/00005344-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. The Journal of Physiology. 2004;557(Pt. 1):147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre JA. Functional upregulation of Ca(2+)-activated K(+) channels in the development of substantia nigra dopamine neurons. PloS One. 2012;7(12):e51610. doi: 10.1371/journal.pone.0051610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz EA, Hausser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. The Journal of Neuroscience. 2006;26(20):5428–5437. doi: 10.1523/JNEUROSCI.5284-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehak R, Bartoletti TM, Engbers JD, Berecki G, Turner RW, Zamponi GW. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PloS One. 2013;8(4):e61844. doi: 10.1371/journal.pone.0061844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart PH, Chung S, Levitan IB. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989;2(1):1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Riazi MA, Brinkman-Mills P, Johnson A, Naylor SL, Minoshima S, Shimizu N, …McDermid HE. Identification of a putative regulatory subunit of a calcium-activated potassium channel in the dup(3q) syndrome region and a related sequence on 22q11.2. Genomics. 1999;62(1):90–94. doi: 10.1006/geno.1999.5975. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. Journal of Neuroscience. 1992;12(1):297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11(4):645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Romani AM. Magnesium homeostasis and alcohol consumption. Magnesium Research. 2008;21(4):197–204. [PubMed] [Google Scholar]
- Runden-Pran E, Haug FM, Storm JF, Ottersen OP. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: A study in organotypical hippocampal slice cultures. Neuroscience. 2002;112(2):277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Kogler M, Chen L, Sausbier U, Ottersen OP, … Knaus HG. Immunolocalization of BK channels in hippocampal pyramidal neurons. The European Journal of Neuroscience. 2006;24(2):442–454. doi: 10.1111/j.1460-9568.2006.04936.x. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Ishikane H, Tachibana M. Ca2+-activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neuroscience Research. 1997;27(3):219–228. doi: 10.1016/s0168-0102(97)01155-3. [DOI] [PubMed] [Google Scholar]
- Sakagami Y, Yamamoto K, Sugiura S, Inokuchi K, Hayashi T, Kato N. Essential roles of Homer-1a in homeostatic regulation of pyramidal cell excitability: A possible link to clinical benefits of electroconvulsive shock. The European Journal of Neuroscience. 2005;21(12):3229–3239. doi: 10.1111/j.1460-9568.2005.04165.x. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Sokolowski B. The large conductance calcium-activated potassium channel affects extrinsic and intrinsic mechanisms of apoptosis. Journal of Neuroscience Research. 2015;93(5):745–754. doi: 10.1002/jnr.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann M, Seidel KN, Bernard R, Pruss H, Veh RW, Derst C. BKbeta1 subunits contribute to BK channel diversity in rat hypothalamic neurons. Cellular and Molecular Neurobiology. 2010;30(6):967–976. doi: 10.1007/s10571-010-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake H, Saunders JC, Greene MI, Navaratnam DS. Ca(2+) and K(+) (BK) channels in chick hair cells are clustered and colocalized with apical-basal and tonotopic gradients. The Journal of Physiology. 2004;560(Pt. 1):13–20. doi: 10.1113/jphysiol.2004.069856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samengo I, Curro D, Barrese V, Taglialatela M, Martire M. Large conductance calcium-activated potassium channels: Their expression and modulation of glutamate release from nerve terminals isolated from rat trigeminal caudal nucleus and cerebral cortex. Neurochemical Research. 2014;39(5):901–910. doi: 10.1007/s11064-014-1287-1. [DOI] [PubMed] [Google Scholar]
- Santos AR, Trentin AP, Ferreira J, Yunes RA, Calixto JB. Mechanisms involved in the antinociception caused by compound MV8612 isolated from Mandevilla velutina in mice. Brain Research. 2003;961(2):269–276. doi: 10.1016/s0006-8993(02)03968-9. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos CD, McCallum JB, Rigaud M, Fuchs A, Kwok WM, Hogan QH. Opposing effects of spinal nerve ligation on calcium-activated potassium currents in axotomized and adjacent mammalian primary afferent neurons. Brain Research. 2007;1132(1):84–99. doi: 10.1016/j.brainres.2006.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, …Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier U, Sausbier M, Sailer CA, Arntz C, Knaus HG, Neuhuber W, Ruth P. Ca2+-activated K+ channels of the BK-type in the mouse brain. Histochemistry and Cell Biology. 2006;125(6):725–741. doi: 10.1007/s00418-005-0124-7. [DOI] [PubMed] [Google Scholar]
- Savinaa TA, Levina SG, Poletaevab II, Fedotovab IB, Shchipakinaa TG. Audiogenic kindling changes the subunit composition of BK channels in dentate gyrus of Krushinskii–Molodkina rats. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology. 2014;8(1):111–115. [Google Scholar]
- Schilling T, Eder C. Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Research. 2007;1186:21–28. doi: 10.1016/j.brainres.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. Microglial K(+) channel expression in young adult and aged mice. Glia. 2015;63(4):664–672. doi: 10.1002/glia.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. The Journal of Physiology. 1998;513(Pt. 1):55–69. doi: 10.1111/j.1469-7793.1998.055by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry. 1994;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, Brown SA. Testing the level of response to alcohol: Social information processing model of alcoholism risk—A 20-year prospective study. Alcoholism, Clinical and Experimental Research. 2004;28(12):1881–1889. doi: 10.1097/01.alc.0000148111.43332.a5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Wilhelmsen K, Smith TL, Feiler HS, Lind P, Lange LA, Kalmijn J. Autosomal linkage analysis for the level of response to alcohol. Alcoholism, Clinical and Experimental Research. 2005;29(11):1976–1982. doi: 10.1097/01.alc.0000187598.82921.27. [DOI] [PubMed] [Google Scholar]
- Seidel KN, Derst C, Salzmann M, Holtje M, Priller J, Markgraf R, …Pruss H. Expression of the voltage- and Ca2+-dependent BK potassium channel subunits BKbeta1 and BKbeta4 in rodent astrocytes. Glia. 2011;59(6):893–902. doi: 10.1002/glia.21160. [DOI] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servais L, Bearzatto B, Schwaller B, Dumont M, De Saedeleer C, Dan B, … Cheron G. Mono- and dual-frequency fast cerebellar oscillation in mice lacking parvalbumin and/or calbindin D-28k. The European Journal of Neuroscience. 2005;22(4):861–870. doi: 10.1111/j.1460-9568.2005.04275.x. [DOI] [PubMed] [Google Scholar]
- Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(23):9858–9863. doi: 10.1073/pnas.0607037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. The Journal of Physiology. 1999;521(Pt. 1):135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JJ, Benedetti BL, Barth AL. Anticonvulsant effects of the BK-channel antagonist paxilline. Epilepsia. 2009;50(4):711–720. doi: 10.1111/j.1528-1167.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- Shi J, He HQ, Zhao R, Duan YH, Chen J, Chen Y, …Ji YH. Inhibition of martentoxin on neuronal BK channel subtype (alpha+beta4): Implications for a novel interaction model. Biophysical Journal. 2008;94(9):3706–3713. doi: 10.1529/biophysj.107.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh CC, Turner SC, Zhang XF, Milicic I, Parihar A, Jinkerson T, … Gopalakrishnan M. A-272651, a nonpeptidic blocker of large-conductance Ca2+-activated K+ channels, modulates bladder smooth muscle contractility and neuronal action potentials. British Journal of Pharmacology. 2007;151(6):798–806. doi: 10.1038/sj.bjp.0707278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi-zand Z, Ahmad-Molaei L, Motamedi F, Naderi N. The role of potassium BK channels in anticonvulsant effect of cannabidiol in pentylenetetrazole and maximal electroshock models of seizure in mice. Epilepsy & Behavior. 2013;28(1):1–7. doi: 10.1016/j.yebeh.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Shoudai K, Nonaka K, Maeda M, Wang ZM, Jeong HJ, Higashi H, …Akaike N. Effects of various K+ channel blockers on spontaneous glycine release at rat spinal neurons. Brain Research. 2007;1157:11–22. doi: 10.1016/j.brainres.2006.09.097. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem RL, Barth AL. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiology of Disease. 2008;30(3):323–330. doi: 10.1016/j.nbd.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruti S, Urban-Ciecko J, Fitzpatrick JA, Brenner R, Bruchez MP, Barth AL. The brain-specific Beta4 subunit downregulates BK channel cell surface expression. PloS One. 2012;7(3):e33429. doi: 10.1371/journal.pone.0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Li M, Hall L, Chen S, Sukur S, Lu R, …Toro L. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience. 2016;317:76–107. doi: 10.1016/j.neuroscience.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. MitoBK (Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):10836–10841. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skafidas E, Testa R, Zantomio D, Chana G, Everall IP, Pantelis C. Predicting the diagnosis of autism spectrum disorder using gene pathway analysis. Molecular Psychiatry. 2014;19(4):504–510. doi: 10.1038/mp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. Journal of Neurophysiology. 2002;87(4):2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PloS One. 2011;6(12):e28532. doi: 10.1371/journal.pone.0028532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro CR, Lingle CJ. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 1992;257(5077):1694–1698. doi: 10.1126/science.1529355. [DOI] [PubMed] [Google Scholar]
- Song B, Marvizon JC. N-methyl-D-aspartate receptors and large conductance calcium-sensitive potassium channels inhibit the release of opioid peptides that induce mu-opioid receptor internalization in the rat spinal cord. Neuroscience. 2005;136(2):549–562. doi: 10.1016/j.neuroscience.2005.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Comes N, Ferrer E, Morales M, Escalada A, Pales J, …Gasull X. Modulation of aqueous humor outflow by ionic mechanisms involved in trabecular meshwork cell volume regulation. Investigative Ophthalmology & Visual Science. 2004;45(10):3650–3661. doi: 10.1167/iovs.04-0060. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. The Journal of Physiology. 1987a;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Research. 1987b;435(1–2):387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiological Reviews. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Stumpff F, Strauss O, Boxberger M, Wiederholt M. Characterization of maxi-K-channels in bovine trabecular meshwork and their activation by cyclic guanosine monophosphate. Investigative Ophthalmology & Visual Science. 1997;38(9):1883–1892. [PubMed] [Google Scholar]
- Sun X, Gu XQ, Haddad GG. Calcium influx via L- and N-type calcium channels activates a transient large-conductance Ca2+-activated K+ current in mouse neocortical pyramidal neurons. The Journal of Neuroscience. 2003;23(9):3639–3648. doi: 10.1523/JNEUROSCI.23-09-03639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. The Journal of Neuroscience. 2003;23(29):9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto N, Sothilingam V, Euler T, Ruth P, Seeliger MW, Schubert T. BK channels mediate pathway-specific modulation of visual signals in the in vivo mouse retina. The Journal of Neuroscience. 2012;32(14):4861–4866. doi: 10.1523/JNEUROSCI.4654-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Kelly ME. Calcium-activated potassium current in cultured rabbit retinal pigment epithelial cells. Current Eye Research. 1996;15(3):237–246. doi: 10.3109/02713689609007617. [DOI] [PubMed] [Google Scholar]
- Thieme H, Stumpff F, Ottlecz A, Percicot CL, Lambrou GN, Wiederholt M. Mechanisms of action of unoprostone on trabecular meshwork contractility. Investigative Ophthalmology & Visual Science. 2001;42(13):3193–3201. [PubMed] [Google Scholar]
- Tian L, Jeffries O, McClafferty H, Molyvdas A, Rowe IC, Saleem F, … Shipston MJ. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):21006–21011. doi: 10.1073/pnas.0806700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, Toro L, Zarei MM. KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience. 2006;142(3):661–669. doi: 10.1016/j.neuroscience.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: And yet they do move! Trends in Neurosciences. 2005;28(3):133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13(6):1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Typlt M, Mirkowski M, Azzopardi E, Ruettiger L, Ruth P, Schmid S. Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PloS One. 2013;8(11):e81270. doi: 10.1371/journal.pone.0081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, … Swanson R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. The Journal of Biological Chemistry. 2000;275(30):23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- van Welie I, du Lac S. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. Journal of Neurophysiology. 2011;105(4):1651–1659. doi: 10.1152/jn.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez-Martinez MC, Vazquez-Torres R, Rojas LV, Sanabria P, Jimenez-Rivera CA. Alpha-1 adrenoreceptors modulate GABA release onto ventral tegmental area dopamine neurons. Neuropharmacology. 2015;88:110–121. doi: 10.1016/j.neuropharm.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Marrero C, Seale GE, Treistman SN, Martin GE. Large conductance voltage- and Ca2+-gated potassium (BK) channel beta4 subunit influences sensitivity and tolerance to alcohol by altering its response to kinases. The Journal of Biological Chemistry. 2014;289(42):29261–29272. doi: 10.1074/jbc.M114.604306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Marrero C, Wynne P, Bernardo A, Palacio S, Martin G, Treistman SN. The relationship between duration of initial alcohol exposure and persistence of molecular tolerance is markedly nonlinear. The Journal of Neuroscience. 2011;31(7):2436–2446. doi: 10.1523/JNEUROSCI.5429-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velinov M, Dolzhanskaya N, Gonzalez M, Powell E, Konidari I, Hulme W, … Zuchner S. Mutations in the gene DNAJC5 cause autosomal dominant Kufs disease in a proportion of cases: Study of the Parry family and 8 other families. PloS One. 2012;7(1):e29729. doi: 10.1371/journal.pone.0029729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Molecular Neurobiology. 2008;38(2):153–166. doi: 10.1007/s12035-008-8039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Jaffe DB, Brenner R. Current understanding of iberiotoxin-resistant BK channels in the nervous system. Frontiers in Physiology. 2014;5:382. doi: 10.3389/fphys.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kang H, Li Y, Shui Y, Yamamoto R, Sugai T, Kato N. Cognitive recovery by chronic activation of the large-conductance calcium-activated potassium channel in a mouse model of Alzheimer’s disease. Neuropharmacology. 2015;92:8–15. doi: 10.1016/j.neuropharm.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Wang GY, Robinson DW, Chalupa LM. Calcium-activated potassium conductances in retinal ganglion cells of the ferret. Journal of Neurophysiology. 1998;79(1):151–158. doi: 10.1152/jn.1998.79.1.151. [DOI] [PubMed] [Google Scholar]
- Wang B, Rothberg BS, Brenner R. Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. The Journal of General Physiology. 2009;133(3):283–294. doi: 10.1085/jgp.200810141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhang Y, Wang L, Sun P, Luo X, Ishigaki Y, …Kato N. Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer’s disease model mice. Neuropharmacology. 2015;97:210–219. doi: 10.1016/j.neuropharm.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Wanner SG, Koch RO, Koschak A, Trieb M, Garcia ML, Kaczorowski GJ, Knaus HG. High-conductance calcium-activated potassium channels in rat brain: Pharmacology, distribution, and subunit composition. Biochemistry. 1999;38(17):5392–5400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- Weiger TM, Holmqvist MH, Levitan IB, Clark FT, Sprague S, Huang WJ, … Curtis R. A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. The Journal of Neuroscience. 2000;20(10):3563–3570. doi: 10.1523/JNEUROSCI.20-10-03563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience. 2009;159(3):1055–1069. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, … Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306(5704):2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- Wimmers S, Halsband C, Seyler S, Milenkovic V, Strauss O. Voltage-dependent Ca2+ channels, not ryanodine receptors, activate Ca2+-dependent BK potassium channels in human retinal pigment epithelial cells. Molecular Vision. 2008;14:2340–2348. [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. The European Journal of Neuroscience. 2002;16(7):1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. The Journal of Neuroscience. 1999;19(2):726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf H, Hay-Schmidt A, Poulsen AN, Klaerke DA, Olesen J, Jansen-Olesen I. Molecular studies of BKCa channels in intracranial arteries: Presence and localization. Cell and Tissue Research. 2008;334(3):359–369. doi: 10.1007/s00441-008-0701-x. [DOI] [PubMed] [Google Scholar]
- Wynne PM, Puig SI, Martin GE, Treistman SN. Compartmentalized beta subunit distribution determines characteristics and ethanol sensitivity of somatic, dendritic, and terminal large-conductance calcium-activated potassium channels in the rat central nervous system. The Journal of Pharmacology and Experimental Therapeutics. 2009;329(3):978–986. doi: 10.1124/jpet.108.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+-and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. The Journal of Neuroscience. 1999;19(13):5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Black DL. A CaMK IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature. 2001;410(6831):936–939. doi: 10.1038/35073593. [DOI] [PubMed] [Google Scholar]
- Xie J, Jan C, Stoilov P, Park J, Black DL. A consensus CaMK IV-responsive RNA sequence mediates regulation of alternative exons in neurons. RNA. 2005;11(12):1825–1834. doi: 10.1261/rna.2171205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280(5362):443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298(5595):1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- Xu JW, Slaughter MM. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. The Journal of Neuroscience. 2005;25(33):7660–7668. doi: 10.1523/JNEUROSCI.1572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ueta Y, Wang L, Yamamoto R, Inoue N, Inokuchi K, …Kato N. Suppression of a neocortical potassium channel activity by intracellular amyloid-beta and its rescue with Homer1a. The Journal of Neuroscience. 2011;31(31):11100–11109. doi: 10.1523/JNEUROSCI.6752-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Krishnamoorthy G, Saxena A, Zhang G, Shi J, Yang H, …Cui J. An epilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+ sensing. Neuron. 2010;66(6):871–883. doi: 10.1016/j.neuron.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CT, Zeng XH, Xia XM, Lingle CJ. Interactions between beta subunits of the KCNMB family and Slo3: Beta4 selectively modulates Slo3 expression and function. PLoS One. 2009;4(7):e6135. doi: 10.1371/journal.pone.0006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Buttigieg J, Wan Y, Wang J, Figley S, Fehlings MG. Expression and functional role of BK channels in chronically injured spinal cord white matter. Neurobiology of Disease. 2012;47(2):225–236. doi: 10.1016/j.nbd.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Ye H, Jalini S, Mylvaganam S, Carlen P. Activation of large-conductance Ca(2+)-activated K(+) channels depresses basal synaptic transmission in the hippocampal CA1 area in APP (swe/ind) TgCRND8 mice. Neurobiology of Aging. 2010;31(4):591–604. doi: 10.1016/j.neurobiolaging.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Yu L, Eaton AF, Yue Q, Bao HF, Ma HP, Cuppoletti J, Eaton DC. Unoprostone activation of BK (KCa1.1) channel splice variants. Biochimica et Biophysica Acta. 2015;1848(11 Pt A):2859–2867. doi: 10.1016/j.bbamem.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, Toro L. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(27):10072–10077. doi: 10.1073/pnas.0302919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, …Frick A. Dendritic channelopathies contribute to neocortical and sensory hyper-excitability in Fmr1(−/y) mice. Nature Neuroscience. 2014;17(12):1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122(4):1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Katz EJ, Gold MS. BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. The European Journal of Neuroscience. 2010;31(3):450–462. doi: 10.1111/j.1460-9568.2009.07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schools GP, Lei T, Wang W, Kimelberg HK, Zhou M. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Experimental Neurology. 2008;212(1):44–52. doi: 10.1016/j.expneurol.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZB, Tian MQ, Gao K, Jiang YW, Wu Y. De novo KCNMA1 mutations in children with early-onset paroxysmal dyskinesia and developmental delay. Movement Disorders. 2015;30(9):1290–1292. doi: 10.1002/mds.26216. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xie M, Schools GP, Feustel PF, Wang W, Lei T, …Zhou M. Tamoxifen mediated estrogen receptor activation protects against early impairment of hippocampal neuron excitability in an oxygen/glucose deprivation brain slice ischemia model. Brain Research. 2009;1247:196–211. doi: 10.1016/j.brainres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Molecular Pharmacology. 2008a;73(2):359–368. doi: 10.1124/mol.107.039743. [DOI] [PubMed] [Google Scholar]
- Zou S, Jha S, Kim EY, Dryer SE. The beta 1 subunit of L-type voltage-gated Ca2+ channels independently binds to and inhibits the gating of large-conductance Ca2+-activated K+ channels. Molecular Pharmacology. 2008b;73(2):369–378. doi: 10.1124/mol.107.040733. [DOI] [PubMed] [Google Scholar]


