Supplemental Digital Content is available in the text.
Key Words: stem cell–like memory T cell, lung cancer, memory T cell
Abstract
Human stem cell–like memory T (Tscm) cells are long-lived, self-renewing memory lymphocytes that can differentiate into effector cells and mediate strong antitumour response in murine model. The distribution and function of Tscm cells in human lung cancer remain unknown. In this study, we investigated the properties of human Tscm cells in the blood and lymph node of non–small cell lung cancer (NSCLC) patients. There were more CD4+ Tscm cells in blood from NSCLC patients than from healthy donors, fewer CD4+ and CD8+ TSCM cells in blood than in lymph node from NSCLC patients. To further analyze their properties, we stimulated peripheral blood mononuclear cells from NSCLC patients by mitogens to examine cytokine production. Our data suggest that both CD4 and CD8 Tscm cells in blood produced interferon-γ significantly increased in NSCLC patients compare with healthy subjects. In addition, fewer Tscm cells produced interferon-γ in lymph node than in blood from NSCLC patients. Our results strongly suggest that the distribution and function of CD4 Tscm cells in NSCLC patients is upregulated. Understanding of the properties of stem-like memory T cells will supply a good rationale for designing the new adoptive immunotherapy in cancer.
Long-lived memory lymphocytes are hallmark features of adaptive immune systems. Naive T cells are activated in response to pathogens and tumors. Most of the activated T cells die of apoptosis after undergoing stimulation and clonal expansion, whereas only a minority of activated T cells become memory T cells. These memory T cells provide rapid and strong protection against recurrent pathogens. The heterogeneity of memory T cells with respect to phenotypic markers, effector function, and tissue-homing capabilities can be categorized into 2 main populations: effector memory T cell (Tem) and central memory T cells (Tcm). Tcm cell preferentially reside in secondary lymphoid organs and express CCR7 and CD62L; whereas Tem reside in extra lymphoid sites but do not express CCR7. Tcm and Tem cells have immediate effector function against polyclonal or antigen stimulation.1–4
Generation and maintenance of the longevity of memory T cells require stem cell–like capacity to self-renew and differentiate into the subsets of memory T cells.5 A new population of memory T cells with enhanced stem cell–like property was recently identified in mouse and humans.6–9 Stem cell–like memory T (Tscm) cells exhibit analogous effector function to memory T cells, but they also have increased proliferation, self-renewal, and strong antitumour function compared with conventional memory T cells in murine model of melanoma.10 One group reported that human memory stem cells occur naturally in peripheral blood mononuclear cell (PBMCs). Researchers also observed that CD8+ Tscm cells exhibit a strong capacity to reject tumor growth both in murine tumor and melanoma model in human mice.8,10 Tscm cells share both naive T cell and memory T cell characteristic phenotypes. Similar to naive T cells, Tscm cells express molecular marker CD45RA, CD62L, CCR7, stem cell–like associate marker CD127 and memory-associated markers CD27, CD28, CD95, and CD122. The CD95 signalling complex induces proapoptosis, and is preferentially expressed by CD45RA–CD45RO+ T cells but not CD45RA+CD45RO– T cells in adults.11 CD122 [interleukin (IL)-2R β, the common β chain of the IL-2 and IL-15 receptors] can undergo homeostatic proliferation in response to IL-15 and IL-7. CD122 may be a relevant factor in controlling the development, programming, and survival of memory T cells.12–14 After being treated with the WNT pathway activator TWS119, the expression of CD95 and CD122 was upregulated in Tscm cells, CD95 and CD122 might be considered as a marker of Tscm cells. Current studies demonstrated that antigen-specific memory T cells are a major target cell type of adoptive cell transfer in tumor immunotherapy and vaccine design,15–20 CD4+ helper T cells may have a critical function in improving cancer immunotherapies.21–23 The antitumour responses of CD8+ Tscm cells have been discovered in animal model,10 but the compartmentalization and function of CD4+ Tscm, CD8+ Tscm in human non–small cell lung cancer (NSCLC) patient still remains unclear.
In this study, we analyze the properties of human Tscm cells and its subsets in PBMCs and lymph node from healthy donors (HDs) and NSCLC patient. The number of CD4+ Tscm cells in blood of NSCLC patients was found to be higher compared with HDs. CD4+ Tscm and CD8+ Tscm cells were low in blood than in lymph nodes of NSCLC patients. The further functional analysis shows that both CD4+ Tscm and CD8+ Tscm cells in blood produced interferon (IFN)-γ in significantly increased amounts in NSCLC patients. In addition, fewer CD4+ Tscm and CD8+ Tscm cells produced IFN-γ in lymph nodes than in blood from NSCLC patients. Our results reveal that the distinct compartmentalization of Tscm cells in NSCLC patients and provide a new strategy for vaccination design and immunotherapy in cancer.
MATERIALS AND METHODS
Ethics Statement
Written informed consent was obtained from all patients and HDs. Ethics approval for this study was obtained from the ethics committees of the Zhong Shan Medical School, Sun Yat-San University (Guangzhou, China) and First Affiliated Hospital of Sun Yat-San University (Guangzhou, China).
Study Participants
Non–small cell lung patients were enrolled in this study from the First Affiliated Hospital of Sun Yet-San University of Guangzhou, China. These patients’ median age was 66 years (ranging from 45 to 75 y). Final diagnosis of NSCLC patient was based on pathologic evidence (detected by histologic staining). Patients whose serology tested positive for HIV, HBV, and HCV were excluded from the study. None of the patients received cancer-related chemotherapy during the period of collecting sample. The blood and lymph nodes from the same NSCLC patients were collected. Healthy donors were recruited for collecting blood and healthy lymph nodes were taken from noncancer patients.
Reagents and mAbs
Fluorescently conjugated antibodies were purchased from BD Biosciences for phenotypic and intracellular cytokine analyses. The following panel of mouse antihuman mAbs was used: APC.cy7-conjugated anti-CD3 (557832), Percp.cy5.5-conjugated anti-CD4 (560650), FITC-conjugated anti-CD45RA (11-0458-42), Alexa Fluor 700A-conjugated CCR7 (561143), PE-CF594-conjugated anti-CD62L (562301), Percp.cy5.5-conjugated anti-CD27 (560612), V450-conjugated anti-CD28 (48-0289-42), APC-conjugated anti-CD127 (17-1278-42), PE.cy7-conjugated anti-CD95 (561633), PE-conjugated anti-CD122 (554525), PE.cy7-conjugated IgG1 and PE-conjugated IgG1, V450-conjugated anti-IFN-γ (560371), APC-conjugated anti-tumor necrosis factor (TNF)-α (17-7349-82), were all purchased from BD Biosciences (San Jose, CA, USA). Phorbol 12-myristate13-acetate (PMA), ionomycin, brefeldin A, bovine serum albumin, and NaN3 were all purchased from Sigma-Aldrich (St Louis, MO).
Preparation of PBMCs and Lymphocytes
PBMCs were isolated by Ficoll-Hypaque (Tian Jin Hao Yang Biological Manufacture Co. Ltd, China; cat. LTS1077) gradient centrifugation of sodium heparin-blood obtained from HDs or lung cancer patients. Lymphocytes were isolated by mashing the lymph node tissue and lysing erythrocytes using ammonium chloride solution, and resuspending in complete RPMI 1640 medium (cat. 11875093; Invitrogen, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (Hang Zhou Si Ji Qing Biological Manufacture Co. Ltd), 100 U/mL penicillin (cat. 15071163), 100 mg/mL streptomycin (15071163), 2 mM L-glutamine (cat. 25030081) and 50 mM 2-mercaptoethanol (cat. 21985023) (Invitrogen).
Flow Cytometry
Phenotypic Characterization
Pooled PBMCs and lymph node cells from HDs and lung cancer patients were stained for flow cytometry. The following antibodies were used for flow cytometry: APC.cy7-conjugated anti-CD3, Percp.cy5.5-conjugated anti-CD4, FITC-conjugated anti-CD45RA, Alexa Fluor 700-conjugated CCR7, PE-CF594-conjugated anti-CD62L, Percp.cy5.5-conjugated anti-CD27, V450-conjugated anti-CD28, APC-conjugated anti-CD127, PE.cy7-conjugated anti-CD95, PE-conjugated anti-CD122, PE.cy7-conjugated IgG1 and PE-conjugated IgG1.
IFN-γ Staining
PBMCs and lymph node cells were incubated at 2×106 cells per well in RP10 media (RPMI, 10% heat-inactivated fetal calf serum) alone or with PMA (20 ng/mL) plus ionomycin (1 μg/mL) for 4–6 hours at 37°C in the presence of brefeldin A (10 μg/mL). Cells were then harvested and stained for flow cytometric analysis and intracellular markers. All samples were collected on FACSAria II BD (Moutain View, CA). Data were analyzed using Flow Jo software (Tree Star; San Carlos, CA).
Statistical Analysis
GraphPad Prism software version 5 was used for statistical analysis. The Mann-Whitney test (2-tailed) and Student t test were performed to determine statistical differences. A value of P≤0.05 was considered statistically significant.
RESULTS
Identification of CD4+CD45RA+CD45RO–CD62L+CCR7+CD127+CD27+CD28+CD95+CD122+T-Cell Subsets in Human Blood and Lymph Nodes
The NSCLC patients recruited to this study had been diagnosed with NSCLC according to the pathologist’s report; patients were all HIV and HBV negative and free of other cancers. Peripheral blood was collected from HDs and NSCLC patients; lymphocytes separated from tumor-free lymph nodes and tumor-infiltrated lymph nodes from each lung cancer patient.
To determine whether Tscm cells exist in lung cancer patients and identify their distribution, we analyzed CD4+Tscm and CD8+Tscm cells in blood and local lymph nodes from NSCLC patients. We detected the Tscm cells with (CD3+CD4+CD45RA+CD45RO–CD62L+CCR7+CD127+CD27+CD28+CD95+CD122+) marker in blood from HDs (HD-PBMC) and NSCLC patients (NSCLC-PBMC) (Fig. 1A), the frequency of CD4+Tscm cells was significantly higher in NSCLC-PBMC (2.59%) than in HD-PBMC (1.74%) (P=0.007) (Fig. 1B); the percentage of CD8+Tscm cells did not differ in HD-PBMC (2.11%) and NSCLC-PBMC (2.18%) (Fig. 1C). The proportion of CD4+Tscm cells were higher in lymph nodes of NSCLC (NSCLC-Ly) (29.91%) than in healthy lymph nodes (Normal-Ly) (4.88%) (P=0.0187) (Fig. 1B); the proportion of CD8+Tscm cells were higher in NSCLC-Ly (18.99%) than in Normal-Ly (1.25%) (P=0.014) (Fig. 1C). In both NSCLC-Ly and Normal-Ly, CD4+TSCM cells outnumbered the CD8+Tscm cells (Fig. 1D). In contrast, the frequency of Tscm cells (CD4+ and CD8+Tscm) was lower in NSCLC-PBMC than in NSCLC-Ly (Fig. 1B–D). Our results indicate that Tscm cells can be detected in healthy lymph node and NSCLC patient, there is remarkably different composition between HD and lung cancer patient in different tissue.
FIGURE 1.

Identification of Tscm CD4+CD45RA+CD45RO−CD62L+CCR7+CD127+CD27+CD28+CD95+CD122+ T (Tscm) cell in human blood and lymph nodes. PBMCs were isolated from the blood of non–small cell lung cancer (NSCLC) patients (n=15) (NSCLC-PBMC) and healthy donors (n=11) (HD-PBMC); lymphocytes were isolated from the tumor-infiltrated lymph node of NSCLC patients who were collected blood at same time (n=7) (NSCLC-Ly); lymphocytes were isolated from the healthy lymph node of non lung cancer patients (n=7) (Normal-Ly), analyzed by flow cytometry. A, Representative flow cytometric analyses of CD4+CD45RA+CD45RO−CD62L+CCR7+CD127+CD27+CD28+CD95+CD122+ T cells, indicating Tscm cells. B, The frequency of the CD4+Tscm cells in the HD-PBMC, NSCLC-PBMC, Normal-Ly, NSCLC-Ly. The events of CD4+Tscm cells in the blood and lymph node from NSCLC patients and healthy donors, expressed as the mean±SEM. C, The frequency of the CD8+Tscm cells in the HD-PBMC, NSCLC-PBMC, Normal-Ly, NSCLC-Ly, expressed as the mean±SEM. D, The events of Tscm of CD4+ and CD8+ cells in the blood and the lymph node from NSCLC patients and healthy donors. HD indicates healthy donors; IFN, interferon; PBMC, peripheral blood mononuclear cells; Tscm cell, stem cell–like memory T cell. *P<0.05; **P<0.005; ***P<0.001; Mann-Whitney test (2-tailed) and Student t test.
Cytokine Production of the Tscm Cell
Memory T cells can be distinguished from naive T cells by their ability to produce effector cytokines on antigen rechallenge or stimulate with polyclonal reagents. Tscm cells in blood produce cytokines (IFN-γ, IL-2, and TNF-α) with super antigen (Staphylococcus enterotoxin B) or anti-CD3/CD2/CD28 stimulation.8 To observe whether the functional capacities of Tscm cells is different in tissue from NSCLC patient and HD, we stimulated Tscm cells with PMA plus ionomycin for 4 hours, 55.49% of CD4+Tscm cells in NSCLC-PBMC produced IFN-γ, and it was significantly higher than in HD-PBMC (30.7%) (P=0.03) (Fig. 2B); only a small portion of (3.94%) CD4+Tscm cells in Normal-Ly produced IFN-γ (Figs. 2A, B); 11.55% of CD4+Tscm cells in NSCLC-Ly produce IFN-γ (Fig. 2B). We also observed that the proportion of IFN-γ-expressing Tscm cells (CD4+ and CD8+Tscm) was higher in blood than in lymph node from NSCLC patients (Figs. 1B, D). Comparison of the ratio of IFN-γ producing CD4+Tscm cells in HD-PBMC, NSCLC-PBMC, Normal-Ly, and NSCLC-Ly were shown in pie chart (Fig. 2C). The IFN-γ-secreting CD4+Tscm cells outnumbered in blood from NSCLC patient than from the HD (Fig. 2E). TNF-α-producing Tscm cells was observed in blood and lymph node from NSCLC patient and HD, and there is no significant difference between 2 groups (Supplementary 1a, Supplemental Digital Content 1, http://links.lww.com/JIT/A434; Supplementary 1b, Supplemental Digital Content 1, http://links.lww.com/JIT/A434). Our results demonstrate the different pattern of IFN-γ producer of CD4+Tscm and CD8+Tscm cells in blood and lymph node from lung cancer patients.
FIGURE 2.
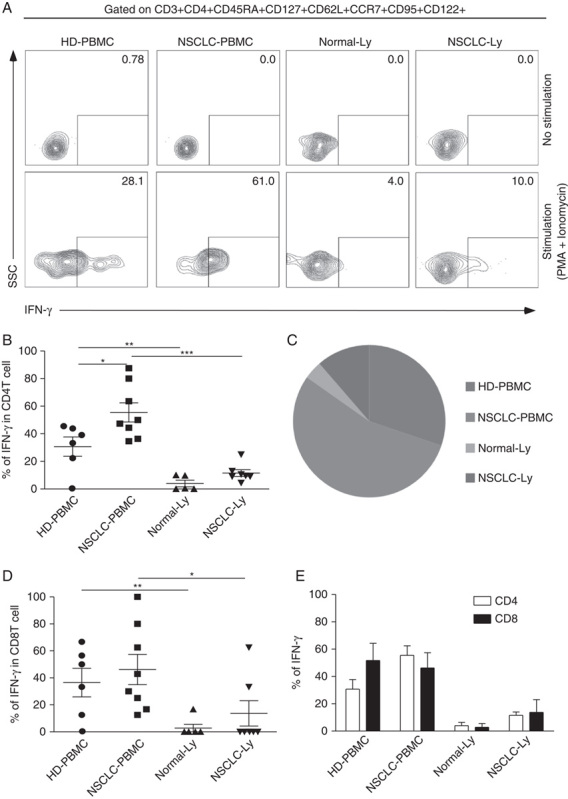
Cytokine production of the Tscm cell. HD-PBMCs (n=6), non–small cell lung cancer (NSCLC)-PBMCs (n=8) from the blood or lymphocytes isolated from Normal-Ly (n=5), NSCLC-Ly (n=7) were stimulated for 4–6 hours with PMA+ionomycin, and cytokine production was assessed by intracellular cytokine staining. A, Representative intracellular cytokine staining analysis of IFN-γ production by CD4+Tscm and CD8+Tscm cells in the blood and the lymph node relative to the unstimulated controls. B, The mean IFN-γ production (±SEM) of the CD4+ Tscm cells in the blood and lymph node from the nNSCLC patients and healthy donors. C, Pie charts depicting the quality of the IFN-γ-expressing CD4+Tscm cells in the blood and the lymph node from NSCLC patients and healthy donor. D, The mean IFN-γ production (±SEM) of the CD8+Tscm cells in the blood and lymph node from the NSCLC patients and healthy donors. E, The graph shows the relative frequencies of the IFN-γ-producing CD4+Tscm and CD8+Tscm cells in the HD-PBMC, LC-PBMC, Normal-Ly, NSCLC-Ly. HD indicates healthy donors; IFN, interferon; PBMC, peripheral blood mononuclear cells; PMA, phorbol 12-myristate13-acetate; Tscm cell, stem cell–like memory T cell; SSC, side scatter. *P<0.05; **P<0.005; ***P<0.001; Mann-Whitney test (2-tailed) and Student t test.
Fraction of CD4+CD45RA+CD45RO–CD95–CD122+CD127+T Cell Population Displays Different Phenotypes in Human Blood and Lymph Nodes
CD8+CD122+ T cells have a potent capacity to produce IFN-γ after bacterial infection, and enhance antitumour activities against liver metastatic lymphoma in mouse models.24,25 Mouse stem cell–like CD8+ memory T cells expressed high levels of CD122. IL-7 is essential for memory T cell survival. High expressions of IL-7R-α-chain (CD127) on effector T cells have preferentially developed into long-lived memory cells.26,27 The exact properties of CD4+CD122+CD127+T cells need to be determined. We observed the frequency of CD4+CD45RA+CD45RO–CD127+CD122+CD95– T cells in different anatomic locations from NSCLC patients and HDs. A total of 2.2% of CD4+CD45RA+CD45RO−CD95−CD122+CD127+T cells were examined in NSCLC-PBMC, whereas only 0.93% of CD4+CD45RA+CD45RO–CD95–CD122+CD127+T cells were examined in NSCLC-Ly (P=0.0056) (Figs. 3A, B). The frequency of CD8+CD45RA+CD45RO–CD95–CD122+CD127+ T cells was higher in blood than in lymph node from both the NSCLC patient (P=0.013) and the HD (P=0.0006) (Supplementary 2a, Supplemental Digital Content 2, http://links.lww.com/JIT/A435). We verified that Tscm cells had potent capacity to produce IFN-γ from NSCLC patients (Figs. 2B, D). IFN-γ-producing CD4+CD45RA+CD45RO–CD95–CD122+CD127+ cells in NSCLC-PBMC (56.14%) exceeded that in NSCLC-Ly (33.83%) (Fig. 3C); IFN-γ secreting CD8+CD45RA+CD45RO–CD95–CD122+CD127+ cells were more in NSCLC-PBMC (57.64%) than in NSCLC-Ly (10.45%) (P=0.0001) (Supplementary 2b, Supplemental Digital Content 2, http://links.lww.com/JIT/A435); in case of the TNF-α production in CD4+CD45RA+CD45RO–CD95–CD122+CD127+ cells, higher level of TNF-α were produced in NSCLC-PBMC than in HD-PBMC (P=0.0002) (Supplementary 2c, Supplemental Digital Content 2, http://links.lww.com/JIT/A435), as well as the TNF-α-producing in CD8+CD45RA+CD45RO–CD95–CD122+CD127+ cells (P=0.0063) (Supplementary 2d, Supplemental Digital Content 2, http://links.lww.com/JIT/A435); no TNF-α production were detected in CD8+CD45RA+CD45RO–CD95–CD122+CD127+ cells from lymph node (Supplementary 2d, Supplemental Digital Content 2, http://links.lww.com/JIT/A435). Together, these results show significant decreased of frequency and IFN-γ produced by CD4+/CD8+CD45RA+CD45RO–CD95–CD122+CD127+ cells in lymph node from lung cancer patients.
FIGURE 3.

CD4+CD45RA+CD45RO−CD95−CD122+CD127+T cell population displays different phenotypes in human blood and lymph nodes. A, Flow cytometry plots showing IFN-γ expression in the CD4+CD45RA+/CD45RO−CD95−CD122+CD127+ T cells from the blood and lymph node of the non–small cell lung cancer (NSCLC) patients and healthy donors. B, The mean frequency (±SEM) of the events of CD4+CD45RA+/CD45RO−CD95−CD122+CD127+ T cells. C, The mean IFN-γ production (±SEM) of the CD4+CD45RA+/CD45RO−CD95−CD122+CD127+ T cells. HD indicates healthy donors; IFN, interferon; PBMC, peripheral blood mononuclear cells; PMA, phorbol 12-myristate13-acetate. *P<0.05; **P<0.005; paired Student t test.
Different Subsets of CD4+CD95+ T Cells Show Distinct Capacity for IFN-γ Production
The majority of the activated antigen-specific T cells were eliminated by activation-induced cell death after clonal expansion and differentiation into effector cells. Only a small portion of these T cells survived and became long-lived memory cells. CD95 (APO-1/Fas) has a major function in activation-induced cell death and mediated T-cell apoptosis.28,29 However, a research group found that CD95 has a contradictory function; CD95 not only triggers apoptosis in terminally differentiated neurons, but also promotes cell proliferation in neural progenitors and cancer stem cells by inducing T cell factor-β-catenin signalling in the adult brain.30,31 CD95 is one of the surface markers in the formation and maintenance of memory T cells,32 Gattinoni et al’s8 research revealed that CD95+ naive-like CD8+T cell produced IFN-γ, IL-2, TNF-α after exposure to Staphylococcus enterotoxin B, whereas the naive T cell did not produce cytokine. To determine whether CD95+CD4+/CD8+ naive T cells possesses same properties from lung cancer patient, we analyzed the cytokine production in CD95+ naive T cells and CD95+ memory T cells from different anatomic positions. We gated CD4+CD45RA+CD95+ (Fig. 4A) and CD4+CD45RA-CD95+ (Fig. 4C) T cells, IFN-γ and TNF-α were detected in response to PMA and ionomycin stimulation in blood and lymph node. Both in CD4+CD45RA+CD95+ T cells (Fig. 4B) and CD4+CD45RA-CD95+ T cells (Fig. 4D), IFN-γ producer cells was remarkably reduced in NSCLC-Ly (Fig. 4B, P=0.033; Fig. 4D, P=0.0003). In contrast, IFN-γ production in CD4+CD45RA–CD95+ T cells was significantly higher than in CD4+CD45RA+CD95+ T cells in NSCLC-Ly (Fig. 4E, P=0.047). We observed the similar phenomena for CD8+CD45RA+CD95+ T cells and CD8+CD45RA–CD95+ T cells in lymph node (Supplementary 3a, b, Supplemental Digital Content 3, http://links.lww.com/JIT/A436). TNF-α production was similar to the IFN-γ production in CD95+ naive T cells and CD95+ memory T cells in NSCLC patients (Supplementary 3c–h, Supplemental Digital Content3, http://links.lww.com/JIT/A436). Thus, CD45RA+CD95+T cells are different from the naive T cells and had distinct capacity to produce cytokine in different position from lung cancer patients.
FIGURE 4.

CD4+CD45RA+CD95+ T cells and CD4+CD45RA−CD95+ T cells show distinct capacity for IFN-γ production. A, Representative analysis of IFN-γ production by CD4+CD45RA+CD95+ and CD4+CD45RA−CD95+T cells in the blood and the lymph node relative to the unstimulated controls from non–small cell lung cancer (NSCLC) patients and healthy donors. B, The mean IFN-γ production (±SEM) in the CD4+CD45RA+CD95+ T cells from the blood and lymph node of NSCLC patients and healthy donor. C, Representative analysis of IFN-γ production by CD4+CD45RA−CD95+ T cells in the blood and lymph node relative to the unstimulated controls from NSCLC patients and healthy donors. D, The percentage of IFN-γ-producing CD4+CD45RA−CD95+ T cells in the blood and lymph node from NSCLC patients and healthy donors, expressed as the mean±SEM. E, The plot shows the relative frequencies of the IFN-γ-producing CD4+CD45RA+CD95+ T cells and CD4+CD45RA−CD95+ T cells in the HD-PBMC, LC-PBMC, Normal-Ly, NSCLC-Ly. HD indicates healthy donors; IFN, interferon; PBMC, peripheral blood mononuclear cells; PMA, phorbol 12-myristate13-acetate; SSC, side scatter. *P<0.05; **P<0.005; ***P<0.001 Mann-Whitney test (2-tailed) and Student t test.
DISSCUSION
In this study, we confirmed the existence of CD4+Tscm cells in blood from HDs, and our data were consistent with those of previous reports. To investigate whether CD4+Tscm cells have a special distribution, we examined the frequency of CD4+Tscm cells in healthy lymph nodes, but there is no difference compared with that of in blood from HD s. The antitumor function of CD8+Tscm cells has significant effects,8,33,34 but the number and function of CD4+Tscm cells in tumor-bearing patients remains unclear. We investigated the frequency of CD4+Tscm cells in blood and tumor-infiltrating local lymph nodes from NSCLC patients, and discovered that the number of CD4+Tscm cells in NSCLC-PBMC was significantly higher than that in HD-PBMCs. The frequency of CD4+Tscm cells in NSCLC-Ly was higher than that in Normal-Ly. In NSCLC patients, the distribution of Tscm cells varied between in tissue. The number of CD4+ and CD8+Tscm cells increased in lymph nodes than in blood from NSCLC patients, and this result may be explained by the characterization of Tscm cells. Tscm cells possess the stem cell–like ability to self-renew, proliferate, and potently differentiate into all memory T cell subsets and effector T cells. The numbers of Tcm cells and Tem cells in blood from NSCLC patients decreased than those in HDs (data not shown). On the basis of these results, we hypothesize that the reduction in Tcm cells and Tem cells both in blood and lymph nodes may induce the proliferation and differentiation of Tscm cells to replenish the memory T cell pool (data not shown). However, we still lack direct evidence to support this hypothesis.
Different murine and human tumor model revealed that Tem cells are dominant in tumor microenvironments. These viable cells fail to kill and attack tumor cells mainly because of the existence of suppressive tumor microenvironments, such as cytokines produced by tumor cells, suppressive function of Tregs, imbalance of costimulation and coinhibition signals on effector T cells. Whether these negative tumor immunity factors will influence the function of Tscm cells in tumor-infiltrating lymph nodes remains unclear. We observed that IFN-γ production upregulated in CD4+Tscm cells from lymph nodes. We also detected IFN-γ production of CD4+Tscm cells with respect to PMA and ionomycin stimulation in NSCLC-PBMCs and NSCLC-Ly, the ability of CD4+Tscm cells to produce IFN-γ was stronger in blood than in lymph nodes from NSCLC patients. A reducing trend in IFN-γ production of CD4+Tscm cells was observed in blood from HDs compared with that of NSCLC patient. The animal and humanized mouse melanoma models demonstrated that the efficiency of tumor regression induced by Tscm cells was the strongest among Tcm cells and Tem cells transfer experiments.8 Therefore, our data indicate that the increased number and function of CD4+Tscm cells in local lymph nodes from NSCLC patients may be related to the exposure of persistent antigen stimulation or tumor microenvironments. The mechanism by which CD4+Tscm cells exert antitumour functions requires further indepth research.
CD122 (IL-2Rb) is a receptor subunit for IL-2 and IL-15. Its expression is undetectable in naive CD4+T cells, whereas naive CD8+T cells have low expression levels of CD122. The expression level of CD122 is low in memory CD4+T cells, but high in memory CD8+T cells. Memory T cell development primarily occurs by providing survival signals. Weak CD122 signals readily support Tcm, whereas stronger CD122 signals are required for Tem development.35 CD122 controls the proliferation and survival of CD8+CD122+T cells, which possess potent IFN-γ-producing capacity and antitumour function in mouse models.36 In this study, we observed CD122 expression in the CD4+CD45RA+CD95– naive-like T cell subset. IFN-γ production increased in lymph nodes from NSCLC patients than that in healthy lymph nodes. This result shows that the CD4+CD45RA+CD95+CD122+ T cell subset possessed certain memory cell phenotypes and antitumor functions.
Fas (Apo-1/CD95) is a cell-surface protein belonging to the TNF receptor superfamily. Fas activates T-cell apoptosis by initiating a cascade of proteases. Resting human T cells only express low level of CD95, which is rapidly upregulated during activation.32,37 CD95 is a discriminant naive and memory T cell marker.38,39 We investigated IFN-γ production in CD4+CD45RA-CD95+ T cells and CD4+CD45RA+CD95+ T cells. These 2 populations exhibited the memory phenotype. Our study showed that these 2 T cell populations had different abilities to produce IFN-γ in different anatomic positions both in HDs and lung cancer patients. IFN-γ production in CD4+CD45RA-CD95+ T cells was higher than in CD4+CD45RA+CD95+ T cells in blood from NSCLC patients. Moreover, IFN-γ production of CD4+CD45RA−CD95+ T cells was higher in lymph nodes from NSCLC patient than that of in HD. These results indicate that CD4+CD45RA−CD95+ T cells from lung cancer patients exhibited stronger antitumor function.
Finally, we characterized the heterogeneity of CD4 memory T cells, which is critical for the immunotherapy of lung cancer and vaccine design.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.immunotherapy-journal.com.
ACKNOWLEDGMENTS
The authors thank Xiao Ying Fu and Hu Wang for their technical assistance with flow cytometry.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by Sun Yat-Sen University “985” (Grant No. 50000-3281305).
All authors have declared there are no financial conflicts of interest with regard to this work.
Footnotes
H.H. and Y.G. are co-first authors.
REFERENCES
- 1.Campbell JJ, Murphy KE, Kunkel EJ, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–884. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. [DOI] [PubMed] [Google Scholar]
- 4.Sathaliyawala T, Kubota M, Yudanin N, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu XZ, Anasetti C. Memory stem cells sustain disease. Nat Med. 2005;12:1282–1283. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Joe G, Hexner E, et al. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Zhong XS, Palmer DC, et al. WNT signaling arrests effector T cell differentiation and generates CD8 memory stem cells. Nat Med. 2009;15:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Yang Q, Zhu Y, et al. T-bet and eomes regulate the balance between the effector/central memory T cells versus memory stem like T cells. PloS One. 2013;8:e67401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Ji Y, Restifo NP. WNT/β-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung F, Zheng L, Lenardo MJ. Maintenance of clonotype specificity in CD95/Apo-1/Fas mediated apoptosis of mature T lymphocytes. J Immunol. 1997;159:3816–3822. [PubMed] [Google Scholar]
- 12.Blaser BW, Roychowdhury S, Kim DJ, et al. Donor-derived IL-15 is critical for acute allogeneic graft-versus-host disease. Blood. 2005;105:894–901. [DOI] [PubMed] [Google Scholar]
- 13.Castro I, Yu AX, Dee MJ, et al. The basis of distinctive IL-2-and IL-15-dependent signaling: weak CD122-dependent signaling favors CD8 +T central-memory cell survival but not effector-memory T cell development. J Immunol. 2011;187:5170–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–584. [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Teichgräber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. [DOI] [PubMed] [Google Scholar]
- 18.Willinger T, Freeman T, Hasegawa H, et al. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–5903. [DOI] [PubMed] [Google Scholar]
- 19.Stemberger C, Huster KM, Koffler M, et al. A single naive CD8+T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. [DOI] [PubMed] [Google Scholar]
- 20.Sukumar M, Liu J, Ji Y, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. [DOI] [PubMed] [Google Scholar]
- 22.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhardt RL, Khoruts A, Merica R, et al. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa R, Inui T, Nagafune I, et al. Essential role of bystander cytotoxic CD122+CD8 +T cells for the antitumor immunity as induced in the liver of mice by α-Galactosylceramide. J Immunol. 2004;172:6550–6557. [DOI] [PubMed] [Google Scholar]
- 25.Motegi A, Kinoshita M, Inatsu A, et al. IL-15 induced CD8+CD122+T cells increase antibacterial and anti-tumor immune responses: implications for immune function in aged mice. J Leukoc Biol. 2008;84:1047–1056. [DOI] [PubMed] [Google Scholar]
- 26.Maraskovsky E, Teepe M, Morrissey PJ, et al. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 27.Kaech SM, Tan JT, Wherry EJ, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. [DOI] [PubMed] [Google Scholar]
- 28.Glass A, Walsh CM, Lynch DH, et al. Regulation of the Fas lytic pathway in cloned CTL. J Immunol. 1996;156:3638–3644. [PubMed] [Google Scholar]
- 29.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. [DOI] [PubMed] [Google Scholar]
- 30.Knight JC, Scharf EL, Mao-Draayer Y. Fas activation increases neural progenitor cell survival. J Neurosci Res. 2010;88:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight JC, Hackett C, Breton J, et al. Cross-talk between CD4+ T-cells and neural stem/progenitor cells. J Neurol Sci. 2011;306:121–128. [DOI] [PubMed] [Google Scholar]
- 32.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. [DOI] [PubMed] [Google Scholar]
- 33.Lugli E, Dominguez MH, Gattinoni L, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugli E, Gattinoni L, Roberto A, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gett AV, Sallusto F, Lanzavecchia A, et al. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach C, Rohr JC, Perié L, et al. Heterogeneous differentiation patterns of individual CD8+T cells. Science. 2013;340:635–639. [DOI] [PubMed] [Google Scholar]
- 37.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. [DOI] [PubMed] [Google Scholar]
- 38.Miyawaki T, Uehara T, Nibu R, et al. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral. J Immunol. 1992;149:3753–3758. [PubMed] [Google Scholar]
- 39.Uehara T, Miyawaki T, Ohta K, et al. Apoptotic cell death of primed CD45RO+T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood. 1992;80:452–458. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.immunotherapy-journal.com.


