Abstract
Prodrugs that release hydrogen sulfide upon esterase-mediated cleavage of an ester group followed by lactonization are described herein. By modifying the ester group and thus its susceptibility to esterase, and structural features critical to the lactonization rate, H2S release rates can be tuned. Such prodrugs directly release hydrogen sulfide without the involvement of perthiol species, which are commonly encountered with existing H2S donors. Additionally, such prodrugs can easily be conjugated to another non-steroidal anti-inflammatory agent, leading to easy synthesis of hybrid prodrugs. As a biological validation of the H2S prodrugs, the anti-inflammatory effects of one such prodrug were examined by studying its ability to inhibit LPS-induced TNF-α production in RAW 264.7 cells. This type of H2S prodrugs shows great potential as both research tools and therapeutic agents.
Keywords: Hydrogen sulfide prodrugs, Enzyme trigger, Trimethyl lock, H2S-NSAIDs, Lactonization, Anti-inflammatory
H2S is an endogenously produced signaling molecule in mammals and is critical to human health and disease.[1] Many studies have also shown the various therapeutic effects of H2S, which include protection against myocardial ischemia injury, cytoprotection against oxidative stress, mediation of neurotransmission, inhibition of insulin signaling, regulation of inflammation and dilation of blood vessel.[1a, 1b, 1d, 1e, 2]
In studying the physiological and pathological properties of H2S, H2S gas or inorganic sulfide salts such as Na2S and NaHS have been widely used. However, the uncontrollable release of H2S from sulfide salts and the toxic effects of excessive H2S limit their potential as possible therapeutic agents.[3] Moreover, inorganic sulfide salts could not mimic the slow and continuous H2S production in the biological system, which further limits their usage. Therefore, new H2S releasing agents (H2S donors) are of great clinical and research interests.[3–4] Currently, there are seven types of H2S donors that have been reported:[5] 1) garlic and related sulfur compounds;[6] 2) Lawensson’s reagent and analogs (GYY4137);[7] 3) 1,2-dithiole-3-thiones (DTTs) and hybrids of H2S and non-steroidal anti-inflammatory drugs;[8] 4) thiol-activated H2S donors;[9] 5) photo-induced H2S donors;[10] and 6) thiol amino acid;[11] and 7) polysulfide (SG-1002).[12] There are some obvious limitations among these H2S donors. First of all, the release rates of H2S from most donors such as GYY4137 and DTTs are hard to control, and the effect of their byproducts associated with H2S release is unclear. Furthermore, existing H2S donor systems lack well defined negative controls. Some H2S donors, such as thiol-activated H2S donors would generate the perthiol intermediates, which may also have certain physiological effect, making it hard to deconvolute the experimental results. Almost all existing donors release precursors of H2S. Thus the correlation of H2S release and the amount of donor added is difficult. H2S donors that can mimic endogenous H2S production through a single enzymatic step are currently not available. Recently, Moore et al [13] tested the effect of GYY4137 on the release of pro- and anti-inflammatory mediators in lipopolysaccharide (LPS)-treated murine RAW264.7 macrophages, and found that the effects of H2S on inflammatory processes are complex and dependent not only on H2S donor concentration but also on the rate of H2S generation. Therefore, there is a need for new H2S prodrugs, which directly generate H2S with well-defined and tunable release rates that are not directly affected by the redox balance of the cellular environment and the presence of other thiol species. Such H2S donors/prodrugs will be very important research tools in delineating the functions of H2S and lay a foundation for the future development of H2S-based therapeutics.
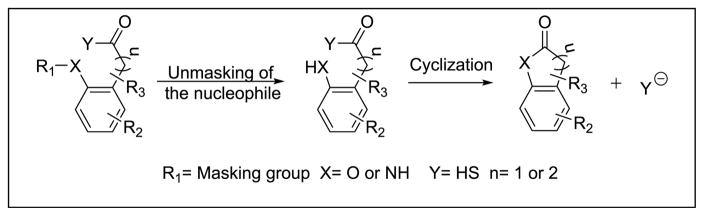
Our lab has long-standing interests in prodrugs based on intramolecular cyclizations.[14]. We took advantage of one such lactonization prodrug systems (Scheme 1), and designed esterase-sensitive prodrugs of H2S. Specifically, the nucleophilic hydroxyl or amino group can be masked as an ester or amide, and the drug, H2S, can be conjugated to the carbonyl carbon in the form of a thioacid. After hydrolysis of the masking group, the nucleophile can attack the carbonyl group and undergo a lactonization reaction, and thus release hydrogen sulfide.
Scheme 1.
The general concept of cyclization driven prodrugs of hydrogen sulfide
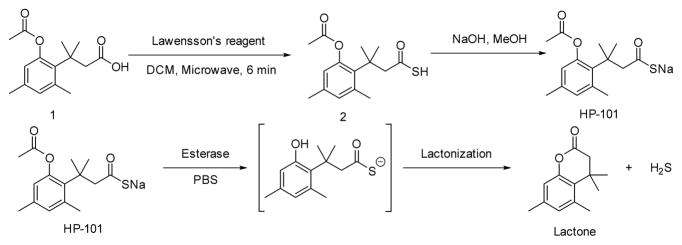
To test the idea, we first synthesized prodrug BW-HP-101, which uses a “trimethyl lock” and thus stereochemical control to facilitate lactonization.[14a, 15] The synthesis of BW-HP-101 was accomplished by treating compound 1,[16] with Lawensson’s reagent under microwave conditions,[17] followed by reaction with one equivalent of sodium hydroxide (Scheme 2).
Scheme 2.
Synthesis of “trimethyl lock”-based H2S prodrug HP-101 and the mechanism of esterase triggered H2S release
The novel BW-HP-101 is a very stable white and odorless solid, and has very good water solubility, allowing 10 mM stock solutions to be prepared in aqueous buffer. The prodrug showed no obvious decomposition during storage at room temperature for 3 days and at −20 °C for 3 month.
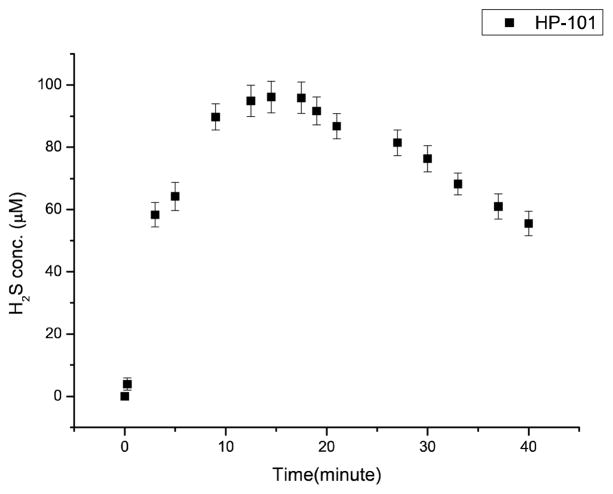
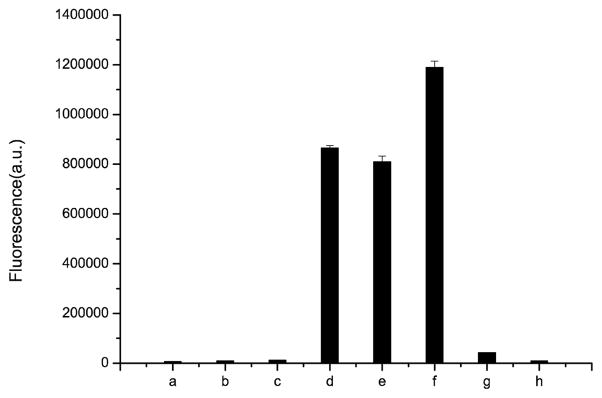
To examine the feasibility of concept described in Schemes 1 and 2, we studied whether esterase can catalyze H2S release from BW-HP-101 by using a H2S-selective microelectrode, and found time-dependent H2S release in the presence of porcine liver esterase (PLE) (Figure 1) with a peak concentration at about 15 min. H2S release was further confirmed by using a well-known hydrogen sulfide fluorescent probe WSP-5[18] (Figure 2). Strong fluorescence was detected when WSP-5 was incubated with the prodrug in the presence of PLE in phosphate buffer saline (PBS) or cell culture media containing fetal bovine serum (FBS). Such results indicate that BW-HP-101 indeed releases H2S in PBS with esterase catalysis, or cell culture media containing FBS. In contrast, incubation in PBS alone did not lead to H2S formation, indicating the chemical stability of the prodrug. Lactone formation was also confirmed by NMR (SI). As another piece of evidence on the stability of the thioacid group, thioactic acid was incubated in aqueous solution; no decomposition was observed within 48 h (SI).
Figure 1.
Hydrogen sulfide generation from BW-HP-101. 200 μM the prodrug in PBS (1% DMSO) at 37 °C with 1 unit/mL of PLE. (p=0.95, n=3.)
Figure 2.
Qualitatively detection of hydrogen sulfide releasing from BW-HP-101 by WSP-5. The concentration of WSP-5 is 50 uM, and the intensities of fluorescence were recorded after 5 min of incubation of WSP-5 with different substrates at room temperature. a: WSP-5 only in PBS; b: WSP-5+ 200 μM prodrug in PBS; c: WSP-5+ 200 μM prodrug in DMEM (No FBS), no cells; d: WSP-5+ 200 μM prodrug in DMEM (with FBS)+ cells e: WSP-5+ 200 μM prodrug in DMEM (with FBS), no cells; f: WSP-5+ 200 μM prodrug + 1 unit/mL of esterase; g: WSP-5+200 μM GYY1437; h: WSP-5+1unit/mL esterase.
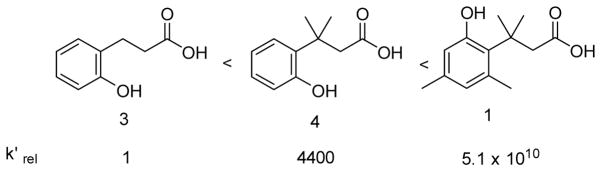
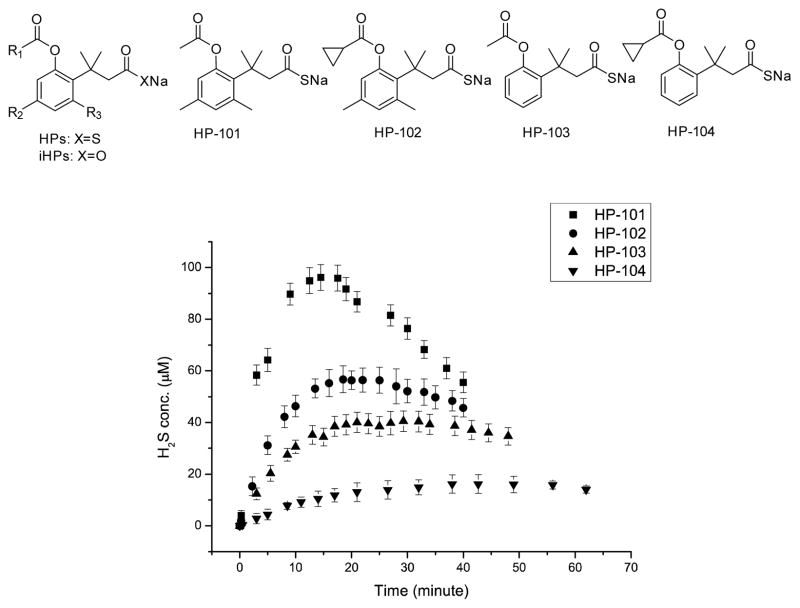
All these studies demonstrate the expected enzyme-catalyzed release of hydrogen sulfide from the prodrug system. We then further studied the tunability of the release rates by varying the ester group and factors controlling the lactonization step. Variations of the ester group allows for tuning the rate of the unmasking step. For ester hydrolysis, it has been shown that increasing the size of the acyl moiety results in decreased catalytic hydrolysis rate.[19] Thus we reasoned that modifying the acyl moiety should help tune the hydrolysis rates. The second direction in tuning H2S releasing rates is based on controlling the lactonization rate by varying the number of methyl groups in the system. It is well known that the lactonization of compound 1 is much faster than that of o-hydroxydihydrocinnamic acids 3 and 4, which lack pendant methyl groups (Scheme 3) and thus has decreased entropic control of the conformation favorable for lactonization.[15b] Therefore, BW-HP-102, -103, and -104 were synthesized to tune the release rates. BW-HP-102 and BW-HP-104 contain a large acyl moiety cyclopropanecarbonyl ester and BW-HP-103 and BW-HP-104 lack two methyl groups on phenyl ring.
Scheme 3.
Relative rate constants for lactonization of various hydroxydihydrocinnamic acids. [20]
H2S release from these prodrugs was studied (Figure 3). As designed, these prodrugs show very different H2S release rates. For 200 μM of the prodrug in PBS at 37 °C with 1unit/mL PLE, the peak H2S concentration for the fastest one, BW-HP-101, was about 95 μM at 15 min; and for the slowest one, BW-HP-104, it was about 13 μM at 43 min. Such results bring out an important issue, i.e. the same concentration of the prodrug may mean very different effective H2S concentrations, depending on the release rates. This issue is especially important in delivering a gaseous molecule because of its volatility nature and the lack of “accumulation,” which is in direct contrast to the delivery of non-volatile drugs. In the latter case, a slower release may only affect the onset time, but not the final concentration. With a gaseous molecule, release rates affect the onset time, the peak concentration, and the eventual concentration significantly. Thus when comparing the results using various H2S prodrugs/donors, particular attention needs to be paid to the release rate and effective concentration issue.
Figure 3.
H2S generation curves. 200 μM prodrugs in PBS (1% DMSO) with 1 unit/mL esterase at 37 °C. (p=0.95, n=3.)
We also monitored the lactone product formation by HPLC (Table 1), and found t1/2 ranging from 13 to 99 min for 200 μM prodrugs in the presence of PLE. Such results further demonstrated the concept of tuning the H2S release rates.
Table 1.
The half-lives of various prodrugs. 200 μM prodrugs in PBS with esterase 1 unit/mL at 37 °C, p=0.95, n=3.
| BW-HP-101 | BW-HP-102 | BW-HP-103 | BW-HP-104 | |
|---|---|---|---|---|
| T1/2 (min) | 13.0 ±2.4 | 28.7±1.5 | 44.5±2.1 | 99.0 ±8.9 |
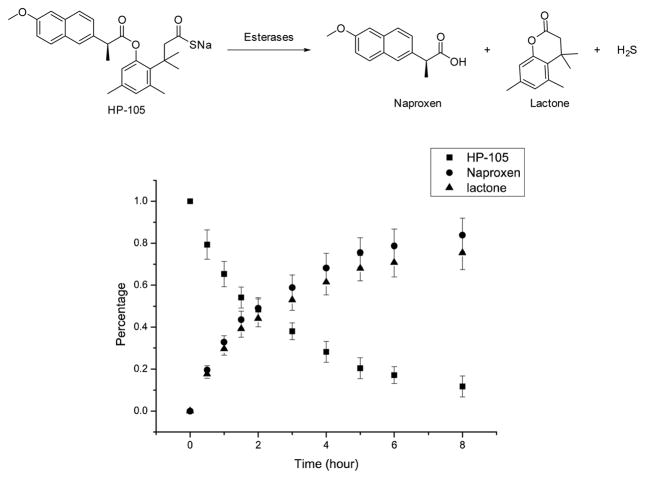
The conjugation of two drugs with the same therapeutic indication, but different mechanisms, is attracting a great deal of attention in the hybrid drug field.[21] Especially interesting is the idea of conjugating a non-steroidal anti-inflammatory drug (NSAID) to a H2S donor,[22] which has known anti-inflammatory effects. H2S-NSAIDs have shown remarkable improvements in activity and tolerability as compared with the related parent compounds.[21] However, few of the hydrogen sulfide prodrugs could be successfully applied to hybrid drug preparation for chemistry reasons, and existing H2S-NSAIDs also suffer from the lack of control in H2S release. Because most NSAIDs have a free carboxyl group, their conjugation to one of our prodrugs is not only easy, but also leads to a hybrid prodrug, which uses the same mechanism to release both drugs. This is very unique among all known H2S hybrid drugs. Thus, we also synthesized BW-HP-105, which is formed by coupling the novel H2S release system to naproxen (Figure 4). HPLC kinetic studies showed that BW-HP-105 could generate naproxen and H2S by treatment with an esterase. Compared to other H2S prodrugs with the same stereochemical control (BW-HP-101, 102), BW-HP-105 showed a slower hydrolysis rate as expected because of the larger masking group (naproxen as the acyl group). The H2S-NSAID hybrid shown here is the first example of controllable H2S release employing the same mechanism to “activate” both drugs, and will be a very useful research tool and potential therapeutic agent.
Figure 4.
200 μM HP-105 in PBS (1% DMSO) with esterase 20unit/mL at 37 °C. (p=0.95, n=3.)
We next tested whether such compounds also produced H2S-associated biological effects in vitro. For such studies, negative control compounds are always important. However, there are no good control compounds for existing H2S prodrugs. To address this issue, we used the inactive oxyacid version of the prodrugs (iHPs, Figure 3) as the control compounds. Compared to thioacid prodrugs, iHPs have the same chemical structural frame except replacing sulfur with oxygen.
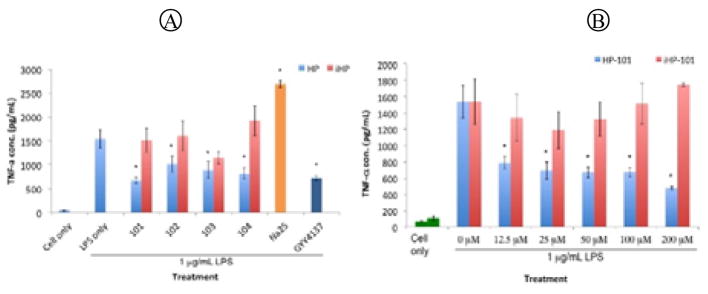
We first tested the cytotoxicity of these prodrugs on RAW264.7 macrophages. None showed any toxicity at 200 μM (See Supporting Information). We then examine the effect of the prodrugs on TNF-α production after co-treatment of the cell with the prodrugs and 1 μg/mL LPS for one hour, using an ELISA kit (Figure 5). The results showed that only the prodrugs and GYY 4137 effectively inhibited TNF-α secretion, and Na2S showed pro-inflammatory effect, which is similar to literature results.[13] None of the iHPs showed the same effect, which clearly demonstrated that the inhibition effect on TNF-α production came from the H2S released from the respective prodrug.
Figure 5.
TNF-α concentrations of RAW 264.7 cell culture media after 1-hour co-treatment with H2S prodrugs and LPS. (A) Treatment with 50 μM HPs, iHPs Na2S and GYY4137. (B) Treatment with various concentrations of BW-HP-101 and iHP-101 (n=4 *: p<0.05)
In conclusion, we successfully investigated a new strategy of making H2S prodrugs by using an esterase catalyzed lactonization prodrug system. Compared to existing H2S prodrugs/donors, the new H2S prodrugs described show several unique features. First of all, they have controlled H2S release rates. This aspect seems to be the most challenging and important issue in the field of H2S donors. Secondly, the trigger is an enzyme ubiquitous in the biological system.[23] Thirdly, the prodrugs require a specific type of enzyme to trigger H2S release, which afford the potential for controlled release at preferred sites. Fourthly, as research tools, the prodrugs described have well-defined negative controls. Fifthly, this strategy provides the first H2S-NSAIDs hybrids with controllable release rates. We believe that these new H2S prodrugs will be very useful research tools to others working in this field.
Supplementary Material
Acknowledgments
Financial support from the NIH (CA180519) is gratefully acknowledged.
Footnotes
Patent pending covering Hydrogen Sulfide Precursors and Drug Conjugates Thereof
Supporting information for this article is given via a link at the end of the document
References
- 1.a) Vandiver MS, Snyder SH. J Mol Med (Berl) 2012;90:255–263. doi: 10.1007/s00109-012-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Szabo C. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]; c) Wang R. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]; d) Blackstone E, Morrison M, Roth MB. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]; e) Lowicka E, Beltowski J. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]; f) Bohlender C, Glaser S, Klein M, Weisser J, Thein S, Neugebauer U, Popp J, Wyrwa R, Schiller A. J Mater Chem B. 2014;2:1454–1463. doi: 10.1039/c3tb21649g. [DOI] [PubMed] [Google Scholar]
- 2.a) Calvert JW, Coetzee WA, Lefer DJ. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gadalla MM, Snyder SH. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Martelli A, Testai L, Citi V, Marino A, Bellagambi FG, Ghimenti S, Breschi MC, Calderone V. Vascul Pharmacol. 2014;60:32–41. doi: 10.1016/j.vph.2013.11.003. [DOI] [PubMed] [Google Scholar]; d) Testai L, Martelli A, Marino A, D’Antongiovanni V, Ciregia F, Giusti L, Lucacchini A, Chericoni S, Breschi MC, Calderone V. Biochem Pharmacol. 2013;85:1634–1643. doi: 10.1016/j.bcp.2013.03.018. [DOI] [PubMed] [Google Scholar]; e) Ahmad A, Olah G, Szczesny B, Wood ME, Whiteman M, Szabo C. Shock (Augusta, Ga) 2015 doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Kashfi K, Olson KR. Biochem Pharmacol. 2013;85:689–703. doi: 10.1016/j.bcp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhao Y, Biggs TD, Xian M. Chem Commun (Camb) 2014;50:11788–11805. doi: 10.1039/c4cc00968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Song ZJ, Ng MY, Lee ZW, Dai W, Hagen T, Moore PK, Huang D, Deng LW, Tan CH. Med Chem Commun. 2014;5:557–570. [Google Scholar]; b) Pluth MD, Bailey TS, Hammers MD, Hartle MD, Henthorn HA, Steiger AK. Synlett. 2015;26:2633–2643. [Google Scholar]
- 5.Zheng Y, Ji X, Ji K, Wang B. Acta Pharm Sin B. 2015;5:367–377. doi: 10.1016/j.apsb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Proc Natl Acad Sci U S A. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liang D, Wu H, Wong MW, Huang D. Org Lett. 2015;17:4196–4199. doi: 10.1021/acs.orglett.5b01962. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 8.a) Baskar R, Sparatore A, Del Soldato P, Moore PK. Eur J Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]; b) Qandil AM. Int J Mol Sci. 2012;13:17244–17274. doi: 10.3390/ijms131217244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Zhao Y, Wang H, Xian M. J Am Chem Soc. 2011;133:15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhao Y, Bhushan S, Yang C, Otsuka H, Stein JD, Pacheco A, Peng B, Devarie-Baez NO, Aguilar HC, Lefer DJ, Xian M. ACS Chem Biol. 2013;8:1283–1290. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Martelli A, Testai L, Citi V, Marino A, Pugliesi I, Barresi E, Nesi G, Rapposelli S, Taliani S, Da Settimo F, Breschi MC, Calderone V. ACS Med Chem Lett. 2013;4:904–908. doi: 10.1021/ml400239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Devarie-Baez NO, Bagdon PE, Peng B, Zhao Y, Park CM, Xian M. Org Lett. 2013;15:2786–2789. doi: 10.1021/ol401118k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fukushima N, Ieda N, Sasakura K, Nagano T, Hanaoka K, Suzuki T, Miyata N, Nakagawa H. Chem Commun (Camb) 2014;50:587–589. doi: 10.1039/c3cc47421f. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, von Wantoch Rekowski M, Coletta C, Szabo C, Bucci M, Cirino G, Topouzis S, Papapetropoulos A, Giannis A. Bioorg Med Chem. 2012;20:2675–2678. doi: 10.1016/j.bmc.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Gojon G, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. Antioxid Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Shan D, Nicolaou MG, Borchardt RT, Wang B. J Pharm Sci. 1997;86:765–767. doi: 10.1021/js970069d. [DOI] [PubMed] [Google Scholar]; b) Wang B, Zhang H, Wang W. Bioorg Med Chem Lett. 1996;6:945–950. [Google Scholar]; c) Zych L, Yang W, Griffin K, Liao Y, Wang B. Bioorg Chem. 2004;32:109–123. doi: 10.1016/j.bioorg.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 15.a) Borchardt RT, Cohen LA. J Am Chem Soc. 1972;94:9175–9182. doi: 10.1021/ja00781a031. [DOI] [PubMed] [Google Scholar]; b) Borchardt RT, Cohen LA. J Am Chem Soc. 1972;94:9166–9174. doi: 10.1021/ja00781a030. [DOI] [PubMed] [Google Scholar]; c) Amsberry KL, Borchardt RT. J Org Chem. 1990;55:5867–5877. [Google Scholar]; d) Amsberry KL, Borchardt RT. Pharm Res. 1991;8:323–330. doi: 10.1023/a:1015885213625. [DOI] [PubMed] [Google Scholar]; e) Schowen KB, Schowen RL, Borchardt SE, Borchardt PM, Artursson P, Audus KL, Augustijns P, Nicolazzo JA, Raub TR, Schöneich C, Siahaan TJ, Takakura Y, Thakker DR, Wolfe MS. J Pharm Sci. 2016;105 doi: 10.1002/jps.24687. published on-line. [DOI] [PubMed] [Google Scholar]
- 16.Amsberry KL, Gerstenberger AE, Borchardt RT. Pharm Res. 1991;8:455–461. doi: 10.1023/a:1015890809507. [DOI] [PubMed] [Google Scholar]
- 17.Rao Y, Li X, Nagorny P, Hayashida J, Danishefsky SJ. Tetrahedron lett. 2009;50:6684–6686. doi: 10.1016/j.tetlet.2009.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng B, Chen W, Liu C, Rosser EW, Pacheco A, Zhao Y, Aguilar HC, Xian M. Chemistry. 2014;20:1010–1016. doi: 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Elleby B, Sjoblom B, Lindskog S. Eur J Biochem. 1999;262:516–521. doi: 10.1046/j.1432-1327.1999.00400.x. [DOI] [PubMed] [Google Scholar]; b) Roberts TK, Masson PL, Lauwerys R, Heremans JF. Andrologia. 1976;8:67–71. doi: 10.1111/j.1439-0272.1976.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 20.a) Levine MN, Raines RT. Chem Sci. 2012;3:2412–2420. doi: 10.1039/C2SC20536J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shan D, Nicolaou MG, Borchardt RT, Wang B. J Pharm Sci. 1997;86:765–767. doi: 10.1021/js970069d. [DOI] [PubMed] [Google Scholar]; c) Milstien S, Cohen LA. Proc Natl Acad Sci U S A. 1970;67:1143–1147. doi: 10.1073/pnas.67.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Milstien S, Cohen LA. J Am Chem Soc. 1972;94:9158–9165. doi: 10.1021/ja00781a029. [DOI] [PubMed] [Google Scholar]
- 21.Sparatore A, Santus G, Giustarini D, Rossi R, Del Soldato P. Expert Rev Clin Pharmacol. 2011;4:109–121. doi: 10.1586/ecp.10.122. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Bahar FG, Ohura K, Ogihara T, Imai T. J Pharm Sci. 2012;101:3979–3988. doi: 10.1002/jps.23258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.