Supplemental Digital Content is available in the text
Abstract
Disease progression in HIV-1 infected children is faster than in adults. Less than 5% of the infected children maintain stable CD4 counts beyond 7 years of infection and are termed long-term nonprogressors (LTNPs). Delineating the host immune response in antiretroviral naïve (ART) and treated HIV-1 infected children at different disease stages will help in understanding the immunopathogenesis of the disease.
A total of 79 asymptomatic, perinatally HIV-1 infected children (50 ART naïve and 29 ART treated) and 8 seronegative donors were recruited in this study. T- and B-cell activation PCR arrays were performed from the cDNA, using total RNA extracted from the peripheral blood mononuclear cells (PBMCs) of 14 HIV-1 infected children at different stages of the disease. The differentially expressed genes were identified. Quantitative RT-PCR was performed for the (interleukin-8) IL-8 gene and its transcriptional mediators, that is, SHP2, GRB2, and IL-8R (IL-8 receptor/CXCR1). Plasma levels of IL-8 were measured by flow cytometry.
Gene array data revealed a higher expression of IL-8 in the ART naïve HIV-1 infected progressors and in ART nonresponders than LTNPs and ART responders, respectively. Quantitative RT-PCR analysis demonstrated a significant higher expression of IL-8 (P < 0.001), its receptor CXCR1 (P = 0.03) and the upstream signaling molecule SHP2 (P = 0.04) in the progressors versus LTNPs. Plasma levels of IL-8 were significantly higher in progressors versus LTNPs (P < 0.001), and ART nonresponders versus ART responders (P < 0.001). A significant negative correlation of plasma levels of IL-8 with CD4 counts (cells/μL) was observed in HIV-1 infected ART naïve subjects (r = −0.488; P < 0.001), while the IL-8 levels positively correlated with viral load in the ART treated children (r = 0.5494; P < 0.001). ART naïve progressors on follow up demonstrated a significant reduction in the mRNA expression (P = 0.05) and plasma levels of IL-8 (P = 0.05) post 6 months of ART initiation suggesting the beneficial role of ART therapy in reducing inflammation in infected children.
Our data suggest that IL-8 may serve as a potential prognostic marker in adjunct with CD4 counts to monitor disease progression in the HIV-1 infected children and the efficacy of ART.
INTRODUCTION
A number of host and viral factors contribute to the extensive variability in susceptibility to HIV-1 infection and disease progression.1–3 Among those infected with HIV-1, 5% to 15% adults,4–7 and <5% children,8,9 who are antiretroviral therapy (ART) naïve maintain a stable CD4 count (>500 cells/μL) for more than 7 years and are termed long-term nonprogressors (LTNPs). Slower disease progression in HIV-1 infection has been shown to be associated with some of the host factors like the 32 base pair deletion of the coreceptor CCR5,10,11 and the presence of specific HLA (human leukocyte antigen) class I alleles (HLA-B57 and HLA-B27).11,12 However, not all the LTNPs have protective alleles, thereby suggesting contribution of immune factors in the maintenance of T- and B-cell homeostasis and effective antiviral immunity. In perinatal HIV infection, the immune responses of the infected children may depend on a number of factors, one of them being the maternal viral load. Thus the host genetic and immune responses to HIV-1 in infants in early life are complex. Understanding the various immune parameters involved in the pathogenesis, such as chemokines and their receptors, will help us identify critical components of protective immunity and the factors involved in rapid disease progression in the infected children.13
During disease progression in HIV-1, a complex set of interactions between viral and host factors takes place leading to a chronic immune activation state.14,15 Recent observations suggest that inflammation is a crucial contributing factor, in addition to T cell activation, to predict disease progression and mortality.16–19 HIV-1 triggers several immunological changes like decreased CD4 T cells, polyclonal B cell activation and high levels of proinflammatory cytokines.20 A panel of plasma inflammatory biomarkers consisting of CXCL9, CXCL10, sIL-2R, and sCD14 has been suggested to serve as a surrogate prognostic marker to monitor immune activation in both viremic and aviremic HIV patients on ART.21 In a study conducted by Stacey et al22 the increase in plasma viremia in acute HIV-1 infection was found to be associated with elevations in plasma levels of multiple cytokines and chemokines such as IFNα, IL-15, TNFα, and MCP-1, more slowly initiated elevations in levels of IL-6, IL-8, IL-18; and a late-peaking increase in levels of the immunoregulatory cytokine IL-10.
The introduction of ART, by reducing viral load and increasing the CD4 count, has dramatically improved survival in HIV-1 infected individuals. However, despite ART, there is incomplete immune reconstitution due to the persistent immune activation, although at a lower level.23–28 Furthermore, some patients cannot tolerate therapy, due to cytotoxicity caused by the available ART drugs,29,30 and in others, nonadherence to the treatment regimen or development of resistance to the drugs constitutes a limiting factor in controlling the infection.31,32 In a recent study, we have reported mutations in the reverse transcriptase and protease genes of the virus from both antiretroviral naïve (ART) and treated HIV-1 infected children.33 The investigations for immunology-based therapies, new viral or cellular targets, that in combination with ART,32,34 would bring the infection under control are underway. Profiling the cytokines and their receptors will plausibly enable identification of factors that could complement the information from viral load and CD4 counts in the analysis of the disease status,35 thus aiding in better management of the disease and therapeutic interventions.36
Both, humoral and cell-mediated immune responses contribute to the protective immunity in LTNPs and cytokines are integral components of the immune response.37,38 It is important to evaluate the modulation of cytokine responses in HIV-1 infection as cytokines are not only crucial for cell-mediated immunity,38–40 but can also stimulate HIV-1 replication in latently infected cells.38,41 The role of immune activation at different stages of HIV-1 infection and disease progression in ART naïve and treated children has not been investigated so far. In HIV-1 infected children, persistent higher viremia and limited immunological memory contribute to faster disease progression than in adults,9,42 thereby warranting therapeutic interventions at an early age. This study is aimed to assess the expression of various immune response genes as a correlate of disease progression in HIV-1 infected children.
METHODS
Subjects
The study was approved by the Institute Ethics Committee (IEC/NP-269/2012 and RP-39/2012). A total of 79 HIV-1 infected children (50 ART naïve and 29 ART treated) and 8 seronegative donors were recruited in this study. After obtaining written informed consent from parents/guardians, blood samples (2–4 mL) of HIV-1 infected children and seronegative donors, scheduled for unrelated surgeries were collected in Ethylenediaminetetraacetic acid (EDTA) vacutainers from the Pediatrics Out Patient Department (OPD) and Pediatrics Surgery OPD, respectively, at the All India Institute of Medical Sciences (AIIMS), New Delhi. The HIV-1 infected children recruited in the study were documented to have acquired the infection through perinatal mode of transmission. The HIV-1 infected ART naïve children were grouped into 24 LTNPs and 26 progressors; the ART treated children were sub-grouped into 17 ART responders and 12 ART nonresponders. All the infected children were asymptomatic at the time of recruitment. Children with coinfections like respiratory, gut infections or tuberculosis were excluded from the study. HIV-1 infected asymptomatic ART naïve children with >7 years of infection, with CD4 counts ≥500 cells/μL at the time of recruitment, and stable for the last 18 months, were categorized as LTNPs. Progressors were defined as infected children (age <5 years) with CD4 count ≤25% and children (age >5 years) with CD4 counts ≤500 cells/μL or declining for 18 months before recruitment.43–45 Majority of the progressors in this study were below 5 years of age (rapid progressors), while in only 3 children, the onset of disease progression was at a later age (>5–15 years) (slow progressors). Due to limited number of slow progressors, we have clubbed both rapid and slow progressors under the category of progressors. ART responders were defined as infected children on ART for at least 6 months, with controlled viremia, that is, ≤2000 viral RNA copies/mL. ART nonresponders were defined as children on ART for at least 6 months, with uncontrolled viremia, that is, ≥2000 viral RNA copies/mL.7 Fifteen of the 26 ART progressors were followed up post 6 months initiation of ART during the span of this study, while a few of the infected children were lost to follow up.
The ART regimen for the infected children on treatment included a combination of 2 nucleoside reverse transcriptase inhibitors (NRTIs)—lamivudine with zidovudine or stavudine and 1 nonnucleoside reverse transcriptase inhibitor (NNRTI)—nevirapine or ffavirenz, as per the national guidelines.46
CD4 Count and Viral Load Measurement
The plasma viral load was determined by quantitative reverse transcriptase polymerase chain reaction (quantitative RT-PCR) (Roche COBAS TaqMan HIV-1 v2.0; Roche Diagnostics, Indianapolis, IN), according to the manufacturer's instructions. The lower detection limit of the assay was 47 HIV-1 copies/mL. The CD4+ T cell counts were assessed by flow cytometric analysis (BD Biosciences, Sparks, MD) at the Department of Microbiology, AIIMS.
Isolation of PBMCs, RNA, and cDNA Synthesis
Blood (2–4 mL) drawn from the HIV-1 infected children and seronegative donors was centrifuged within 30 minutes at 3000 rpm for 10 minutes. The separated plasma samples were stored frozen at −80°C for assessment of cytokine levels. PBMCs were isolated using the Ficoll (Histopaque) gradient centrifugation method. RNA was extracted using RNeasy kit (Qiagen Ltd, Crawley, UK), quantified by nanospectrophotometry NanoDrop, ND-1000, (Thermo Scientific, Wilmington, DE, USA). The cDNA was prepared using 500 ng RNA by Reverse Transcriptase (Genei, Bangalore, India) in the presence of RNAse inhibitor (Genei) and Random Hexamer Primers (Genei).
T- and B-Cell Activation PCR Array
Quantitative RT-PCR array was performed for T- and B-cell activation genes and for various cytokines using Qiagen T and B cell activation array kit (Qiagen Ltd, cat no.-PAHS-053Z). Reactions were set up using the cDNA from HIV-1 infected children, of the following categories; 5 LTNPs, 3 progressors, 3 ART responders, 3 ART nonresponders, and 3 HIV-1 seronegative donors. The array data were analyzed using the software (RT Profiler PCR Array Data analysis version 3.5).
Gene Expression Analysis by Real-Time PCR
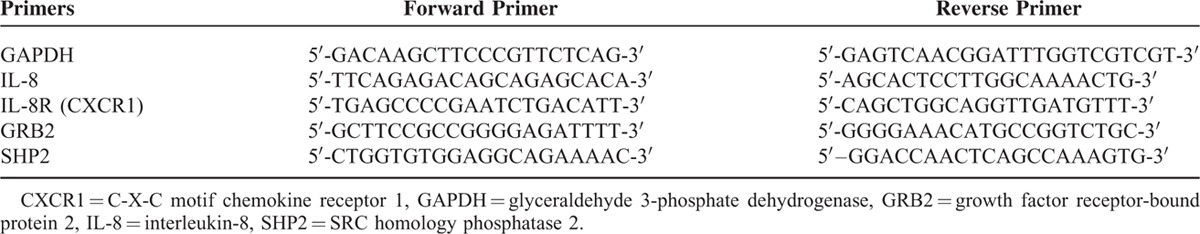
The array data were validated by performing quantitative RT-PCR analysis of the selected differentially expressed genes. Gene expression profiling of SHP2 and GRB2 involved in the induction of IL-8 and IL-8R (CXCR1) was done by qRT-PCR using specific primers (Table 1), Taq Polymerase (Invitrogen, Thermo Scientific, Wilmington, DE, USA) and Sybr Green dye (Bio Rad CFX96 Touch™ Real-Time PCR Detection SystemCalifornia, USA). Fold expression was calculated by the 2−ΔΔCT method.
TABLE 1.
List of Primers
Measurement of Plasma Levels of IL-8
Plasma levels of IL-8 were measured using Cytometric Bead Flex Set (BD Biosciences, Sparks, MD, USA) in the HIV-1 infected children and seronegative donors. Briefly, 50 μL of beads coated with capture anti IL-8 antibodies (capture beads), were mixed with 50 μL of sample and incubated for 1 hour in dark at room temperature. After 1 hour, 50 μL of phycoerythrin conjugated detection antibody was added to this sample-bead mixture, incubated for 2 hours in the dark at room temperature. The samples were washed with 1 mL of wash buffer (300 g, 5 minutes) and were then acquired on the FACS Accuri C6 and analyzed using FCAP Array Software (BD Biosciences, Sparks, MD, USA). Calibration curves were constructed using serial dilutions of cytokine standard provided with the kit. Instrument calibration was done (BD FACS Accuri C6) with SPHERO 8-peak Rainbow Particles to ensure accuracy/validity of the data.
In order to evaluate the changes in the relative mRNA expression and plasma levels of IL-8 with time, we followed up 15 ART progressors post 6 months’ initiation of ART.
Statistical Analysis
Fold changes in the expression of T- and B-cell genes in the HIV-1 infected children of different groups were quantitated by qRT-PCR array and analyzed using RT Profiler PCR Array Data analysis version 3.5). Relative mRNA expression of the P38, SHP2, GRB2, IL-8, CXCR1 genes, and plasma levels of IL-8 were calculated between the different groups using the Mann–Whitney U test (GraphPad Software, San Diego, CA). Results are given as median and interquartile ranges. Spearman Rank test was used to evaluate the correlation between plasma levels of IL-8 and immune parameters in the infected children. P-values <0.05 were considered significant. For analysis of paired samples of HIV-1 infected children before and after initiation of ART, paired t test was used to calculate the relative IL-8 mRNA expression, and alterations in plasma levels of IL-8.
RESULTS
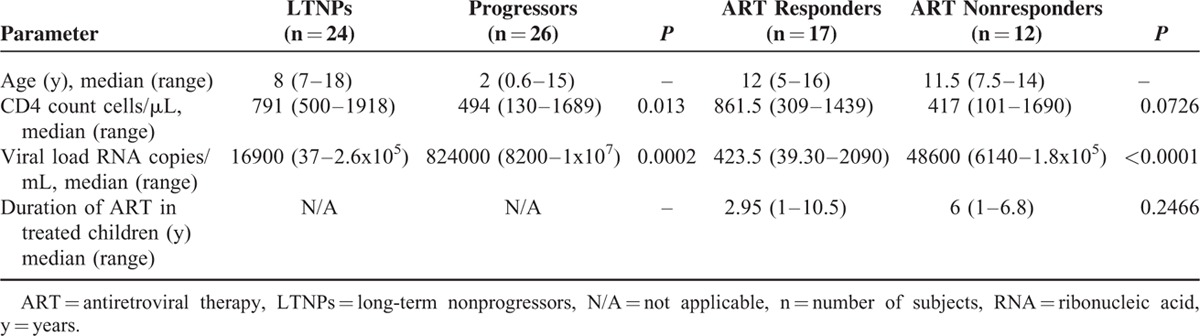
A total of 79 asymptomatic, perinatally HIV-1 infected children, including 50 ART naïve, 29 ART treated, and 8 healthy seronegative donors were recruited for the study. The median age of LTNPs was 8 years, while that of progressors was 2 years. The CD4 counts were significantly lower and viral load was significantly higher in progressors than LTNPs. Among the ART treated group, ART non-responders showed lower CD4 counts and significantly higher viral load than ART responders. The demographic profile of the HIV-1 infected children is summarized in Table 2.
TABLE 2.
Demographic and Clinical Profile of the HIV-1 Infected Children
Following the categorization of the infected children into the different groups, 5 LTNPs, 3 progressors, 3 ART responders, and 3 ART nonresponders were analyzed for the expression of immune response genes by qRT-PCR arrays (Figure 1). The array layout is given in Fig. S1 and the differentially expressed genes in the ART naïve and ART treated groups of HIV-1 infected children are provided in Table 3.
FIGURE 1.
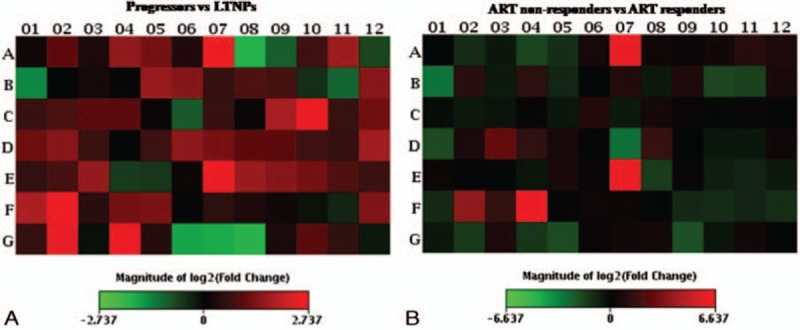
Visualization of log2 (fold change) of HIV-1-specific qRT-PCR array of T and B cell activation genes. F4 represents relative mRNA expression of IL-8 gene in (A), progressors (n = 3) vs long-term nonprogressors (LTNPs) (n = 5) (B), ART nonresponders (n = 3) vs ART responders (n = 3).
TABLE 3.
List of Genes Differentially Expressed in T and B Cell Activation PCR Array (A)
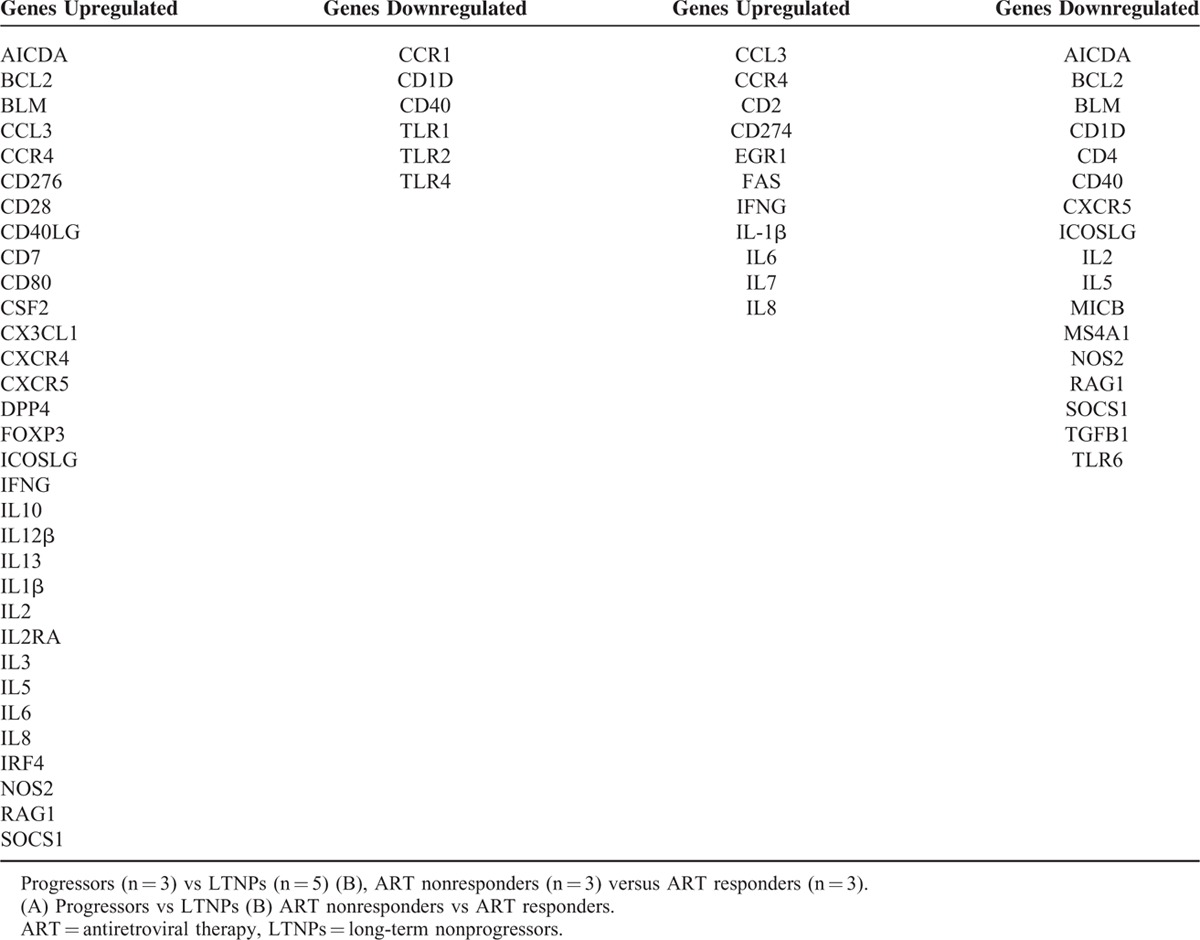
Alterations in T- and B-Cell Activation Genes Array in ART Naïve Progressors Versus LTNPs and ART Nonresponders Versus ART Responders
A >2-fold upregulation was observed in a number of genes, predominantly those encoding proinflammatory cytokines such as IL-8, IL-1β, CCL3, and IL-6 in the 3 progressors versus 5 LTNPs and 3 ART responders versus 3 ART nonresponders. Downregulation of expression of CD1d and CD40 was observed in progressors and ART nonresponders. Among the B-cell response genes, a lower expression of BCL-2 and RAG-1 was observed in ART non-responders versus ART responders (Table 3).
IL-8 Upregulation in ART Naïve Progressors Versus LTNPs and ART Nonresponders Versus ART Responders in T- and B-Cell Activation Array
The expression of IL-8 was 2.4-fold higher in HIV-1 infected 3 progressors as compared to 5 LTNPs (Figure 1A) and 85.4-fold higher in 3 HIV-1 infected ART nonresponders than in the 3 ART responders (Figure 1B).
Higher Expression of IL-8, IL-8R, and the Mediators of IL-8 in ART Naïve Progressors Versus LTNPs Confirmed by qRT-PCR
The differential expression of the IL-8 gene observed between the different study groups was validated by qRT-PCR analysis of IL-8, its receptor CXCR1 (IL-8R), and the upstream signaling molecules, that is, SHP2 and GRB2 in 12 LTNPs and 16 progressors, including the samples analyzed by gene arrays. Relative mRNA expression of IL-8 (P < 0.001), CXCR1 (P = 0.03) and SHP2 (P = 0.04) were significantly higher in progressors compared to LTNPs (Figure 2 A, B, and D). The expression of GRB2 was comparable between the progressors and LTNPs (P = 0.65) (Figure 2C).
FIGURE 2.
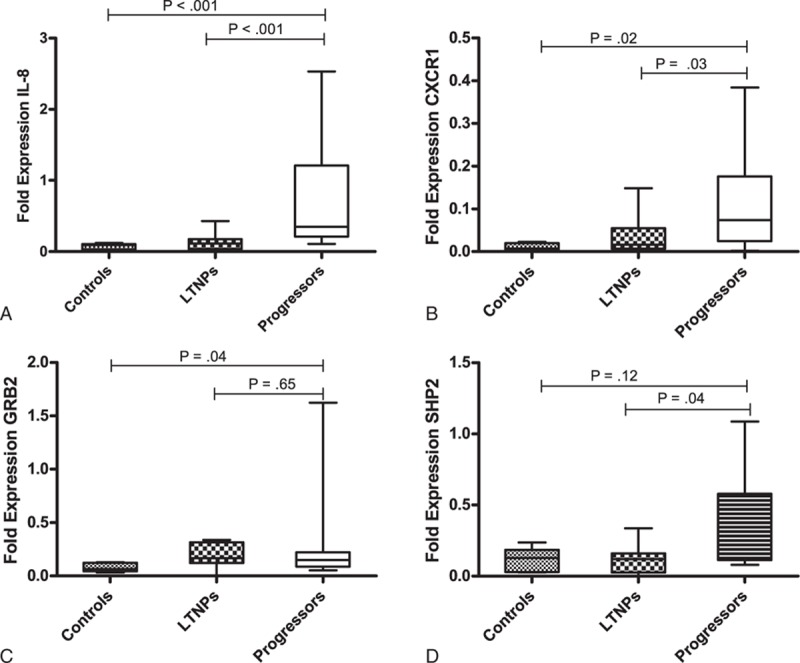
Relative mRNA expression of (A), IL-8 (B), CXCR1 (C), GRB2, (D), SHP2, in PBMCs of HIV infected ART naïve children at different stages of the disease, that is, long-term nonprogressors (LTNPs) (n = 12) vs progressors (n = 16). Lines indicate the median, boxes interquartile ranges, and whiskers indicate the minimum–maximum.
High Levels of Plasma IL-8 in Antiretroviral Naïve Progressors Versus LTNPs and ART Nonresponders Versus ART Responders
The plasma levels of IL-8, as determined by CBA Flex set, were found to be significantly higher in 26 progressors versus 24 LTNPs (P < 0.001) and 12 ART nonresponders versus 17 ART responders (P < 0.001) (Figure 2). Median plasma levels of IL-8 were 46.24, 5.34, 22.9, and 4.05 pg/mL, in progressors, LTNPs, ART nonresponders, ART responders, respectively. The median plasma IL-8 levels in the LTNPs and ART responders were comparable to that in the control samples (Table 4, Figure 3).
TABLE 4.
Plasma IL-8 Levels in Different Categories of HIV-1 Infected Children
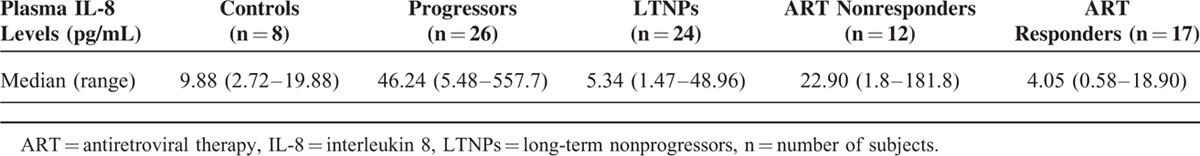
FIGURE 3.
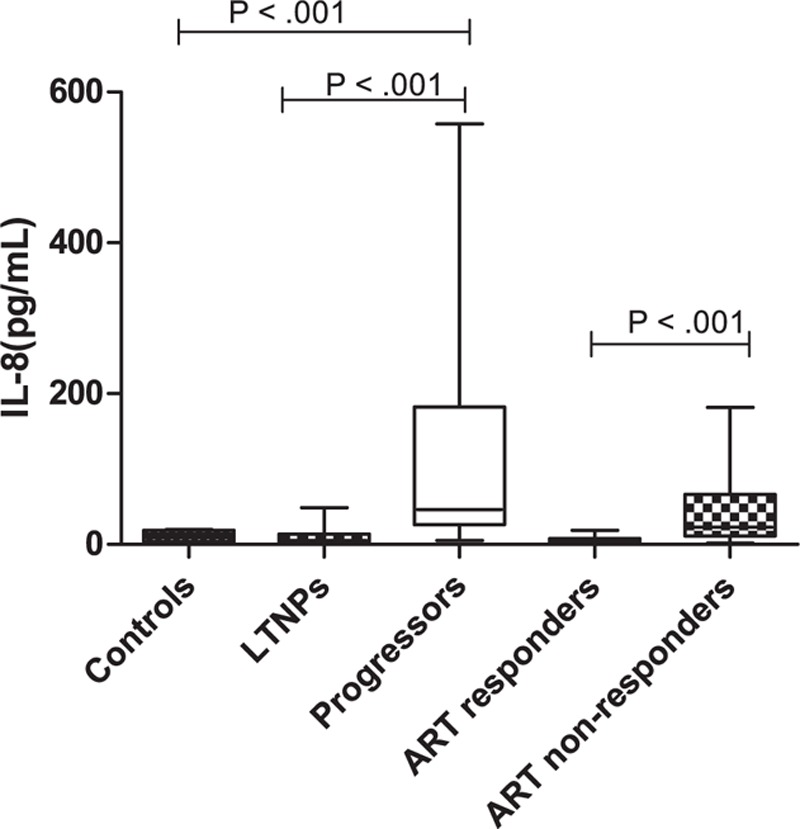
Plasma levels of IL-8 (pg/mL) in HIV infected children at different stages of the disease, that is, HIV infected ART naïve, long-term nonprogressors (LTNPs) (n = 24) versus progressors (n = 26) and ART responders (n = 17) versus ART nonresponders (n = 12). Lines indicate the median, boxes interquartile ranges, and whiskers indicate the minimum–maximum.
Plasma Levels of IL-8 Correlated Positively With CD4 Counts and Inversely With Viremia
Plasma levels of IL-8 showed significant negative correlation with CD4 count (cells/μL) in HIV-1 infected 50 ART naïve subjects (r = −0.488; P < 0.001) (Figure 4 A). Among the 29 ART treated children, the plasma levels of IL-8 positively correlated with viral load (r = 0.5494; P < 0.001) (Figure 4B).
FIGURE 4.
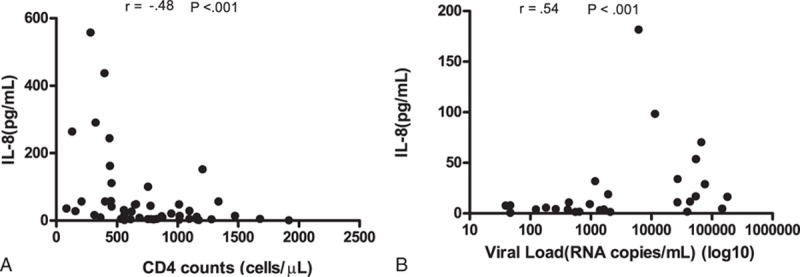
(A) Correlation of CD4 counts with plasma levels of IL-8 in ART naïve (n = 50) HIV-1 infected children. (B) correlation of viral load with plasma levels of IL-8 in ART treated (n = 29) HIV-1 infected children.
Reduced Plasma Levels and Relative mRNA Expression of IL-8 in Progressors, 6 Months Post-ART
On following up 15 ART naïve progressors, we observed a significant reduction in the plasma levels of IL-8 (P = 0.05), after 6 months of initiation of ART (Figure 5A). Similarly, a significant reduction in the relative mRNA expression of IL-8 (P = 0.05) was found in 8 children post 6 months of ART as compared to their ART naïve status (Figure 5B).
FIGURE 5.
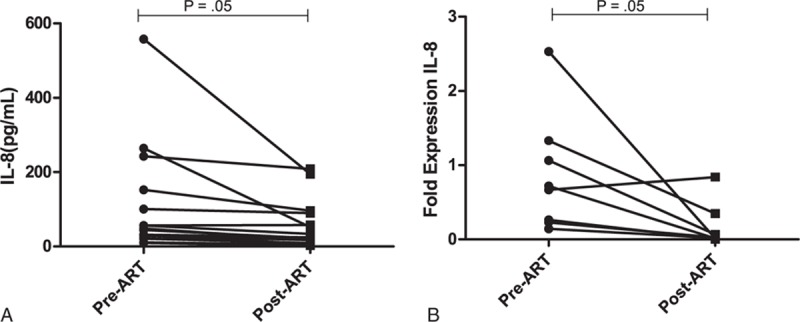
(A), Plasma levels of IL-8 (pg/ml) in progressors (n = 15) (B), relative mRNA expression in progressors (n = 8), pre- and postinitiation of ART.
DISCUSSION
According to the recent guidelines of the National AIDS Control Organization (NACO), India,47 all children <24 months, tested to be seropositive for HIV-1 should be started on ART, irrespective of clinical or immunological stage. Therefore, the present existing cohort of LTNPs and ART-naïve progressors included in this study are potential candidates to study the immunopathogenesis of HIV. The perinatally infected children recruited here comprised of ART naïve and treated children that have been systematically followed up since the year 2009. Hence we have identified 24 potential LTNPs demonstrating stable CD4 counts for at least more than 7 years of infection. Majority of the ART naïve progressors included here (23 of the 26 progressors) were younger than the LTNPs as expected. As is also well documented in literature that disease progression is faster in children than adults,42 most of the progressors in the ART naïve group progressed rapidly within 5 years of infection. To the best of our knowledge, this is the first systematic study conducted in HIV-1 infected children wherein we assessed the array of T- and B-cell activation genes and evaluated the alterations in the modulated genes at different stages of disease progression.
The array represents the host immune response, with key genes encoding mediators of both innate and adaptive immunity. A change of >2-fold was taken into consideration. The expression of several genes, such as the proinflammatory cytokines were higher in progressors versus LTNPs and ART non-responders versus ART responders. IL-8, a proinflammatory cytokine, serves as a chemoattractant involved in the activation of neutrophils, basophils, and some subpopulation of lymphocytes.48,49 During HIV-1 infection, IL-8 plays an important role in the recruitment of CD4-positive T cells to the lymph nodes, thus generating more targets for viral replication.50 Elevated levels of IL-8 are reported in the serum of HIV-1 infected individuals and in the lymphoid tissue of patients with AIDS.51 The precise role of IL-8 in disease progression needs to be established. Majority of the studies addressing the cytokine profile in HIV-1 infection are conducted in adults, with no information available in the infected children. This study focused on assessing alterations in IL-8 and its related signaling molecules in the infected children at different disease stages. A salient finding of our study is the several fold higher genotypic and phenotypic expression of IL-8, as observed from the PCR array and plasma levels, in the HIV-1 infected progressors and ART nonresponders as compared to LTNPs and ART responders, respectively. Our findings are in concordance with earlier studies demonstrating the in vitro induction of IL-8 by HIV-1 envelope glycoprotein gp120.52 The high viremia in the progressors and ART non-responders could be one of the factors contributing to the elevated expression and plasma levels of IL-8, in addition to a number of other host factors.15 Moreover, we observed that high IL-8 levels correlates with lower number of CD4 T cells (in progressors) and high viremia (in ART nonresponders), the factors contributing to faster disease progression, irrespective of age. Further, the reduction in IL-8 levels in progressors who were initiated on ART treatment suggests the beneficial effects of antiretroviral therapy in reducing inflammation, which may plausibly be due to the effect of the therapy in controlling viremia.
The high expression of IL-8, IL-8R (CXCR1), and SHP2 along with elevated plasma levels of IL-8 in the progressors as compared to LTNPs implicates the involvement of the IL-8 pathway in the immunopathogenesis of HIV-1. The major biological effects of IL-8 are mediated via its receptor, rhodopsin-like guanine-protein-coupled receptors (GPCRs): CXCR1 (IL-8RA).53,54 Also, our findings are in accordance with an earlier report implicating the role of SHP2, GRB2 as mediators of IL-8 induction in HIV and HCV infection.55
In vitro studies have demonstrated that IL-8 stimulates HIV-1 replication in monocyte derived macrophages (MDM) and T lymphocytes.32 Currently, an IL-8-specific monoclonal antibody is in use in clinical trial of patients with psoriasis, a disease marked by chronic inflammation.56 Antibodies that neutralize IL-8 activity, or block IL-8 receptors have been shown to inhibit HIV-1 replication in T-lymphocytes.32
In a study conducted by Roberts et al,57 HIV infected adult participants were divided into low, medium, or high risk groups according to their risk scores calculated using CD4 counts by the β-coefficients of the Cox proportional-hazards model. However, there is wide variation in CD4 counts in children of different age groups, thereby warranting the need to identify additional immune based parameters, which along with CD4 counts can be useful for calculating the risk score for better prognosis of the disease. Cytokines are potential candidates to be considered for prognostic purposes.
One of the limitations of the present study is that we have focused on a single cytokine IL-8 and its related signaling molecules, although changes were observed in a number of proinflammatory cytokines such as IL-1β, CCL3, IL-6 and its receptor in the different study groups. These cytokines and their associated molecules could not be evaluated by qRT-PCR at the baseline or in the follow up samples due to limited sample availability from the children. The inflammatory responses in HIV-1 infection are mediated by a complex interaction between several cytokines. The role of other cytokines and immune response genes that promote inflammation and disease progression remain to be addressed. Further studies should be conducted in a larger sample size of HIV-1 infected children at various disease stages and in different populations to confirm our findings.
To conclude, our data suggest that IL-8 levels are indicative of the varying inflammatory status of the HIV-1 infected children at different stages of the disease. The study identifies IL-8 as one of the potential cytokines that may be used among a panel of cytokines that can be designed in future to evaluate disease progression in HIV-1 infected children. The role of inhibitors of IL-8 and its receptor CXCR1, to serve as candidates for therapeutic intervention, needs to be evaluated.
Supplementary Material
Footnotes
Abbreviations: AIDS = acquired immune deficiency syndrome, ART = antiretroviral therapy, BCL-2 = B-cell lymphoma 2, CCL3 = chemokine (C-C motif) ligand 3, CCR5 = C-C chemokine receptor type 5, CD40 = cluster of differentiation 40, cDNA = complementary DNA, CXCR1 = C-X-C motif chemokine receptor 1, GAPDH = glyceraldehyde 3-phosphate dehydrogenase, GRB2 = growth factor receptor-bound protein 2, HIV-1 = human immunodeficiency virus-1, IFN-α = interferon alpha, IL-8 = interleukin 8, LTNPs = long-term nonprogressors, MCP-1 = monocyte chemoattractant protein-1, PBMCs = peripheral blood mononuclear cells, RAG-1 = recombination activating gene 1, RNA = ribonucleic acid, RT-PCR = reverse transcription polymerase chain reaction, SHP2 = SRC homology phosphatase 2, sIL-2R = soluble IL-2 receptor, TNF-α = tumor necrosis factor-alpha.
ANP and HA contributed equally to this work.
This work was funded by the Department of Biotechnology (DBT) # BT/PR5758/MED/29/591/2012. We thank Professor Subrata Sinha for his valuable scientific inputs. Ambili Pananghat Nair and Heena Aggarwal are supported by a Junior Research Fellowship from the Council of Scientific and Industrial Research, Govt of India.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Miller CJ. Host and viral factors influencing heterosexual HIV transmission. Rev Reprod 1998; 3:42–51. [DOI] [PubMed] [Google Scholar]
- 2.Mackelprang RD, Carrington M, Thomas KK, et al. Host genetic and viral determinants of HIV-1 RNA set point among HIV-1 seroconverters from sub-saharan Africa. J Virol 2015; 89:2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yue L, Prentice HA, Farmer P, et al. Cumulative impact of host and viral factors on HIV-1 viral-load control during early infection. J Virol 2013; 87:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med 1995; 332:209–216. [DOI] [PubMed] [Google Scholar]
- 5.Sheppard HW, Lang W, Ascher MS, et al. The characterization of non-progressors: long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS Lond Engl 1993; 7:1159–1166. [PubMed] [Google Scholar]
- 6.Madec Y, Boufassa F, Avettand-Fenoel V, et al. Early control of HIV-1 infection in long-term nonprogressors followed since diagnosis in the ANRS SEROCO/HEMOCO cohort. J Acquir Immune Defic Syndr 1999 2009; 50:19–26. [DOI] [PubMed] [Google Scholar]
- 7.Okulicz JF, Marconi VC, Landrum ML, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 2009; 200:1714–1723. [DOI] [PubMed] [Google Scholar]
- 8.Warszawski J, Lechenadec J, Faye A, et al. Long-term nonprogression of HIV infection in children: evaluation of the ANRS prospective French Pediatric Cohort. Clin Infect Dis Off Publ Infect Dis Soc Am 2007; 45:785–794. [DOI] [PubMed] [Google Scholar]
- 9.Prendergast AJ, Klenerman P, Goulder PJR. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol 2012; 12:636–648. [DOI] [PubMed] [Google Scholar]
- 10.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 1996; 273:1856–1862. [DOI] [PubMed] [Google Scholar]
- 11.Luque MC, Santos CC, Mairena EC, et al. Gene expression profile in long-term non progressor HIV infected patients: in search of potential resistance factors. Mol Immunol 2014; 62:63–70. [DOI] [PubMed] [Google Scholar]
- 12.Kiepiela P, Leslie AJ, Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 2004; 432:769–775. [DOI] [PubMed] [Google Scholar]
- 13.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev 2013; 254:143–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stellbrink H-J, Baldus S, Behrens G, et al. HIV-induced immune activation: pathogenesis and clinical relevance—summary of a workshop organized by the German AIDS Society (DAIG e.v.) and the ICH Hamburg, Hamburg, Germany, November 22, 2008. Eur J Med Res 2010; 15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 2013; 254:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klatt NR, Chomont N, Douek DC, et al. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 2013; 254:326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquelin B, Petitjean G, Kunkel D, et al. Innate immune responses and rapid control of inflammation in African green monkeys treated or not with interferon-alpha during primary SIVagm infection. PLoS Pathog 2014; 10: doi:10.1371/journal.ppat.1004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 1999 2010; 55:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther 2007; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malherbe G, Steel HC, Cassol S, et al. Circulating Biomarkers of Immune Activation Distinguish Viral Suppression from Nonsuppression in HAART-Treated Patients with Advanced HIV-1 Subtype C Infection. Mediators Inflamm 2014; 2014: doi:10.1155/2014/198413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009; 83:3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalfamo M, Le Saout C, Lane HC. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev 2012; 23:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS Lond Engl 2002; 16:1859–1866. [DOI] [PubMed] [Google Scholar]
- 26.Tasca KI, Calvi SA, Souza Ldo R. Immunovirological parameters and cytokines in HIV infection. Rev Soc Bras Med Trop 2012; 45:663–669. [DOI] [PubMed] [Google Scholar]
- 27.McComsey GA, Kitch D, Sax PE, et al. Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Acquir Immune Defic Syndr 1999 2014; 65:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcon-Neyra L, Benmarzouk-Hidalgo OJ, Madrid L, et al. No differences of immune activation and microbial translocation among HIV-infected children receiving combined antiretroviral therapy or protease inhibitor monotherapy. Medicine (Baltimore) 2015; 94:e521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciccosanti F, Corazzari M, Soldani F, et al. Proteomic analysis identifies prohibitin down-regulation as a crucial event in the mitochondrial damage observed in HIV-infected patients. Antivir Ther 2010; 15:377–390. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez M, del M, Mateo MG, et al. The toxicogenetics of antiretroviral therapy: the evil inside. Curr Med Chem 2011; 18:209–219. [DOI] [PubMed] [Google Scholar]
- 31.Souza MPD, Cairns JS, Plaeger SF. Current evidence and future directions for targeting HIV entry: therapeutic and prophylactic strategies. JAMA 2000; 284:215–222. [DOI] [PubMed] [Google Scholar]
- 32.Lane BR, Lore K, Bock PJ, et al. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol 2001; 75:8195–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bure D, Makhdoomi MA, Lodha R, et al. Mutations in the reverse transcriptase and protease genes of human immunodeficiency virus-1 from antiretroviral naïve and treated pediatric patients. Viruses 2015; 7:590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Science 1996; 271:324–328. [DOI] [PubMed] [Google Scholar]
- 35.Imami N, Antonopoulos C, Hardy GA, et al. Assessment of type 1 and type 2 cytokines in HIV type 1-infected individuals: impact of highly active antiretroviral therapy. AIDS Res Hum Retroviruses 1999; 15:1499–1508. [DOI] [PubMed] [Google Scholar]
- 36.Noel N, Lerolle N, Lécuroux C, et al. Immunologic and virologic progression in HIV controllers: the role of viral “blips” and immune activation in the ANRS CO21 CODEX Study. PloS One 2015; 10:e0131922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science 1993; 262:1011–1018. [DOI] [PubMed] [Google Scholar]
- 38.Than S, Hu R, Oyaizu N, et al. Cytokine pattern in relation to disease progression in human immunodeficiency virus-infected children. J Infect Dis 1997; 175:47–56. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med 1995; 332:567–575. [DOI] [PubMed] [Google Scholar]
- 40.Clerici M, Lucey DR, Berzofsky JA, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science 1993; 262:1721–1724. [DOI] [PubMed] [Google Scholar]
- 41.Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses 1992; 8:191–197. [DOI] [PubMed] [Google Scholar]
- 42.Martinez DR, Permar SR, Fouda GG. Contrasting Adult and Infant Immune Responses to HIV Infection and Vaccination. Clin Vaccine Immunol CVI 2015; 23:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips AN. CD4 lymphocyte depletion prior to the development of AIDS. AIDS Lond Engl 1992; 6:735–736. [DOI] [PubMed] [Google Scholar]
- 44.Hoover DR, Graham NM, Chen B, et al. Effect of CD4+ cell count measurement variability on staging HIV-1 infection. J Acquir Immune Defic Syndr 1992; 5:794–802. [PubMed] [Google Scholar]
- 45.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126:946–954. [DOI] [PubMed] [Google Scholar]
- 46.Naco Guidelines for HIV Care and Treatment in Infants and Children, November 2006. Available online: http://www.Whoindia.Org/link-files/hiv-aids_naco_guidelines_on_art_for_paediatric_hiv_aids.Pdf (accessed on 4 November 2015).
- 47.Pediatric Antiretroviral Therapy (ART) Guidelines. Available online: naco.gov.in/upload/2014%20mslns/CST/Pediatric_14-03-2014.pdf.
- 48.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett 1992; 307:97–101. [DOI] [PubMed] [Google Scholar]
- 49.Taub DD, Anver M, Oppenheim JJ, et al. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest 1996; 97:1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ott M, Lovett JL, Mueller L, et al. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-kappaB factors. J Immunol (Baltim Md) 19501998; 160:2872–2880. [PubMed] [Google Scholar]
- 51.Matsumoto T, Miike T, Nelson RP, et al. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol 1993; 93:149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto T, Miike T, Nelson RP, et al. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol 1993; 93:149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vasilescu A, Terashima Y, Enomoto M, et al. A haplotype of the human CXCR1 gene protective against rapid disease progression in HIV-1+ patients. Proc Natl Acad Sci U S A 2007; 104:3354–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liou J-W, Chang F-T, Chung Y, et al. In silico analysis reveals sequential interactions and protein conformational changes during the binding of chemokine CXCL-8 to its receptor CXCR1. PLoS ONE 2014; 9.doi:10.1371/journal.pone.0094178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem 2003; 278:35755–35766. [DOI] [PubMed] [Google Scholar]
- 56.Yang XD, Corvalan JR, Wang P, et al. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease states. J Leukoc Biol 1999; 66:401–410. [DOI] [PubMed] [Google Scholar]
- 57.Roberts L, Passmore J-AS, Williamson C, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS Lond Engl 2010; 24:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


