Supplemental digital content is available in the text.
Key Words: Diameter, Dry Needling, Myofascial Pain Syndrome, Trigger Points
ABSTRACT
Objective
To investigate the impact of diameter of needles on the effect of dry needling treatment of chronic lumbar myofascial pain syndrome.
Design
Forty-eight patients with chronic lumbar myofascial pain syndrome were randomly allocated to 3 groups. They received dry needling with needles of diameter 0.25 (group A), 0.5 (group B), and 0.9 mm (group C). Visual analog scale evaluation and health survey were conducted at baseline and 3 months after the treatment.
Results
Visual analog scale scores were significantly different in all groups from baseline to 3 months. Visual analog scale scores at 3 months showed differences between group C and the other 2 groups. When baseline and 3 months after treatment (0 day and 3 months) in each of the 3 groups was compared, there was a difference between group C and group B. The Short Form (36) Health Survey scores from baseline to 3 months were different within the treatment groups.
Conclusions
Visual analog scale score evaluations at 3 months showed efficacy in all groups. Results of 3 months showed that efficacy of treatment with larger needles (0.9-mm diameter) was better than that of smaller ones (0.5-mm diameter). The Short Form (36) Health Survey scores at 3 months indicated that treatments with needles of varying diameters were all effective, and when the results of 3 months were compared, there was no difference between the 3 groups.
Myofascial pain syndrome (MPS) is a common nonarticular local musculoskeletal pain syndrome caused by myofascial trigger points (MTrPs) located in the muscle, fascia, or tendinous insertions.1 Myofascial syndrome affects approximately 95% of people with chronic pain disorders2 and has been found to be the principal cause of pain in 85% of patients attending a pain center.3 Travell and Simons first used the term myofascial trigger point, and it is defined as a palpable and most sensitive point of tenderness in the muscle fiber tension (hyperirritable). Pressure that stimulates the typical MTrPs may induce pain, referred pain, and local twitch response.4 There are many factors that have been proposed to result in the development and persistence of MTrP pain. These factors include anatomic abnormalities; various postural habits; vocational activities causing excessive strain on a particular muscle, tendon, or ligament; endocrine dysfunctions; psychological stressors; sleep disorders; and lack of exercise.5–7
Postural habits contribute to the development of myofascial pain by causing excessive overload on specific muscle groups, the quadratus lumborum being the most commonly involved.8
There are many MPS clinical treatment methods, and dry needling (DN) therapy has become popular in recent years. Dry needling therapy is also known as intramuscular stimulation, Western acupuncture, and medical acupuncture.9 It does not use drug intervention and is based on an aggressive therapy through needle lesions (such as MPS needle in MTrPs). Dry needling found widespread application after the publication by Lewit (1979). In this article, he brought up the idea that a traditional drug injection therapy consists of 2 parts, the role of the drug itself and the effect of needling, both of which work independently.10 Since then, research on the effect of needling has increased gradually. The technique is called “dry needling” to distinguish from the traditional drug injection. The application range of DN includes treatment of MPS,11,12 and tendon lesions and prevention of joint replacement pain.13 There are studies on the short-term and long-term efficacy, pathophysiologic basis, and complications of the needle therapy and different evaluation methods. Vulfsons et al.14 believed that the treatment of MTrP with DN is effective. Kietrys et al.,15 through a systematic review and meta-analysis, showed that DN therapy for upper extremity myofascial pain was more effective than traditional physical therapy. However, results from Tough et al.16 showed that the dry needle therapy was no better in the treatment of MTrPs than traditional methods. There are many possible reasons for these inconsistent findings, one of which is that different needles were used in these studies. In the literature, the authors have not provided the rationale for choosing a particular needle (such as Chinese acupuncture needles, syringes, needles, or special needles), and these different types of dry needles were of varying diameters. Hsieh et al.,17 in animal studies, and Barbara et al.,18 in clinical studies in healthy volunteers, used 30G acupuncture needles (0.3-mm diameter). Huang et al.19 studied prognostic factors of DN treatment of myofascial pain using acupuncture needles with a diameter of 0.25 mm, and John et al.20 also adopted identical needles for his study. A 25G (needle diameter, 0.5 mm) syringe needle was applied to treat patients in studies by Hsieh et al.21 and Tsai et al.22 Travell and Simons8 recommended using dry needles of 0.8-mm diameter while initiating treatment in the thick tissues as gluteus maximus. In other studies, needles with thicker diameters (>0.5 mm) were also used. Will these differences produce different efficacy for DN treatment of myofascial pain? In fact, some scholars have already studied this issue. In 2009, Yoon et al.23 reported application of 3 syringe needles with different diameters of 0.8, 0.6, and 0.5 mm to inject the same drug (0.5% lidocaine) to MTrPs in the same conditions (only the diameter of the syringe needle was different). By Short Form (36) Health Survey (SF-36) score evaluation, they found that the 0.8-mm diameter group was better than the effect of the 0.6- and 0.5-mm groups. This suggests that the diameter of the needle may have an impact on DN treatment of myofascial pain. Unfortunately, the study was for drug injection and did not strictly belong to the DN treatment; hence, it cannot be used as direct evidence. However, the result provides information for further research on the therapeutic effect of different needle diameters.
In this study, by comparing before and after treatment effects, we evaluated the long-term therapeutic efficacy of DN treatments of chronic lumbar MPS with needles of 3 different diameters (0.25, 0.5, and 0.9 mm). We also investigated the percentage of the patients who were willing to accept a second-time treatment in each group as a secondary parameter.
METHODS
Patients
Patients were recruited from the Chinese People’s Liberation Army (PLA) General Hospital. Male or female patients aged between 20 and 60 years with a diagnosis of lumbar MPS, with a disease history of more than 12 months and a visual analog scale (VAS) score between 5 and 10 (0, no pain; 10 points, worst pain) were included. Patients had normal cognitive function, body mass index between 18 and 25 kg/m2. Myofascial pain syndrome was diagnosed if all three of the following criteria were met: (1) presence of a taut band, (2) tender point within the taut band, and (3) recognition of pain.24
The study protocol was approved by the ethics committees of our hospital (No. S2014-017-012), and all participants provided written informed consent.
The main exclusion criteria were the following: (1) previous DN therapy (including acupuncture) or trigger point injections within 6 months; (2) surgery history on lumbar, central nervous system diseases; malignant diseases; skin diseases in the lumbar region; blood system diseases; mental disease or cognitive dysfunction; pregnancy; application of anticoagulant drugs; immune system disorders; history of fainting, alcoholism, or drug addiction; (3) patients received other treatment (including trigger point injections, medication, physical therapy, etc.) for the lumbar MPS within the time period between the DN therapy and the last follow-up (3 months).
Before the study, each patient meeting the inclusion criteria was provided with an informed consent form, explained about the basic procedure to be performed for treatment and evaluation and potential risk. The patients were informed of their rights to exit treatments at any time without undertaking any consequence. Those who agreed to participate in the treatment and subsequent follow up were required to sign the informed consent form for the study.
Sample Size
G-power 3.1.9.2 was used to calculate the sample size. The statistical test method was set as repeated-measures analysis of variance, the number of groups was set as 3, and the effect size was set as 0.3. All the other values were set as default. A final sample size was calculated as 48, 16 in each group.
Randomized Grouping
A total of 48 patients with chronic lower back MPS were randomly allocated to 3 groups, 16 in each group; patients in group A received DN treatment with needles of 0.25-mm diameter, and groups B and C received treatment with needles of 0.5 and 0.8 mm, respectively (Fig. 1).
FIGURE 1.
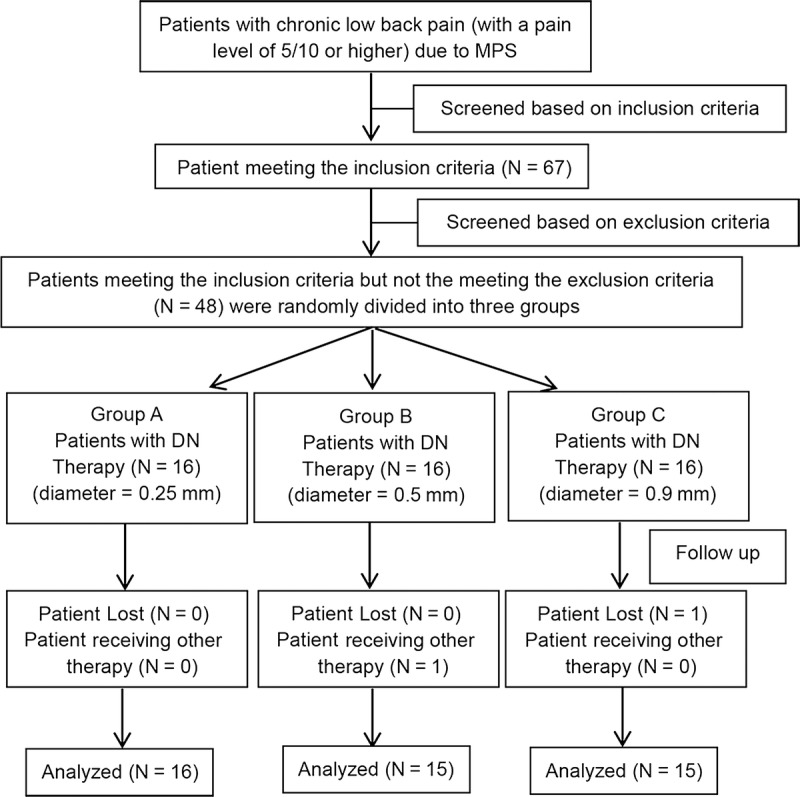
Patients’ disposition. N, number.
Blinding
This was a double-blind study. Pretreatment evaluation and the evaluation after treatment were performed by specially trained physicians who were unaware of the grouping of patients and treatments. After the admissions, the physicians confirmed the diagnosis and determined whether the patient could be treated with DN therapy and ensured that no exclusion criteria were met. The patients were informed that they would receive DN treatment. Furthermore, they were informed about the DN treatment with needles of 3 different diameters by random allocation. Patient information was then sealed. Patients received a card with only a serial number, and the triage nurse determined the patient’s treatment group according to the randomization table and the number, and informed the treating physician. The physicians described the procedure and precautions to the patients before performing the treatment, but not information on the diameter of the needle. The needle was not shown to the patient. The person who performed the data analysis was also blinded.
Treatments
Dry needles with a diameter of 0.25, 0.5, and 0.9 mm were used for treatment (Fig. 2). Every patient received the treatment only once, and no further treatments were received. The first 2 types of needles were single-use needles, and the last one was a special recyclable needle. The treatment procedures were identical for all 3 groups. Patients in groups A, B, and C were treated with dry needles of diameter 0.25, 0.5, and 0.9 mm, respectively, and 20 dry needles were used per patient. With the patient prone on the table, the lumbar myofascial tenderness points were determined and marked by an experienced and licensed physician, which was followed by disinfection. Wearing sterile gloves, the physician determined the location of tender points using the left hand, fixed the skin with the thumb and index finger, and then needled into the skin vertically with the right hand until the needle reached the point of maximum tenderness. The assessment of correct positioning of the needle point is based on the symptoms induced by usual pain or local twitch response.24 Every tender point was treated as previously described, and the needle was removed after10 minutes (Fig. 3). To reduce the pain during needling, and to improve the patient’s tolerance, vertical stretching of the skin may be needed using the left hand. According to our experience, a small amount of blood would ooze from the needle point with a needle of 0.9-mm diameter. To reduce bleeding and prevent infection, the area around the needle point was compressed for 5 minutes with sterile gauze and then covered with another sterile gauze and fixed with adhesive tape. The gauze could be removed by the patient 24 hours later. To maintain a double-blind state, and taking into consideration the impact of the pressure to the efficacy, patients of the other 2 groups (0.25 and 0.5 mm) could also perform the aforementioned action after needle injection, although this was not mandatory. No medication or physical therapy was performed within the first 3 months after treatment, and the patient was asked to avoid water contact at the treatment site for 24 hours.
FIGURE 2.
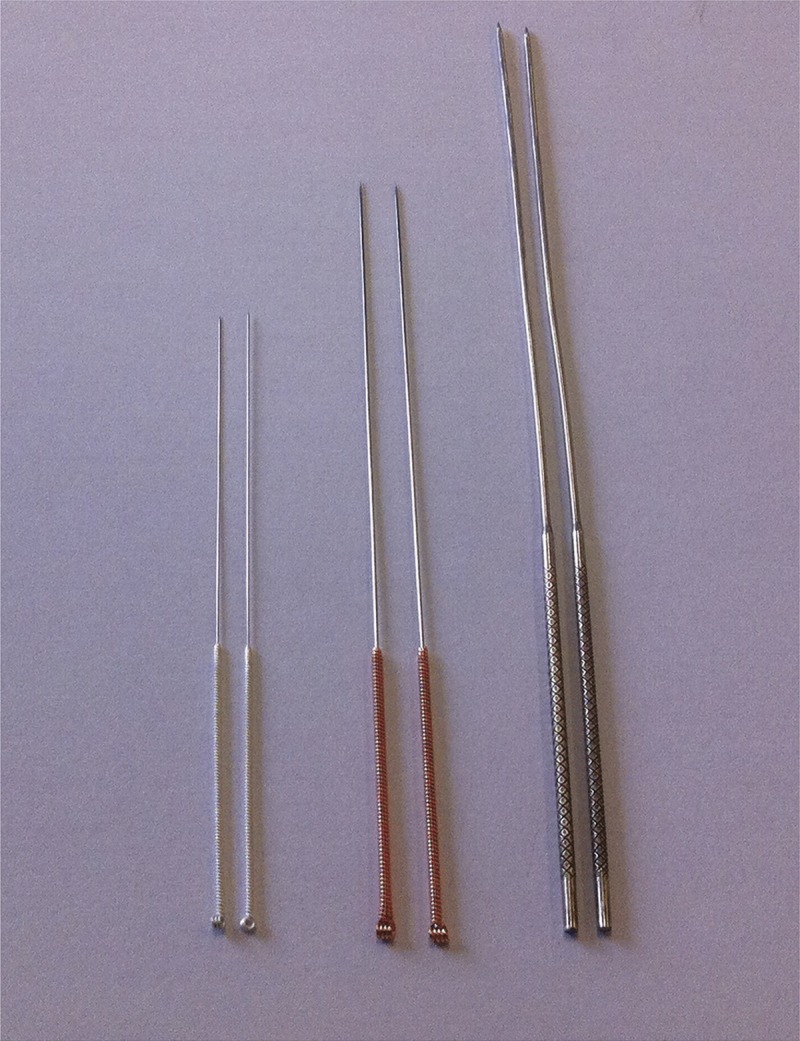
Dry needles of 3 different diameters.
FIGURE 3.
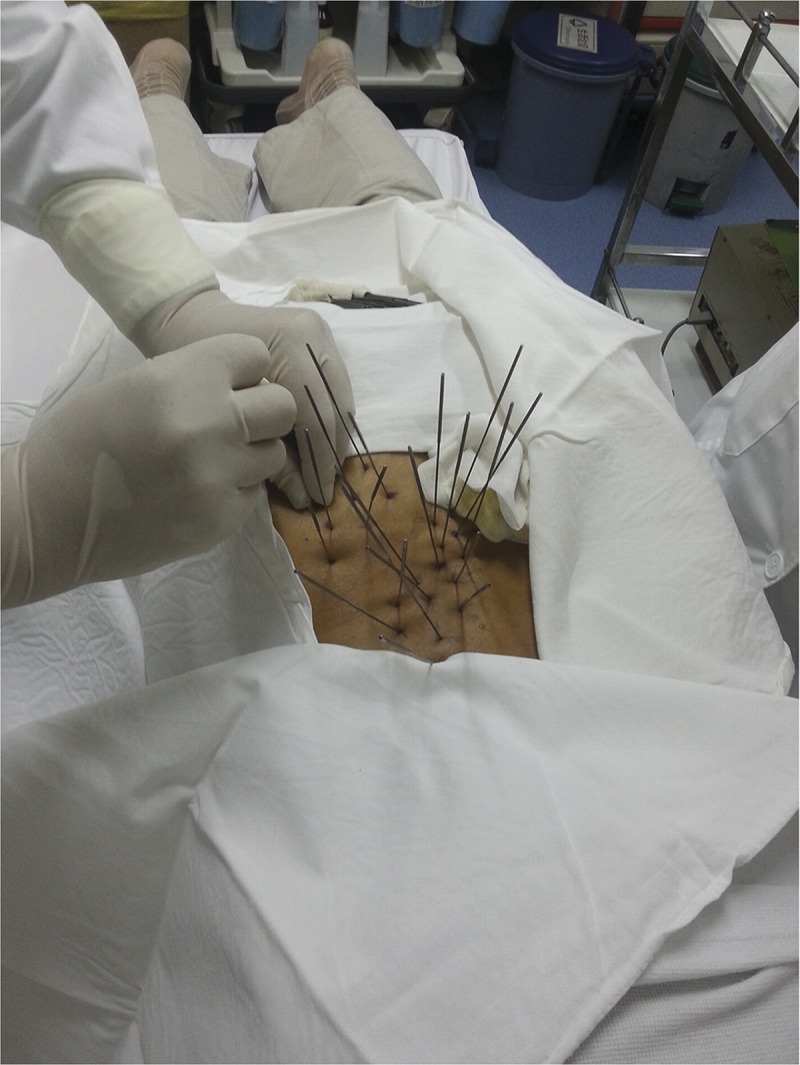
Dry needle (0.9 mm in diameter) treatment of lumbar myofascial pain.
Assessment
The Main Assessment
There are no laboratory tests or diagnostic images that can serve as a criterion standard for trigger point identification.1 We used VAS score and health survey (the medical outcome study item short form health survey, SF-36) as evaluation methods, based on myofascial pain treatment by acupuncture literature of Tekin et al.25
The main evaluation time points were before (0 day) treatment and 3 months after treatment. We also evaluated the VAS and SF-36 after treatment at 7 days and 1 month.
Secondary Assessment
The degree of pain of each patient receiving treatment and the willingness to accept the same treatment again, if necessary, were assessed. The VAS was used for pain intensity assessment during the treatment and evaluated immediately after the treatment. Furthermore, patients were asked the following question: Considering the discomfort associated with this treatment, would you consider undergoing this treatment again, if needed? The patient chose one from alternative answers: accept or not accept. Taking into account that treatment efficacy may affect a patient’s response to this question, we conducted the survey at 3 subsequent points in time (7 days, 1 month, 3 months afterwards), in addition to the one right after the treatment.
Statistical Analysis
Data analysis was performed on the Statistical Package for Social Science version 13 (SPSS 13). There were 4 aspects of the statistics: (i) internal comparison of data before and after each treatment to evaluate the efficacy of 3 dry needles with different diameters, (ii) comparison between treatment groups of the same time point after treatment to evaluate whether the diameter of the needle body influenced the treatment effect; (iii) comparison between treatment groups on pain intensity immediately after the treatment for patients to explore the perceived pain differences when the patients were treated with needles of different diameters; (iv) comparison of patients’ acceptance of DN therapy with different diameters. We used paired-samples t test to compare the therapeutic effect of before and 3 months after treatment (i), and the difference between the 3 groups before and after treatment was tested with repeated-measures analysis of variance; data in 2 time points (0 day and 3 months) were compared (ii). Analysis of variance was used to test the difference of pain intensity immediately after the treatment between the 3 groups (iii). The fourth was done using R × C table χ2 test. Statistical significance was considered if P < 0.05.
RESULTS
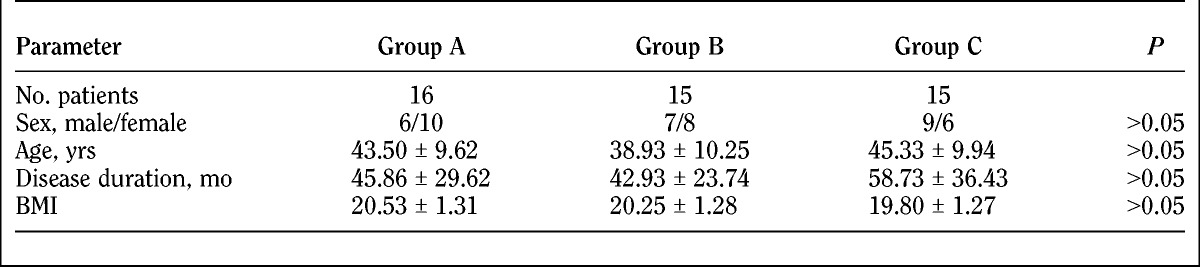
The treatment groups were well balanced in sex, age, and duration of disease. Table 1 shows the baseline demographic characteristics of patients.
TABLE 1.
Demographic characteristics of study participants
The VAS evaluation is as follows (Table 2, Fig. 4).
TABLE 2.
Visual analog scale results before and after treatment (mean ± SD)
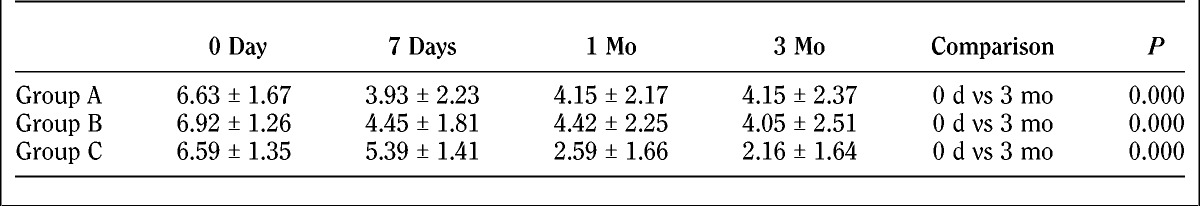
FIGURE 4.
Visual analog scale score evaluation before and after treatment for patients in 3 groups.
There were no differences (P > 0.05) of the baseline degree of pain in patients of the 3 groups. Significant differences were found on horizontal comparison (P < 0.05) in all 3 groups comparing the results of pain levels of 7 days, 1 month, and 3 months after treatment with baseline. There were no significant differences between 7 days, 1 month, and 3 months after treatment with regard to pain intensity (P > 0.05) of groups A and B, which suggested a similar degree of improved pain level in patients after 7 days to 3 months of treatment. In group C patients, degrees of pain were significantly different for 1 month and 3 months compared with the results of 7 days after treatment (P < 0.05), and no significant difference (P > 0.05) was observed between 1 month and 3 months of treatment. The degree of pain was decreased in patients of group C on the seventh day of survey and decreased more from the seventh day to 1 month and 3 months. For longitudinal comparison, during the first 7 days, no significant differences (P > 0.05) were found between groups A and B and groups B and C for the degree of pain after treatment. However, a significant difference (P > 0.05) was found between groups A and C for the degree of pain after 7 days, which indicated that improvement of the pain intensity was greater for patients in group A than in group C. After 1 month and 3 months of treatment, there was no significant difference of pain intensity for patients in groups A and B (P > 0.05). There was significant difference at 7 days, 1 month, and 3 months in group C versus group A; and significant difference at 1 month and 3 months for group C versus group B. Comparison of the difference of the 3 groups between 0 day and 3 months resulted in the following: group A versus group B, P = 0.858; group A versus group C, P = 0.064; group B versus group C, P = 0.047. There was difference between groups C and B.
The SF-36 evaluation before and after treatment is as follows (Tables 3 and 4; Fig. 5).
TABLE 3.
Short Form-36 Health Survey evaluation results before and after treatment (mean ± SD)
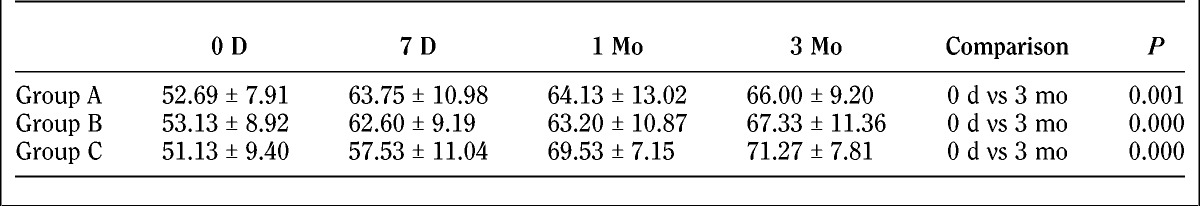
TABLE 4.
Subscale results of the SF-36
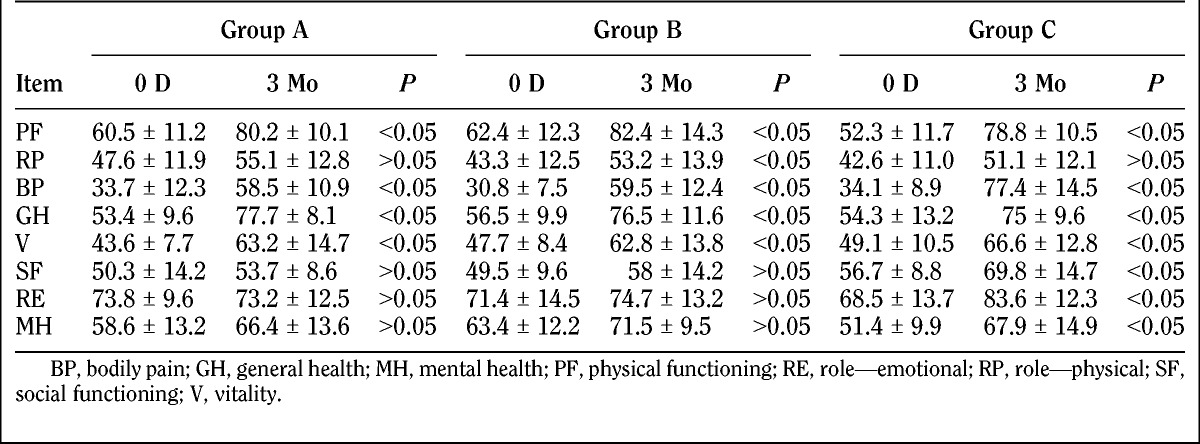
FIGURE 5.
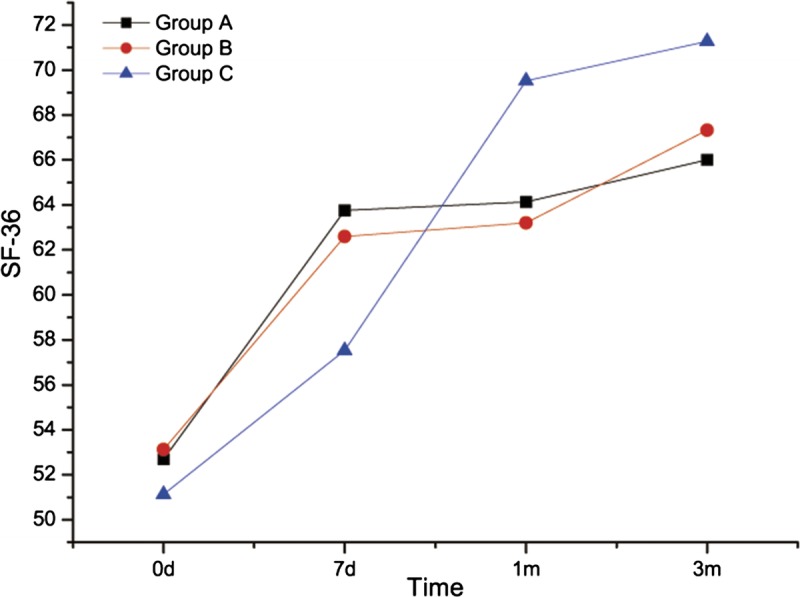
Short Form-36 Health Survey score evaluation before and after treatment for patients in 3 groups.
The SF-36 score evaluation at baseline was comparable between the treatment groups (P > 0.05). For horizontal comparison, significant differences were found (P < 0.05) in patients in groups A and B with regard to pain levels at 7 days, 1 month, and 3 months after treatment compared with baseline. The pain improved in all treatment groups. There were no significant differences between 7 days, 1 month, and 3 months after treatment with regard to pain intensity (P > 0.05), which indicated a similar degree of pain level improvement in patients from 1 day to 3 months after treatment. There was no difference between baseline and day 7 after treatment in pain-related condition of patients in group C. However, significant difference was found at month 1 and month 3 compared with baseline (P < 0.05), and also compared with data at 7 days (P < 0.05). This gave us a hint that no improvement of the overall health of group C was observed at 7 days after treatment, and improvements were observed at month 1 and month 3 after treatment, including same degree of improvements for month 1 and month 3. For longitudinal comparison, there were no differences (P > 0.05) in patients’ overall health at 7 days, 1 month, and 3 months after treatment, which indicated that the overall health of patients in the 3 groups was the same from 7 days to 3 months. Comparison of the difference of the 3 groups between 0 day and 3 months resulted in the following: group A versus group B, P = 0.739; group A versus group C, P = 0.487; group B versus group C, P = 0.721. There was no difference between the 3 groups.
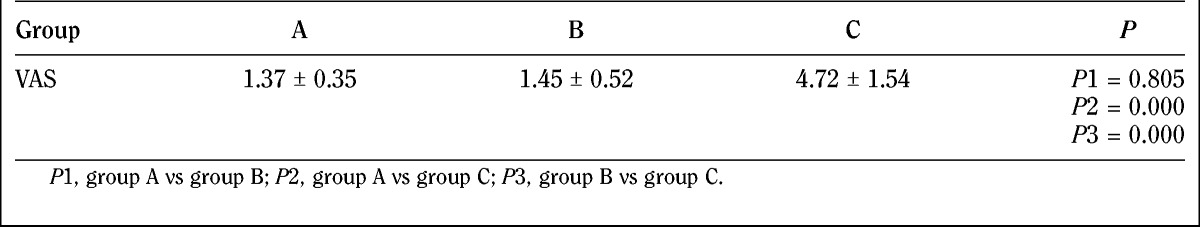
Results of the pain intensity differences of needling for patients in the 3 groups are demonstrated in Table 5.
TABLE 5.
Pain intensity of needling (mean ± SD)
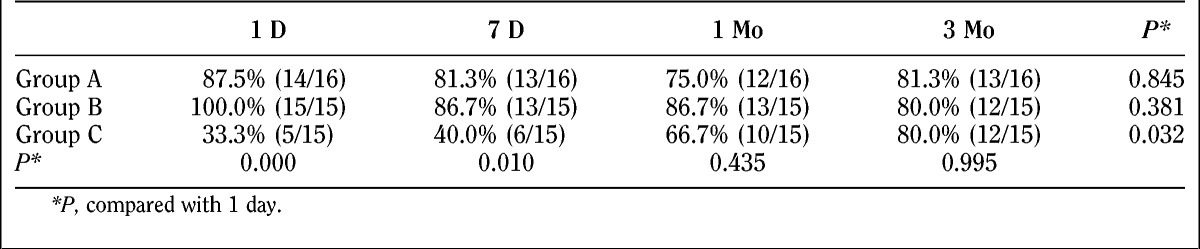
Percentages of patients willing to accept the treatment again at various times after the treatment for 3 groups are demonstrated in Table 6.
TABLE 6.
Percentage of patients willing to accept the treatment again
DISCUSSION
When selecting a needle for DN, the needle should be at least long enough to reach the depth of the myofascial trigger/tender point. With regard to needle diameter, Travell and Simons8 believed that the needle diameter is more of a matter of personal preference and skill developed through practice. In general, needles with larger diameters cannot be easily bent and can arrive at the predetermined trigger points more accurately. Moreover, larger needles may provide better feeling-perceived feedback when the needle pierces through layers of the tissue. The needle with small diameters can guarantee small tissue damage during special operations.26
In our literature review, the present study is the first to investigate the effect of needle diameter on treatment efficacy and pain intensity during trigger point DN in MPS of the low back muscles.
In this study, we used regular acupuncture needles with diameters of 0.25 and 0.5 mm and a special needle of 0.9 mm in diameter. The first 2 needle diameters were selected because they have been commonly used in prior studies. Studies using these needles have been published previously,27–29 and the needle of 0.25-mm diameter was the thinnest needle used in the published literature. Yoon et al.23 and other researchers showed that the efficacy of thicker acupuncture needle treatment may be better than that with smaller ones. Therefore, in this study, we also chose a thicker needle. However, since the thicker needle treatment may lead to more pain and low acceptance by patients, larger needle size was a limitation. We chose a special needle with a diameter of 0.9 mm. Another important reason for choosing this type of needle was that our hospital had adopted this type of needle for treatment of chronic soft tissue injury, MPS, and other diseases for more than 30 years, and had accumulated a wealth of clinical experience. This type of needle was developed decades earlier, which helped accumulate vast experience. The needle was cast using 85% mainly silver and a little copper, chromium, nickel, and other metal smelting together, and the length of pin shank was 5 cm. There were 4 types of needles of different lengths: 6, 8, 10, and 12 cm, with a diameter of 0.9 mm. We have already used this treatment method in clinical and animal studies to treat cervical disease, low back pain, tennis elbow, heel pain and knee pain, and other pain-related diseases, and it also showed good efficacy in treating MPS.30–32 However, our previous studies have focused on the comparison of this DN therapy with other treatments, especially with physical therapy, and did not conduct studies about the relationship between the diameter of the needle and therapeutic effects.
Results from the VAS score evaluation showed that pain levels of patients were all improved from baseline when treated with 3 dry needles with different diameters at 7 days, 1 month, and 3 months. Similar pain level improvements were achieved in both groups A and B at 7 days, 1 month, and 3 months; and in group C patients, pain improvements were better at 1 month and 3 months than at 7 days after treatment. After 7-day treatment, difference of pain levels were not observed between groups A and B and between groups B and C but was observed between groups A and C. The results showed that the pain level improvement was better in group A than in group C. However, at 3 months after the treatment, the degree of improvement in group C patients were better than in group B, which indicated that the efficacy of thicker dry needle (0.9 mm in diameter) treatment was better than the 0.5-mm diameter needles at 3 months after treatments.
Trigger point inactivation mechanism. One of the important principles of DN therapy is that the trigger point is mechanically crushed (mechanical disruption).33 During the DN therapy, palpation of the trigger point was first determined, followed by needling. Needles with small diameters are soft and are deflected easily with changes in texture during needling, leading to inaccurate arrival at the trigger points. In addition, since the trigger point itself is a nodule contraction8 with a higher density than the surrounding tissues, the needle may slide off, leading to incomplete inactivation of the trigger points, thereby affecting the therapeutic effects. Needles with larger diameter are relatively stiffer and can reach the trigger point more easily, leading to accurate piercing and complete inactivation, which may reflect as better clinical results.
Damage repair mechanisms. Animal studies have shown that the needle may cause damage of the muscle and nerves during treatment,34 and repairing this damage may be one of the mechanisms for the DN therapy. Muscle regeneration after TrP-DN is expected to be complete in approximately 7 to 10 days.35 Efficacy of patients in group C is better at 1 month and 3 months than at 7 days, which may be because the tissue repair was not accessed fully. Dry needles with different diameters may cause different extents of tissue damage,8 which involves different natural repair processes and could be the reason for efficacy differences.
The most likely mechanism of pain relief by needle stimulation is hyperstimulation analgesia via the descending pain inhibitory system.36 The strong pressure stimulation to the MTrP loci can provide strong neural impulses to the dorsal horn cells in the spinal cord, which may then break the vicious cycle of the MTrP circuit.37 Strong stimulation was more likely to affect the trigger point, and the dry needles with larger diameters may have stronger stimulation to the tissue than the smaller ones. This increase in the intensity of stimulation may improve the therapeutic effect of DN.
Limited mechanical stretch. An accurately placed needle may also provide a localized stretch to the contracture of cytoskeletal structures, which allow the involved sarcomeres to resume their resting length by reducing the degree of overlap between actin and myosin filaments.38 Usually, to obtain better efficacy, we only can improve the limited traction force by rotating the body of the needle.39 One would expect that a thicker needle body would provide greater traction force than the thin needles.
Needles with diameters of 0.9 mm may have some more potential treatment mechanisms than the smaller ones. Animal studies40 showed that capillary proliferation of the soft tissue occur after acupuncture with 0.9-mm-diameter needles in rats. The senescent capillaries would increase the blood supply of the injured site, thereby improving clinical symptoms. It differs from the inactivated trigger points, which was realized at the same time of the treatment, and the formation of new blood capillaries needs further time. This may also explain why the efficacy of pain alleviation is more obvious after 1 month and 3 months than after 7 days in patients of group C (0.9 mm in diameter).
The discussion earlier may provide theoretical basis for the better efficacy of treatment of chronic lower back MPS using needles of 0.9 mm in diameter compared with needles of 0.5 mm in diameter.
The SF-36 scores evaluation showed that health condition of patients had improved 7 days, 1 month, and 3 months after treatment with dry needles of 0.25- and 0.5-mm diameters. No improvement in the health status of the patient in group C after 7 days of treatment. After 7 days, 1 month, and 3 months of treatment, the degree of health status changes were similar between the 3 groups.
Inconsistent results were shown between the SF-36 and VAS evaluations. Possible reasons for this may be that the content of SF-36 is more extensive and a small degree of change in pain intensity may not be reflected in the SF-36 overall score evaluation. Another possible reason may be the small sample size of this study.
Needling Pain Intensity and the Extent of the Patient’s Acceptance
In theory, the larger the diameter of the needle, the stronger was the pain intensity during needling. But this theory is still controversial. Jørgensen41 compared the needling pain intensity of 30G and 27G needles, and the results showed that the intensity of needles of 0.3-mm diameter (30G) was weaker than that of 0.4-mm (27G) needles. Robb and Kanji42 found that there was no difference of needling pain intensity between 0.3-mm-diameter (30G) needles and 0.45-mm-diameter (26G) needles. Arendt-Nielsen et al.43 compared the proportion of patients who felt pain and discomfort during injection with needles of the 3 diameters (27G, 30G, 32G). Results showed that 54% of the patients of the 27G needle group, 45% of the patients of the 30G needle group, and 31% of the patients of 32G needle group felt obvious pain, and the number of people was statistically different. In patients who felt obvious pain, the pain intensity had no difference. In our study, average needling pain of 0.5-mm-diameter needles was slightly higher than the average pain intensity of 0.25-mm diameter, which was not statistically significant. The needling pain of the 0.9-mm-diameter needle was stronger than the other 2 needles, and the difference was statistically significant (P < 0.01). The results of this study show that the needling pain was not related to the diameter of the needle within a certain size. However, beyond a certain size, the pain of the patient will increase with the increase of the needle diameter.
We obtained interesting results with regard to patients’ potential willingness to accept additional treatments when surveyed at the various time points (immediately afterward, 7 days, 1 month, and 3 months). Most of the patients in groups A and B showed willingness to accept the treatment again, which was independent of all 4 time points (P > 0.05). In patients who accepted treatment in group C, the acceptance ratio changed significantly over time (P < 0.01). Only 33.3% of the patients were willing to accept the treatment just after the treatment and then gradually increased with time to 80.0% after 3 months. The willingness of patients to accept another treatment is different in 3 groups immediately after treatment and 7 days later. Ratio of the group C is much less than the other 2 groups (P < 0.05). This is consistent with the needling pain results, which indicated that the degree of pain is stronger in group C than groups A and B, hence the low proportion of patients willing to accept the treatment again. After 1 month and 3 months of treatment, there was no difference between the 3 groups. Presumably, part of the reason was the recognition of the therapeutic efficacy in group C, and they then changed their willingness to accept the treatment again.
Taking into account the intensive needling pain and low acceptance during the early phase after treatment, promoting clinical usage of dry needles with a diameter of 0.9 mm still needs further discussion. As mentioned previously, we have used this type of needles for treatment of soft tissue injuries (including MPS) for many years in our department. In clinical practice, we usually use 0.5% lidocaine to anesthetize the site of injection before inserting the dry needles, and this can significantly reduce the pain of needling. In this study, anesthesia was not required for the 0.25- and 0.5-mm dry needles. Therefore, to eliminate the effects of local anesthetics, no anesthesia was performed in the 0.9-mm-diameter group.
Limitations of the Study
The present study has some limitations. First, there was no natural recovery group or other treatments (eg, physiotherapy) used as control groups. Changes in the VAS or SF-36 evaluation may be due to the outcome of the natural course of the disease itself. Second, this study was designed especially for patients with lumbar myofascial pain. Whether the results apply to myofascial pain of other parts remain unclear. Third, this study only used 3 needle diameters and cannot represent all diameters of needles. There is no conclusion with regard to the needle with the best efficacy in this setting.
CONCLUSION
All the 3 different pin-diameter dry needles showed therapeutic effect on lumbar chronic MPS 7 days, 1 month, and 3 months after treatment. At 3 months after the treatment, needles with a diameter of 0.9 mm had better therapeutic effects than that of 0.5 mm. The diameter of the needle had a certain effect on the needling pain, and needles with a diameter of 0.9 mm could induce stronger needling pain than that of 0.25 and 0.5 mm in diameters. Patients had a higher acceptance of dry needles with diameters of 0.25 mm and basically did not change with time. Patients’ acceptance of dry needles of 0.9-mm diameter changed over time; most patients were reluctant to accept the treatment again in the early phase, although they were willing to accept after 3 months. Based on this study, needle diameter does seem to influence the effects of DN therapy in the treatment of MPS. However, more research is warranted to determine the optimal needle diameter.
Supplementary Checklist
CONSORT Checklist: http://links.lww.com/PHM/A158
Footnotes
This study was supported by a grant from National Natural Science Foundation of China (Grant No. 61172007, 010810).
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ajpmr.com).
REFERENCES
- 1.Malanga GA, Cruz Colon EJ: Myofascial low back pain: a review. Phys Med Rehabil Clin N Am 2010;21:711–24 [DOI] [PubMed] [Google Scholar]
- 2.Fishbain DA, Goldberg M, Meagher BR, et al. Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain 1986;26:181–97 [DOI] [PubMed] [Google Scholar]
- 3.Tough EA, White AR, Richards S, et al. Variability of criteria used to diagnose myofascial trigger point pain syndrome—evidence from a review of the literature. Clin J Pain 2007;23:278–86 [DOI] [PubMed] [Google Scholar]
- 4.Hong CZ: Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil 1994;73:256–63 [DOI] [PubMed] [Google Scholar]
- 5.Vedolin GM, Lobato VV, Conti PC, et al. The impact of stress and anxiety on the pressure pain threshold of myofascial pain patients. J Oral Rehabil 2009;36:313–21 [DOI] [PubMed] [Google Scholar]
- 6.Chien JJ, Bajwa ZH: What is mechanical back pain and how best to treat it? Curr Pain Headache Rep 2008;12:406–11 [DOI] [PubMed] [Google Scholar]
- 7.Gerwin RD: A review of myofascial pain and fibromyalgia—factors that promote their persistence. Acupunct Med 2005;23:121–34 [DOI] [PubMed] [Google Scholar]
- 8.Simons DG, Travell JG, Simons LS. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. Vol 1 Pennsylvania, PA: Williams & Wilkins, 1999 [Google Scholar]
- 9.Kalichman L, Vulfsons S: Dry needling in the management of musculoskeletal pain. J Am Board Fam Med 2010;23:640–6 [DOI] [PubMed] [Google Scholar]
- 10.Lewit K: The needle effect in the relief of myofascial pain. Pain 1979;6:83–90 [DOI] [PubMed] [Google Scholar]
- 11.Dıraçoğlu D, Vural M, Karan A, et al. Effectiveness of dry needling for the treatment of temporomandibular myofascial pain: a double-blind, randomized, placebo controlled study. J Back Musculoskelet Rehabil 2012;25:285–90 [DOI] [PubMed] [Google Scholar]
- 12.Nagraba Ł, Tuchalska J, Mitek T, et al. Dry needling as a method of tendinopathy treatment. Ortop Traumatol Rehabil 2013;15:109–16 [DOI] [PubMed] [Google Scholar]
- 13.Mayoral O, Salvat I, Martín MT, et al. Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. Evid Based Complement Alternat Med 2013;2013:694941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vulfsons S, Ratmansky M, Kalichman L: Trigger point needling: techniques and outcome. Curr Pain Headache Rep 2012;16:407–12 [DOI] [PubMed] [Google Scholar]
- 15.Kietrys DM, Palombaro KM, Azzaretto E, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther 2013;43:620–34 [DOI] [PubMed] [Google Scholar]
- 16.Tough EA, White AR, Cummings TM, et al. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain 2009;13:3–10 [DOI] [PubMed] [Google Scholar]
- 17.Hsieh YL, Chou LW, Joe YS, et al. Spinal cord mechanism involving the remote effects of dry needling on the irritability of myofascial trigger spots in rabbit skeletal muscle. Arch Phys Med Rehabil 2011;92:1098–105 [DOI] [PubMed] [Google Scholar]
- 18.Cagnie B, Barbe T, De Ridder E, et al. The influence of dry needling of the trapezius muscle on muscle blood flow and oxygenation. J Manipulative Physiol Ther 2012;35:685–91 [DOI] [PubMed] [Google Scholar]
- 19.Huang YT, Lin SY, Neoh CA, et al. Dry needling for myofascial pain: prognostic factors. J Altern Complement Med 2011;17:755–62 [DOI] [PubMed] [Google Scholar]
- 20.Srbely JZ, Dickey JP, Lee D, et al. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med 2010;42:463–8 [DOI] [PubMed] [Google Scholar]
- 21.Hsieh YL, Kao MJ, Kuan TS, et al. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil 2007;86:397–403 [DOI] [PubMed] [Google Scholar]
- 22.Tsai CT, Hsieh LF, Kuan TS, et al. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil 2010;89:133–40 [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Rah UW, Sheen SS, et al. Comparison of 3 needle sizes for trigger point injection in myofascial pain syndrome of upper- and middle-trapezius muscle: a randomized controlled trial. Arch Phys Med Rehabil 2009;90:1332–9 [DOI] [PubMed] [Google Scholar]
- 24.Abbaszadeh-Amirdehi M, Ansari NN, Naghdi S, et al. The neurophysiological effects of dry needling in patients with upper trapezius myofascial trigger points: study protocol of a controlled clinical trial. BMJ Open 2013;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tekin L, Akarsu S, Durmuş O, et al. The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double-blinded placebo-controlled trial. Clin Rheumatol 2013;32:309–15 [DOI] [PubMed] [Google Scholar]
- 26.Hong C-Z: Considerations and recommendations regarding myofascial trigger point injection. Journal of Musculoskeletal Pain 1994;2:29–59 [Google Scholar]
- 27.Rachlin ES, Rachlin I. Myofascial Pain and Fibromyalgia. St. Louis: Mosby, 1994: 173–88 [Google Scholar]
- 28.Iwama H, Akama Y: The superiority of water-diluted 0.25% to neat 1% lidocaine for trigger-point injections in myofascial pain syndrome: a prospective, randomized, double-blinded trial. Anesth Analg 2000;91:408–9 [DOI] [PubMed] [Google Scholar]
- 29.Ga H, Choi JH, Park CH, et al. Acupuncture needling versus lidocaine injection of trigger points in myofascial pain syndrome in elderly patients—a randomised trial. Acupunct Med 2007;25:130–6 [DOI] [PubMed] [Google Scholar]
- 30.Pang Z, Liu N, Yu SW: On the treatment of 35 cases with chronic neck and back pain by silver needle diathermy. Rheum Arthritis 2014;3:30–7 [Google Scholar]
- 31.Lu Y, Su SH, Wang JQ, et al. Curative effect abservation on lumbar hypo-patella fat pad inflamation treated with painless silver needle. Chinese Journal of Trauma and Disability Medicine 2013;21:37–8 [Google Scholar]
- 32.Wang HD: Clinical observation on intensive acupuncture-moxibustion with silver needles for lumbodorsalmyofascial pain syndrome. Shanghai J Acu-mox 2013;32:664–5 [Google Scholar]
- 33.Simons DG, Travell JG, Simons LS. Travell and Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd Vol 1 Baltimore, MD: Williams & Wilkins, 1999 [Google Scholar]
- 34.Domingo A, Mayoral O, Monterde S, et al. Neuromuscular damage and repair after dry needling in mice. Evid Based Complement Alternat Med 2013;2013:260806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reznik M: Current concepts of skeletal muscle regeneration, in Pearson CM, Mostofy FK. (eds): The Striated Muscle. Baltimore, MD, Williams & Wilkins, 1973, pp. 185–225 [Google Scholar]
- 36.Melzack R: Myofascial trigger points: relation to acupuncture and mechanisms of pain. Arch Phys Med Rehabil 1981;62:114–7 [PubMed] [Google Scholar]
- 37.Hong CZ: Treatment of myofascial pain syndrome. Curr Pain Headache Rep 2006;10:345–9 [DOI] [PubMed] [Google Scholar]
- 38.Dommerholt J: Dry needling in orthopedic physical therapy practice. Orthop Phys Ther Pract 2004;16:15–20 [Google Scholar]
- 39.Langevin HM, Churchill DL, Cipolla MJ: Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. FASEB J 2001;15:2275–82 [DOI] [PubMed] [Google Scholar]
- 40.Yang ZL. The Mechanisms of Inner Heating Needle and Chinese Silver Needle Treating on Chronic Skeletall Muscle Lesion in Rats. Chinese PLA Postgraduate Medical School, 2011 [Google Scholar]
- 41.Jørgensen JT: Improvement of patient convenience in treatment with growth hormone. J Pediatr Endocrinol 1994;7:175–80 [DOI] [PubMed] [Google Scholar]
- 42.Robb DM, Kanji Z: Comparison of two needle sizes for subcutaneous administration of enoxaparin: effects on size of hematomas and pain on injection. Pharmacotherapy 2002;22:1105–9 [DOI] [PubMed] [Google Scholar]
- 43.Arendt-Nielsen L, Egekvist H, Bjerring P: Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res 2006;23:37–43 [DOI] [PubMed] [Google Scholar]


