Abstract
The strong link between stomatal frequency and CO2 in woody plants is key for understanding past CO2 dynamics, predicting future change, and evaluating the significant role of vegetation in the hydrological cycle. Experimental validation is required to evaluate the long-term adaptive leaf response of C3 plants to CO2 conditions; however, studies to date have only focused on short-term single-season experiments and may not capture (1) the full ontogeny of leaves to experimental CO2 exposure or (2) the true adjustment of structural stomatal properties to CO2, which we postulate is likely to occur over several growing seasons. We conducted controlled growth chamber experiments at 150 ppmv, 450 ppmv and 800 ppmv CO2 with woody C3 shrub Betula nana (dwarf birch) over two successive annual growing seasons and evaluated the structural stomatal response to atmospheric CO2 conditions. We find that while some adjustment of leaf morphological and stomatal parameters occurred in the first growing season where plants are exposed to experimental CO2 conditions, amplified adjustment of non-plastic stomatal properties such as stomatal conductance occurred in the second year of experimental CO2 exposure. We postulate that the species response limit to CO2 of B. nana may occur around 400–450 ppmv. Our findings strongly support the necessity for multi-annual experiments in C3 perennials in order to evaluate the effects of environmental conditions and provide a likely explanation of the contradictory results between historical and palaeobotanical records and experimental data.
Introduction
Stomatal frequency analysis is a well-established proxy technique used to reconstruct atmospheric CO2 dynamics from leaf cuticle morphology of fossils and herbarium leaf specimens [1,2], encompassing the broad variation in the estimated Cenozoic CO2 range from glacial minima of 180 ppmv [3] to maxima of over 1000 ppmv during past ‘hothouse’ conditions [4,5]. Based on the strong link between leaf cuticle morphology and CO2, the coupling of stomatal conductance and CO2 has been investigated in an effort to further develop the predictive capacity of CO2 imprints in fossil leaf remains [6–8]. The 120 ppmv industrial CO2 increase is commonly used to calibrate the acclimation of leaf cuticle morphology through the study of historical leaf collections from herbaria [9–11] and leaf fragments preserved in shallow peat sequences [2,9]. In the resulting high-resolution time-series data the majority of woody C3 species studied show a quantifiable reduction of stomatal frequency, changes in stomatal geometry, and down-regulation of structural stomatal conductance [12–14] with increasing atmospheric CO2. Stomatal analysis is particularly valuable for our understanding of CO2 dynamics in periods preceding ice core-based records, the consequences of on-going future CO2 increase [15,16], and to evaluate the active role of vegetation in the hydrological cycle under past and future CO2 dynamics [13].
For additional information and calibration of leaf-level response beyond the historical 280–400 ppmv CO2 range, controlled-environment CO2 experiments are required. While historical datasets show a clear reduction in stomatal parameters over the pre-industrial to present CO2 range, morphological adjustment data from woody C3 plants from experimental free-field and growth chambers for past low and projected future high CO2 concentrations are largely ambiguous [17,18]. Of the experiments with set-ups constraining the stomatal response under sub-ambient CO2, these generally do not show consistent responses in terms of stomatal frequency adjustments to CO2 [17,19].
Experimental validation of structural changes in stomatal conductance, as observed in time-series studies, has become a research focus only recently. The few studies on structural stomatal conductance changes available for the pre-industrial to elevated CO2 range reveal significant down-regulation from sub-ambient to ambient CO2 and levelling off between ambient to elevated CO2 [20] (Hincke et al., submitted). Although the non-linearity of stomatal frequency and associated stomatal conductance response is to date a well-recognised phenomenon [13,16], no comprehensive experimental validation over the complete range of glacial lows to past and potential future high CO2 concentrations is available. Despite the inherent uncertainties in stomatal frequency and structural stomatal conductance records based on fossil or historical leaf samples, all show comparable trends in response to CO2, however, testing and validation of leaf morphological acclimation in C3 woody species in experimental set-ups is difficult. It may be the case that certain aspects of the complementary experimental set-ups themselves do not fully capture the long-term adaptive response of stomatal parameters, particularly in perennial C3 plants, to the full range of CO2 variability.
We identify and investigate two major issues in short-term controlled-CO2 experiments. Firstly, in short-term growth experiments covering less than one full growing season, leaf matureness may be incomplete at the time of sampling. In Osmunda regalis leaves stomatal parameters increased during the first 10–20% of leaf development, stabilizing at 30% of total leaf size [21]. In Eucalyptus regnans stomatal frequency decreased from 56 until 113 days after emergence, with stomatal initiation continuing until 70% of full leaf expansion [22]. Epidermal cells continue to expand with leaf size; thus, leaves which are not mature may not represent the full expression of stomatal parameters required to calibrate a CO2 response. Sampling of leaves during ontogeny, before matureness, potentially does not reflect their full expression and may skew analysis of the stomatal response to CO2.
Secondly, we posit that experiments which cover a single growing season only reflect a limited response to pre-set conditions which are extremely different from ambient CO2 levels. Monitoring studies on annually-collected leaves of mature birch trees show that leaf cuticle morphology adjustments takes place in multiple successive leaf generations in response to annual CO2 increments of approximately 2 ppmv CO2 per year [11,23]. A significant response from mature leaves to the next generation has also been experimentally demonstrated for Arabidopsis using CO2-controlled cuvettes to expose individual leaf generations to high CO2 [24]. While the capacity for generational CO2 signalling is evident from natural and experimentally-grown leaf material [25], there is still no proof that leaf adjustment captures the full change of morphological response to CO2 within one leaf generation. We hypothesize that the apparently ambiguous response in previous free-field and growth chamber studies can partially be explained by the short-term nature of those studies. If the acclimation of leaf-level parameters to experimentally-adjusted CO2 conditions occurs over more than one growth season, and thus over multiple leaf generations, a clearer response is likely to be evident in experiments carried out over multiple growth season with the same individual plants.
In order to improve the interpretation of leaf morphological CO2 signals and validate the stomatal response of woody C3 plants to CO2, we carried out growth experiments to test the within- and between-leaf generation response of stomatal characteristics in Betula nana (dwarf Birch) over a broad range of CO2 concentrations. The experimental CO2 levels of 150 ppmv, 450 ppmv, and 800 ppmv CO2 encompass a range of full glacial low to predicted future high CO2, which is required to provide data supporting palaeoatmospheric CO2 reconstructions, and, importantly, also produces data relevant for modelling efforts of structural stomatal conductance-induced hydrological changes through time. We tested the responsiveness of leaf generations initiated externally and in situ in order to evaluate potential uncertainties arising from short-term CO2 exposure experiments with perennial plants. The repetition of the experiments in a second consecutive year allows us to evaluate the degree to which morphological CO2 acclimation is captured in traditional single-season experiments.
Methods
The Utrecht University Fytotron (REFTECH B.V., Sassenheim) was set up to artificially simulate environmental conditions and monitor plant growth and development. The three available growth chambers are identical and conditions within each chamber are independently controlled. Chambers measured ~3 x ~1.5 m and have a ~2.2 m high ceiling. Each chamber was equipped with four tables measuring 0.75 x 1.5 m and of adjustable height as a surface on which to place plants. CO2 within the chambers was controlled by a molecular sieve and pressure swing adsorption (PSA) technology (PG 1500L, CMC Instruments GmbH, Eschborn), removing CO2 and H2O vapour after the air was filtered to remove water, oil, dust, and aerosols. In the low CO2 chamber, CO2-free air was mixed into the volume of the room air to achieve the desired low CO2 concentration. While working in the chamber, a gas mask attached to a sealed plastic bag to trap exhaled air was used to limit CO2 rise in the chamber. The ambient CO2 chamber was maintained with an input of outside air. In the high CO2 chamber, extra CO2 was added from a tank to achieve the desired CO2 concentration while maintaining ambient air pressure. The CO2 level in the growth chambers was monitored digitally (BMP343 Vaisala GmbH, Bonn). Relative air humidity (rH) was 70%. Light intensity was ~350 μ mol m-2 s-1 with a 10 hour photoperiod, comparable to a March day in the Netherlands [19]. Temperature was set to vary between day/night 21°C/18°C, which was the lowest possible setting achievable in this set-up.
The set points of environmental conditions inside the Fytotron were agreed amongst the researchers performing experiments in the facility, and limited by the capacity of the chambers. CO2 was set at 150, 400, and 750 ppmv. Some variability was inherent in these set points due to design of the chambers, and the actual CO2 levels in the chambers fluctuated around means of 160, 450, and 800 ppmv (±50 ppmv in the ambient and high CO2 chamber). The low CO2 (150 ppmv) setting was selected to replicate absolute minimum CO2 levels during the Last Glacial, where CO2 may have been as low as 160 ppmv [3]. The 400 ppmv setting attempted to replicate ambient atmospheric CO2 level. The 400 ppmv atmospheric CO2 mark was reached at Mauna Loa between April and May 2014 [26]. This mark is thus a realistic comparison for current and near-future atmospheric CO2 levels. The 750 ppmv setting was selected to represent potential future CO2 levels. IPCC scenarios suggest that the 750 ppmv atmospheric CO2 mark may be reached as early as 2075. However, as the actual CO2 levels in the chamber were closer to 450 and 800 ppmv these values are used throughout the paper.
B. nana specimens were obtained from a gardening centre (Plantentuin Esveld, Boskoop) as cuttings in individual 1.5 L pots. They had been exposed to global ambient CO2 conditions for multiple growing years. They were placed in outdoor greenhouses at the Utrecht University Botanische Tuinen (Botanical Gardens) before being moved first to the ~20°C greenhouse to acclimatise to the temperature, and then to the CO2 chambers and control treatment (temperature-controlled greenhouse) prior to budburst. Of a total 50 plants, 13 were placed in each growth chamber and 11 plants in the greenhouse control. Following the growth season in the chambers, when leaves reached senescence and began to drop from the plant, plants were moved first to the ~20°C greenhouse to acclimate to greenhouse conditions for 2–6 weeks and then to an outdoor greenhouse where they were exposed to winter temperatures. This strategy was developed as it was not possible to adjust daylight length or temperature settings to mimic actual long-term seasonal changes in the Fytotron growth chambers. Plants were re-potted in enriched potting soil (Botanische Tuinen, Utrecht) following the 2013 growth season.
Leaves were sampled weekly throughout the growing season. In 2013, five apparently mature leaves were sampled randomly from the plant population in each growth chamber and in the greenhouse. In 2014, 3–5 leaves per plant were sampled. In this study, 141 leaves of B. nana were analysed for leaf area (LA) and cuticle micro-morphological properties. Sampling dates are referred to as days in the experimental chamber, where 0 is the first day the plants were exposed to experimental CO2 conditions (150, 450, and 800 ppmv), on 18/03/2013 and 20/02/2014. Leaves which had reached apparent maturity were sampled rather than newly-developed or developing leaves. Leaves which grew on shoots newly-formed in the growth chambers were preferentially sampled. The leaves were removed from the plant upon sampling and placed into paper envelopes to prevent mould from forming before they could be dried.
LA was measured using a LI-COR LI-3100C Area Meter. Leaves were dried at 70°C for 24–72 hours and dry weight measured with a Sartorius ENTRIS 3202-1S Precision Scale. Approximately 0.5 cm2 of material was removed from the central part of each leaf, avoiding the main hydraulic vein, and soaked in a 4% NaHClO2 solution for 24 hours. The abaxial (stomatal-bearing) cuticle could then be loosened and removed from the mesophyll, stained with saffranine, and mounted with glycerine jelly on microscope slides. Cuticle measurements and stomatal counting was performed on an Olympus BH-2 optical microscope with the AnalySIS Auto image analysis system (Soft Imaging System GmbH, Germany, v. 5.1) at 660x magnification with a digital image size of 0.0575 mm2. Images had a resolution of 2080 x 1544 pixels. Stomatal density (DS) and epidermal cell density (DE) were measured on seven stomatal-bearing alveoles. Pore length (LP), stomatal length (LS), guard cell width (LGC), cell circumference, and epidermal cell area (CAE) were measured on n = 30 cells or stomata for each slide.
Stomatal index (SI) was calculated to correct for the influence of epidermal cell expansion on stomatal frequency [27] (Eq 1). Maximum pore area (amax) was calculated in μm2 (Eq 2) and maximum stomatal conductance to water vapour (gsmax) was calculated using a two-end auto-correction for shell diffusion [28] where DH2O is the diffusivity of water vapour (m2 s-1) and V is the molar volume of air (mol m-2 s-1) calculated as constants based on the ambient temperature in °C (Eq 3).
| (1) |
| (2) |
| (3) |
Results
Leaf ontogeny of B. nana in G1-1 and G1-2
Leaves from weekly sampling were analysed and mean values of leaf morphological parameters (Table 1) were produced for each sampling date. The data from the first experimental year (G1) clustered in two phases: a primary leaf flush, G1-1, sampled up to and including 66 experimental days and a secondary leaf flush, G1-2, sampled from 73 experimental days onwards (Table 2). The abbreviations for successive leaf generations are summarised in Table 3. Changes for all ontogenetically relevant parameters, with significantly lower LA and significantly higher DE and DS values at 73 experimental days compared to 66 days, were observed in all CO2 treatments (Table 2). LP is generally smaller at 73 days than at 66 days. CAE was smaller in the samples from 73 experimental days with the exception of the 450 ppmv treatment where CAE is approximately the same. SI and gsmax did not show comparative variability between the two sampling dates.
Table 1. Leaf morphological and stomatal parameters.
The leaf morphological and stomatal parameters discussed in this paper and their conventional abbreviations and units.
| Parameter | Abbreviation | Unit |
|---|---|---|
| Leaf area | LA | cm2 |
| Stomatal density | DS | n mm-2 |
| Epidermal cell density | DE | n mm-2 |
| Epidermal cell area | CAE | μm2 |
| Stomata index | SI | % |
| Maximum stomatal conductance | gsmax | mol m-2 s-1 |
| Pore length | LP | μm |
| Stomatal length | LS | μm |
| Guard cell width | LGC | μm |
| Maximum pore area | amax | m2 |
Table 2. Mean leaf parameters at experimental CO2 levels in G1-1 and G1-2.
Mean leaf parameters at 66 and 73 experimental days. The leaf response clustered into two distinct stages (G1-1 and G1-2) in the first growing season (G1). Error in standard error of the mean. Tests of significance were two-tailed Student’s T-tests assuming unequal variance.
| Parameter (unit) | CO2 (ppmv) | G1-1 (66 days) | Change | G1-2 (73 days) | P-value |
|---|---|---|---|---|---|
| LA (cm2) | 150 | 0.88 ± 0.13 | 0.59 ± 0.07 | <0.002 | |
| 450 | 0.94 ± 0.12 | > | 0.66 ± 0.06 | <0.002 | |
| 800 | 1.37 ± 0.15 | 0.65 ± 0.09 | <0.002 | ||
| CA (μm2) | 150 | 1115.41 ± 172.17 | 962.21 ± 77.08 | ns | |
| 450 | 945.15 ± 60.03 | > | 998.02 ± 33.42 | ns | |
| 800 | 1212.69 56.91 | 883.26 ± 61.3 | <0.02 | ||
| DE (n mm-2) | 150 | 968.12 ± 70.06 | 1588.69 ±84.18 | <0.001 | |
| 450 | 881.99 ± 76.30 | < | 1497.45 ± 135.64 | <0.001 | |
| 800 | 1013.66 ±61.72 | 1286.29 ± 86.96 | <0.001 | ||
| DS (n mm-2) | 150 | 127.54 ± 12.53 | 162.82 ± 9.24 | <0.002 | |
| 450 | 106.83 ± 9.6 | < | 163.42 ± 21.46 | <0.002 | |
| 800 | 119.88 4.8 | 147.74 ± 11.35 | <0.002 | ||
| LP (μm) | 150 | 20.86 ± 1.31 | 18.46 ± 0.5 | ns | |
| 450 | 26.82 ± 0.96 | > | 17.82 ± 0.66 | <0.003 | |
| 800 | 21.71 ±0.56 | 17.41 ± 0.52 | <0.003 | ||
| gsmax (mol m-2 s-1) | 150 | 1.05 ± 0.06 | 1.09 ± 0.06 | ns | |
| 450 | 1.17 ± 0.05 | 1.08 ± 0.09 | ns | ||
| 800 | 1.1 ± 0.05 | 0.92 ± 0.06 | ns | ||
| SI (%) | 150 | 11.56 ± 0.049 | 9.31 ± 0.16 | <0.04 | |
| 450 | 10.97 ± 0.52 | > | 9.8 ± 0.49 | ns | |
| 800 | 10.74 ± 0.57 | 10.32 ± 0.24 | ns |
Table 3. Growth seasons and leaf generations.
The distinction of growth seasons and leaf generations as discussed in this paper.
| Growing season | Leaf flush | Leaf flush (days) | Data discussed (days) | Leaf generation |
|---|---|---|---|---|
| 2013 | First | 0–66 | 66 | G1-1 |
| Second | 73–94 | 80–94 | G1-2 | |
| 2014 | No distinction | No distinction | 44 | G2 |
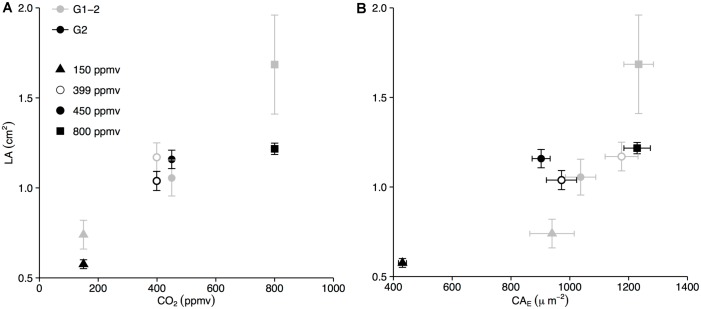
The secondary leaf flush G1-2 is comprised of leaves which developed entirely in situ, that is, inside the growth chamber while exposed only to experimental CO2 concentrations. Data presented for G1-2 is the mean of leaves sampled on experimental days 80 and 94 when the leaves produced in G1-2 had reached full maturity and leaf morphological and stomatal parameters were fully expressed. In G1-2, LA increased significantly under increasing experimental CO2 concentrations with mean LA of 0.74 cm2, 1.06 cm2, and 1.69 cm2 at 150, 450 and 800 ppmv, respectively. The leaves sampled from the control treatment were slightly larger, with mean LA of 1.17 cm2, than leaves from the plants in the ambient experimental CO2 growth chamber (Fig 1A).
Fig 1. LA of B. nana leaves increases with CO2 and CAE in G1-2 and G2.
(A) LA was higher in successive CO2 steps in G1-2 and G2. Greenhouse leaves had slightly, but not significantly, larger LA than leaves grown in the 450 ppmv CO2 chamber. (B) Larger leaves have larger CAE. Error bars represent standard error of the mean.
CAE did not show any clear CO2-related trend in G1-1. In G1-2, CO2 clearly stimulated cell expansion in mature leaves with a CAE of 939.47 μm2 at 150 ppmv, 1036.75 cm2 at 450 ppmv, and 1233.97 cm2 at 800 ppmv. The strong relationship between LA and CAE, as well as the strong relation to CO2, is visualised in Fig 1. Accordingly, the enhanced cell expansion is indicated with lower DE across the CO2 treatments (Fig 2A).
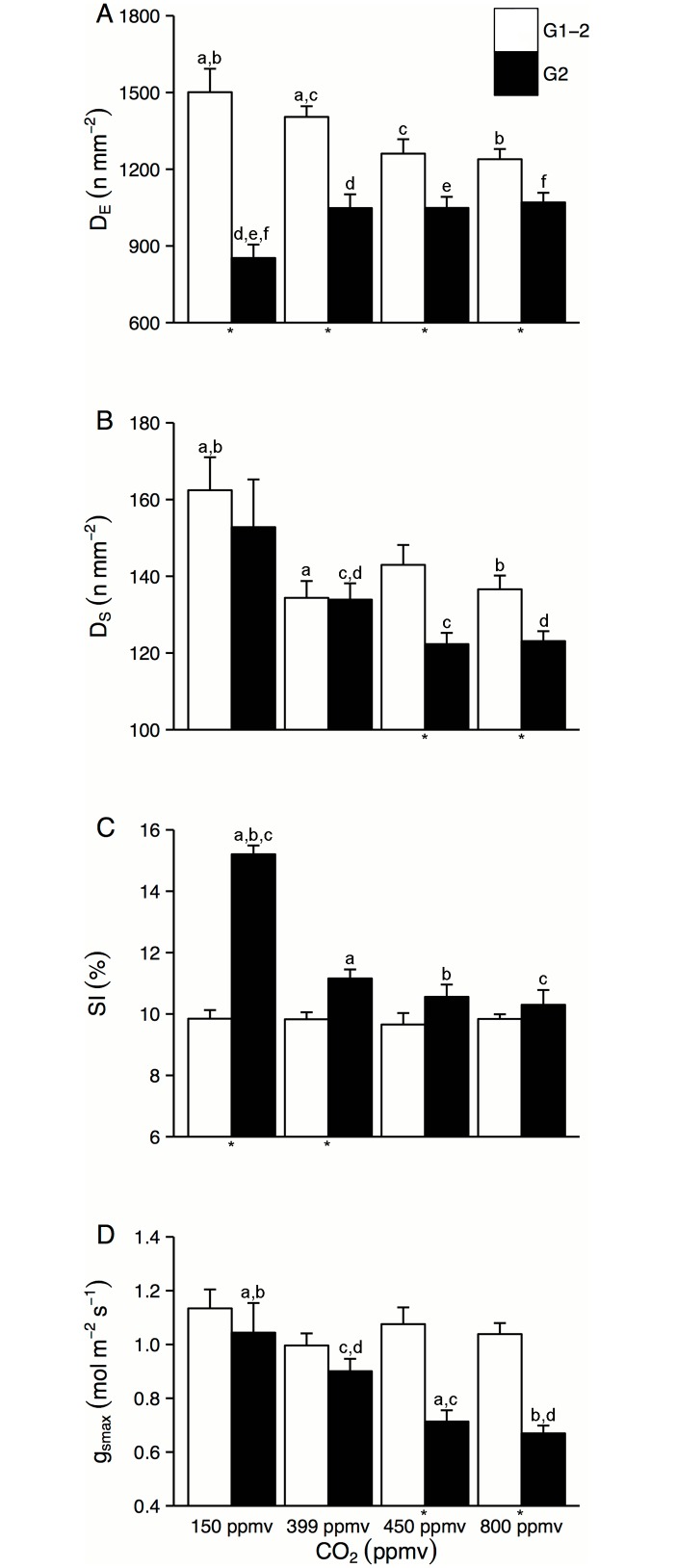
Fig 2. Response of structural stomatal parameters to CO2 in G1-2 and G2.
(A) DE decreased with CO2 in G1-2. In G2, DE is lower at 150 ppmv than in other CO2 treatments. (B) DS responded slightly to CO2 in G1-2 and shows a levelling-off of the response between 399–450 ppmv in G2. (C) SI did not respond to CO2 in G1-2. In G2, the SI response at 150 ppmv was significant with a levelling-off of response around 399 ppmv. (D) The gsmax did not respond to CO2 in G1-2. In G2, gsmax is highest at 150 ppmv, then decreased stepwise with CO2 until 450 ppmv, where the response levelled off. Error bars represent standard error of the mean. Asterisks below the bars indicate a significant difference (P<0.05) between parameter data in G1-2 and G2 for that CO2 level. Letter codes above the bars indicate significant differences; bars which share the same letter are significantly different.
Stomatal geometry is not significantly related to CO2 concentration in G1-1 or G1-2. Mean LP ranges from 18.9 μm to 19.6 μm while mean LS ranges between 28.0 μm and 29.6 μm and mean LGC of around 9.3 μm in all treatments except at 150 ppmv where LGC is slightly larger at 10.5 μm.
DS was highest at low CO2 and decreased successively with CO2 increase in each of the experimental treatments. The decrease was significant in the step between 162 mm-2 at 150 ppmv to 136 mm-2 at 800 ppmv (Fig 2B). SI, calculated from DE and DS, did not have a pronounced response to experimental CO2 conditions in G1-2 (Fig 2C). The absence of a CO2 response of SI in this case was a result of the parallel lowering of DE and DS across the experimental CO2 treatments. Mean gsmax did not vary significantly between the CO2 treatments, however a weak lowering of mean gsmax between 150 ppmv to both 450 and 800 ppmv was observed (Fig 2D).
Leaf response to CO2 in G2
The leaf morphological characteristics and stomatal parameters in the subsequent (2014) growth season, G2, with the same individual plants from the initial experiment, were examined. The repeated exposure during the growing season to experimental CO2 conditions allowed for the evaluation of the response of leaves to experimental CO2 from two consecutive growing seasons.
In G2, the smallest leaves were produced at 150 ppmv with a mean LA of 0.58 cm2 compared to 1.19 cm2 at 450 ppmv, 1.22 cm2 at 800 ppmv, and 1.04 cm2 in the 399 ppmv control treatment (Fig 1A). Mean LA was not significantly different between the ambient experimental CO2 chamber (450 ppmv) and the control greenhouse (399 ppmv). CAE was lowest at 150 ppmv and significantly higher at both 450 and 800 ppmv, but not significantly different in the step between 450 and 800 ppmv. DE is significantly lower at 150 ppmv, with a mean of 852.8 mm-2, than in all other treatments. DE did not significantly differ in the step between 450 and 800 ppmv, nor between 450 ppmv and the 399 ppmv control greenhouse treatment (Fig 2A).
In G2, mean DS was higher in the 150 ppmv chamber with values of 152 mm-2 compared to 450 and 800 ppmv where means of 122 mm-2 and 123 mm-2 were observed, although this difference is not significant (Fig 2B). Plants in the 399 ppmv control greenhouse treatment, however, produced leaves with a significantly higher DS of 133 mm-2 compared to plants in the 450 ppmv experimental CO2 chamber.
SI responded significantly to CO2 at 150 ppmv compared to the 450 and 800 ppmv CO2. SI at 150 ppmv was 15.2%, while at 450 ppmv it was 10.5% and at 800 ppmv, 10.3%. No significant difference in SI between 450 ppmv and the 399 ppmv greenhouse control, where SI was 11.16%, were observed (Fig 2C).
The gsmax of 1.04 mol m-2 s-1 at 150 ppmv was significantly higher than gsmax of 0.71 mol m-2 s-1 at 450 ppmv and 0.66 mol m-2 s-1 at 800 ppmv. In the 399 ppmv greenhouse control treatment, gsmax was significantly higher than in the 450 ppmv experimental CO2 chamber, measuring 0.90 mol m-2 s-1 (Fig 2D).
Stomatal geometry adjusted to experimental CO2 conditions in G2. LP was significantly larger at 150 ppmv, measuring 19.9 μm compared to 17.4 μm at 450 ppmv and 17.3 μm at 800 ppmv. No significant difference was observed between the 450 ppmv and control greenhouse treatment where mean LP was 17.5 μm. LS was highest at 150 ppmv, measuring 32.4 μm, which was significantly larger than LS of 28.9 μm at 450 ppmv. LS was slightly, but insignificantly, larger at 800 ppmv than at 450 ppmv, measuring 30.7 μm. LS was not significantly different between 450 ppmv and the greenhouse control at 27.3 μm. LGC did not vary significantly between experimental CO2 treatments. LGC was significantly smaller in the 399 ppmv control greenhouse treatment, measuring 7.0 μm, compared to 450 ppmv where mean LGC measured 9.3 μm.
Discussion
Changes in leaf morphology and structural stomatal parameters in response to experimental CO2 exposure were observed between G1-1 and G1-2 and between G1 and G2. The adjustment of different leaf parameters occured on a range of time scales; these are discussed in the following sections.
Leaf ontogeny in B. nana in G1
The G1 data was divided into a first and second leaf flush, G1-1 and G1-2, respectively (Tables 2 and 3). The second leaf flush, G1-2, was initiated and formed entirely in the growth chambers under constant environmental and CO2 conditions, and it was thus possible to evaluate the response of leaf morphology and stomatal properties solely related to experimental CO2 exposure. With constant growth conditions we excluded the potential influence of, e.g., higher temperature or changing light conditions during the growth season which potentially alter leaf ontogeny and stomatal expression in naturally-grown lammas leaves [29].
Leaf response in G1-1
Of the plastic and non-plastic leaf morphological parameters evaluated in this experiment, no conclusive or highly significant changes were observed in G1-1. LA was largest at 800 ppmv CO2 and LP is marginally smaller at 150 ppmv. Other stomatal and epidermal cell frequency-related parameters were variable in the experimental CO2 chambers in G1-1, but did not reveal any significant structural trends related to CO2.
The absolute number of stomata expressed on the leaf surface is fixed before initiation of lateral leaf growth and is not sensitive to CO2 during leaf ontogeny [30]. Higher DS at 150 ppmv in G1-1 compared to the other CO2 levels was likely a result of the intrinsic variability of stomatal expression rather than a true adjustment to experimental CO2 conditions, emphasising the required care when interpreting data from experiments of short duration with individual, pre-grown perennial plants.
Leaf response in G1-2
At sampling day 73 significantly lower LA was associated with lower CAE and higher DE and DS. The combination of these parameters demonstrated the development of the next leaf generation, G1-2, which was formed in situ and is fully mature at 80–94 days, the sampling dates on which the data for leaf generation G1-2 were based.
From G1-2 on, a clearer response to CO2 in the plastic and non-plastic leaf and stomatal parameters was observed. LA increased linearly under successively higher CO2, forced by increasing lateral cell expansion which was expressed in the higher CAE and lower DE. CO2 enrichment is associated with an increase in LA in a range of C3 species [31–34]
The extension of this trend to sub-ambient CO2 levels, however, is less well-documented in the literature but has been demonstrated for Arabidopsis thaliana grown under 100 ppmv and 380 ppmv where low CO2 structurally limited leaf expansion [35]. Along the experimental gradient in our study, DS and DE decreased in G1-2, although only the difference between 150 ppmv and 800 ppmv was significant. The trend in DS was seemingly consistent with the expectation that increased CO2 concentration induced a lowering of stomatal frequency, however, in this case a large part must be attributed to higher DE in the experimental treatments. Although Li et al. [35] propose the structurally higher DS in A. thaliana was related to their 100 ppmv CO2 treatment, this signal was likely also—at least partially—related to the extremely restricted leaf expansion at 100 ppmv CO2 rather than a true response to the CO2 signal.
No significant response of SI or gsmax to CO2 was observed in G1-2, however a small but insignificant reduction in gsmax over the experimental CO2 gradient was observed.
Leaf response in G2
The consecutive 2014 growth season, G2, allowed us to evaluate the multi-seasonal response of stomatal properties to long-term controlled-CO2 exposure, which may not be evident in a single-season CO2 experiment. The adjustment of plastic LA as a CO2 response also occurred in G2 with significantly smaller leaves produced at 150 ppmv than at 450 ppmv and 800 ppmv. As in G1, low LA at 150 ppmv was associated with low CAE and higher LA with higher CAE at 450 ppmv and 800 ppmv (Fig 1B) showing that lateral epidermal cell expansion was strongly regulated by CO2 availability as in G1.
In contrast with G1, G2 revealed pronounced responses of non-plastic structural stomatal parameters DS, SI, and gsmax to CO2 along the CO2 gradient between 150, 399, and 450 ppmv CO2. The additional enrichment step from 450 to 800 ppmv, however, demonstrated response levelling-off with only minor additional changes compared to the step from low to ambient CO2 conditions in all relevant parameters.
In studies of European tree birches, Betula pendula and Betula pubescens, analysis of SI in leaves grown over the industrial CO2 increase from 290 to 360 ppmv showed that these species reach their upper CO2 response limit at around 400 to 430ppmv [36]. This hypothesis is based on the observed successive slowdown in SI decrease from ~340ppmv onwards. A comparable pattern was observed in herbarium and modern leaf specimens collected over the period from 1919 to 2002, where the SI response levelled off between ~ 350 to 380 ppmv [11]. Moreover, available data from single-season elevated CO2 experiments and variable nitrogen treatments did not show any significant SI decline over the CO2 doubling from 350 ppmv to 700 ppmv in B. pendula [37]. Our data fully support the assertion that much of the stomatal response of B. nana occurs between 150 and 399 ppmv, with some further adjustment between 399 and 450 ppmv and no additional SI decrease between 450 and 800 ppmv. These observations of response patterns in SI hold for DS in all studies. After pronounced initial decline over the experimental gradient from sub-ambient to ambient CO2, DS and SI levelled off. Stomatal frequency adaptation is species-specific and should be evaluated for individual species; in the case of European tree and shrub birches, however, the CO2 ceiling of the SI at around 400 ppmv is a common feature which occurs independently in naturally-grown as well as in experimental leaf material.
From DS and stomatal geometry, structural maximum stomatal conductance, gsmax, was derived; this is an important hydrological parameter regulating the water exchange between plant and atmosphere [13,14,28]. C3 plants reduce their transpirational water loss in response to increasing atmospheric CO2 concentrations, which may affect global climate by reduced cloud formation and precipitation, thus exerting a physiological feedback on climate and hydrology [38–40].
In B. nana, gsmax showed a strong adjustment to CO2 in G2 compared to G1 with over 30% reduction of gsmax in G2 from 150 ppmv to 450 ppmv. The additional CO2 increase to 800 ppmv induced only a ~-6% further reduction in gsmax. Comparing our results to stomatal conductance to water vapour (gwmax) data deduced from historical B. nana leaf material [12], we calculated structurally lower absolute values which may, however, result from different input parameters used in the gsmax calculation for each dataset. In Florida species collected over the past 150 years from herbarium and naturally-grown specimens, a reduction in gsmax of ~-33% per 100 ppmv for angiosperms and ~-37% per 100 ppmv for conifers was observed [14]. In the Florida study, long-term species adaptation within the limits of phenotypic plasticity was a result of the plants primarily adjusting DS, and to some extent stomatal dimensions, as a response to the anthropogenic CO2 increase [13]. Like these species, experimentally-grown B. nana from our study adjusted DS and, to some extent, stomatal architecture within its phenotypic plasticity in response to changing CO2 conditions, in order to optimise CO2 uptake and reduce transpirational water loss.
One possible way to compare the various datasets is by evaluating the relative response rate of gsmax to CO2. In our B. nana study this is -0.11% ppmv-1 from 150 to 450 ppmv and -0.06% ppmv-1 between 150 and 800 ppmv. This decline in gsmax corresponds with observed rates of change of -0.16% ppmv-1 in naturally grown B. nana over the industrial CO2 increase of 290 to 380 ppmv [12]. In growth experiments with Nothofagus fusca, response rates between -0.21 and -0.31% ppmv-1 were observed over a 260 to 370 ppmv CO2 gradient [Hincke et al., unpub.]. Relatively few studies have been conducted where gsmax is inferred from leaf cuticle analysis of material from growth experiments, herbaria, and well-dated palaeo-cores. The data from G2 is within the range of published response rates, between -0.13 and -0.47% ppmv-1 (see S1 Table for a summary of reported response rates of gsmax in a range of species).
Stomatal adjustment over successive growth seasons
In response to changing atmospheric CO2, plants adjust stomata and leaf diffusive conductance to optimise CO2 uptake and limit water loss [41]. The adjustment, however, does not occur instantaneously and takes place over multiple seasons, although some plastic leaf parameters, such as LA, may adjust within one season of exposure to experimental CO2 conditions. Our data suggest that the majority of stomatal response occurred between 150 and 450 ppmv, but not before a new leaf generation formed completely under experimental conditions, indicating that either the upper phenotypic response limits for this species were reached around 400–450 ppmv, or that no further adjustment was necessary to limit water loss as a response to CO2 enrichment past this level. Data from subsequent experimental growth seasons is required to evaluate whether the response limit for B. nana was achieved in G2 in this experiment, or if further adjustments of SI, gsmax, and structural stomatal parameters as a response to CO2 would occur in G3 onwards.
The data from consecutive growth seasons G1 and G2 in B. nana strongly emphasise the requirement for multi-generational growth experiments with the same plant individuals to observe the full plant response to CO2 conditions. The single-season nature of previous experimental work may be an insufficient length of time to evaluate the true response of structural stomatal parameters to CO2 change.
Lammas leaves provide a method to test the CO2 acclimation response within one season of growth as they are initiated during the growth season under experimental CO2 conditions. Some adjustment of leaf parameters in the lammas generation (G1-2) was observed in B. nana with adjustment of DS, DE, and CAE, however, key structural parameters SI and gsmax did not respond clearly to CO2 in a single experimental season. In A. thaliana newly formed leaves produced significantly lower DS and SI when mature leaves were exposed in cuvettes to elevated CO2 conditions [24], a similar leaf generation response to the effect observed from G1 to G2 in B. nana.
While free-air carbon enrichment (FACE) experiments found some reduction in stomatal properties DS and gsmax under elevated CO2 conditions in some studies, the observed reduction in gsmax was attributed to instantaneous adaptation and not a long-term CO2 effect [17,18,42,43]. Studies conducted evaluating plant response to ambient and elevated CO2 may not capture the full range of structural stomatal response to CO2 as the majority of reduction in DS, SI, and gsmax occurs below the species response limits of ~400–450 ppmv.
Earlier experiments with B. pendula found no clear response in SI, measured over one leaf generation, to elevated CO2 exposure [37]. The lack of SI response in this case was due to the single-season nature of the experiment. Seedlings were germinated, and the leaves initiated, under global ambient CO2 for 40 days prior to exposure to experimental CO2 levels [44]. SI adjustment occurred between 150 and 399 ppmv in our data, and as the B. pendula study examined ambient to high CO2 conditions, it is possible that no further adjustment of SI to CO2 occurred past the species response limits of c. 400 ppmv, and thus was not be captured in the B. pendula study. Single-season experiments such as the B. pendula study potentially do not capture the full CO2 range under which stomatal acclimation occurs, rather, they likely capture changes resulting from plants adjusting within their phenotypic plasticity which does not reflect a true, long-term adjustment. Our results demonstrate that true leaf-level adjustment of stomatal properties in response to altered CO2 levels takes place over at least two successive growth seasons.
Implications of the long-term experimental approach for DS- and SI-based CO2 reconstructions
In G2, both DS and SI were responsive to CO2 across the extensive range of glacial CO2 lows to potential future CO2 highs. The absolute values of DS observed in the experimental data were lower than DS from historical B. nana data, which have values between 300 mm-2 for pre-industrial CO2 levels and approaches 100 mm-2 around 370 ppmv CO2 [11]. SI ranged from 15% in experimental B. nana grown in the 150 ppmv chamber to 11.2% at 399 ppmv, to 10.6% at 450 ppmv and 10.3% at 800 ppmv. Historical SI values of 12% at pre-industrial CO2 concentrations of around 290 ppmv to 9–5.5% at around 370 ppmv CO2 levels are recorded in the literature [11]. The 15% SI from experimental B. nana grown at 150 ppmv would logically extend the dataset to glacial CO2 conditions, however SI at ambient to high CO2 range are higher than expected compared to the historical dataset. Differences in measured parameters between historical and experimentally-grown B. nana may be due to habitat variability between the tested populations or the difference in growth conditions between historical specimens and the growth chamber experiments. In our experiments, the systematic down-regulation of parameter SI was directly related to CO2 due to the consistent conditions in the experimental set-ups. In a study with B. pendula, SI linearly increased in 12°C, 20°C, and 30°C treatments [37]. In our study, SI at 12°C (11%) was still significantly higher than 8.4% SI observed under natural growth conditions at 10°C mean annual temperature in the southern Netherlands [37]. While some offset between experimentally-grown B. nana and naturally-grown sub-arctic plants and growth chamber plants is expected due to higher temperature conditions in the growth chambers compared to natural sub-Arctic conditions, these systematic offsets in absolute values were related to specific experimental conditions and not the response of stomatal parameters to CO2 conditions.
Work with herbarium and down-core sub-fossil leaves and leaf fragments provide the potential for seasonally-resolved stomatal data calibrated against instrumental measurements. Data collected year-to-year automatically cover multiple generations and growth seasons. Where material from the same individual plant or genetic plant population was sampled the adjustment of stomatal parameters can be traced over known atmospheric CO2 ranges. However, these ranges are limited to the CO2 rise between pre-industrial CO2 of approximately 290 ppmv and present values of 400 ppmv, and exclude glacial CO2 conditions and potential future CO2 rise. Incorporating growth chamber data into historical herbarium and well-dated palaeo material datasets can provide a better understanding of plant response to varying atmospheric CO2 levels through the Cenozoic CO2 range.
Comparison of greenhouse and growth chamber conditions
The greenhouses which housed the control (399 ppmv) plants during this study were exposed to open air and natural light conditions through a semi-shaded roof. In the experimental chambers, artificial light conditions and humidity were more strictly controlled. The temperature was controlled in both set-ups (~20°C in the greenhouses compared to 21°C/18°C day/night in growth chambers) so this factor was unlikely to cause any offset in measured parameters. While there was a small offset in measured stomatal parameters between the greenhouse control and ambient experimental growth chamber, the control values of morphology and stomatal features from the greenhouse-grown plants did not significantly differ from the plants grown in the ambient CO2 growth chamber. This confirms that, despite differences in the environmental conditions in both set-ups, for example, exposure to the natural diurnal light cycle in the greenhouses rather than artificial light in the growth chambers, it may still be possible to integrate data generated from long-term experimentally-grown plants with datasets for validation from herbaria or sub-fossil leaf deposits from peat sequences.
Conclusions
Results from our growth experiments with B. nana showed that while a short-term response to CO2 change over one growing season was evident in leaf morphological and stomatal parameters including LA, DS, and DE, the full effect of exposure to experimental CO2 conditions, especially on non-plastic stomatal properties gsmax and SI, was not captured in G1 as these parameters did not fully adjust to CO2 until G2. The leaf-level signal may thus be skewed if leaf sampling occurs during the early stages of ontogeny, potentially concealing the true CO2 response in these plants. Our results validate the seasonal-scale response of leaf morphological parameter LA and stomatal parameters DS and DE to CO2 in B. nana, as postulated by Lake et al. [24].
Further, we confirm that the intensified adjustment or acclimation response of stomatal parameters occurs over several growing seasons under altered CO2. Our data improves the understanding of seasonal response of stomatal parameters in a woody C3 plant by demonstrating that the stomatal response to atmospheric CO2 in B. nana extends over a minimum of two consecutive growth seasons within one population of plants. The documented response provides a likely explanation to the—so far—partially contradictory results between historical and palaeobotanical records and experimental data. It is not possible within the context of this experiment to predict whether the observed response is the full extent of the adjustment of B. nana to experimental CO2 conditions, as further adjustments to stomatal properties may continue in subsequent growing seasons. Our findings very strongly support the necessity for multi-annual experiments to fully quantify the extent of stomatal acclimation to the range of Cenozoic CO2 concentrations in perennial C3 species as short-term growth experiment data may not reflect the full morphological and stomatal response to experimental CO2.
Supporting Information
The error is standard error of the mean. No clear pattern of response of this parameters in G1-2 was observed. In G2, SLA was lower at higher CO2 levels, with a levelling-off of the response between 450 ppmv and 800 ppmv.
(TIF)
The response rates of gsmax of species studied in experimental set-ups and from herbarium records was calculated as % ppmv-1. The response rate of gsmax is negative with increasing CO2 in all cases.
(PDF)
Acknowledgments
We greatly appreciate the support from the Utrecht University Botanische Tuinen, particularly the assistance of Gerard van Buiten. Thank you to Rob Welschen for technical assistance in the Fytotron.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was funded by the Darwin Centre for Biogeosciences (http://www.darwincenter.nl/). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1.Wagner F, Kouwenberg LLR, van Hoof TB, Visscher H. Reproducibility of Holocene atmospheric CO2 records based on stomatal frequency analysis. Quat Sci Rev. 2004;23: 1947–1954. 10.1016/j.quascirev.2004.04.003 [DOI] [Google Scholar]
- 2.Wagner F, Bohncke SJP, Dilcher DL, Kürschner WM, van Geel B, Visscher H. Century-scale shifts in early Holocene atmospheric CO2 concentration. Science (80-). 1999;284: 1971–1973. 10.1126/science.284.5422.1971 [DOI] [PubMed] [Google Scholar]
- 3.Petit JR, Jouzel J, Raynaud D, Barkov NI, Barnola J-M, Basile I, et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399: 429–436. 10.1038/20859 [DOI] [Google Scholar]
- 4.Beerling DJ, Royer DL. Convergent Cenozoic CO2 history. Nat Geosci. 2011;4: 418–420. 10.1038/ngeo1186 [DOI] [Google Scholar]
- 5.Royer DL. CO2-forced climate thresholds during the Phanerozoic. Geochim Cosmochim Acta. 2006;70: 5665–5675. 10.1016/j.gca.2005.11.031 [DOI] [Google Scholar]
- 6.Franks PJ, Casson S. Connecting stomatal development and physiology. New Phytol. 2014;201: 1079–82. 10.1111/nph.12673 [DOI] [PubMed] [Google Scholar]
- 7.Roth-Nebelsick A, Grein M, Utescher T, Konrad W. Stomatal pore length change in leaves of Eotrigonobalanus furcinervis (Fagaceae) from the Late Eocene to the Latest Oligocene and its impact on gas exchange and CO2 reconstruction. Rev Palaeobot Palynol. 2012;174: 106–112. 10.1016/j.revpalbo.2012.01.001 [DOI] [Google Scholar]
- 8.Roth-Nebelsick A, Fernández V, Peguero-Pina JJ, Sancho-Knapik D, Gil-Pelegrín E. Stomatal encryption by epicuticular waxes as a plastic trait modifying gas exchange in a Mediterranean evergreen species (Quercus coccifera L.). Plant Cell Environ. 2013;36: 579–89. 10.1111/j.1365-3040.2012.02597.x [DOI] [PubMed] [Google Scholar]
- 9.Wagner F, Dilcher DL, Visscher H. Stomatal frequency responses in hardwood-swamp vegetation from Florida during a 60-year continuous CO2 increase. Am J Bot. 2005;92: 690–5. 10.3732/ajb.92.4.690 [DOI] [PubMed] [Google Scholar]
- 10.van Hoof TB, Kürschner WM, Wagner F, Visscher H. Stomatal index response of Quercus robur and Quercus petraea to the anthropogenic atmospheric CO2 increase. Plant Ecol. 2006;183: 237–243. 10.1007/s11258-005-9021-3 [DOI] [Google Scholar]
- 11.Finsinger W, Wagner-Cremer F. Stomatal-based inference models for reconstruction of atmospheric CO2 concentration: a method assessment using a calibration and validation approach. The Holocene. 2009;19: 757–764. 10.1177/0959683609105300 [DOI] [Google Scholar]
- 12.Gagen M, Finsinger W, Wagner-Cremer F, Mccarroll D, Loader NJ, Robertson I, et al. Evidence of changing intrinsic water-use efficiency under rising atmospheric CO2 concentrations in Boreal Fennoscandia from subfossil leaves and tree ring δ13C ratios. Glob Chang Biol. 2011;17: 1064–1072. 10.1111/j.1365-2486.2010.02273.x [DOI] [Google Scholar]
- 13.de Boer HJ, Lammertsma EI, Wagner-Cremer F, Dilcher DL, Wassen MJ, Dekker SC. Climate forcing due to optimization of maximal leaf conductance in subtropical vegetation under rising CO2. Proc Natl Acad Sci. 2011;108: 4041–4046. 10.1073/pnas.1100555108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammertsma EI, de Boer HJ, Dekker SC, Dilcher DL, Lotter AF, Wagner-Cremer F. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc Natl Acad Sci. 2011;108: 4035–4040. 10.1073/pnas.1100371108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Burgh J, Visscher H, Dilcher DL, Kürschner WM. Paleoatmospheric signatures in Neogene fossil leaves. Science. 1993;260: 1788–90. 10.1126/science.260.5115.1788 [DOI] [PubMed] [Google Scholar]
- 16.Kürschner WM, Kvacek Z, Dilcher DL. The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proc Natl Acad Sci U S A. 2008;105: 449–53. 10.1073/pnas.0708588105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royer DL. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev Palaeobot Palynol. 2001;114: 1–28. 10.1016/S0034-6667(00)00074-9 [DOI] [PubMed] [Google Scholar]
- 18.Reid CD, Maherali H, Johnson HB, Smith SD, Wullschleger SD, Jackson RB. On the relationship between stomatal characters and atmospheric CO2. Geophys Res Lett. 2003;30: 1983 10.1029/2003GL017775 [DOI] [Google Scholar]
- 19.Temme AA, Liu JC, Cornwell WK, Cornelissen JHC, Aerts R. Winners always win: growth of a wide range of plant species from low to future high CO2. Ecol Evol. 2015;5: 4949–61. 10.1002/ece3.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rico C, Pittermann J, Polley HW, Aspinwall MJ, Fay PA. The effect of subambient to elevated atmospheric CO2 concentration on vascular function in Helianthus annuus: implications for plant response to climate change. New Phytol. 2013;199: 956–65. 10.1111/nph.12339 [DOI] [PubMed] [Google Scholar]
- 21.Wagner F, Visscher H, Kürschner WM, Dilcher DL. Influence of ontogeny and atmospheric CO2 on stomata parameters of Osmunda regalis. CFS Cour Forschungsinstitut Senckenb. 2007;258: 183–189. [Google Scholar]
- 22.England JR, Attiwill PM. Changes in stomatal frequency, stomatal conductance and cuticle thickness during leaf expansion in the broad-leaved evergreen species, Eucalyptus regnans. Trees. 2011;25: 987–996. 10.1007/s00468-011-0573-7 [DOI] [Google Scholar]
- 23.Wagner F, Below R, Klerk PD, Dilcher DL, Joosten H, Kurschner WM, et al. A natural experiment on plant acclimation: lifetime stomatal frequency response of an individual tree to annual atmospheric CO2 increase. Proc Natl Acad Sci. 1996;93: 11705–11708. 10.1073/pnas.93.21.11705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lake JA, Quick WP, Beerling DJ, Woodward FI. Plant development. Signals from mature to new leaves. Nature. Nature Publishing Group; 2001;411: 154 10.1038/35075660 [DOI] [PubMed] [Google Scholar]
- 25.Royer DL, Wing SL, Beerling DJ, Jolley DW, Koch PL, Hickey LJ, et al. Paleobotanical evidence for near present-day levels of atmospheric CO2 during part of the Tertiary. Science (80-). 2001;292: 2310–3. 10.1126/science.292.5525.2310 [DOI] [PubMed] [Google Scholar]
- 26.US Department of Commerce NESRL. ESRL Global Monitoring Division—Global Greenhouse Gas Reference Network.
- 27.Salisbury EJ. On the Causes and Ecological Significance of Stomatal Frequency, with Special Reference to the Woodland Flora. Philos Trans R Soc London Ser B. 1928;216: 1–65. [Google Scholar]
- 28.Franks PJ, Beerling DJ. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci. 2009;106: 10343–10347. 10.1073/pnas.0904209106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beerling DJ, Chaloner WG. The impact of atmospheric CO2 and temperature changes on stomatal density: observation from Quercus robur lammas leaves. Ann Bot. 1993;71: 231–235. 10.1006/anbo.1993.1029 [DOI] [Google Scholar]
- 30.Ticha I. Photosynthetic characteristics during ontogenesis of leaves. 7. Stomata density and sizes. Photosynthetica. 1982;16: 375–471. [Google Scholar]
- 31.Morison J, Gifford R. Plant Growth and Water Use With Limited Water Supply in High CO2 Concentrations. I. Leaf Area, Water Use and Transpiration. Aust J Plant Physiol. 1984;11: 361 10.1071/PP9840361 [DOI] [Google Scholar]
- 32.Radoglou KM, Jarvis PG. Effects of CO2 enrichment on four poplar clones. II. Leaf surface properties. Ann Bot. 1990;65: 627–632. [Google Scholar]
- 33.Epron D, Liozon R, Mousseau M. Effects of elevated CO2 concentration on leaf characteristics and photosynthetic capacity of beech (Fagus sylvatica) during the growing season. Tree Physiol. 1996;16: 425–432. 10.1093/treephys/16.4.425 [DOI] [PubMed] [Google Scholar]
- 34.Ferris R, Sabatti M, Miglietta F, Mills RF, Taylor G. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant, Cell Environ. 2001;24: 305–315. [Google Scholar]
- 35.Li Y, Xu J, Haq NU, Zhang H, Zhu X-G. Was low CO2 a driving force of C4 evolution: Arabidopsis responses to long-term low CO2 stress. J Exp Bot. 2014;65: 3657–67. 10.1093/jxb/eru193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kürschner WM, Wagner F, Visscher H. Predicting the response of leaf stomatal frequency to a future CO2-enriched atmosphere: constraints from historical observations. Geol Rundschau. 1997;86: 512–517. 10.1007/s005310050158 [DOI] [Google Scholar]
- 37.Wagner F. Effects of nitrogen deficiency stress on epidermal cell properties of Betula pendula under ambient and elevated CO2 concentrations The influence of environment on the stomatal frequency in Betula. Utrecht: LPP Foundation; 1998. p. 102. [Google Scholar]
- 38.Betts RA, Boucher O, Collins M, Cox PM, Falloon PD, Gedney N, et al. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature. 2007;448: 1037–41. 10.1038/nature06045 [DOI] [PubMed] [Google Scholar]
- 39.Andrews T, Forster PM, Boucher O, Bellouin N, Jones A. Precipitation, radiative forcing and global temperature change. Geophys Res Lett. 2010;37 10.1029/2010GL043991 [DOI] [Google Scholar]
- 40.Cao L, Bala G, Caldeira K, Nemani R, Ban-Weiss G. Importance of carbon dioxide physiological forcing to future climate change. Proc Natl Acad Sci. 2010;107: 9513–9518. 10.1073/pnas.0913000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franks PJ, Leitch IJ, Ruszala EM, Hetherington AM, Beerling DJ. Physiological framework for adaptation of stomata to CO2 from glacial to future concentrations. Philos Trans R Soc B Biol Sci. 2012;367: 537–546. 10.1098/rstb.2011.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant Cell Environ. 2007;30: 258–270. 10.1111/j.1365-3040.2007.01641.x [DOI] [PubMed] [Google Scholar]
- 43.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. Annual Reviews; 2004;55: 591–628. 10.1146/annurev.arplant.55.031903.141610 [DOI] [PubMed] [Google Scholar]
- 44.Pettersson R, McDonald AJS, Stadenberg I. Effects of elevated carbon dioxide concentration on photosynthesis and growth of small birch plants (Betula pendula Roth.) at optimal nutrition. Plant, Cell Environ. 1992;15: 1115–1121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The error is standard error of the mean. No clear pattern of response of this parameters in G1-2 was observed. In G2, SLA was lower at higher CO2 levels, with a levelling-off of the response between 450 ppmv and 800 ppmv.
(TIF)
The response rates of gsmax of species studied in experimental set-ups and from herbarium records was calculated as % ppmv-1. The response rate of gsmax is negative with increasing CO2 in all cases.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.