Abstract
The Ca2+-responsive phosphatase calcineurin/ protein phosphatase 2B dephosphorylates the transcription factor NFATc3. In the myocardium activation of NFATc3 down-regulates the expression of voltage-gated K+ (Kv) channels after myocardial infarction (MI). This prolongs action potential duration and increases the probability of arrhythmias. Although recent studies infer that calcineurin is activated by local and transient Ca2+ signals the molecular mechanism that underlies the process is unclear in ventricular myocytes. Here we test the hypothesis that sequestering of calcineurin to the sarcolemma of ventricular myocytes by the anchoring protein AKAP150 is required for acute activation of NFATc3 and the concomitant down-regulation of Kv channels following MI. Biochemical and cell based measurements resolve that approximately 0.2% of the total calcineurin activity in cardiomyocytes is associated with AKAP150. Electrophysiological analyses establish that formation of this AKAP150-calcineurin signaling dyad is essential for the activation of the phosphatase and the subsequent down-regulation of Kv channel currents following MI. Thus AKAP150-mediated targeting of calcineurin to sarcolemmal micro-domains in ventricular myocytes contributes to the local and acute gene remodeling events that lead to the down-regulation of Kv currents.
Keywords: A-Kinase anchoring protein AKAP, calcineurin, acute transcriptional response, myocardial infarction
1. Introduction
Calcineurin (CaN) is a Ca2+/calmodulin dependent phosphatase that dephosphorylates a myriad of phospho-proteins (1; 2). Although this enzyme is expressed in a variety of tissue and cell types, its action is particularly relevant for the modulation of nuclear signaling events that proceed through the family of NFAT transcription factors (3; 4). Upon activation by Ca2+/calmodulin, it forms a bimolecular complex with NFAT’s. This prompts nuclear translocation of NFAT’s with concomitant effects on the transcriptional control of certain genes. NFATc3 target genes include Kv4.3, Kv4.2, Kv2.1, and Kv1.5 subunits of the voltage gated K+ (KV) channels (5). The health relevance of this transcriptional remodeling pathway is underscored by evidence that rapid activation of NFAT’s is responsible for the down-regulation of KV channels following myocardial infarction (MI) (6). One pathophysiological consequence is an aberrant modification of ventricular action potentials to increase the probability of lethal arrhythmia after myocardial infarction (7).
Thus, understanding the signaling events that underlie this acute cardiac comorbidity that occurs after heart attack is of considerable value. The mechanism by which calcineurin is activated in the cardiovascular system is a subject of intensive investigation. We have previously suggested that a local Ca2+ signal originating from the repetitive opening of a single or clusters of L-type Ca2+ channels (“Ca2+ sparklets”) induced nuclear NFAT translocation in smooth muscle (8; 9). Subsequently it was shown that inhibition of a subpopulation of myocyte L-type Ca2+ channels associated with caveolin also prevents NFAT’s activation and hypertrophy (10). These studies are consistent with a model in which a local Ca2+ signal originating from the persistent opening of L-type Ca2+ channels drives the activation of calcineurin. Thus, the spatiotemporal organization of calcineurin and its interplay with NFAT may be a contributing factor to acute transcriptional remodeling events in ventricular and arterial myocytes (8; 10).
An additional regulatory element in the process may be the A-Kinase Anchoring Protein AKAP150 (the rodent ortholog of human AKAP79). This multifunctional anchoring protein sequesters protein kinase A (PKA), protein kinase C (PKC), and calcineurin at plasma membranes (11; 12). Recent evidence suggests that targeting of calcineurin via association with AKAP150 is required for NFAT activation in neurons (13). AKAP150 is also present in ventricular myocytes where it is believed to physically associate with caveolin (14). Collectively, these findings raise the intriguing possibility that AKAP79/150 targets this calcium responsive phosphatase to specific regions of the sarcolemma of ventricular myocytes where it can respond to calcium influx through L-type Ca2+ channels (15; 16).
From a pathophysiological perspective, it is becoming increasingly clear that abnormal utilization of localized cAMP and calcium signaling pathways underlie a variety of cardiac disorders (17–25). Anomalies in the β adrenergic signaling cascade are linked to altered regulation of [Ca2+]i in ventricular myocytes (26; 27). Although acute activation of β adrenergic receptors (βAR) can increase heart function, a well known and long standing paradox is that chronic activation of the same signaling cascade causes hypertrophy, electrical remodeling, and arrhythmogenesis after myocardial infarction (28–30). Likewise, NFAT mediated transcriptional remodeling is a critical event in certain heart diseases including the attenuation of KV channel function that is associated with QT prolongation and increased susceptibility to arrhythmia (6). Yet, the role of AKAP150-mediated signaling events in the activation of calcineurin and NFAT in the down-regulation of Kv channel function after myocardial infarction is less clear.
Using a combination of primary cell lines from genetically modified mice we report that an anchored pool of calcineurin is required for down-regulation of Kv channels following myocardial infarction. We demonstrate that AKAP150 and calcineurin exist as a macromolecular complex in the sarcolemma of ventricular myocytes whereas functional studies in samples from AKAP150 knockout and knockin mice imply that the anchoring protein is required NFATc3 responsive cardiac remodeling. Our data infer that loss of AKAP150 is protective against down regulation of Kv currents following MI.
2. Materials and Methods
2.1. Adult and neonatal Cardio Myocyte Isolation
Except for neonatal cardiomyocytes isolation, all experiments were performed using 6 to 12 week old mice. Detailed description about generation of AKAP150 knock out and AKAP150-ΔPIX mice can be found in previous publications (31; 32). All animal protocols were approved by the University of Washington Institutional Animal Care and Use Committee. Adult mice and/or neonatal mice (<72 hrs post-partum) were euthanized by a lethal IP injection of pentobarbital (100 mg/Kg) or decapitation, respectively, to harvest the hearts. Single adult and neonatal ventricular myocytes were isolated as described elsewhere (33–35). Short-term culture of these myocytes was performed using published protocols (33–35). For electrophysiology experiments isolated single ventricular myocytes were stored at room temperature in Tyrode solution in mM (140 NaCl, 5 KCl, 10 HEPES, 10 glucose, 2 CaCl2, and 1MgCl2; pH 7.4) until used.
2.2. Myocardial infarction (MI) surgery
Procedure for MI surgery was as described in elsewhere (36). Briefly, mice were anesthetized using isofluorane (3 to 5%) and maintained them on spontaneous ventilation. Subsequently, small incision was made on ventral cervical region to intubate the trachea for initiating mechanical ventilation. A small incision was made on left side of the thoracic wall between 4th intercostal space to expose the heart. Then, the left anterior descending coronary artery was ligated using 8-0 prolene suture, and chest was closed using 6-0 suture.
2.3. Electrophysiology
KV currents were recorded using an Axopatch 200B amplifier. Myocytes were patched on physiological buffer with the following composition in mM; 5 KCl, 140 NaCl, 1 MgCl, 2 CaCl2, 10 Glucose, 10 HEPES (pH = 7.4). After achieving giga-seal the extracellular solution was changed to a solution containing in mM, 140 N-methyl-D-glucamine, 5 KCl, 10 HEPES, 10 glucose, 0.1 CaCl2, 2 MgCl, and 0.01 nifedipine (pH = 7.4). The intracellular solution contained (in mM): 110 K-aspartate, 30 KCl, 10 HEPES, 5 ATP-Mg and 10 EGTA (pH = 7.3). KV currents were elicited by 1.5 s depolarizing step from holding potential of −80mV to test potential of −50 to +60 in increments of 10 mV. The series resistance compensation circuitry of the Axopatch was used to compensate for about 60% of the series resistance. Current analysis was performed using Clampfit 10 software (Axon Instruments).
2.4. Western blot and co-immunoprecipitation
Heart and brain tissue samples were homogenized in RIPA protein lysis buffer (RLB) (25 Tris-HCl 25 mM, NaCl 150 mM, NP-40 1 %, Sodium deoxycholate 1 %, SDS 0.1 %; pH 7.6) containing protease inhibitors (Complete Mini, Roche). Homogenates were centrifuged at 10,000 × g for 10 minutes to remove larger tissue debris. Supernatants from this step were used for western blot analysis. Protein contents in the supernatant were measured using BCA protein quantification method.
For comparison of protein expression between samples, equal amounts of tissue lysates were loaded onto polyacrylamide gels (gradient or 8, 10 to 12%), electrophoretically separated by size, and transferred on to nitrocellulose membranes. Membranes were washed in Tris-buffered saline containing 0.1% Tween 20 (TBS-T), and blocked with 5 % non-fat dried milk in TBS-T for an hour in room temperature. Membranes were incubated with primary antibodies (1:1000; to 1:250 dilution) overnight and then subjected to five 10-minute washes in TBS-T. Primary antibodies used in this study were rabbit anti-AKAP150 (V088), goat anti-AKAP150 (C-20; Santa Cruz), anti-GAPDH (clone 71.1; Sigma), mouse anti-PP2BB (clone CN-B1; Abcam) (32). Membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG in 5% milk/TBS-T for 1 hour and washed. The bound secondary antibody was detected by enhanced chemiluminescence (ECL) on MultiImage III (Alpha Innotech) imager, and immunoreactivity densitometry was calculated using NIH imageJ software tool.
Immunoprecipitation (IP) studies were performed using previously published method (37). Briefly, protein lysates (1 ml, concentration 1 mg/ml) were incubated with 25 µL protein-A agarose beads, and 2 µg of primary antibody for 2 hr at the 4° C. Following incubation, immune complexes were washed 3 times in IP buffer and protein-A bound protein complexes were eluted by boiling in 2 × NuPage sample buffer with protein denaturing agent such as DTT (0.5 M). Eluted protein complexes were used to quantify AKAP150 and CaNB association in heart and brain tissue using conventional western blot technique.
2.5. Immunofluorescence
Isolated cardiac myocytes from WT and AKAP150−/− mice were plated on BD Cell-Tak coated cover slips. Cells were allowed to attach for 4 hrs. at 37 °C in M199 media (Sigma-Aldrich). Following removal of media cells were rinse twice with PBS and fixed in 1% paraformaldehyde, washed three times in PBS, and permeabilized with 0.075% Triton X-100/PBS solution. Following permeabilization cells were incubated in blocking buffer containing 2% donkey serum, 20% goat serum, and 1% bovine serum albumin in antibody dilution buffer (0.1% Triton X-100 and 1% IgG free BSA in PBS). Cells were incubated overnight (4 °C) with primary antibodies; mouse anti-α-actinin (A7811, Sigma-Aldrich, 1:500) and goat anti-AKAP150 (AKAP150-C20, sc-6445, Santa Cruz Biotech, 1:500). Secondary antibodies Alexa Fluor 488-congugated donkey anti-mouse and Alexa Fluor 568-conjugated donkey antigoat (Molecular Probes) were use to detect α-actinin and AKAP150 respectively (1h at 27°C). Secondary Ab incubation was followed by PBS wash and the cells were mounted on slides. Primary antibodies were omitted in negative control experiments to test labeling specificity. Cells were visualized (512×512 pixel images) using a Radiance 2100 confocal system coupled to a Nikon TE300 inverted microscope equipped with a Nikon 60x oil immersion lens (NA=1.4) lens and a zoom of 3.5 (pixel size=0.1 µm).
2.6. Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was isolated WT and AKAP150−/− control and MI myocytes. RNA was reverse transcribed using the SuperScript III First strand cDNA synthesis (Invitrogen) as instructed by manufacturer. RT product was used for Real-time PCR reactions, performed using SYBER green (QuantiTect SYBER green PCR; Qiagen) as the fluorescence probe on an ABI 7700 sequence detector (PE; Applied Biosystems). QuantiTect Primer assays (Qiagen) were used to detect KV4.3 (NM_001039347; QT00166229), KV4.2 (NM_019697; QT00170443), KV1.5 (NM_145983; QT00268387) and KV2.1 (NM_008420; QT00285971). β-actin (NM_007393; QT00095242) expression was used as an internal control. Product quantization was performed using the relative quantification method (6). Primer efficiency was determined by regression analysis of series dilution of RT product. Relative transcript abundance was normalized to β-actin.
2.7. Cell Culture and NFATc3 translocation assay
NFAT translocation in response to adrenergic agonists was assessed in primary cultured WT or AKAP150−/− neonatal cardio myocytes. Ventricular myocytes from 2–3 days old WT or AKAP150−/− were cultured in cell-tak coated cover slips and infected with NFATc3 fused to EGFP (NFATc3-EGFP). Cells were cultured 48 hours. Twelve hours after infection 100 µM phenylephrine was added to the culture media. A Radiance 2100 confocal system coupled to a Nikon TE300 inverted microscope equipped with a Nikon 60x oil immersion lens (N.A.= 1.4) lens was used to image cells. The ratio of nuclear to cytosolic GFP fluorescence was determined in control and PE treated cells.
3. Results
3.1. AKAP150 anchors low levels of calcineurin in the heart
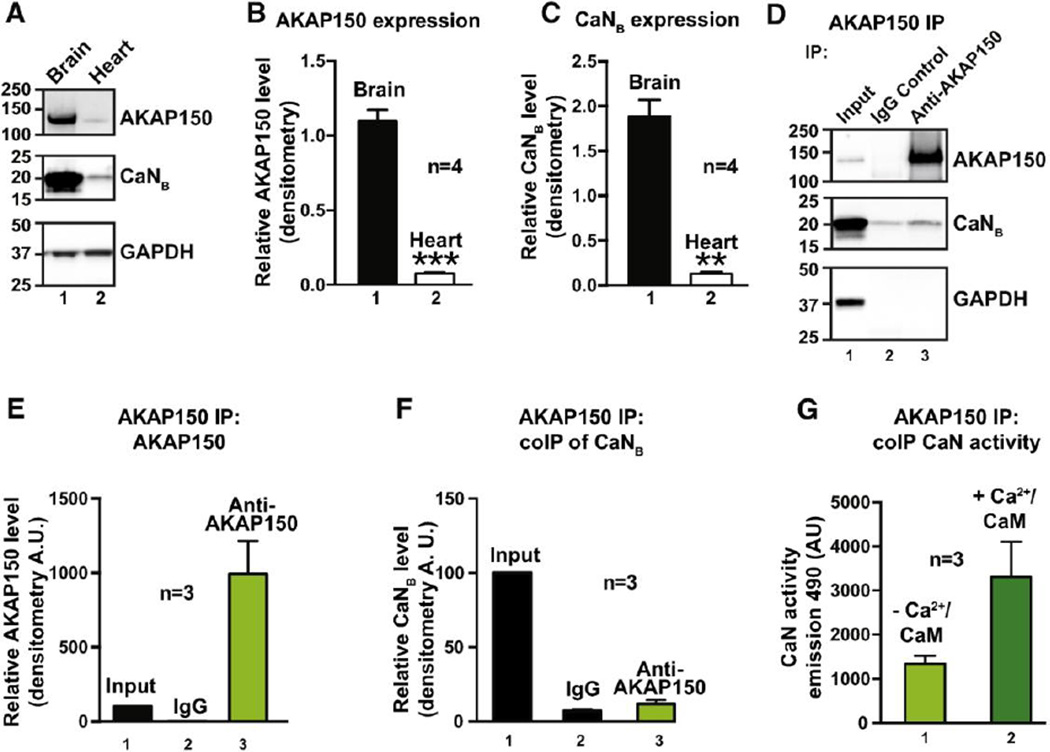
Previous studies have defined and characterized the calcineurin-AKAP79/150 interface in neurons, skeletal muscle and β-islets (32; 38–40). Moreover, functional analyses reveal that loss of this anchoring protein promotes a redistribution of its enzyme binding partners including protein kinase A and calcineurin with concomitant changes in a variety of vital cellular processes including excitatory synaptic transmission, glucose homeostasis and vascular tone (13; 31; 41; 42). However, much less is known about the AKAP150-calcineurin interface in the heart (43–46). Therefore, we sought to measure the amount of calcineurin associated with AKAP150 in the heart. As a prelude to these studies we compared the expression levels of AKAP150 and calcineurin in heart and brain lysates from mice (Fig 1A–C). Quantitation by densitometry revealed that whole heart lysates expressed 6.9 ± 0.4% of the AKAP150 (n=4; Fig 1B) and 7.2 ± 1.7% of calcineurin compared to the levels of both proteins in mouse brain (n = 4; Fig 1C). Thus extensive biochemical characterization of the endogenous proteins in mouse heart lysates was not technically feasible.
Figure 1. AKAP150 anchors low levels of calcineurin in the heart.
A–C, Evaluation of expression levels of AKAP150 (A top panel and B) and calcineurin (A mid panel and C). D, Immunoprecipitation of AKAP150 followed by immunoblot detection of AKAP150 (top panel) and CaNB (mid panel) in heart lysates from wildtype animals. E–F, Densitometry analysis of AKAP150 (E) and CaNB (F) enrichment. G, Bar plot of phosphatase activity in AKAP150 enriched fractions.
Yet, using enrichment methods it was possible to capture AKAP150 immune complexes on protein-A agarose and demonstrate co-fractionation of the phosphatase by immunoblot detection of the calcineurin B subunit (Fig 1D, top and mid panels, lane 3). Quantitation by densitometry estimated a significant enrichment of the AKAP150 and calcineurin B subunit signals over background IgG control (Fig 1E and F). Parallel experiments confirmed that phosphatase activity toward the synthetic substrate PnPP was elevated in AKAP150 enriched fractions (Fig 1G). In addition, Ca2+/calmodulin stimulated PnPP phosphatase activity a further 2.3 ± 0.01 n=3 fold. This is consistent with previous reports that association with the AKAP79/150 scaffold may somehow constrain a small, but inactive pool anchored phosphatase (47–49).
3.2. AKAP150 sequesters calcineurin at the T-tubules of ventricular myocytes
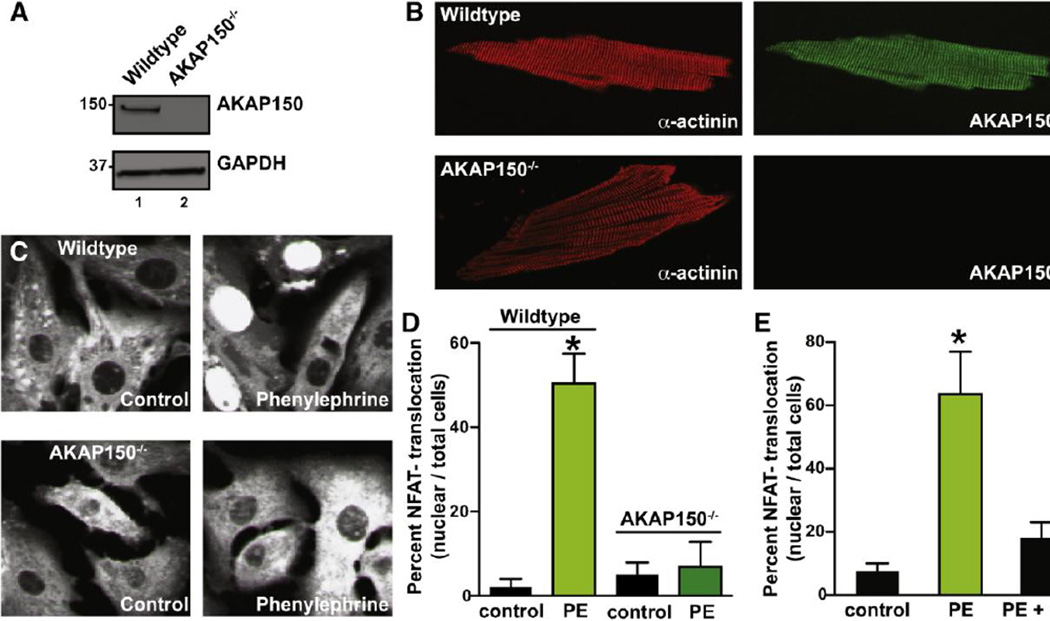
Since AKAP150 is expressed at low levels in cardiac tissues, it was imperative to resolve the spatial organization of the anchoring protein in ventricular myocytes (Fig 2). As a prelude to these studies control experiments determined the specificity of our AKAP150 antibody in mouse ventricular myocyte lysates. Reassuringly, the AKAP150 antibody detected a single protein band at the expected molecular weight in wildtype (WT) tissue samples, but not from AKAP150−/− hearts (Fig 2A, top panel). Monitoring Glyceraldehyde 3-phosphate dehydrogenase levels served as a loading control (Fig 2A, bottom panel). Confocal imaging of ventricular myocytes detected a periodic, Zlike staining pattern for AKAP150 in wildtype cells (Fig 2B, top right panel). This staining pattern resembled the subcellular distribution of the microfilament protein α-actinin, a recognized marker protein for the Z-lines of ventricular myocytes (Fig 2B, left panels). As expected the anchoring protein was not detected in ventricular myocytes isolated from AKAP150−/− mice (Fig 2B). Confocal images of α-actinin and AKAP150, suggest that these proteins are targeted to the same compartment. The Pearson co-localization of AKAP150 and α-actinin-associated fluorescence was 0.90 ± 5. Accordingly, we can conclude that AKAP150 is concentrated near the T-tubules of ventricular myocytes. Antibody compatibility issues precluded the double labeling of AKAP150 and calcineurin these cells.
Figure 2. Blunted phenylephrine (PE)-induced NFATc3 translocation in AKAP150−/− cardiomyocytes.
A, Western blot and B, immunofluorescence evaluation of AKAP150 expression and distribution, respectively, in adult cardiomyocytes from Wildtype (WT) and AKAP150−/− mice. C, Representative images of neonatal cardiomyocytes from WT and AKAP150−/− mice expressing an NFATc3-tagged with GFP that were cultured under control conditions and in the presence of 100 µM PE. D, Amalgamated data of the percentage of neonatal cardiomyocytes showing NFATc3-GFP nuclear accumulation relative to total number of cells from WT and AKAP150−/− mice that were treated under control conditions (WT: n = 100; AKAP150−/−: n = 95) and in the presence of 100 µM PE (WT: n = 226; AKAP150−/−: n = 160). E, Inhibition of PP2B activity decreases PE-induced NFATc3-GFP nuclear accumulation in WT cardiomyocytes. Bar plot shows percent of WT neonatal cardiomyocytes with NFATc3-GFP nuclear accumulation relative to total number of cells in control (n = 20), PE (n = 35) and PE + CsA (n = 71). *P < 0.05.
3.3. AKAP150 associated calcineurin is required for nuclear translocation of NFATc3
Next, we investigated the functional significance of AKAP150-targeted calcineurin in ventricular myocytes. One recognized role for this phosphatase is to catalyze the dephosphorylation of NFAT family of transcription factors. This facilitates the nuclear translocation of certain NFAT isoforms (50). Therefore, we monitored NFATc3-GFP translocation in cultured ventricular myocytes isolated from wildtype and AKAP150−/− mice. Nuclear translocation of NFATc3-GFP was scored by fluorescent detection of the transcription factor in the nuclei of these cells (Fig 2C).
In wildtype myocytes application of the α-adrenergic receptor agonist phenylephrine (PE, 100 µM) promoted a significant increase in the detection of nuclear NFATc3-GFP (Fig 2C, top panels). The amalgamated data from 4 independent experiments are shown in figure 2D. In contrast application of phenylephrine had no qualitative effect on the nuclear translocation of NFATc3-GFP in AKAP150−/− myocytes (Fig 2C, bottom panels and Fig 2D). These results infer that AKAP150 participates in this process, but do not directly implicate calcineurin.
The immunosuppressive drug cyclosporin (CsA) binds to the cytosolic protein cyclophilin to form a potent inhibitor complex that selectively blocks calcineurin activity (4; 50). Thus, administering CsA in vivo is frequently used as a diagnostic tool to identify cellular events regulated by this phosphatase (51). In keeping with this notion, application of CsA prevented phenylephrineresponsive translocation of NFATc3-GFP in wildtype ventricular myocytes (Fig 2E). These data implicate AKAP150 and calcineurin in the nuclear translocation of NFATc3 in cardiac myocytes.
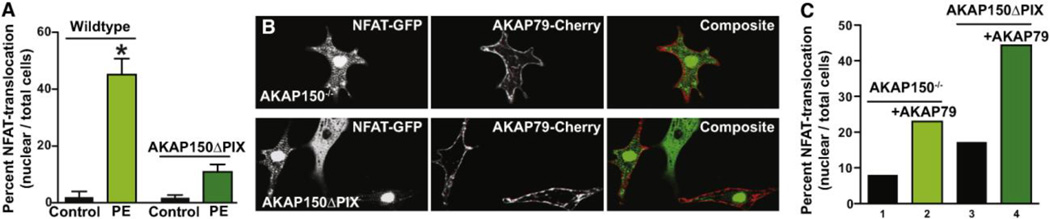
Our cumulative data infer that AKAP150 and calcineurin are mediators of NFATc3 translocation to the nucleus. The next step in this process was to establish if AKAP150 anchored calcineurin was a key effector. In order to test this hypothesis we isolated ventricular myocytes from a knockin mouse strain genetically engineered to express an AKAP150 form that lacks a binding site for calcineurin (AKAP150-ΔPIX) (32; 52; 53). Phenylephrine (100 µM) responsive translocation of NFATc3-GFP to the nucleus was dramatically reduced in ventricular myocytes isolated from AKAP150-ΔPIX mice when compared to wildtype controls (Fig 3A). In addition we performed rescue experiments to rule out the possibility that ablation of the AKAP150 gene or expression of AKAP150-ΔPIX protein evoked compensatory cellular changes that rendered myocytes incapable of sustaining NFATc3-GFP translocation. Rescue upon transfection of plasmids encoding AKAP79-Cherry restored nuclear translocation of NFATc3-GFP in myocytes from AKAP150−/− mice (Fig 3B). Similar results were obtained when rescue experiments were conducted in myocytes isolated from AKAP150-ΔPIX mice (Fig 3B). Amalgamated data from three independent experiments is presented in figure 3C. Thus, we can conclude that binding of calcineurin to AKAP150 is required for the nuclear translocation of NFATc3 in cardiac myocytes.
Figure 3. Expression of AKAP79 in AKAP150−/− and AKAP150ΔPIX neonatal cardiomyocytes rescues NFATc3 activation.
A, Bar plot of NFAT-GFP nuclear accumulation in response to PE stimulation after over-expression of AKAP79-mCherry in AKAP150ΔPIX neonatal cardiomyocytes (n = 190). B, Representative images showing AKAP150−/− (top) and AKAP150ΔPIX (bottom) cardiomyocytes co-expressing NFATc3-GFP and AKAP79-mCherry. C, Amalgamated data of NFATc3-GFP nuclear accumulation in response to PE stimulation in AKAP150−/− and AKAP150ΔPIX neonatal cardiomyocytes over-expressing AKAP79-mCherry (n = 140 cells per condition). *P < 0.05.
3.4. An AKAP150-calcineurin-NFAT pathway influences transcriptional reprogramming of voltage-gated K+ (Kv) channel genes during myocardial infarction
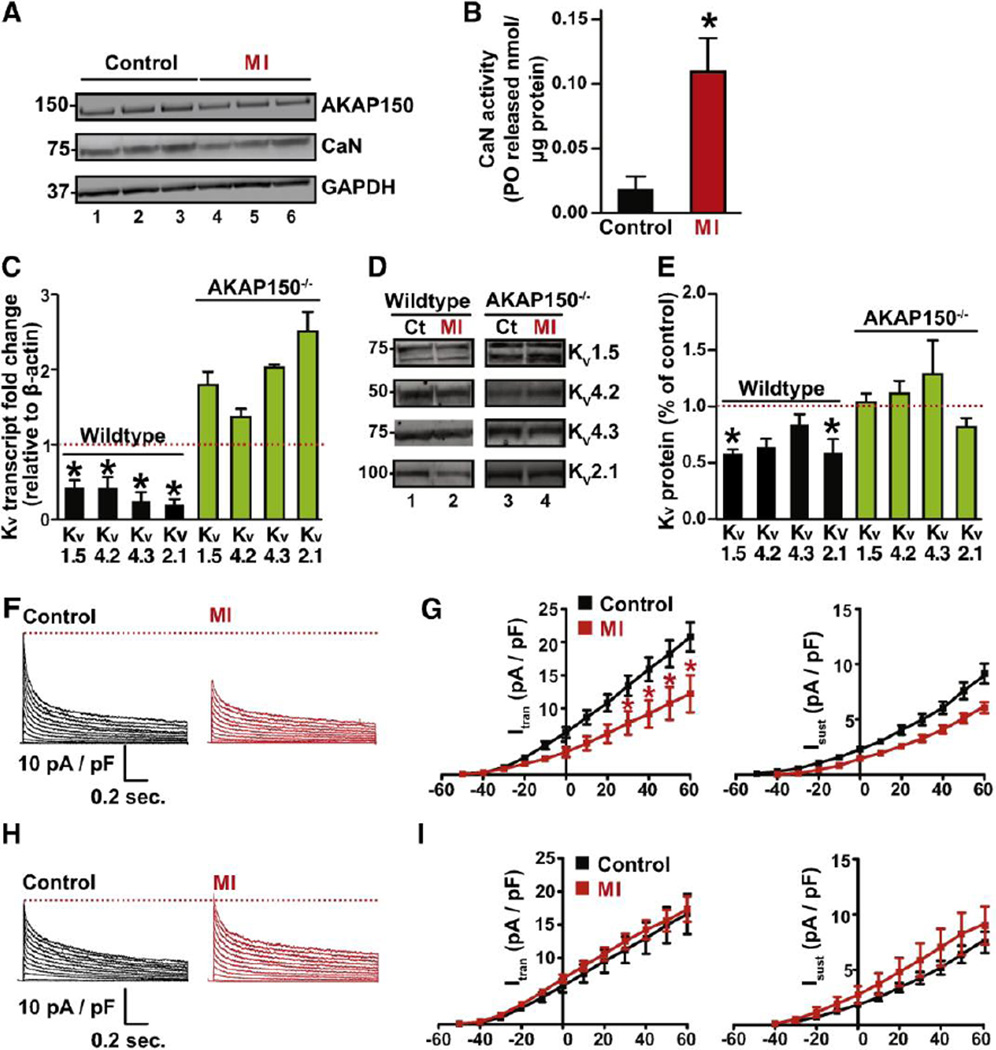
Acute myocardial infarction (MI), commonly referred to as a heart attack, occurs when blood flow stops causing damage to ventricular myocytes. Myocardial infarction can be simulated by ligation of the left descending coronary artery in mice (27). The subsequent isolation of cardiomyocytes provides experimental samples of diseased cells. Immunoblot analyses revealed minimal reductions in the levels of AKAP150 and calcineurin in myocytes isolated from MI simulated animals when compared to controls (Fig 4A, top and middle lanes). Immunoblot detection of GAPDH served as a loading control (Fig 4A, bottom panel). These results imply that the acute cardiac stress associated with myocardial infarction may adversely affect the protein levels of AKAP150 and calcineurin. Yet paradoxically, phosphatase activity measurements detected an increase in calcineurin activity upon induction of myocardial infarction when compared to untreated controls (Fig 4B). One speculative explanation for this result is that under conditions of extreme cardiac stress such as myocardial infarction the composition of AKAP150 complexes change to favor the release of the active calcineurin into the cytoplasm.
Figure 4. Ablation of AKAP150 prevents KV remodeling in adult cardiomyocytes after MI.
A, Western Blot analysis of AKAP150 and PP2B protein expression in WT (n = 3) control and MI (n = 3) hearts. B, Bar plot of cellular PP2B activity (PO released / µg protein) in WT control (n = 3) and MI hearts (n = 3). C, Summary data for transcript levels of KV1.5, KV4.2, KV4.3 and KV2.1 subunits in WT and AKAP150−/− control (n = 3) and MI (n =3) hearts. D, Representative blots of immunoreactive bands for KV1.5, KV4.2, KV4.3 and KV2.1 subunits in control (n = 3) and MI (n =3) hearts from WT and AKAP150−/− mice and (E) corresponding densitometry data. Representative whole-cell KV currents and current-voltage relationship of Itrans and Isust in WT (F and G) and AKAP150−/− (H and I) control and MI cells (WT: n = 10 and 8 cells for control and MI respectively) from 3 hearts; AKAP150−/−: n = 8 cells for both control and MI group, from 3 hearts). *P < 0.05.
One pathophysiological consequence of NFATc3 transcriptional remodeling during myocardial infarction is the silencing of voltage-gated K+ (Kv) channels (5). Accordingly, mRNA transcript levels and protein expression profiles of the Kv1.5, Kv4.2, Kv4.3, and Kv2.1 subunits of the Kv channels were reduced in wildtype myocytes subjected to the MI protocol (Fig 4C–E, black columns). In contrast, parallel studies conducted in AKAP150−/− myocytes detected normal levels of each transcript and protein product (Figs 4C–E, green columns). Collectively, these data argue that AKAP150 participates in the down-regulation of Kv channel subunits following myocardial infarction.
Finally, we tested the notion that AKAP150-calcineurin-NFATc3 signaling attenuates Kv currents as a consequence of myocardial infarction. To this end, we tested the hypothesis that AKAP150 is required for a diminution of these currents following myocardial infarction. Accordingly, Kv currents were recorded from wildtype (Fig 4F) and AKAP150−/− (Fig 4H) myocytes isolated from control and infarcted hearts 48 hours after coronary artery ligation (Fig 4F–I). Kv currents were evoked by 1.5 seconds depolarizations from the holding potential of −80 mV to test potentials ranging from −50 to +60 mV (Fig 4F–I). During analysis, we measured the amplitude of the transient (Ito) and sustained (Isust) components of these currents. Ito was defined as the difference between the peak current, immediately after the depolarizing step, and the sustain component measured at the end of the 1.5-s pulse. In control myocytes the Ito and Isust were similar in cells from both genotypes (at +60 mV, Ito = 23 ± 3 pA/pF, Isust = 8 ± 1 pA/pF; AKAP150−/− at +60 mV, Ito = 20.3 ± 3 pA/pF, Isust = 9 ± 1 pA/pF). However, wildtype cells subjected to the MI protocol exhibited a significantly decreased Ito (+60 mV, Ito = 9 ± 2) and Isust (+60 mV = 5 ± 1) amplitude (Fig 4F and G). In contrast, the amplitude of Ito and Isust was similar in myocytes from control and infarcted (at +60 mV, Ito = 16.5 ± 3 pA/pF, Isust = 8.3 ± 1 pA/pF) AKAP150−/− hearts (Fig 4H and I). Collectively these data are consistent with the view that AKAP150 anchored calcineurin participates in NFAT3c mediated down-regulation of Kv channels currents in response to acute cardiac stress.
4. Discussion
Myocardial infarction occurs when ischemia, a diminished blood supply to the heart, overwhelms cellular repair mechanisms that are designed to preserve the normal cardiac function and homeostasis. Our combined, biochemical, imaging and electrophysiological approaches have uncovered calcineurin micro-domains in ventricular myocytes that are implicated in pathophysiological changes that occur post myocardial infarction (54). We have discovered that the multivalent anchoring protein AKAP150 targets calcineurin to the sarcolemma of these cells. Although the data in figure 1 infer that only a small fraction (≈0.2%) of the total calcineurin activity is associated with the anchoring protein in the heart, formation of a AKAP150-calcineurin signaling dyad contributes to NFAT mediated transcriptional reprogramming events to signal the down-regulation of Kv channel currents following acute cardiac stress such as myocardial infarction. These findings offer additional mechanistic insight into how an anchored pool of this protein phosphatase is altered during acute myocardial infarction (MI) a leading cause of morbidity and mortality worldwide (55).
A-kinase anchoring proteins are local effectors of the cardiovascular system by virtue of their ability to cluster different classes of second messenger-regulated enzymes. For example mAKAP confers bi-directional control of ryanodine receptor phosphorylation during excitation-contraction (EC) coupling; AKAP18γmanages calcium reuptake through the SERCA2 pump and AKAP-Lbc directs the nuclear export of class 2 histone deacetylases in response to hypertrophic signals (16; 20; 56–64). A common feature of these anchored signaling complexes is the ability to synchronize calcium and cAMP signaling pathways (15). The AKAP79/150 family of anchoring proteins embodies this property through their capacity to direct protein kinase A (PKA), protein kinase C (PKC) and calcineurin toward selected substrates (11; 31). In mouse ventricular myocytes it has been shown that AKAP150 targets its cohort of calcium and cAMP responsive enzymes to CaV1.2 channels (44). Calcium influx through this ion channel not only permits local activation of PKC and calcineurin, but also ensures that bi-directional control of the phosphorylation status and activity of the ion channel (27; 53). The efficacy and speed of this vital process is enhanced because signaling can occur within the confines of the AKAP150-CaV1.2 channel macromolecular assembly (15). Moreover, since AKAP150 can form dimers while still attached to the carboxyl tail of the ion channel we propose that this anchoring protein not only clusters effector enzymes with the channel, but also operates as an allosteric modulator that stabilizes the open conformation of CaV1.2 (65; 66).
Although the AKAP150-CaV1.2 channel macromolecular assembly is not the sole source of the [Ca2+]i signal responsible for the activation of the calcineurin/NFAT pathway, it is likely that rapid calcium influx through CaV1.2 channels known as Ca2+ sparklets contribute to some of the gene reprogramming events that we have observed. Experimental support for this model is provided by the data in figure 2 showing that NFAT translocation is impaired in ventricular myocytes from AKAP150−/− mice. More sophisticated analyses presented in figure 3 consolidate this notion by showing that removal of a seven amino acid calcineurin anchoring sequence abolishes nuclear translocation of NFATc3 in AKAP150-ΔPIX knockin mice. Thus, we contend that anchored pools of calcineurin that are sequestered close to the plasma membrane somehow influence the dephosphorylation of cytoplasmic NFATc3. Consequently dephosphorylated NFATc3 translocates into the nucleus of ventricular myocytes where it modulates gene expression.
In healthy ventricular muscle, calcineurin and NFATc3 activities are low. This is in part because myocytes display low persistent Ca2+ sparklet activity and sustain high basal levels of phospho-NFATc3 in the cytoplasm (8). However, these parameters are reversed after myocardial infarction when the onset of ischemia for an extended period causes irreversible myocardial cell damage or death. Molecular responses include heightened β adrenergic receptor (βAR) signaling and enhanced persistent Ca2+ sparklet activity within the vicinity of diseased or dead myocytes. This in turn stimulates calcineurin to dephosphorylate NFATc3. The subsequent nuclear accumulation of this transcription factor drives pathological gene remodeling events that include down-regulation of voltage-gated K+ (KV) channel transcripts. Thus an adverse consequence of NFAT responsive gene silencing is a net decrease in KV currents. The ensuing changes in myocyte membrane depolarization contribute to the prolongation of ventricular action potentials and increase the probability of post myocardial infarction arrhythmia (55).
On the basis of data presented in figure 4, we have reason to believe that changes in the activity and localization of calcineurin underlie the downregulation of myocyte KV currents following myocardial infarction. Three observations support this hypothesis. First, western blot analyses reveal slight reductions in the total AKAP150 and calcineurin levels in myocardium extracts from MI simulated mice, yet enzymological measurements record an increase in soluble calcineurin activity in the diseased tissue. This apparent paradox can be explained if one considers that AKAP150 constrains calcineurin in an inactive conformation. Hence, loss of the anchoring protein in MI tissues could de-repress phosphatase activity to augment cytoplasmic dephosphorylation events.
Second, indirect evidence for the latter postulate is provided by the profiling data in figures 4 D–E showing that KV channel subunit transcripts and protein levels are reduced in MI tissue. Yet, normal transcript levels and expression patterns of KV channel subunits are detected in samples isolated from AKAP150−/− mice subjected to the MI protocol. One intriguing outcome of these latter results is that the AKAP150-assocated pool of calcineurin appears to have a preferred role in NFAT responsive gene silencing to promote a net decrease in KV currents. This notion is further supported by evidence in figure 3 showing that NFAT3c translocation to the nucleus is impaired in the presence of the calcineurin-binding-defective mutant AKAP150-ΔPIX. Thus the synchronization of second messenger signals that proceed through the AKAP79/150 signaling complex has a unique influence on NFAT responsive gene silencing.
Third, we found that NFATc3 is required for Ito downregulation during sustained activation of βAR signaling as evidenced by changes in the whole cell recording of KV currents in isolated myocytes from normal and disease simulated mice (Fig 4F–I). Indeed, a gradient of [Ca2+]i and calcineurin/NFATc3 signaling has been suggested to underlie differential KV4 expression across the mouse left ventricular free wall (6; 67). A further extrapolation of our data is the postulate that chronic isoproternol infusion which is known to exacerbate coronary heart diseases trough chronic stimulation of the β adrenergic pathway may act to dissipate an KV4.2 to Ito gradient by increasing [Ca2+]i. Thus protracted β adrenergic stimulation at plasma membrane fosters changes in the composition of AKAP150 signaling complexes that drive NFATc3 toward the interior of the cell. Ironically, nuclear accumulation of this transcription factor ultimately feeds back on potassium current density at the plasma membrane. Thus AKAP150 and calcineurin act synergistically to shuttle cellular signals to and from the nucleus in a manner that is pertinent to the damaged heart.
Finally, all of the experiments in this study were performed in cells from genetically engineered mice. However, a recent study reports that NFATc3 modulates KV4.3 expression and currents in canine ventricular myocytes (68). Thus the NFAT-signaling axis could decrease KV current density and expression in larger mammals. That being said, experiments need to be performed to establish the relationship between β1AR, calcineurin/NFAT, and KV4.3 expression in human and canine hearts. Moreover it has been reported that related KV currents are regulated by another transcription factor IRX5 (69). Therefore, future studies should investigate the relationship between AKAP150, calcineurin, IRX5, NFATc3, and KV4 expression in ventricular myocytes. In conclusion, our genetic profiling and electrophysiological studies point toward a specific role for the anchoring protein in the modulation of NFAT activity under conditions of acute myocardial stress. Hence the targeting the molecular interface between calcineurin and AKAP150 may have therapeutic potential in the prevention of arrhythmogenesis as a consequence of heart attack.
Highlights.
AKAP150 sequesters calcineurin at the T-tubules of ventricular cardiomyocytes.
Dephosphorylation of NFAT promotes nuclear translocation
An AKAP-calcineurin-NFAT pathway attenuates Kv currents after myocardial infarction
Acknowledgments
Funding sources
This work was supported in part by the following grants from the National Institutes of Health DK054441 (JDS), DK105542 (JDS), HL085686 (LFS), MH102338 (MLD) and HL098200 (MN), and the American Heart Association -14GRNT18730054 (MN). Partial support was also provided by the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80) FEBS Lett. 1982;137:80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- 2.Klee CB, Draetta GF, Hubbard MJ. Calcineurin. Adv. Enzym. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- 3.McCaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Farmer JDJ, Lane WL, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 5.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 6.Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circ Res. 2004;94:1340–1350. doi: 10.1161/01.RES.0000128406.08418.34. [DOI] [PubMed] [Google Scholar]
- 7.Qin D, Zhang ZH, Caref EB, Boutjdir M, Jain P, el-Sherif N. Cellular and ionic basis of arrhythmias in postinfarction remodeled ventricular myocardium. Circ Res. 1996;79:461–473. doi: 10.1161/01.res.79.3.461. [DOI] [PubMed] [Google Scholar]
- 8.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 10.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, et al. A caveolae-targeted L-type Ca(2)+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 12.Coghlan V, Lester L, Scott JD. A targeting model for reversible phosphorylation. Advances in Protein Phosphatases. 1995;6:51–61. [Google Scholar]
- 13.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley CJ, Nichols L, Jeong K, Moore CG, Ragni MV. Coronary atherosclerosis and cardiovascular mortality in hemophilia. J Thromb Haemost. 2010;8:208–211. doi: 10.1111/j.1538-7836.2009.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol. 2015;16:232–244. doi: 10.1038/nrm3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61:394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 18.Zoccarato A, Surdo NC, Aronsen JM, Fields LA, Mancuso L, et al. Cardiac Hypertrophy Is Inhibited by a Local Pool of cAMP Regulated by Phosphodiesterase 2. Circ Res. 2015;117:707–719. doi: 10.1161/CIRCRESAHA.114.305892. [DOI] [PubMed] [Google Scholar]
- 19.Lee LC, Maurice DH, Baillie GS. Targeting protein-protein interactions within the cyclic AMP signaling system as a therapeutic strategy for cardiovascular disease. Future Med Chem. 2013;5:451–464. doi: 10.4155/fmc.12.216. [DOI] [PubMed] [Google Scholar]
- 20.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negro A, Dodge-Kafka K, Kapiloff MS. Signalosomes as Therapeutic Targets. Prog Pediatr Cardiol. 2008;25:51–56. doi: 10.1016/j.ppedcard.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Negro A, Lopez J, Bauman AL, Henson E, et al. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010;48:387–394. doi: 10.1016/j.yjmcc.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, et al. The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circulation. Heart failure. 2014;7:663–672. doi: 10.1161/CIRCHEARTFAILURE.114.001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baisamy L, Cavin S, Jurisch N, Diviani D. The ubiquitin-like protein LC3 regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem. 2009;284:28232–28242. doi: 10.1074/jbc.M109.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diviani D, Maric D, Perez Lopez I, Cavin S, Del Vescovo CD. A-kinase anchoring proteins: Molecular regulators of the cardiac stress response. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbamcr.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Mika D, Bobin P, Pomerance M, Lechene P, Westenbroek RE, et al. Differential regulation of cardiac excitation-contraction coupling by cAMP phosphodiesterase subtypes. Cardiovasc Res. 2013;100:336–346. doi: 10.1093/cvr/cvt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bristow MR, Ginsburg R, Strosberg A, Montgomery W, Minobe W. Pharmacology and inotropic potential of forskolin in the human heart. J Clin Invest. 1984;74:212–223. doi: 10.1172/JCI111404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 30.Bristow MR. Myocardial beta-adrenergic receptor downregulation in heart failure. Int J Cardiol. 1984;5:648–652. doi: 10.1016/0167-5273(84)90179-7. [DOI] [PubMed] [Google Scholar]
- 31.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, et al. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinke SA, Navedo MF, Ulman A, Whiting JL, Nygren PJ, et al. Anchored phosphatases modulate glucose homeostasis. EMBO J. 2012;31:3991–4004. doi: 10.1038/emboj.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtypespecific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 34.Shioya T. A simple technique for isolating healthy heart cells from mouse models. J Physiol Sci. 2007;57:327–335. doi: 10.2170/physiolsci.RP010107. [DOI] [PubMed] [Google Scholar]
- 35.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 36.Kolk MV, Meyberg D, Deuse T, Tang-Quan KR, Robbins RC, et al. LAD-ligation: a murine model of myocardial infarction. J Vis Exp. 2009 doi: 10.3791/1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol. Cell. 2010;37:541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Pink MD, Murphy JG, Stein A, Dell'acqua ML, Hogan PG. Balanced interactions of calcineurin with AKAP79 regulate Ca(2+)-calcineurin-NFAT signaling. Nature structural & molecular biology. 2012;19:337–345. doi: 10.1038/nsmb.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dell'Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315–360. J Biol Chem. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisenhaus M, Allen ML, Yang L, Lu Y, Nichols CB, et al. Mutations in AKAP5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS One. 2010;5:e10325. doi: 10.1371/journal.pone.0010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samelson BK, Gore BB, Whiting JL, Nygren PJ, Purkey AM, et al. A-Kinase Anchoring Protein 79/150 recruits Protein Kinase C to phosphorylate Roundabout receptors. J Biol Chem. 2015 doi: 10.1074/jbc.M115.637470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott JD, Santana LF. A-kinase anchoring proteins: getting to the heart of the matter. Circulation. 2010;121:1264–1271. doi: 10.1161/CIRCULATIONAHA.109.896357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 45.Nystoriak MA, Nieves-Cintron M, Nygren PJ, Hinke SA, Nichols CB, et al. AKAP150 Contributes to Enhanced Vascular Tone by Facilitating Large-Conductance Ca2+-Activated K+ Channel Remodeling in Hyperglycemia and Diabetes Mellitus. Circ Res. 2014;114:607–615. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lester LB, Faux MC, Nauert JB, Scott JD. Targeted protein kinase A and PP-2B regulate insulin secretion through reversible phosphorylation. Endocrinology. 2001;142:1218–1227. doi: 10.1210/endo.142.3.8023. [DOI] [PubMed] [Google Scholar]
- 47.Dell'Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membranetargeting sequences on AKAP79 bind phosphatidylinositol-4, 5- bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveria SF, Gomez LL, Dell'Acqua ML. Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. J. Cell Biol. 2003;160:101–112. doi: 10.1083/jcb.200209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, et al. A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABA(A) receptors by cAMP-dependent protein kinase via selective interaction with receptor beta subunits. Mol Cell Neurosci. 2003;22:87–97. doi: 10.1016/s1044-7431(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 50.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 51.Sim AT, Scott JD. Targeting of PKA, PKC and protein phosphatases to cellular microdomains. Cell Calcium. 1999;26:209–217. doi: 10.1054/ceca.1999.0072. [DOI] [PubMed] [Google Scholar]
- 52.Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, et al. AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci. 2012;32:15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveria SF, Dittmer PJ, Youn DH, Dell'Acqua ML, Sather WA. Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. J Neurosci. 2012;32:15328–15337. doi: 10.1523/JNEUROSCI.2302-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 56.Edwards HV, Scott JD, Baillie GS. The A-kinase-anchoring protein AKAP-Lbc facilitates cardioprotective PKA phosphorylation of Hsp20 on Ser(16) Biochem J. 2012;446:437–443. doi: 10.1042/BJ20120570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCartney S, Little BM, Langeberg LK, Scott JD. Cloning and characterization of A-kinase anchor protein 100 (AKAP100): a protein that targets A-kinase to the sarcoplasmic reticulum. J. Biol. Chem. 1995;270:9327–9333. doi: 10.1074/jbc.270.16.9327. [DOI] [PubMed] [Google Scholar]
- 58.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 59.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehnart SE, Marks AR. Phosphodiesterase 4D and heart failure: a cautionary tale. Expert Opin Ther Targets. 2006;10:677–688. doi: 10.1517/14728222.10.5.677. [DOI] [PubMed] [Google Scholar]
- 62.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 2007;104:10140–10145. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Del Vescovo CD, Cotecchia S, Diviani D. A-kinase-anchoring protein-Lbc anchors IκB kinase β to support interleukin-6-mediated cardiomyocyte hypertrophy. Mol Cell Biol. 2013;33:14–27. doi: 10.1128/MCB.00887-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, et al. Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc Natl Acad Sci U S A. 2011;108:6426–6431. doi: 10.1073/pnas.1014400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintron M, et al. Restoration of normal L-type Ca2+ channel function during Timothy syndrome by ablation of an anchoring protein. Circulation research. 2011;109:255–261. doi: 10.1161/CIRCRESAHA.111.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossow CF, Dilly KW, Santana LF. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ Res. 2006;98:1306–1313. doi: 10.1161/01.RES.0000222028.92993.10. [DOI] [PubMed] [Google Scholar]
- 68.Dixon JE, Shi W, Wang HS, McDonald C, Yu H, et al. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- 69.Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, et al. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell. 2005;123:347–358. doi: 10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]