Abstract
The purpose of this study was to determine the toxicity profile of dendritic cell (DC) vaccination in stage III and IV melanoma patients, and to evaluate whether there is a correlation between side effects and immunologic and clinical outcome. This is a retrospective analysis of 82 stage III and 137 stage IV melanoma patients, vaccinated with monocyte-derived or naturally circulating autologous DCs loaded with tumor-associated antigens gp100 and tyrosinase. Median follow-up time was 54.3 months in stage III patients and 12.9 months in stage IV patients. Treatment-related adverse events occurred in 84% of patients; grade 3 toxicity was present in 3% of patients. Most common adverse events were flu-like symptoms (67%) and injection site reactions (50%), and both correlated with the presence of tetramer-positive CD8+ T cells (both P<0.001). In stage III melanoma patients experiencing flu-like symptoms, median overall survival (OS) was not reached versus 32.3 months in patients without flu-like symptoms (P=0.009); median OS in patients with an injection site reaction was not reached versus 53.7 months in patients without an injection site reaction (P<0.05). In stage IV melanoma patients (primary uveal and mucosal melanomas excluded), median OS in patients with or without flu-like symptoms was 13.1 versus 8.9 months, respectively (P=0.03); median OS in patients with an injection site reaction was 15.7 months versus 9.8 months in patients without an injection site reaction (P=0.003). In conclusion, DC vaccination is safe and tolerable and the occurrence of the immune-related side effects, such as flu-like symptoms and injection site reactions, correlates with immunologic and clinical outcome.
Key Words: dendritic cell vaccination, melanoma, immune-related adverse events, immune response
Melanoma is considered to be one of the most immunogenic tumors and is susceptible to immunotherapy.1 Immunotherapeutic approaches consist of immune checkpoint inhibitors, cytokines, and vaccination strategies.2 The vaccination strategy studied by our group is dendritic cell (DC) vaccination. DCs were first discovered by Ralph Steinman in 1973, and play a crucial role in the induction of antitumor immunity.3 Autologous DCs can be generated ex vivo, activated, and loaded with tumor antigens, and then injected into patients with the intention to induce tumor-specific effector T cells to kill tumor cells and induce immunologic memory to control tumor relapse.4,5 We and others have treated stage III and IV melanoma patients with DC-based vaccines in different trials over the past years, and noted numerous tumor-specific immune responses. Long-lasting clinical responses are, thus far, limited in stage IV patients treated with DC vaccination monotherapy.6,7 Intriguingly, in a retrospective study with stage III patients, who received autologous DCs loaded with gp100 and tyrosinase, overall survival (OS) was significantly better compared with matched controls, but these results have to be confirmed in a prospective randomized clinical trial.8
As in the field of melanoma therapeutic options are growing rapidly, the choice of the right individual treatment plan for each patient is becoming more challenging. Among the currently approved treatments for metastatic melanoma are the immune checkpoint inhibitors ipilimumab, nivolumab, and pembrolizumab,9–12 the BRAF inhibitors vemurafenib13 and dabrafenib, and the MEK inhibitor trametinib.14 Stage III melanoma patients are at high risk of relapse, despite a radical lymph node dissection, and until recently there was a lack of an effective adjuvant systemic treatment.15,16 In 2015, the Food and Drug Administration gave approval for adjuvant ipilimumab (10 mg/kg), based on improvement of recurrence-free survival compared with placebo in a phase III trial.17 However, data on OS have not been reported yet. Anti-PD-1 monoclonal antibodies (mAbs) are currently under investigation in stage III melanoma patients (NCT02388906, NCT02362594). The probability of a durable clinical response is certainly the most important factor in the choice of treatment in stage IV melanoma patients in a good clinical condition. Furthermore, it is essential to consider side effects in treatment decisions, especially when treatments are equally effective or when combination therapy is considered. Trials with ipilimumab and anti-PD-1 mAbs showed considerable grade 3–4 adverse events, particularly when the combination of both treatments was given.9,12,17,18 Immune-related adverse events, such as colitis and dermatitis, are the most common forms of toxicity after checkpoint inhibition. Attia and colleagues showed a correlation between the induction of autoimmunity and durable objective responses in patients treated with anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) in combination with peptide vaccination. A partial or complete response was seen in 36% of patients with grade 3–4 autoimmune toxicity, compared with 5% of patients with no signs of autoimmunity.19 Furthermore, a recent meta-analysis including 5737 stage III/IV melanoma patients treated with immunotherapy showed that vitiligo was associated with a clear survival benefit.20 Therefore, we hypothesized that there might also be a correlation between immune-related side effects and immunologic and clinical outcomes in DC vaccinated patients.
The aim of this retrospective study was to determine the toxicity profile of DC vaccination in a large cohort of stage III and IV melanoma patients, and to evaluate whether there is a correlation between the occurrence of immune-related adverse events and both immunologic and clinical outcome.
MATERIALS AND METHODS
Patient Characteristics
We retrospectively analyzed a cohort of stage III and stage IV melanoma patients, who were enrolled in our DC vaccination trials between June 1999 and March 2014. Stage III patients received adjuvant DC vaccination within 2 months after radical lymph node dissection. DC vaccination in stage IV patients was given as any line of treatment, although mostly as first-line treatment. Inclusion criteria were: histologically proven regional (stage III) or distant (stage IV) metastatic disease, World Health organization performance status of 0 or 1, and melanoma expressing the melanoma-associated antigens gp100 (compulsory) and tyrosinase (noncompulsory). Patients with a second malignancy in the previous 5 years or serious concomitant disease were excluded. All studies were approved by the appropriate Medical Ethical Review Board and written informed consent was obtained from all patients.
DC Vaccination
All patients were vaccinated with DCs loaded with tumor-associated antigens (TAA) of gp100 and tyrosinase according to a schedule of 3 biweekly vaccinations, followed by a delayed type hypersensitivity (DTH) skin test within 2 weeks after each vaccination cycle. Some patients received an extra vaccination before radical lymph node dissection for additional imaging studies. Patients received a maximum of 2 additional cycles of vaccinations at 6-month intervals when no signs of recurrent or progressive disease were present.
Patients were treated with DC vaccination monotherapy in different treatment protocols (Table 1). DC vaccination is still an investigational product in melanoma. Stage III patients were all vaccinated with monocyte-derived autologous DCs (moDCs), as were most stage IV patients. Monocytes were enriched from leukapheresis products by plastic adherence of blood mononuclear cells or by counterflow centrifugation using Elutra-cell separator (Gambro BCT, Lakewood, CO) and single-use, functionally sealed disposable Elutra sets, as described before.21 Monocytes were cultured in the presence of interleukin (IL)-4 (500 U/mL), granulocyte macrophage colony-stimulating factor (GM-CSF) (800 U/mL; both Cellgenix, Freiburg, Germany), and keyhole limpet hemocyanin (KLH) (10 μg/mL; Calbiochem, Darmstadt, Germany or Immucothel, Biosyn Arzneimittel GmbH, Fellbach, Germany). Most protocols used DCs matured with autologous monocyte-conditioned medium (30%, vol/vol) supplemented with prostaglandin E2 (PGE2) (10 μg/mL; Pharmacia & Upjohn, Puurs, Belgium) and tumor necrosis factor-α (10 ng/mL; Cellgenix) for 48 hours or a cocktail of tumor necrosis factor-α (10 ng/mL), IL-1β (5 ng/mL), IL-6 (15 ng/mL; all Cellgenix), and PGE2 (10 μg/mL).22 DCs in protocols 5 and 8B were matured with a cocktail of prophylactic vaccines including BCG vaccine SSI (4% vol/vol; Nederlands Vaccin Instituut, Bilthoven, The Netherlands), Typhim Vi (4% vol/vol; Sanofi Pasteur MSD, Brussels, Belgium), and Act-HIB (4% vol/vol; Aventis Pasteur, Brussels, Belgium), supplemented with PGE2 (10 μg/mL) for 48 hours (VAC-DC).23 In protocol 8A, DCs were matured through electroporation with mRNA encoding CD40L, CD70, and constitutively active TLR4.24 DCs were pulsed with major histocompatibility complex (MHC)-I-restricted peptides gp100:154–162, gp100:280–288, and tyrosinase:369–377, or electroporated with mRNA encoding gp100 or tyrosinase.25 DCs in protocol 4A were also pulsed with MHC-II-restricted peptides gp100:44–59 and tyrosinase:448–462.7
TABLE 1.
Dendritic Cell Vaccination Protocol
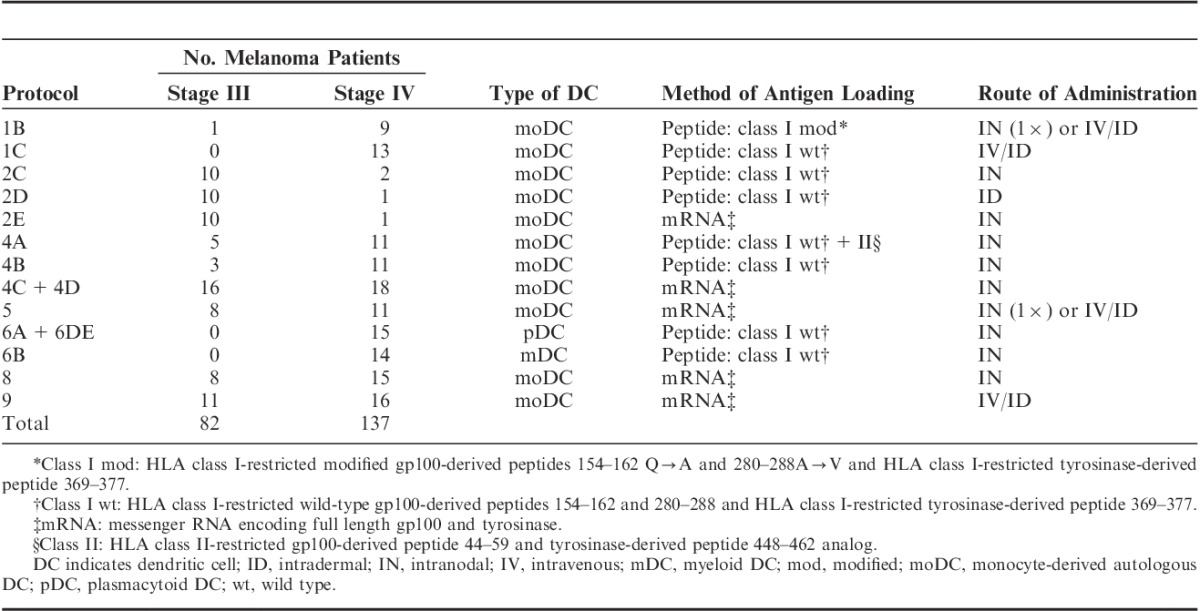
A minority of stage IV patients were treated with naturally occurring plasmacytoid DCs (pDCs) or myeloid DCs (mDCs).26,27 pDCs and mDCs were directly isolated from apheresis products using the fully closed immunomagnetic CliniMACS isolation system (Miltenyi Biotec, Bergisch Gladbach, Germany). GMP-grade magnetic bead–coupled antibodies were used, following the manufacturer’s guidelines. Following apheresis and CliniMACS isolation, pDCs and mDCs were cultured overnight at a concentration of 106 cells/mL in X-VIVO 15 (Cambrex, East Rutherford, NJ) containing pooled human serum (2%; Sanquin, Amsterdam, The Netherlands), supplemented with recombinant human IL-3 (10 ng/mL; Cellgenix) for pDCs, and recombinant human GM-CSF (800 U/mL; Cellgenix) and KLH (1 mg/mL; Immucothel, Biosyn Arzneimittel GmbH, Fellbach, Germany) for mDCs. pDCs were activated by addition of FSME-IMMUN (1:10 vol/vol; Baxter AG, Vienna, Austria). pDCs and mDCs were loaded with the melanoma-associated HLA-A*0201-restricted peptides gp100:154–162, gp100:280–288, and tyrosinase:369–377.
Toxicity and Response Evaluation
Safety evaluations were performed at the outpatient clinic in all patients before each vaccination, DTH, and 2 follow-up visits. Adverse events were scored using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Grade 3 and 4 adverse events were considered to be serious adverse events. Flu-like symptoms include fever, fatigue, chills, body aches, malaise, loss of appetite, and headache. Fatigue was mentioned separately when it lasted at least 1 day longer than the other flu-like symptoms or when present without other flu-like symptoms.
Tumor evaluation was performed at baseline and every 3 months by physical examination in stage III patients and by CT scan according to RECIST version 1.1 in stage IV patients. DTH skin tests were used for immunologic response evaluation in all treatment protocols. Briefly, DCs loaded with either gp100, tyrosinase, or both antigens were injected intradermally in the skin of the back of patients at different sites, 4 cm apart from each other (max.1×106 DC each). After 48 hours, punch biopsies (6 mm) were taken. Skin-test infiltrating lymphocytes (SKILs) were analyzed for antigen-specific T cells by staining them with tetrameric-major MHC complexes containing the gp100 and tyrosinase epitopes (HLA-A*02:01-positive patients) and TAA-specific functional responses by specific production of type 1 T-helper (Th1) cytokines and no type 2 T-helper (Th2) cytokines to TAA, as described before.28
Statistical Analysis
OS was calculated from the date of apheresis to the date of death. The Kaplan-Meier method was used to evaluate the correlation between adverse events and clinical outcome. Primary uveal and mucosal melanomas were excluded from survival analyses of stage IV patients to improve uniformity. Statistical significance was evaluated using a log-rank test. Cox proportional-hazard models were used to estimate hazard ratios (HR). Cramér’s V scores (measure of association between nominal variables) were calculated to measure the association between the occurrence of adverse events and immunologic outcome; Pearson χ2 tests were used to evaluate statistical significance. Ulceration was assumed absent if not reported in the pathology report. P-values <0.05 were considered significant. SPSS version 22 software (SPSS Inc, Chicago, IL) and GraphPad Prism 5.03 (GraphPad Software Inc., San Diego, CA) were used for statistical analysis.
RESULTS
Patient Characteristics
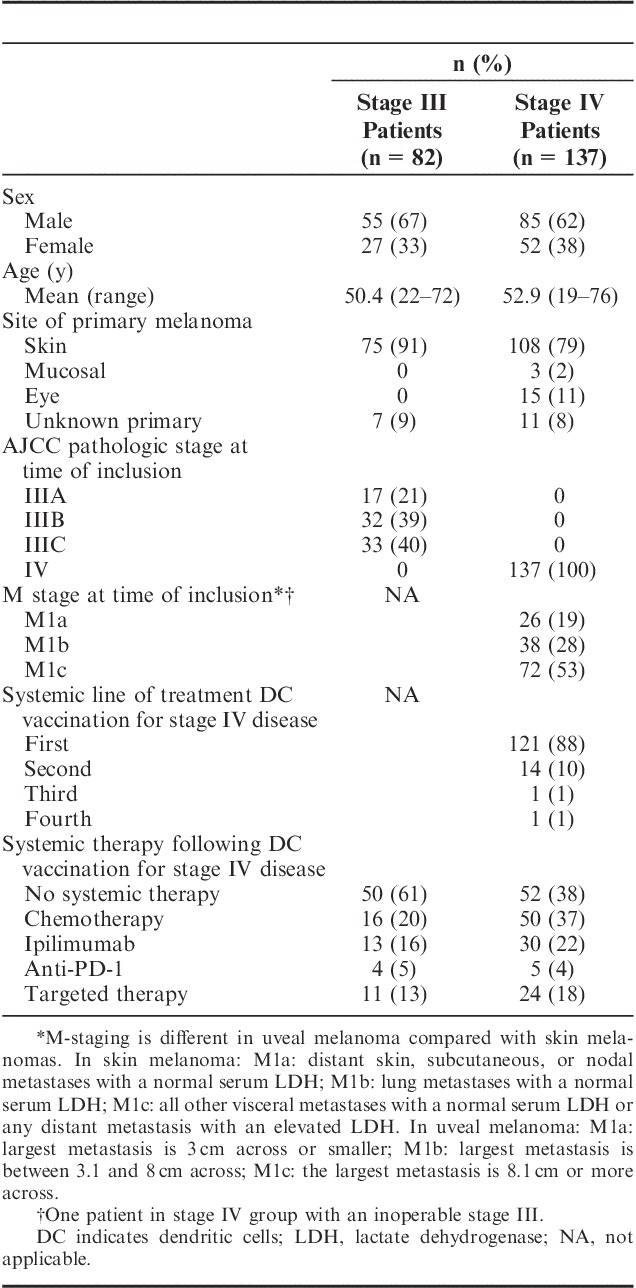
A total of 82 stage III and 137 stage IV melanoma patients treated with DC vaccination monotherapy between June 1999 and July 2015 were included in the analysis. DCs were predominantly administered intranodally (64%), and the most commonly used method of antigen loading was electroporation with mRNA (52%, Table 1). Patient characteristics of all included patients are listed in Table 2. All stage III patients were treated with moDCs, whereas among patients with stage IV disease, 79% received moDCs, 11% were treated with pDCs, and 10% of patients with mDCs. A total of 59% of stage III patients and 10% of stage IV patients completed all 3 cycles of vaccinations, 20% of patients with stage III disease and 7% of stage IV patients received 2 cycles, and only 1 cycle was given to 22% of stage III patients and 83% of stage IV patients. The median follow-up time from apheresis to patient death or censoring was 54.3 months (range, 3.7–162.4 mo) in stage III patients and 12.9 months (range, 2.0–179.7 mo) in stage IV patients.
TABLE 2.
Patient Characteristics
Toxicity Profile
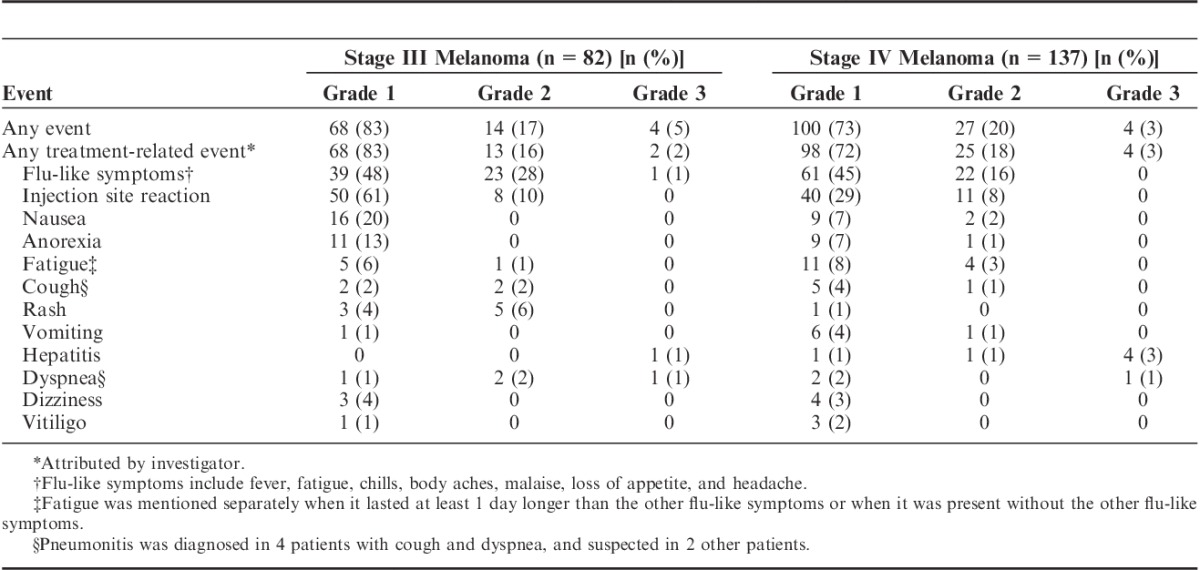
A total of 183 patients (84%) had any treatment-related adverse event. The adverse events reported are listed in Table 3. The most common adverse events related to DC vaccination were flu-like symptoms (67%) and injection site reactions (50%). Flu-like symptoms usually lasted up to 48 hours and consisted of fever, fatigue, chills, body aches, malaise, loss of appetite, and headache, although generally only a few of these symptoms were present. Significantly more stage III than stage IV patients had flu-like symptoms (77% vs. 61%, P=0.024) and injection site reactions (71% vs. 37%, P<0.001). When flu-like symptoms were present, they occurred already during the first cycle in 87% of stage III and 93% of stage IV patients. Of patients with an injection site reaction, 68% of stage III and 97% of stage IV patients showed this during the first cycle of vaccinations. Stage IV patients with a raised lactate dehydrogenase (LDH) at baseline, a bad prognostic factor,29 showed less injection reactions than patients with a normal LDH (10% vs. 29%, P=0.021), but no difference was seen for flu-like symptoms (P=0.457). Treatment-related grade 3 adverse events were present in 3% of patients, consisting of hepatitis (5 patients), pneumonitis (2 patients), and flu-like symptoms (1 patient). All grade 3 adverse events were seen in patients treated in protocols 5 and 8B who received DCs matured with cocktail of prophylactic vaccines.30 Vaccinations were stopped due to toxicity in 3 patients (1%) in these protocols and other patients in these protocols received no subsequent cycles or received a dose reduction. In only 2 patients (1%) in the other vaccination protocols, vaccinations were stopped on patients request due to a temporary grade 2 rash.
TABLE 3.
Adverse Events
As aforementioned, 29 stage IV patients received naturally circulating DCs intranodally, whereas the other 108 stage IV patients received moDCs intranodally, intradermally, or intravenously and intradermally. Patients receiving naturally circulating DCs experienced significantly less treatment-related adverse events compared with patients treated with moDCs (59% vs. 85%, P=0.008). Injection site reactions and flu-like symptoms were less present in patients treated with naturally circulating DCs in comparison with patients treated with moDCs; 7% versus 45% (P=0.001) and 28% versus 69% (P<0.001), respectively. Although intranodal administration induced less immune-related adverse events compared with intravenous/intradermal administration (P<0.001), significant differences in injection site reactions and flu-like symptoms remained when natural DCs were only compared with intranodally injected moDCs; 7% versus 55% (P<0.001) and 28% versus 64% (P=0.001), respectively.
In conclusion, DC vaccination is safe and tolerable in both stage III and IV melanoma patients and naturally circulating DCs induce less immune-related adverse events than moDCs.
Immune-related Adverse Events Correlate With Immunologic and Clinical Outcome
DTH skin tests were performed after each cycle of vaccinations, in which punch biopsies were taken 48 hours after injecting DCs loaded with either gp100, tyrosinase, or both antigens. Lymphocytes out of these biopsies (SKILs) were tested for antigen-specific T cells by staining them with tetrameric-MHC complexes containing the gp100 and tyrosinase epitopes in 178 HLA-A*02:01 positive stage III and IV patients. Furthermore, SKILs of 207 patients were available for analysis of TAA-specific functionality. Tetramer-positive CD8+ T cells were found in SKILs of 85 patients (48%), and in 68 patients (33%) a functional response was present. A significant correlation was found between the occurrence of flu-like symptoms and presence of tetramer-positive CD8+ T cells (Cramér’s V 0.264, P<0.001) or a TAA-specific functional T-cell response (Cramér’s V 0.239, P=0.001). A correlation was also found between injection site reactions and the presence of tetramer-positive CD8+ T cells (Cramér’s V 0.303, P<0.001) or a TAA-specific functional T-cell response (Cramér’s V 0.257, P<0.001).
Median OS in all stage III patients was 81.2 months. OS was significantly longer in 63 stage III patients (77%) with flu-like symptoms (any grade) compared with 19 patients (23%) without these symptoms (HR=0.43; 95% confidence interval (CI), 0.22–0.83; P=0.012), with a corresponding median OS of not reached versus 32.2 months (95% CI, 7.1–55.3; P=0.009; Fig. 1A). Similarly, a longer OS was found in 58 stage III patients (71%) with an injection site reaction (any grade) compared with 24 patients (29%) without an injection site reaction (HR=0.53; 95% CI, 0.28–1.00; P=0.053); median OS was not reached versus 53.7 months (95% CI, 2.8–104.7; P<0.05; Fig. 1B). The correlation between flu-like symptoms and OS remained, when only 48 patients, who completed 3 cycles of vaccinations, were analyzed (HR=0.24; 95% CI, 0.09–0.65; P=0.005); median OS was not reached in 38 patients with flu-like symptoms versus 53.7 months (95% CI, 8.6–98.9) in 10 patients without these symptoms (P=0.002). A trend for a better OS in 38 patients with an injection site reaction was seen (HR=0.41; 95% CI, 0.15–1.11; P=0.078).
FIGURE 1.
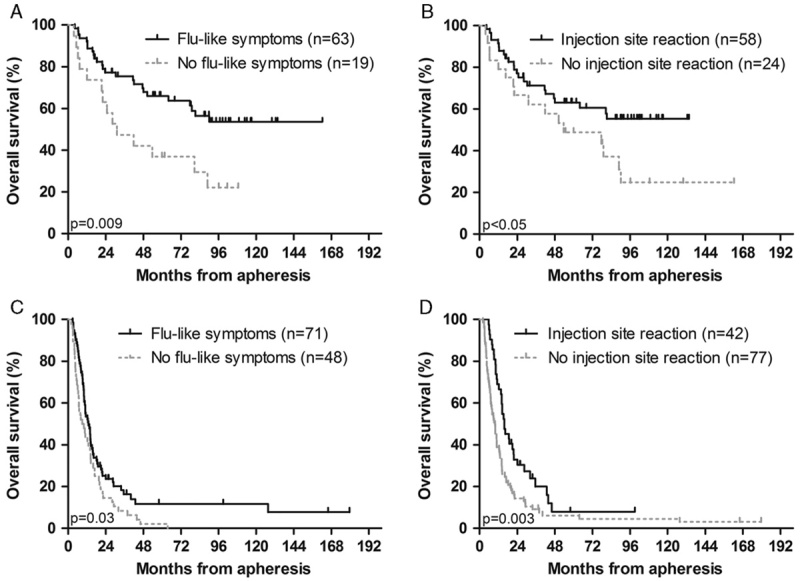
Immune-related adverse events correlate with clinical outcome. Kaplan-Meier curves of overall survival (OS) according to different side effects. The occurrence of flu-like symptoms and injection site reactions correlate significantly with OS in stage III (A, B) and stage IV (C, D) melanoma patients. Patients with a primary uveal or mucosal melanoma were excluded from this analysis.
Median OS in 119 stage IV patients with a primary cutaneous melanoma or unknown primary was 11.3 months. As in stage III patients, flu-like symptoms and injection site reactions correlated with OS in stage IV patients; HR=0.66 (95% CI, 0.45–0.97; P=0.033) and HR=0.55 (95% CI, 0.37–0.83; P=0.004), respectively. Median OS in 71 patients (60%) with flu-like symptoms was 13.1 months (95% CI, 10.1–16.1) versus 8.9 months (95% CI, 4.4–13.3) in 48 patients (40%) without these symptoms (P=0.03; Fig. 1C). In patients with (35%) or without (65%) an injection site reaction, median OS was 15.7 months (95% CI, 11.3–20.1) and 9.8 months (95% CI, 7.2–12.4; P=0.003; Fig. 1D), respectively. The correlation between injection site reactions and OS remained when a conditional landmark analysis was performed after the first cycle of vaccinations (HR=0.62; 95% CI, 0.42–0.94; P=0.022), whereas it was no longer seen for flu-like symptoms (HR=0.94; 95% CI, 0.64–1.38; P=0.754). A significant difference in OS between patients with or without an injection site reaction remained when only patients with a normal LDH were analyzed (median OS 16.3 mo vs. 10.7 mo, P=0.01).
In conclusion, immune-related adverse events correlate with immunologic and clinical outcome in stage III and IV melanoma patients.
DISCUSSION
This analysis of a large patient cohort shows that DC vaccination in stage III and IV melanoma patients is tolerable and safe. Furthermore, it indicates that the occurrence of immune-related adverse events correlates with immunologic and clinical outcome.
The most common treatment-related adverse events of DC vaccination were grade 1–2 flu-like symptoms and injection site reactions at the place of intradermal or intranodal vaccination. Flu-like symptoms lasted up to 48 hours and patients were allowed to take acetaminophen to minimize complaints. Injection site reactions, consisting of redness, swelling, pain, and itch, were usually small and self-limiting within 2–7 days. Patients treated with naturally circulating DCs experienced significantly less injection site reactions and flu-like symptoms than patients receiving moDCs. This difference might be explained by the amount of DCs per vaccination, an average of 6×106 mDCs or 2.6×106 pDCs per vaccination versus up to 30×106 moDCs. However, naturally circulating DCs could induce less side effects themselves independent of the number of injected cells. Treatment-related grade 3 adverse events occurred only in 3% of patients; among them 5 patients had hepatitis and 2 patients had an interstitial pneumonitis. All these patients were treated in protocols using a cocktail of prophylactic vaccines as TLR ligands to mature the DCs, which generates mature DCs that produce IL-12 and optimizes T-cell help.23 The BCG vaccine was probably the reason for these side effects.30 Despite frequent induction of immune responses, this form of DC maturation will not be used in future trials due to its toxicity. So, DC vaccination monotherapy gives mild toxicity and can safely be administered in stage III and IV melanoma patients.
Flu-like symptoms and injection site reactions are considered to be immune-related side effects and they showed a weak, yet significant, correlation with the presence of tetramer-positive CD8+ T cells and a TAA-specific functional T-cell response in stage III and IV patients combined. This correlation does not prove that these side effects are solely caused by activated TAA-specific T cells. Theoretically, KLH-specific responses may have played a role in the occurrence of immune-related side effects. However, no correlation was found between the degree of T-cell proliferation upon stimulation with KLH and these adverse events (data not shown). Furthermore, it is possible that the presence of these immune-related adverse events may indicate a more general raised immune activation, which might be beneficial for a good TAA-specific T-cell response. This would be an explanation for the observation that patients with distant metastases showed less flu-like symptoms and injection site reactions than stage III melanoma patients, as these patients have a more immunosuppressive environment due to a higher tumor burden.31,32
The occurrence of adverse events at any point during the vaccinations correlated with an improved OS in both stage III and stage IV patients. However, there is a risk for guarantee-time bias when correlating side effects with survival, as patients who completed more cycles of vaccinations may have had a higher chance of developing side effects.33 To correct for this possible bias, we performed a conditional landmark analysis in stage III patients after the third cycle of vaccinations, in which only patients were included who completed 3 cycles. The correlation between flu-like symptoms and survival remained in this analysis and a clear trend was still observed for injection site reactions. In stage IV patients, a conditional landmark analysis was performed after the first cycle of vaccinations, as the majority of patients only received 1 cycle of vaccinations, and it showed a remaining correlation between the presence of an injection site reaction and OS, whereas it was not detected for flu-like symptoms. This indicates that the correlation between flu-like symptoms at any point during the vaccinations and OS might have been caused by guarantee-time bias. In conclusion, stage III and IV melanoma patients with immune-related adverse events of DC vaccination show a better immunologic and clinical outcome.
Toxicity profiles play an important role in the choice of treatment for individual melanoma patients with the upcoming of many new agents. Hodi et al9 showed grade 3–4 adverse events in 20%–25% of stage IV melanoma patients treated with ipilimumab and death related to the study drugs in 2.1% of patients. In a phase III trial in stage III melanoma patients treated with adjuvant ipilimumab, 49% of patients discontinued treatment because of therapy-related adverse events and 5 participants (1%) died due to drug-related adverse events.17 Studies with anti-PD-1 mAb in metastatic melanoma showed a favorable toxicity profile compared with ipilimumab. However, toxicity was still substantial with grade 3–4 adverse events in 10%–16% of patients, and 1 treatment-related death was noted in the first-line nivolumab trial.12,18 Immune-related adverse events, for example, colitis, dermatitis, hepatitis, and hypophysitis, are the most common side effects of these checkpoint inhibitors.34 As aforementioned, DC vaccination shows minimal toxicity compared with the checkpoint inhibitors, which could be explained by the fact that DC vaccination, in contrast to checkpoint inhibitors, induces an antigen-specific immune response instead of stimulating the immune system in general. In stage IV melanoma patients the search for effective combinations of treatments is warranted, whereas ipilimumab and anti-PD-1 mAb result in long-term survival in a minority of patients.9,12,18 However, more severe adverse events can occur when therapies are combined. Recent examples of increased toxicity of combination therapy are a phase 3 trial with ipilimumab and nivolumab, with 55% grade 3–4 adverse events, and a phase 1 trial with vemurafenib and ipilimumab, which was terminated due to hepatic toxicity.18,35 With currently available treatment options, DC vaccination monotherapy is not preferred in stage IV melanoma patients, as it sporadically gives long-lasting clinical responses.6,7 However, DC vaccination might be a good candidate to combine with checkpoint inhibitors, both seen from a toxicity as well as from a immune-modulating perspective. Checkpoint inhibitors block immune checkpoints, such as CTLA-4 and PD-1, which could attenuate proliferation and effector function of tumor-specific T cells induced by DC vaccination.36 A recent phase 2 study combining autologous DCs and ipilimumab in pretreated advanced melanoma patients showed that the combination was tolerable and resulted in an encouraging overall response rate of 38%.37 Currently, no trials with a combination of DC vaccination and anti-PD-1 mAb are ongoing in melanoma patients, but there are trials in progress with this combination in other tumor types, for example, brain tumors (NCT02529072). The results on efficacy of a prospective randomized clinical trial with adjuvant DC vaccination in stage III patients have to be awaited, before this mild toxicity profile can plead for DC vaccination in these patients.
In conclusion, DC vaccination is safe and tolerable, and it shows much less toxicity compared with immune checkpoint inhibitors currently available or in trial in both stage III and IV melanoma patients. Furthermore, the occurrence of the immune-related adverse events, such as flu-like symptoms and injection site reactions, correlates with immunologic and clinical outcome.
ACKNOWLEDGMENT
The authors thank the patients who participated in the studies and the involved technicians Nicole Scharenborg, Annemiek de Boer, Mandy van de Rakt, Michel Olde Nordkamp, Jeanette Pots, Tom van Oorschot, and Tjitske Duiveman-de Boer.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
This work is supported by a Radboudumc PhD grant, and EU grants ENCITE (HEALTH-F5-2008-201842) and Cancer Immunotherapy (LSHC-CT-2006-518234) and DC-THERA (LSB-CT-2004-512074). I.J.M.d.V. is recipient of NWO-Vici grant 016.140.655. C.G.F. received an NWO Spinoza award, ERC Advanced Grant PATHFINDER (269019), and KWO grant 2009-4402.
All authors have declared there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19:275–282. [DOI] [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aarntzen EH, Schreibelt G, Bol K, et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res. 2012;18:5460–5470. [DOI] [PubMed] [Google Scholar]
- 7.Aarntzen EH, De Vries IJ, Lesterhuis WJ, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73:19–29. [DOI] [PubMed] [Google Scholar]
- 8.Bol KF, Aarntzen EHJG, in’t Hout FEM, et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology. 2016;5:e1057673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 11.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 13.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheatley K, Ives N, Hancock B, et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–252. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. [DOI] [PubMed] [Google Scholar]
- 18.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–781. [DOI] [PubMed] [Google Scholar]
- 21.Berger TG, Feuerstein B, Strasser E, et al. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods. 2002;268:131–140. [DOI] [PubMed] [Google Scholar]
- 22.de Vries IJ, Adema GJ, Punt CJ, et al. Phenotypical and functional characterization of clinical-grade dendritic cells. Methods Mol Med. 2005;109:113–126. [DOI] [PubMed] [Google Scholar]
- 23.Schreibelt G, Benitez-Ribas D, Schuurhuis D, et al. Commonly used prophylactic vaccines as an alternative for synthetically produced TLR ligands to mature monocyte-derived dendritic cells. Blood. 2010;116:564–574. [DOI] [PubMed] [Google Scholar]
- 24.Bol KF, Figdor CG, Aarntzen EH, et al. Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients. Oncoimmunology. 2015;4:e1019197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuurhuis DH, Verdijk P, Schreibelt G, et al. In situ expression of tumor antigens by messenger RNA-electroporated dendritic cells in lymph nodes of melanoma patients. Cancer Res. 2009;69:2927–2934. [DOI] [PubMed] [Google Scholar]
- 26.Tel J, Aarntzen EH, Baba T, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. [DOI] [PubMed] [Google Scholar]
- 27.Schreibelt G, Bol KF, Westdorp H, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res. 2015. [Epub ahead of print] 10.1158/1078-0432.CCR-15-2205. [DOI] [PubMed] [Google Scholar]
- 28.Aarntzen EH, Bol K, Schreibelt G, et al. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res. 2012;72:6102–6110. [DOI] [PubMed] [Google Scholar]
- 29.Weide B, Richter S, Buttner P, et al. Serum S100B, lactate dehydrogenase and brain metastasis are prognostic factors in patients with distant melanoma metastasis and systemic therapy. PLoS One. 2013;8:e81624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bol KF, Aarntzen EH, Pots JM, et al. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer immunology, immunotherapy : CII. 2016;65:327–339. 10.1007/s00262-016-1796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–240. [DOI] [PubMed] [Google Scholar]
- 32.Lesokhin AM, Hohl TM, Kitano S, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber JS, Yang JC, Atkins MB, et al. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–1366. [DOI] [PubMed] [Google Scholar]
- 36.Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine. 2015;33:7377–7385. [DOI] [PubMed] [Google Scholar]
- 37.Wilgenhof S, Corthals J, Heirman C, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]


