Supplemental Digital Content is available in the text
Abstract
Recent studies have proposed that the ASAP1 gene participates in regulating the adaptive immune response to Mycobacterium tuberculosis infection. A GWAS study has reported that ASAP1 polymorphisms (rs4733781 and rs10956514) were associated with the risk of tuberculosis (TB) in Russians. But due to population heterogeneity, different races would have different causative polymorphisms, and the aim of this study was to investigate the association between single nucleotide polymorphisms (SNPs) of the ASAP1 gene and TB risk in Chinese population.
A total of 7 SNPs in the ASAP1 gene were genotyped in 1115 Western Chinese Han and 914 Tibetan population using an improved multiplex ligation detection reaction (iMLDR) method. The associations of SNPs with TB risk and clinical phenotypes were determined based on the distributions of allelic frequencies and different genetic models. A meta-analysis was carried out to further assess the relationship between ASAP1 polymorphism and TB risk.
Statistical comparisons of cases and controls after correction for multiple testing did not yield any significant associations with the risk of TB via analyses of a single locus, haplotype, and subgroup differences. Meta-analysis showed no evidence supporting association between rs10956514 and overall risk for TB. Subsequent analysis referring to the genotypes of SNPs in relationship to clinical phenotypes identified that rs4236749 was associated with different serum C-reactive protein levels, suggesting a role of this locus in influencing the inflammatory state of Western Chinese Han patients with TB.
Our present data revealed that ASAP1 polymorphisms are unlikely to confer susceptibility to TB in the Western Chinese Han and Tibetan populations, which challenges the promising roles of the ASAP1 gene in the development of TB and highlights the importance of validating the association findings across ethnicities.
INTRODUCTION
Tuberculosis (TB) remains a major cause of morbidity and mortality worldwide, with approximately 9.6 million new TB cases, 0.48 million multi-drug resistant tuberculosis (MDR-TB) and 1.5 million deaths occurring in 2014.1 Host genetic factors play a central role in determining resistance or susceptibility to TB disease.2–4 Understanding these genetic variants could lead to novel insights for diagnostic advances and even for vaccine development. For years, genetic epidemiological association studies have become a predominant orientation in TB research, with several susceptible loci being identified, such as genes of human leukocyte antigen,5 cytokines and receptors,6 vitamin D receptor,7 and others.8 However, the molecular basis of the genetic predisposition to TB remains largely unknown, and investigations into more powerful TB-related loci are still warranted to clarify the detailed pathogenesis underlying this disease.
ASAP1 (also known as AMAP1/DDEF1), located at 8q24.1–8q24.2, is a gene encoding the multifunctional scaffold protein “ADP-ribosylation factor (Arf) GTPase-activating protein (ASAP1),” whose major functions are to regulate membrane trafficking, cytoskeletal remodeling, and cellular motility.9 Abundant evidence has indicated that genetic alterations and abnormal expression of the ASAP1 gene are involved in the processes of invasion and metastasis of various cancers, including breast neoplasms,10 prostate cancer,11 melanomas,12 and colorectal carcinomas,13 providing diagnostic or therapeutic targets for malignancies. Recently, the role of the ASAP1 gene has been explored in other fields except oncology; for instance, in inflammation and infectious diseases. Haque et al reported that ASAP1 negatively mediated inflammatory responses by suppressing several inflammatory signal pathways, including lipopolysaccharide-related signaling,14 NF-κB and mitogen-activated protein kinase activities,15 illuminating its role as a new candidate target for anti-inflammatory treatments. Davidson et al16 revealed that Salmonella bacteria utilized the interplay between ASAP1 and Arf guanine nucleotide exchange factors to determine cycles of Arf GTPase activation and inactivation, which eventually enabled the pathogen to invade into the host cells and cause infection. A study by Curtis et al observed both in vivo and in vitro that ASAP1 gene expressions in dendritic cells (DCs) were dramatically reduced after exposure to Mycobacterium tuberculosis (MTB) infection. They further demonstrated that ASAP1 protein correlated with the function of podosomes in DCs, and ASAP1 depletion resulted in impaired matrix degradation and migration of DCs, which may induce a delayed adaptive immune response to MTB infection.17
Importantly, Curtis's study17 revealed that 2 single nucleotide polymorphisms (SNPs) in the ASAP1 gene, rs4733781 and rs10956514, were significantly associated with the susceptibility to TB in a Russian population: the A alleles of both rs4733781 and rs10956514 were found to increase the risk of developing TB. They further observed that AA homozygote of rs10956514 tended to have a more significant reduction of ASAP1 expression in MTB-infected DCs than GG homozygote, suggesting a potential etiological role of ASAP1 gene in the predisposition to TB. Disease etiology is common generally, but the risk variants are often population specific. The association between ASAP1 polymorphisms and TB susceptibility should been validated in other vulnerable populations, and the detail regulatory effect of the ASAP1 gene in the immunopathogenesis of TB disease warrants further elaboration.
China ranks the leading 2nd among the 22 highest-burdened countries and has the highest annual number of cases of MDR-TB worldwide,18 with the higher incidence of TB in Tibetans and Han population.19 To assess the association of ASAP1 genetic variants with TB susceptibility comprehensively, we selected a set of SNPs within the entire ASAP1 gene and investigated their clinical relevance in relationship to the development of TB in Western Chinese Han and Tibetan population.
MATERIALS AND METHODS
Subjects
In total, 2029 subjects, including 1115 Western Chinese Han (554 patients with TB and 561 healthy controls) and 914 Tibetans (477 Tibetan patients and 437 Tibetan controls), were enrolled. All the participants were consecutively recruited from West China Hospital of Sichuan University and People's Hospital of Tibet Autonomous region from November 2011 to September 2015. Eligible cases were newly diagnosed patients with TB who were eventually established by 2 independent experienced respiratory physicians according to clinical research and microbiological/pathological examinations of TB (positive culture/smear/TB-DNA results). Patients with HIV-infection, hepatitis virus infection, immunodeficiency disease, and other lung diseases were excluded from this cohort. Controls were enrolled from a vast pool of healthy blood donors free from any positive TB-related examinations and history of TB infection or symptoms of TB disease. Demographic data and clinical presentations of Western Chinese Han population were obtained from medical records or qualified interviews. A 2- to 3-mL EDTA-anticoagulated peripheral blood sample was collected from every participant. Ethical approval of this study was obtained from the Committee on Human Research, Publications and Ethics, West China Hospital, Sichuan University, and written informed consent was obtained from the participants or their next of kin.
SNP Selection and Genotyping
The full sequence of the studied gene in the present study included the full-length human ASAP1 gene (39.156 kb in total, Accession Number: NC_000008.11, Reference GRCh38.p2 Current). Genetic variation data for the entire ASAP1 gene were obtained from the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) and candidate SNPs for this study were obtained from a thorough search of this database. SNPs were selected if they were located in potentially functional regions (exon, promoter, and untranslated region) and represented with a minor allele frequency (MAF) ≥ 0.05 in Chinese Han Beijing population. In addition, rs4733781 and rs10956514 were enrolled in this study due to their promising roles in the predisposition to TB based on the GWAS study by Curtis et al.17 Finally, a total of 7 SNPs (rs4733781, rs6470796, rs4236749, rs966185, rs10956514, rs150691716, and rs76377766) remained for subsequent genotyping (details in Table S1).
Genomic DNA was isolated from whole blood samples using the QIAamp DNA blood Mini kit (Qiagen, Hilden, Germany). SNPs were genotyped using an improved multiplex ligation detection reaction method (Genesky Biotechnologies Inc., Shanghai, China). The multiplex polymerase chain reaction (PCR) reaction was designed to amplify fragments covering all 7 SNP loci. The primer information is presented in Tables S2. The 20-μL PCR reaction system included 1 U HotStart Taq DNA polymerase (Takara, Dalian, China), 1× GC I buffer, 3.0 mM Mg2+, 0.3 mM dNTP, 1 μL primer mixture, and 20 ng genomic DNA. The cycling program for PCR consisted of 95°C for 2 min, followed by 11 cycles of 94°C for 20 s, 65°C–0.5°C/cycle for 40 s, 72°C for 150 s, and then 24 cycles of 94°C for 20 s, 59°C for 30 s, 72°C for 150 s, with a final extension at 72°C for 2 min and a hold at 4°C. After amplification, the PCR products were purified by digestion of 1 U shrimp alkaline phosphatase and 2 U Exonuclease I enzyme at 37°C for 1 h and at 75°C for 15 min. The ligation reaction was performed in a 10-μL mixture, including 1 μL of 10× ligation buffer, 0.2 μL of 80 U/μL Taq DNA ligase (New England Biolabs), 0.4 μL of labeling oligo mixture, 0.4 μL of probe mixture, 2 μL purified PCR products, and 6 μL ddH2O. The oligos and probes are described in Table S3. The thermal conditions were 38 cycles × (94°C 1 min, 56°C 4 min) and a hold at 4°C. A 0.5-μL ligation product was fractionated and loaded in ABI 3730XL, and the raw data were analyzed using GeneMapper v4.1 software. The alleles of each SNP were differentiated by different fluorescent labels of allele-specific oligo probe pairs, and different SNPs were distinguished by different extended lengths at the 3′ end.
Genotyping was performed in a blinded fashion without knowledge of the case–control status. Furthermore, ddH2O was considered to be the negative control in each reaction to monitor the genotyping quality. Approximately 10% of random samples were genotyped in duplicate with a concordance rate of 100%, and for a more accurate validation, we further sequenced these 7 SNPs in several samples using the ABI 3500 sequencer, and the genotype results showed 100% reproducibility.
Laboratory Examination
Routine laboratory examinations were performed as follows: serum albumin was evaluated by using the automatic Biochemistry Analyzer Modular P800 (Roche Diagnostics, Germany); blood routine examination was conducted by using the fully automated hematology analyzer XE-5000TM (Sysmex, Japan); C-reactive protein (CRP) was measured with the fully automated analyzer IMMAGE 800 (Beckman Coulter); and erythrocyte sediment rate (ESR) was detected in automatic ESR analyzer test 1 (ALIFAX, Italy), according to the manufacturer's instructions.
MTB-related laboratory tests included MTB-DNA, smear microscopy, and MTB culture. MTB-DNA was determined by real-time PCR using “Care TB” real-time PCR kit (Qiagen China [Shenzhen] Co Ltd, Shenzhen, China). Smear microscopy was carried out with Ziehl–Neelsen acid–fast staining method, and MTB culture was conducted with the Bactec MGIT960 system (Becton Dickinson, MD).
Statistical Analysis
Categorical variables and continuous variables were analyzed using the Chi-squared test and the Mann–Whitney U test, respectively. Hardy–Weinberg equilibrium (HWE) among the controls was assessed using goodness-of-fit Chi-squared test. The differences in data for clinical characters among different genotypes were evaluated using the Kruskal–Wallis tests. Statistical tests above were calculated using SPSS v22.0, (IBM, Chicago, IL). Associations between SNPs and disease status were determined based on the distributions of allelic frequencies and genetic models (additive, dominant, and recessive model), and odds ratios (ORs) and 95% confidence intervals (CIs) were performed in unconditional logistic regression analysis using PLINK v1.07,20 adjusting for age and gender. Correction for multiple testing was further performed using the Bonferroni method. The linkage disequilibrium (LD) was estimated by calculating the pairwise r2 coefficient.21 Haplotype analysis was performed on the basis of the expectation–maximization clustering algorithm using Haploview v4.2.22 Power calculations were performed using PASS Statistical Software v11 prior to data collection.23 All tests were 2-sided, and nominal statistical significance was established at an alpha level of 0.05.
Meta-Analysis
Studies on association between rs10956514 and susceptibility of TB were identified by a systematic search (until April 1, 2016) using the terms “(rs10956514 or ASAP1) and tuberculosis.” Besides, cited references of eligible studies and relevant review articles were screened to find additional publications that were not initially identified. ORs and 95% CIs based on allelic distributions and initial data from studies were extracted to calculate the corresponding ORs and 95% CIs. Heterogeneity in eligible studies was tested by Chi-squared based Q-test and the I2 statistic. The heterogeneity was regarded statistically significant when P < 0.10 or I2 > 50%, and the pooled ORs were calculated by using random-effect model (the DerSimonian and Laird method). Meta-analysis was performed using Stata Softwar v11 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Western Chinese Han Population
Basic characteristics of the cases and controls in Western Chinese Han are summarized in Table 1. Generally, TB cases and control subjects were well-matched by age and gender, and both groups mainly consisted of middle-age males. There were significant differences in Bacillus Calmette–Guerin scar, body mass index, and smoking between 2 groups (P < 0.001 for all).
TABLE 1.
Demographic and Clinical Characteristics of Study Participants in Western Chinese Han Population
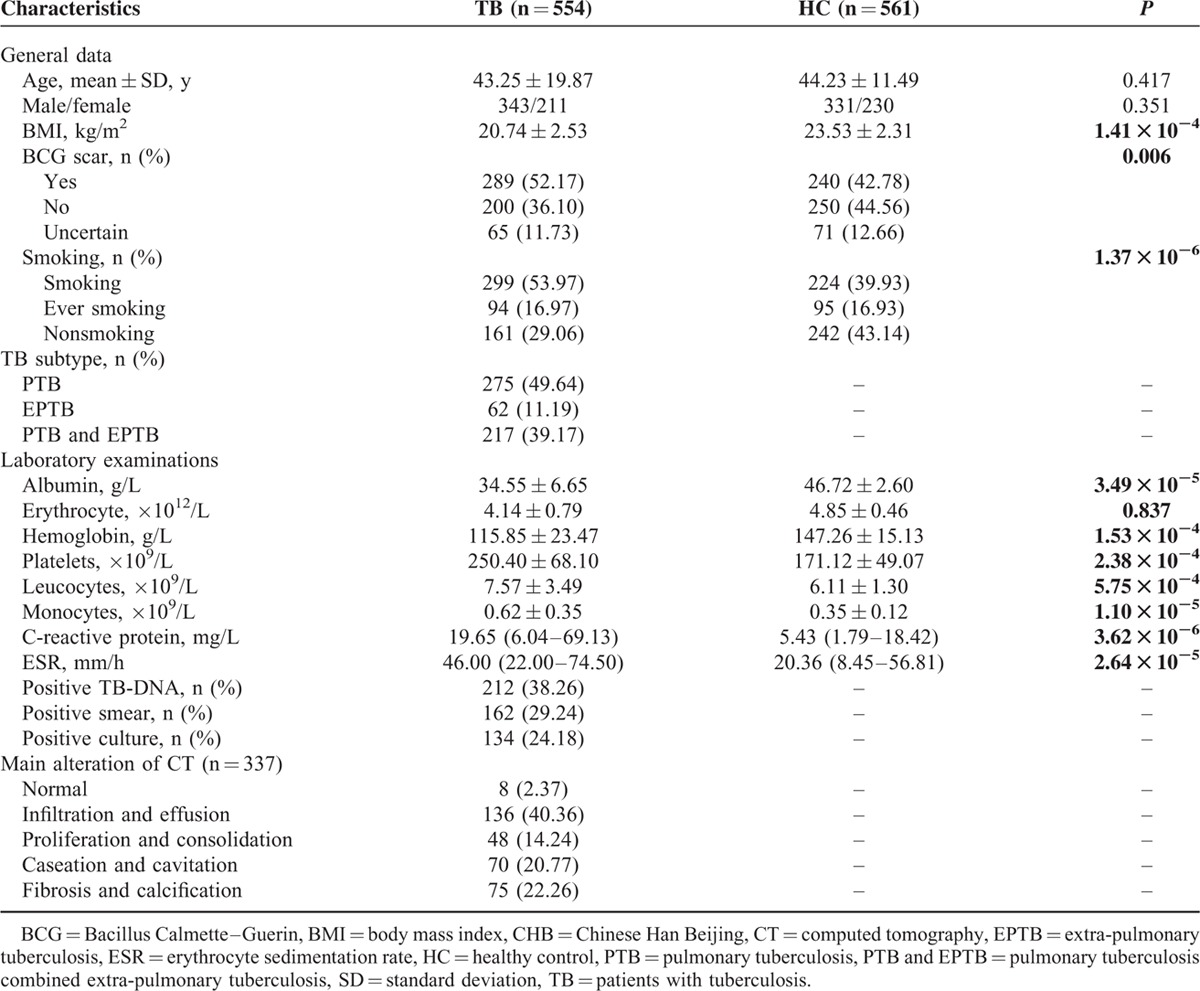
As for the quantitative traits of the participants shown in Table 1, when compared to healthy controls, patients with TB had significantly higher levels of CRP, erythrocyte sedimentation rate (ESR), leukocytes, platelet, and monocyte counts, and presented an obvious reduction in the indices of albumin and hemoglobin (P < 0.001 for all).
A series of TB-related characteristics were also calculated, including TB subtype, TB-related microbiological examinations and computed tomography (CT) scan. All 554 Han TB cases consisted of 275 pulmonary tuberculosis (PTB), 62 extra-pulmonary tuberculosis (EPTB) and 217 PTB with EPTB patients. For the 337 patients with TB who had undergone a CT scan, 40.36% of patients involved infiltration-based and effusion-based presentation, followed by 22.26% primarily for fibrosis and calcification, and 20.77% mainly for caseous and cavernous lesion. The positive rate for TB-DNA results among patients was a little higher than those of smear and culture identification (38.26% vs 29.24% and 24.18%, respectively).
Association of SNPs Genotypes and Alleles With Susceptibility to TB
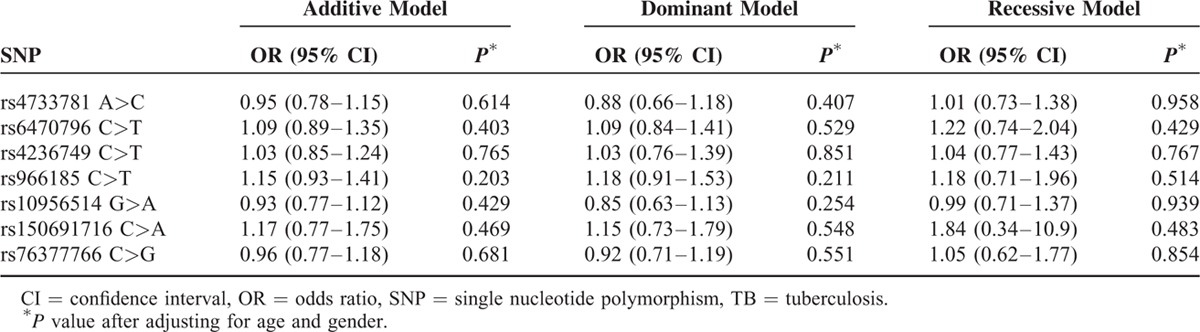
MAFs of the SNPs were similar to in Western Chinese Han and Tibetan population except for rs150691716, with a markedly lower MAF of 0.003 in Han people and relatively higher 0.05 in Tibetans. Genotype distributions of 7 SNPs in the ASAP1 gene among the controls were all consisted with HWE. Information of MAF and HWE P value for all SNPs can be observed in Table S1. Tables 2 and 3 show the genotype and allele frequencies of cases and controls after adjusting for age and gender. Frequencies of alleles and genotypes were similar between the patients and controls for all SNP loci in both populations. Genetic models for all patients with TB and healthy controls in 2 populations are summarized in Tables 4 and 5, respectively. Interestingly, the recessive model of rs4236749 (TT vs TC + CC) in Western Chinese Han tended to be related with a lower TB risk, although this trend was not significant (P = 0.052 after Bonferroni method). No other significant differences in the genetic model analysis were also obtained for these 2 populations.
TABLE 2.
Genotype Distributions of ASAP1 Polymorphisms of Patients With TB in Western Chinese Han Population

TABLE 3.
Genotype Distributions of ASAP1 Polymorphisms of Patients With TB in Tibetan Population

TABLE 4.
Comparison of ASAP1 Gene Polymorphisms in Relation to TB Risk in Western Chinese Han Population
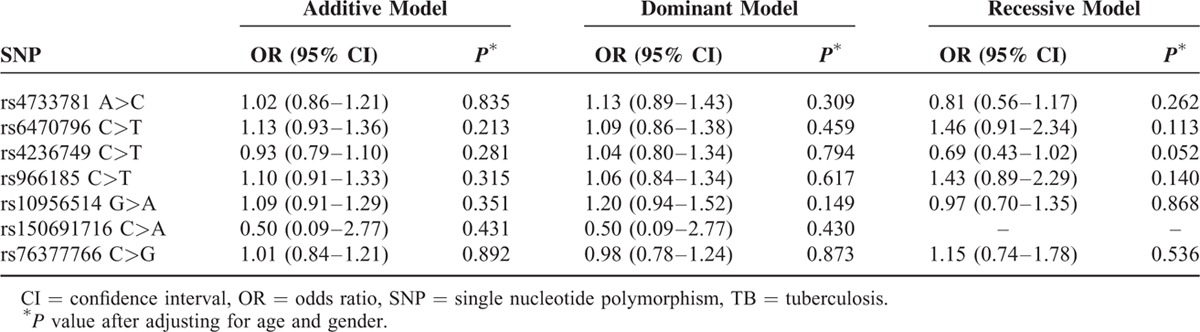
TABLE 5.
Comparison of ASAP1 Gene Polymorphisms in Relation to TB Risk in Tibetan Race
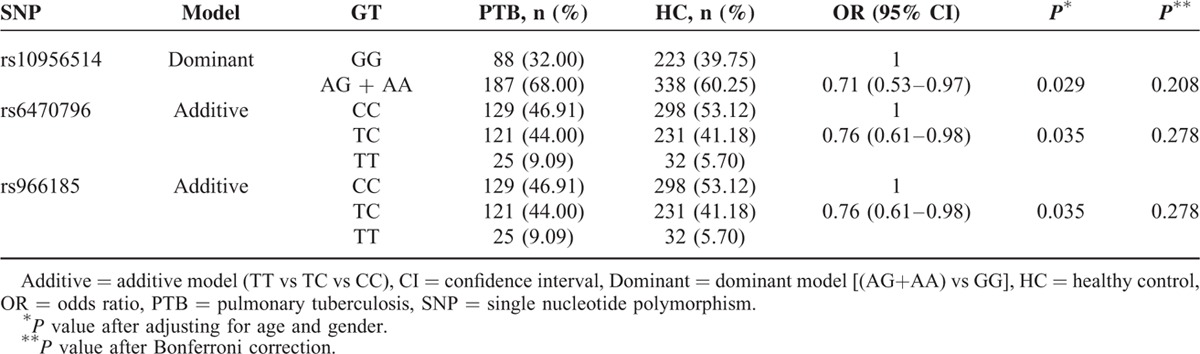
Next, subgroup analysis was stratified by gender and TB subtype in Western Chinese Han. TB subtype included PTB, EPTB, and PTB with EPTB. When patients with PTB and controls were compared (see Table 6), the protective roles of gene effects in PTB were potentially endowed to the T allele at both rs6470796 and rs966185 under the additive model, as well as the A allele-containing genotype at rs10956514 by applying the dominant model. However, none of the findings remained statistically significant after Bonferroni correction for multiple testing (P = 0.278, 0.278, and 0.208, respectively). No other subgroup analysis showed significant differences between the patients and controls.
TABLE 6.
Association of ASAP1 Polymorphisms With Pulmonary Tuberculosis in Western Chinese Han Population
We further performed the age-stratified analysis in Chinese Western Han TB population as described by Hijikata et al.24 When the patients are 10 years younger at the time of diagnosis, ORs for having a decreased risk of TB in each SNP were shown in Table S6, and all the trends were not statistically significant.
Haplotype Construction
Figure 1 shows the LD plot of SNPs in the ASAP1 gene. With a threshold of pairwise r2 > 0.8, in Western Chinese Han, 4 polymorphisms of the ASAP1 gene (rs6470796, rs4236749, rs966185, and rs10956514) were in strong LD with 1 another, suggesting a strong recombination block. Haplotype analysis identified 5 haplotypes in this block, that is, CTCG, TCTA, CCCG, CCCA, and CTCA (Table S4). The ASAP1 haplotype blocks in Tibetans were constructed between rs150691716 and rs76377766, identifying TC, TG, and AG haplotypes (Table S5). When comparing the frequencies between cases and controls, no significant association was observed for these haplotypes (Tables S4 and S5).
FIGURE 1.
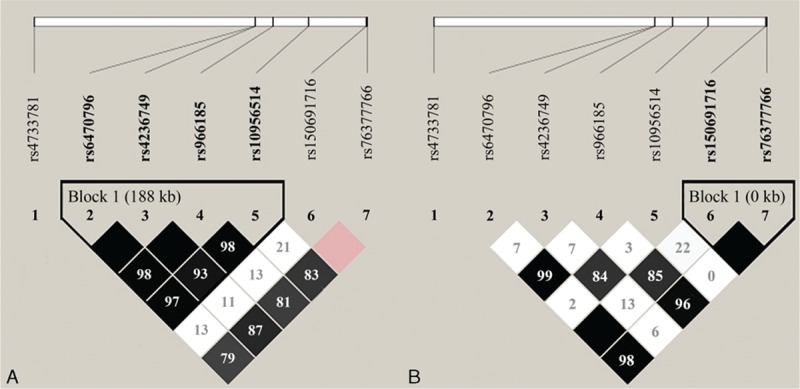
Linkage disequilibrium patterns for the cluster of 7 SNPs in the ASAP1 gene genotyped in Western Chinese Han (A) and Tibetan population (B). Pairwise r2 values for all pairs of SNPs are presented as percentages in diamonds, and shading from white to black indicates the intensity of r2 from 0 to 1. Strong linkage disequilibrium is represented by a high percentage (>80%) and darker square. The darkest squares without a number mean 100%. SNP = single nucleotide polymorphism.
Correlation Between Genotypes and Clinical Indices of Western Chinese Han Patients With TB
Subsequently, we investigated whether these SNPs were correlated to the Western Chinese Han patients with TB clinical manifestation and progression. Common indices of disease severity for TB consisted of symptom intensity (fever, night sweat, and hemoptysis), tubercular subtype, positive findings in CT imaging, and TB-related laboratory abnormality. These results only indicated that rs4236749 were significantly associated with serum CRP level (P = 0.002, Figure 2), and patients carrying the TC and CC genotypes were related to a higher level of CRP (Figure 2). None of the other SNP loci showed clinical relevance in relationship to the development of TB diseases (data are not shown).
FIGURE 2.
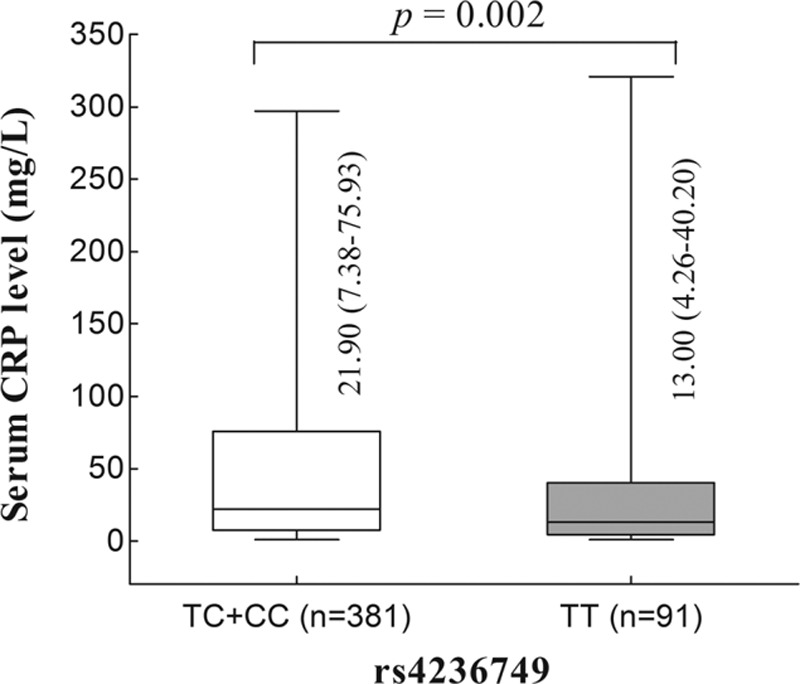
Serum concentration of C-reactive protein in relation to rs4236749 in Western Chinese Han patients with tuberculosis.
Meta-Analysis on the Association of rs10956514 With TB Risk
Following the case–control study, we conducted a meta-analysis on the association of rs10956514 with TB risk by pooling our results with reported results (Figure 3). Overall, 4 studies were identified,17,25–27 and a total of 6 separate populations were included in the meta-analysis together with our present study. Significant heterogeneity was observed among the 6 populations (I2 = 63.7%, P = 0.017). Subgroup analysis was based on ethnicity, and no significant heterogeneity was detected among 3 independent Chinese collections (I2 = 18.1%, P = 0.295). As the forest plot shown in Figure 3, the quantitative synthesis result showed no significant association of rs10956514 and overall risk for TB among overall populations or Chinese.
FIGURE 3.

Meta-analysis of the association between rs10956514 and TB risk.
DISCUSSION AND CONCLUSIONS
Our present study, initially inspired by the GWAS reported association of rs4733781 and rs10956514 in the ASAP1 gene and TB risk in Russians, failed to discover any associations between 7 polymorphisms of ASAP1 and susceptibility to TB from independent Western Chinese Han and Tibetan population. Subsequent analysis identified that rs4236749 was correlated to different serum CRP levels in Han, suggesting a role of this locus in influencing the inflammatory state of patients with TB.
The mononuclear phagocyte system is the first line of defense during the invasion of pathogenic microorganisms, such as MTB. MTB is known to be able to suppress migration and other functions of DCs, which delays the activation of innate immunity and cell-mediated immunity.28–31 Several studies have proven that ASAP1 is important for cell adhesion and migration. Murphy et al initially discovered in NIH 3T3 fibroblasts that decreased expression of ASAP1 inhibited the formation of podosomes and invadopodia, which suppressed the migration of specific cells, such as macrophages and DCs.32 Curtis et al17 also demonstrated that ASAP1 reduction resulted in the impaired migration of DCs upon MTB infection, which may mount a new mechanism of TB predisposition.
In Curtis's GWAS research,17 a strong association between TB and variants in the ASAP1 gene (rs10956514 and rs4733781) was identified in Russians. Although the ASAP1 SNPs were common, they showed no associations with TB in other GWAS data sets from European Icelanders33 and African Ghanaian and Gambian populations.25,26 Following case–control studies by Miao et al and our team also did not replicate significant ASAP1 polymorphisms in 3 dependent Chinese populations. The discrepancies among different studies may be ascribed to ethnic heterogeneity or even different environmental exposures. Subsequent meta-analysis showed no evidence supporting association between rs10956514 and overall risk for TB. Besides, the allele frequencies of SNPs (rs10956514 and rs4733781) were divergent among different populations, for example, the frequency of the C allele at rs4733781 was 27.8% to 31.4% in Russians, only 9.2% to 11.6% in Ghanaians and Gambians, but relative higher in Chinese (33.7% for Western Chinese Han and 46.9% for Chinese Tibetans). In general, the relative higher the frequencies could lead to the more power to detect potential association; however, we failed to find the association between these polymorphisms and TB risk, which might be limited by relative small sample size in the present study. Also, combined with the meta-analysis result, we could not totally exclude that the possible association of the ASAP1 polymorphisms described by Curtis et al as a possible false positive association.
Curtis et al17 further identified that the AA of rs10956514 was related to an excessive reduction in ASAP1 expression, consistent with its higher risk of TB. AliBaba 2.1 Software (http://www.gene-regulation.com/pub/programs/alibaba2/index.html) indicated that rs10956514 lies in a binding site for the activating transcription factor (ATF), a transcriptional regulator that is essential to modulate the secretion of some cytokines.34 The high-affinity ATF-binding motif would be abrogated by rs10956514 G-to-A transition. One might predict that rs10956514 would affect the transcriptional responses of ATF, influencing the expression of inflammatory cytokines and the development of TB, similar to the findings obtained by Barthel et al.35,36 Combined with Curtis's findings and prediction, further investigations of rs10956514 in a large-scale interethnic population and subsequent functional studies are still mandatory to confirm the effect of this polymorphism on TB.
For the remaining 5 SNPs located in the functional or regulatory region of ASAP1 gene, although statistical comparisons of a single locus and haplotype did not yield any significant results, the investigation of these polymorphisms complemented the known association of the ASAP1 gene with the risk of disease. The relatively low MAF of rs150691716 (compared with 0.036 in the dbSNP database) and correspondingly limited number of sample size in our cohort, made it difficult to approve the effect of rs150691716 in Chinese population. rs4236749 posed a potential increased TB risk under the recessive model, and SNPs rs6470796 and rs966185 were found to correlate with decreased susceptibility to PTB risk before multiple correction. Considering that the effects of common polymorphisms predisposing to TB infection are usually mild and the present study may be statistically underpowered (anticipated powers of 0.52–0.75) for the detection of slight significant effects, we could not completely exclude their potential role as putative TB-related targets.
Importantly, Han patients with TB carrying the TT/TC genotype at rs4236749 had a higher CRP level, which was consistent with its potential tendency for a higher TB risk, indicating a role of rs4236749 involved in the regulation of the inflammatory reaction in patients with TB. However, the pathophysiological mechanism underpinning this association remains unknown. rs4236749, in the 3′ untranslated region of the ASAP1 gene, lies in the seed-binding site of miR-138 according to the SNPinfo bioinformatics tool (http://snpinfo.niehs.nih.gov/), and the C-to-T mutation at rs4236749 may sharply diminish the binding capacity of miR-138. Recent data have implicated that miR-138 plays a vital role in the pathophysiological process, including cell migration, pathogenic invasion, and pro-inflammatory cytokine production.37–41 This finding led us to hypothesize that rs4236749 may interact with miR-138 and affect the function or regulation of miR-138, ultimately altering the inflammatory response in patients with TB. This assumption may provide a clue for the potential molecular target in the modulation of mycobacterial infection and requires further studies.
To the best of our knowledge, this study presents the first data to interrogate the association of ASAP1 polymorphisms with TB in Western Chinese Han population and Tibetan population. Our failure to assign significance to the 7 SNPs and haplotypes of ASAP1 gene should be interpreted with caution because of the following considerations: First, accounting for the uncorrelated TB risk among these loci, we did not further analyze the gene–environment or gene–gene interactions in our study. Second, we mainly focused on a finite number of SNPs in the ASAP1 functional region. Intronic SNPs and regulatory variants distant from the gene might be missed. The susceptibility to TB is the result of a combination of numerous genetic polymorphisms, which exert the need for more comprehensive explorations of the ASAP1 polymorphism. Third, we limited the patients enrolled into this study to a racially uniform Han Chinese, which reduced selection bias caused by population admixture, as well as minimizing the likelihood of discovering a meaningful genetic association. It is necessary to further expand the sample size to validate these findings. And finally, we lacked detailed clinical information of Tibetans, thus further analysis on the association with clinical characteristics was missing.
In conclusion, our results, specifically, the absence of associations of polymorphisms of ASAP1 with TB risk, suggest that ASAP1 may not be a susceptible gene for TB in Western Chinese Han and Tibetan population. The lack of significant results challenges the promising roles of the ASAP1 gene in the development of TB and highlights the importance of dissecting the association findings across ethnicities.
Supplementary Material
Acknowledgments
The participation of the patients and healthy controls is gratefully acknowledged, as well as the contributions of the field workers and physicians involved in the recruitment of subjects. The authors also thank Rui Chen for his guidance in the statistics analysis and are grateful to Shanghai Genesky Bio-Tech Genetic Core Lab for providing assistance in genotyping techniques.
Footnotes
Abbreviations: Arf = ADP-ribosylation factor, ATF = activating transcription factor, BCG = Bacillus Calmette–Guerin, BMI = body mass index, CHB = Western Chinese Han Beijing, CI = confidence interval, CRP = C-reactive protein, CT = computed tomography, DC = dendritic cell, EPTB = extra-pulmonary tuberculosis, ESR = erythrocyte sedimentation rate, GEF = guanine nucleotide exchange factor, HWE = Hardy–Weinberg equilibrium, iMLDR = improved multiplex ligation detection reaction, LD = linkage disequilibrium, LPS = lipopolysaccharide, MAPK = mitogen-activated protein kinase, MDR-TB = multidrug resistant tuberculosis, MTB = Mycobacterium tuberculosis, OR = odds ratio, PTB = pulmonary tuberculosis, SNP = single nucleotide polymorphism, TB = Tuberculosis, vs = versus.
XH, WP, and XC contributed equally to this article.
XH, WP, and XC wrote the manuscript and participated in all aspects of the study; ZZ, JZhang, JZhou, and YZ participated in modifying the manuscript and preparing the tables and figures; WP, BC, and JC participated in the data analysis; XL, BY, and XC engaged in the acquisition of data (laboratory or clinical); XL and BY designed the study. All authors have reviewed the manuscript and approve its submission.
This work was supported by grants obtained from the National Natural Science Foundation of China (81472026 and 81202375) and the Projects in the Science and Technology Department of Sichuan Province Pillar Program (2014SZ0208).
This article has not been published in part, or in full, in any form and is not under consideration for any publication.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.WHO. Global tuberculosis report. 2015. http://www.who.int/tb/publications/global_report/en/ (accessed May 13, 2016).
- 2.Rodríguez-Castillo JA, Arce-Mendoza AY, Quintanilla-Siller A, et al. Possible association of rare polymorphism in the ABCB1 gene with rifampin and ethambutol drug-resistant tuberculosis. Tuberculosis (Edinb) 2015; 95:532–537. [DOI] [PubMed] [Google Scholar]
- 3.Daya M, van der Merwe L, Gignoux CR, et al. Using multi-way admixture mapping to elucidate TB susceptibility in the South African coloured population. BMC Genomics 2014; 15:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Möller M, Flachsbart F, Till A, et al. A functional haplotype in the 3′ untranslated region of TNFRSF1B is associated with tuberculosis in two African populations. Am J Respir Crit Care Med 2010; 181:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombard Z, Dalton DL, Venter PA, et al. Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol 2006; 67:643–654. [DOI] [PubMed] [Google Scholar]
- 6.Larcombe LA, Orr PH, Lodge AM, et al. Functional gene polymorphisms in Canadian aboriginal populations with high rates of tuberculosis. J Infect Dis 2008; 198:1175–1179. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 2000; 355:618–621. [DOI] [PubMed] [Google Scholar]
- 8.Grant AV, El Baghdadi J, Sabri A, et al. Age-dependent association between pulmonary tuberculosis and common TOX variants in the 8q12-13 linkage region. Am J Hum Genet 2013; 92:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue H, Ha VL, Prekeris R, et al. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell 2008; 19:4224–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabe H, Hashimoto S, Morishige M, et al. The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis. Traffic 2009; 10:982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin D, Watahiki A, Bayani J, et al. ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res 2008; 68:4352–4359. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers JP, Worley L, Onken MD, et al. DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin Cancer Res 2005; 11:3609–3613. [DOI] [PubMed] [Google Scholar]
- 13.Muller T, Stein U, Poletti A, et al. ASAP1 promotes tumor cell motility and invasiveness, stimulates metastasis formation in vivo, and correlates with poor survival in colorectal cancer patients. Oncogene 2010; 29:2393–2403. [DOI] [PubMed] [Google Scholar]
- 14.Haque A, Noman AS, Koide N, et al. An ADP ribosylation factor-GTPase activating protein negatively regulates the production of proinflammatory mediators in response to lipopolysaccharide. Cancer Immunol Immunother 2011; 60:1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tien DN, Kishihata M, Yoshikawa A, et al. AMAP1 as a negative-feedback regulator of nuclear factor-(B under inflammatory conditions. Sci Rep 2014; 4:5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson AC, Humphreys D, Brooks AB, et al. The Arf GTPase-activating protein family is exploited by Salmonella enterica serovar Typhimurium to invade nonphagocytic host cells. MBio 2015; 6:e02253-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis J, Luo Y, Zenner HL, et al. Susceptibility to tuberculosis is associated with variants in the ASAP1 gene encoding a regulator of dendritic cell migration. Nat Genet 2015; 47:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med 2012; 366:2161–2170. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y. The Compilation of the Fifth National Tuberculosis Epidemiological Sampling Survey. Beijing: Military Medical Science Press; 2011. 23–25. [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardlie KG, Kruglyak L, Seielstad M. Patterns of linkage disequilibrium in the human genome. Nat Rev Genet 2002; 3:299–309. [DOI] [PubMed] [Google Scholar]
- 22.Liang B, Guo Y, Li Y, et al. Association between IL-10 gene polymorphisms and susceptibility of tuberculosis: evidence based on a meta-analysis. PLoS ONE 2014; 9:e88448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hintze J. PASS 11. NCSS, LLC. Kaysville. Utah, USA. 2011. http://www.ncss.com/ (accessed October 28, 2015).
- 24.Hijikata M, Shojima J, Matsushita I, et al. Association of IFNGR2 gene polymorphisms with pulmonary tuberculosis among the Vietnamese. Hum Genet 2012; 131:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thye T, Vannberg FO, Wong SH, et al. Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet 2010; 42:739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thye T, Owusu-Dabo E, Vannberg FO, et al. Common variants at 11p13 are associated with susceptibility to tuberculosis. Nat Genet 2012; 44:257–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao R, Ge H, Xu L, et al. Genetic variants at 18q11.2 and 8q24 identified by genome-wide association studies were not associated with pulmonary tuberculosis risk in Chinese population. Infect Genet Evol 2016; 40:214–218. [DOI] [PubMed] [Google Scholar]
- 28.Wolf AJ, Linas B, Trevejo-Nuñez GJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol 2007; 179:2509–2519. [DOI] [PubMed] [Google Scholar]
- 29.Wolf AJ, Desvignes L, Linas B, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med 2008; 205:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin PL, Ford CB, Coleman MT, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med 2014; 20:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts LL, Robinson CM. Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion and migration to chemokines. Immunology 2014; 141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy DA, Courtneidge SA. The “ins” and “outs” of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011; 12:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sveinbjornsson G, Albrechtsen A, Zink F, et al. Weighting sequence variants based on their annotation increases power of whole-genome association studies. Nat Genet 2016; 48:314–317. [DOI] [PubMed] [Google Scholar]
- 34.Samten B, Townsend JC, Weis SE, et al. CREB, ATF, and AP-1 transcription factors regulate IFN-gamma secretion by human T cells in response to mycobacterial antigen. J Immunol 2008; 181:2056–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthel R, Tsytsykova AV, Barczak AK, et al. Regulation of tumor necrosis factor alpha gene expression by mycobacteria involves the assembly of a unique enhanceosome dependent on the coactivator proteins CBP/p300. Mol Cell Biol 2003; 23:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samten B, Wang X, Barnes PF. Mycobacterium tuberculosis ESX-1 system-secreted protein ESAT-6 but not CFP10 inhibits human T-cell immune responses. Tuberculosis (Edinb) 2009; 89:S74–S76. [DOI] [PubMed] [Google Scholar]
- 37.Jiang L, Liu X, Kolokythas A, et al. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer 2010; 127:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B, Xie HF, Li WZ, et al. Asymmetrical dimethylarginine promotes the senescence of human skin fibroblasts via the activation of a reactive oxygen species-p38 MAPK-microRNA-138 pathway. J Dermatol Sci 2015; 78:161–164. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Shi W, Gao Y, et al. Analysis of microRNA expression profiles in human hepatitis B virus-related hepatocellular carcinoma. Clin Lab 2013; 59:1009–1015. [DOI] [PubMed] [Google Scholar]
- 40.Pan D, Flores O, Umbach JL, et al. A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency. Cell Host Microbe 2014; 15:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen A, Most P, Peppel K. Induction of microRNA-138 by pro-inflammatory cytokines causes endothelial cell dysfunction. FEBS Lett 2014; 588:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


