Abstract
Ankylosing spondylitis (AS) is a chronic inflammatory disease involing spine and enthesis. The primary aim of this study is to investigate the autonomic nervous system (ANS) function and the association between ANS and the functional status or disease activity in AS.
The study included 42 AS patients, all fulfilling the modified New York criteria. All the patients are totally symptom free for ANS involvement and had normal neurological findings. These AS patients and 230 healthy volunteers receive analysis of 5 minutes heart rate variability (HRV) in lying posture. In addition, disease activity and functional status of these AS patients are assessed by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), and Bath Ankylosing Spondylitis Global Score (BAS-G).
Both groups were age and sex-matched. Although the HRV analysis indicates that the peaks of total power (TP, 0–0.5 Hz) and high-frequency power (HF, 0.15–0.40 Hz) are similar in both groups, the activities of low-frequency power (LF, 0.04–0.15 Hz), LF in normalized units (LF%), and the ratio of LF to HF (LF/HF) in AS patients are obviously lower than healthy controls. The erythrocyte sedimentation rate and C-reactive protein revealed negative relationship with HF. The AS patients without peripheral joint disease have higher LF, TP, variance, LF%, and HF than the patients with peripheral joint disease. The AS patients without uvetis have higher HF than the patients with uvetis. The total scores of BASDI, BASFI, and BAS-G do not show any association to HRV parameters.
AS patients have significantly abnormal cardiac autonomic regulation. This is closely related with some inflammatory activities. Reduced autonomic function may be one of the factors of high cardiovascular risk in AS patients.
INTRODUCTION
Ankylosing spondylitis (AS) is an inflammatory spondyloarthritis that mainly affects spine and sacroiliac joints of young men. Besides, extra-articular manifestations involving cardiac and nervous system are well recognized for patients with AS.1–3 Cardiovascular complications, including aortic valvular disease, cardiomyopathy, pericarditis, and intraventricular conduction anomalies were noted in some AS patients.4 Autonomic neuropathy was found to be the third most common neurological disease of rheumatic and autoimmune disease,5 such as rheumatoid arthritis (RA) and Sjogren syndrome.6,7
The spectral analysis of heart rate variability (HRV) has been proven to be a potent, noninvasive, sensitive, and reproducible tool for the diagnosis of cardiovascular autonomic dysfunction. It is well established that power spectrum can be quantified into total power (TP, 0–0.5 Hz), high-frequency power (HF, 0.15–0.40 Hz), low-frequency power (LF, 0.04–0.15 Hz), and very low-frequency power (0.003–0.04 Hz) components.8 The HF component is related to the respiratory sinus arrhythmia and is thought to reflect parasympathetic activity.9 The TP, LF, or variance are components mediated by both sympathetic and parasympathetic modulation.10 The ratio of LF to HF (LF/HF) and LF in normalized units (LF%) have been used as sympathetic tone; the HF in normalized units (HF%) reflex sympathetic inhibition.8,11
Several studies of the effects of AS on autonomic nervous system (ANS) reported controversial and inconclusive results.3,5,12–14 One study showed no difference of HRV for AS patients and healthy controls.3 However, some scholars demonstrated that AS patients had lower HRV and their HRV values were correlated with Bath ankylosing spondylitis disease activity index (BASDAI) and serum level of C-reactive protein (CRP).12 Another study found lower ultra low-frequency power and root mean square recessive difference for AS patients.14 In addition, the relationship of autonomic dysfunction with clinical characteristics, HLA B-27, serum inflammatory marker including CRP and erythrocyte sedimentation rate (ESR), as well as disease activity score has not been well elucidated in the past literature.
To solve the unmet medical need, the primary aim of this study is to investigate autonomic function and clinical characteristics of patients with AS in comparison with control group. The secondary aim is to analyze the association of HRV and clinical characteristics, HLA-B27, disease activity (BASDAI, Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G) score), and findings on physical examination. The tertiary aim is to search for correlations between HRV and complications of AS.
METHODS
Ethical Experimentation
The protocol of the study was approved by the Chung Shan Medical University Research Ethics Review Committee. All patients provided informed consent before participation.
Cases and Controls
Forty-two AS outpatients (32 male, 10 female), aged 38.12 ± 12.37 years who took part in the research were all from the Division of Allergy, Immunology and Rheumatology in Chung Shan Medical University Hospital. All the patients satisfied the modified New York criteria for AS.15 Patients with a history of arrhythmia, systemic hypertension, diabetes mellitus, thyroid disease, or other rheumatic diseases were excluded. We also recruited 230 healthy subjects, sex and age matched (175 male and 57 female, aged 38.08 ± 13.57), as control group who were free of physical illness and were not taking any medications at the time of the study. All the subjects were totally symptom free for ANS involvement and had normal neurological examination findings.
Measurement of Clinical, Laboratory, and Imaging Data
Demographic characteristics including age, sex, height, body weight, drug intake, and disease duration were recorded. Detailed physical and neurological examinations were performed in the AS patients. Blood samples of the AS patients were taken to determine 1 hour ESR by Westergren method, CRP by turbidimetric method, and HLA-B27 by flow cytometry.16 We used validated Chinese version of BASDAI,17 Bath Ankylosing Spondylitis Functional Index (BASFI),3 and Bath Ankylosing Spondylitis Global Score (BAS-G)18 to determine the activity of disease. Plain radiographs of the pelvis were taken in all patients, and diagnosis of AS was made according to the modified New York criteria.15
HRV Analysis
Five-minute frequency-domain analysis using a HRV analyzer (WG-MD-ANSA01, We Gene Co Ltd, Taipei, Taiwan) was performed at approximately morning time of the day on each AS patient and healthy subject while the subject lays quietly and breathed normally. The power spectrum was subsequently quantified into standard frequency-domain measurements as defined previously,8 including LF, HF, TP, variance, LF%, HF%, and LF/HF.
Statistical Analysis
Sociodemographic and clinical characteristics were summarized using a descriptive statistical method. The complications of the AS patients were revealed by a frequency distribution method. Continuous variables considered in this paper including age, body mass index (BMI), and clinical characteristics were reported as mean ± standard deviation. Categorical variables including sex, HLA-B27, and complications were reported as numbers and percentage. The relationship of mean differences between groups was analyzed with Wilcoxon rank sum test. Pearson correlation analysis was used to evaluate the correlation between ANS parameters and clinical entity parameters. A 2-sided P <0.05 was considered statistically significant. All data were analyzed using the statistical package SAS for Windows, version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
In this study, the frequency of HLA-B27-postive (52.4%) was a little greater than the HLA-B27-negative. There was a high rate in the complication of radiographic sacroiliitis (81%), low back pain (76.2%), and peripheral joint involvement (78.6%). About one-third of AS patients suffered from psoriasis. Twelve patients had uveitis and 4 patients had inflammatory bowel disease. There were only 2 patients with heart involvement and none with lung involvement. There is no remarkable difference of autonomic functions in HLA-B27 or other complications, including inflammatory bowel disease, psoriasis, hematuria, urinary tract infection, digestion system, heart or lung involved. The detailed clinical characteristics and complications of the AS patients are summarized in Table 1.
TABLE 1.
Demographics and Clinical Characteristics of Ankylosing Spondylitis Patients
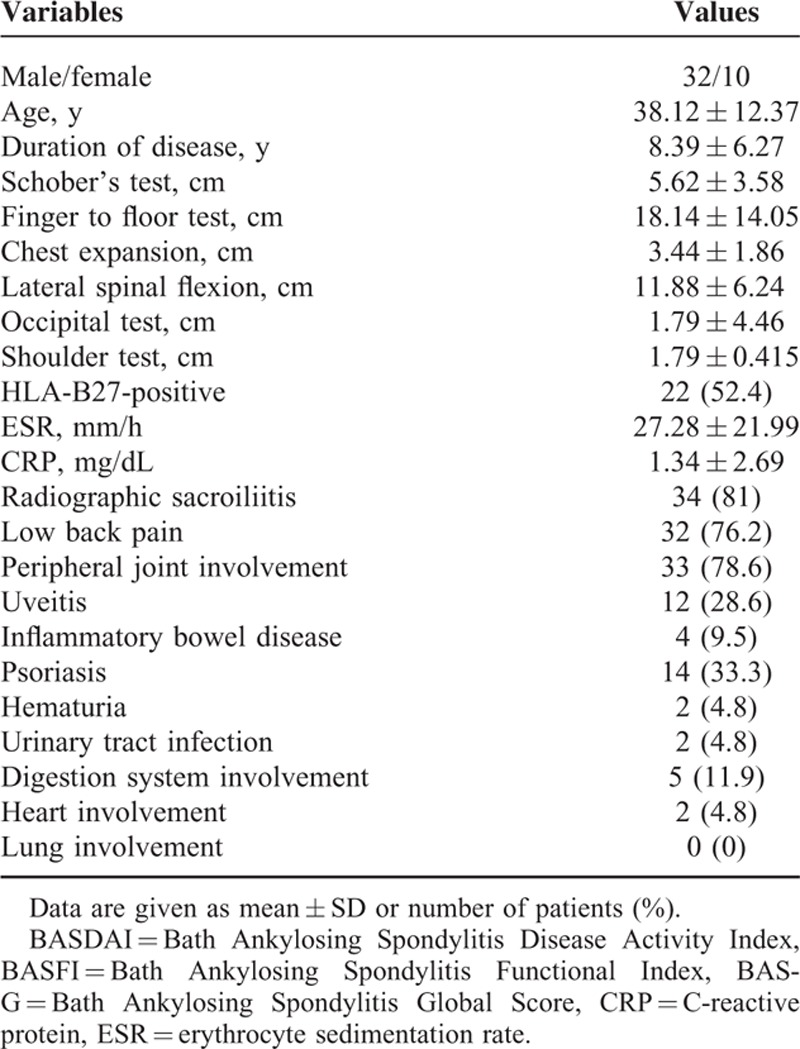
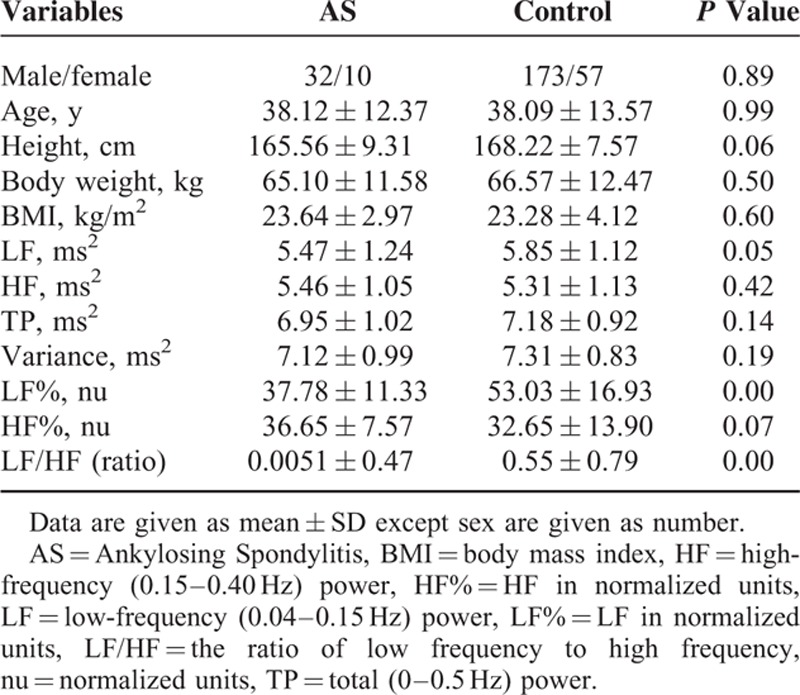
Forty-two patients with AS (32 male, 10 female) with a mean age of 38.12 ± 12.37 years and 230 healthy control subjects (173 male, 57 female) with a mean age of 38.09 ± 13.57 years were included in the study. There was no any significant difference in sex, age, height, body weight, or BMI between these 2 groups. The sociodemographic and autonomic indexes of the AS and control subjects are summarized in Table 2. Compared with control group, the AS group had significantly lower LF, LF%, and LF/HF. The results meant that the AS patients had lower autonomic function including sympathetic and parasympathetic tones.
TABLE 2.
Comparison of Sociodemographic and Autonomic Indexes Between Ankylosing Spondylitis Patients and Control Group
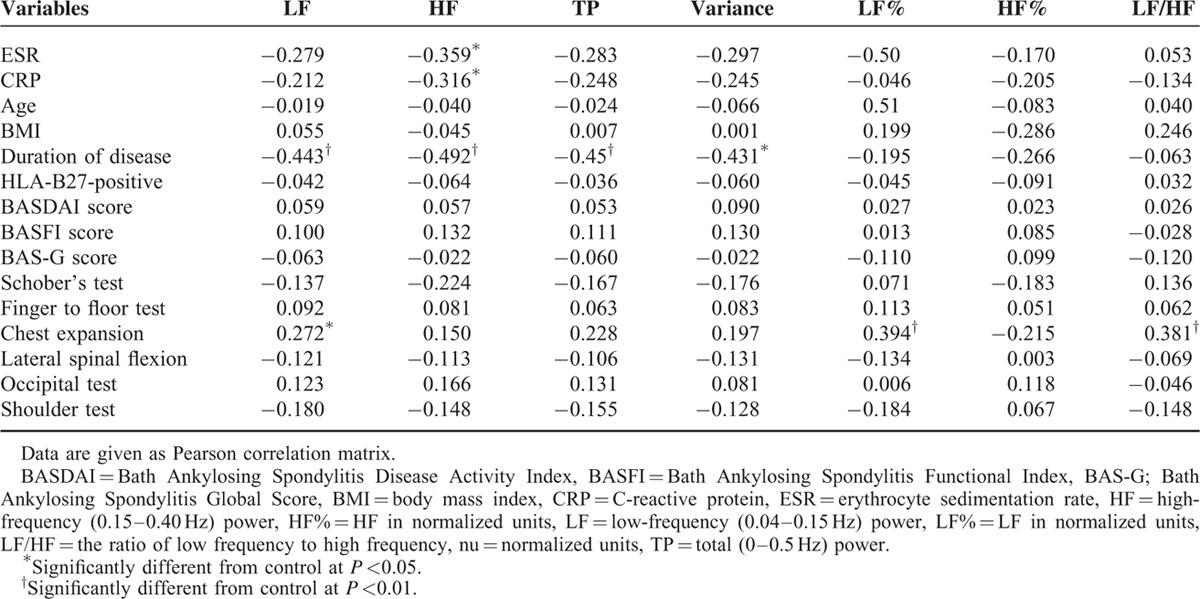
One hour ESR and CRP revealed obvious negative relationship with HF. These negative relationships meant lower parasympathetic tone in association to inflammatory conditions. The global scores of BASDAI, BASFI, and BASG did not show significant relationship with HRV parameters. The duration of disease revealed significant negative association with LF, TP, variance, and HF. The results meant the longer the disease duration, the lower the sympathetic and parasympathetic functions. We found obvious positive correlation between chest expansion and LF or LF%. But other physical examinations, including Schober's test, finger to floor test, lateral spinal flexion, occipital test, or Shoulder test, did not show any relationship with HRV. This meant lower vagal tone. The relationship between autonomic indexes and clinical manifestations is summarized in Table 3.
TABLE 3.
Relationship Between Autonomic Indexes and Clinical Manifestations in Ankylosing Spondylitis Patients
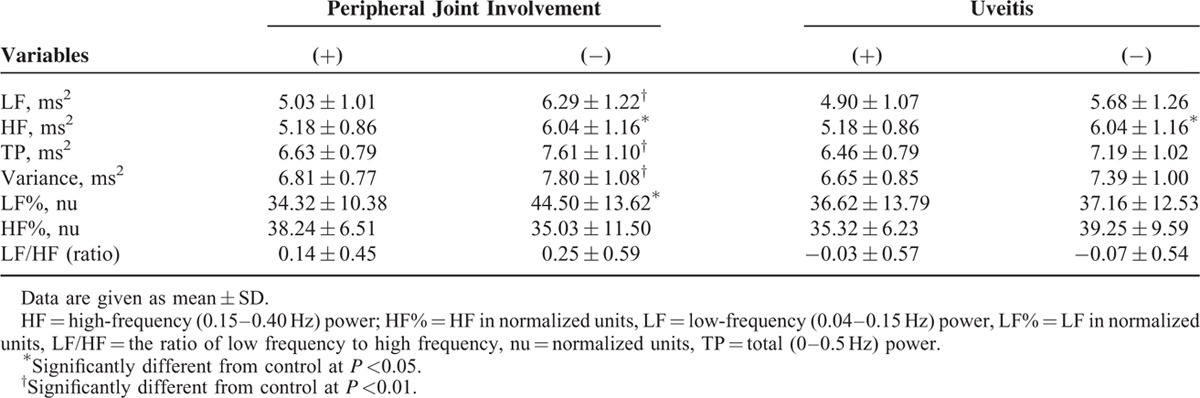
The AS patients with peripheral joint disease (PJD) showed significant lower autonomic functions including LF, TP, variance, LF%, and HF compared with AS patients without PJD. PJD means an ANS dysfunction including both sympathetic and parasympathetic functions. The AS patients with complication of iritis also revealed significant lower in HF. This meant lower vagal tone. Comparison between autonomic indexes and related complications of AS patients is summarized in Table 4.
TABLE 4.
Comparison of Autonomic Indexes and Clinical Features of Ankylosing Spondylitis Patients
DISCUSSION
The autonomic control of the heart is obviously related to age and sex,19,20 so we collected more health subjects for comparing the ANS condition between AS and health groups (42:230). The ANS according to our results as compared with healthy volunteers, patients with AS had significantly lower LF, LF%(nu), and LF/HF, suggesting that dysregulation of autoimmunity in patients with AS might lead to ANS involvement and consequently declined sympathetic activity initially during the course of disease. Nonetheless, while disease progressed with time and extraspinal disease activity increased, the effect of alteration of ANS by AS shifted from predominance of decreasing sympathetic tone to mitigating parasympathetic activity.
It is evidenced by several findings from our study. First, HF was inversely correlated with 1-hour ESR rate and serum level of CRP, which implies that as the serum marker of disease activity increased, the parasympathetic tone decreased significantly. Second, only was duration of disease associated with HRV, including LF, HF, TP, and variance respectively, but not age, BMI, HLA-B27, BASDAI score, BASFI score, or BAS-G score. It suggested that the duration of disease was associated with universal impairment of sympathetic and parasympathetic nerve activity. Third, our study also demonstrated that patients with PJD had significantly lower LF, HF, TP, variance, and LF% while patients with iritis had significantly lower HF only. It also indicated that patients with higher extraspinal disease activity had lower parasympathetic activity. Finally, it revealed positive correlation of chest expansion among findings of physical examination and LF, LF%, and LF/HF, respectively.3
Abnormal balance of sympathetic and parasympathetic system was found in 30% to 50% of patients with RA and Systemic Lupus Erythematosus.6,21,22 Despite lack of convincing evidence supporting immunological mechanism of autonomic system dysfunction, consistent with numerous previous findings, our study revealed that decreasing parasympathetic tone in patients with AS was associated with disease activity and disease duration. Toussirot et al5 found that decreased parasympathetic activity as indicated by higher heart rate and lower baroreflex slope was mainly observed in patients with higher disease activity, such as higher BASDAI score, ESR, or CRP. Gunes et al23 demonstrated that patients with AS had significantly lower root mean square of difference between adjacent normal-to-normal interval (RMSSD), percentage of R-R intervals with more than 50 milliseconds variation (pNN50), and standard deviation of difference between adjacent normal-to-normal interval (SDNN) compared with normal control despite that no significant structural abnormalities of heart were noted, which was a feature of decreased parasympathetic activity and might be partially explained by inflammation. Accordingly, the ultralow frequency power and RMSSD was reported to be lower in patients with AS.14 Although the interactions of disease activity and autonomic nerve system activity remain unanswered, similar phenomenon was also observed in RA and Systemic Lupus Erythematosus.6,21 Kaya et al reported that patients with AS have significantly reduced SDNN, RMSSD, and pNN50. They also noted that patients with AS also had higher LF, and LF/HF.13
Interestingly, Borman et al demonstrated subclinical dysfunction of parasympathetic autonomic system in patients with AS evidenced by significantly lower heart rate variation, heart rate and blood pressure to standing and exercise, sympathetic skin response (SSR), and R-R interval variation. They also found that the dysfunction was related to BASDAI score CRP level.12 However, some contrary data exists that there was no significant difference with regard to LF, LF% (nu), HF, HF% (nu), and LF/HF for patients with AS. Yildirir et al3 thought that it might be due to younger age, short disease duration, anti-inflammatory medications, such as NSAIDs or sulfasalazine.
There were several limitations in our study. First, the duration of disease ranges from 2 to 14 years and it partially explained that there were no significant associations between HRV and BASDAI because it has been considered more appropriate when the disease activity was assessed by BASDAI, BAS-G, or BASFI at initial diagnosis.15 Second, patients and healthy control received their HRV analysis only in the morning only but not around the clock. HRV may also be influenced by circadian rhythm,24 but we did not know the differences in the AS patients. Third, there are some factors that possibly mediate HRV that were not analyzed in the study, including the sleep quality and physical activity of each participant.25,26 Fourth, this work is a cross-sectional study, which limits our ability to assess causality; thus, longitudinal studies and repeated measurements are necessary.
On the other hand, there were several strengths of our study. We combined the electrophysiological analysis, findings of physical examination, disease assessment using BASDAI, BAS-G, or BASFI, and serum markers and clinical characteristics to study patients with AS. In addition, the population size of our study was bigger than that of previous literatures, not to mention healthy controls. Moreover, to our best knowledge, this is the first research to indicate that uveitis and PJD were correlated with decreased vagal tone.
In conclusion, AS patients had significantly lower sympathetic tone as compared with the healthy control group. This is the first study to demonstrate that extra-articular manifestations of AS, including uveitis and PJD, were associated with reduced parasympathetic activity. These findings may benefit our comprehensive care in AS patients. To prevent cardiovascular accident, HRV analysis is needed, especially with longer duration or higher inflammatory condition.
Footnotes
Abbreviations: ANS = autonomic nervous system, AS = ankylosing spondylitis, BASDAI = Bath Ankylosing Spondylitis Disease Activity Index, BASFI = Bath Ankylosing Spondylitis Functional Index, BAS-G = Bath Ankylosing Spondylitis Global Score, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, HF = high-frequency power, HF% = HF in normalized units, HRV = heart rate variability, LF = low-frequency power, LF% = LF in normalized units, LF/HF = the ratio of LF to HF, PJD = peripheral joint disease, TP = total power.
The authors alone are responsible for the content and writing of the paper.
C-YW and W-MK both contributed equally to this work as co-first authors. The authors acknowledged research grant from Chung Shan Medical University Hospital, No. CSH-2009-C-006.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mitchell MJ, Sartoris DJ, Moody D, et al. Cauda equina syndrome complicating ankylosing spondylitis. Radiology 1990; 175:521–525. [DOI] [PubMed] [Google Scholar]
- 2.Pillay N, Hunter T. Delayed evoked potentials in patients with ankylosing spondylitis. J Rheumatol 1986; 13:137–141. [PubMed] [Google Scholar]
- 3.Yildirir A, Aksoyek S, Calguneri M, et al. No evidence of cardiac autonomic involvement in ankylosing spondylitis, as assessed by heart rate variability. Clin Rheumatol 2001; 20:185–188. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill TW, Bresnihan B. The heart in ankylosing spondylitis. Ann Rheum Dis 1992; 51:705–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toussirot E, Bahjaoui-Bouhaddi M, Poncet JC, et al. Abnormal autonomic cardiovascular control in ankylosing spondylitis. Ann Rheum Dis 1999; 58:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekkelund SI, Jorde R, Husby G, et al. Autonomic nervous system function in rheumatoid arthritis. A controlled study. J Rheumatol 1996; 23:1710–1714. [PubMed] [Google Scholar]
- 7.Mandl T, Jacobsson L, Lilja B, et al. Disturbances of autonomic nervous function in primary Sjogren's syndrome. Scand J Rheumatol 1997; 26:253–258. [DOI] [PubMed] [Google Scholar]
- 8.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93:1043–1065. [PubMed] [Google Scholar]
- 9.Fouad FM, Tarazi RC, Ferrario CM, et al. Assessment of parasympathetic control of heart rate by a noninvasive method. Am J Physiol 1984; 246:H838–H842. [DOI] [PubMed] [Google Scholar]
- 10.Berger RD, Saul JP, Cohen RJ. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am J Physiol 1989; 256:H142–H152. [DOI] [PubMed] [Google Scholar]
- 11.Montano N, Ruscone TG, Porta A, et al. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90:1826–1831. [DOI] [PubMed] [Google Scholar]
- 12.Borman P, Gokoglu F, Kocaoglu S, et al. The autonomic dysfunction in patients with ankylosing spondylitis: a clinical and electrophysiological study. Clin Rheumatol 2008; 27:1267–1273. [DOI] [PubMed] [Google Scholar]
- 13.Kaya EB, Okutucu S, Aksoy H, et al. Evaluation of cardiac autonomic functions in patients with ankylosing spondylitis via heart rate recovery and heart rate variability. Clin Res Cardiol 2010; 99:803–808. [DOI] [PubMed] [Google Scholar]
- 14.Kazmierczak J, Peregud-Pogorzelska M, Biernawska J, et al. Cardiac arrhythmias and conduction disturbances in patients with ankylosing spondylitis. Angiology 2007; 58:751–756. [DOI] [PubMed] [Google Scholar]
- 15.Robertson LP, Davis MJ. A longitudinal study of disease activity and functional status in a hospital cohort of patients with ankylosing spondylitis. Rheumatology 2004; 43:1565–1568. [DOI] [PubMed] [Google Scholar]
- 16.Chou CT, Tsai YF, Liu J, et al. The detection of the HLA-B27 antigen by immunomagnetic separation and enzyme-linked immunosorbent assay-comparison with a flow cytometric procedure. J Immunol Methods 2001; 255:15–22. [DOI] [PubMed] [Google Scholar]
- 17.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21:2286–2291. [PubMed] [Google Scholar]
- 18.Jones SD, Steiner A, Garrett SL, et al. The Bath Ankylosing Spondylitis Patient Global Score (BAS-G). Br J Rheumatol 1996; 35:66–71. [DOI] [PubMed] [Google Scholar]
- 19.Kuo TB, Lin T, Yang CC, et al. Effect of aging on gender differences in neural control of heart rate. Am J Physiol 1999; 277:H2233–H2239. [DOI] [PubMed] [Google Scholar]
- 20.Voss A, Schroeder R, Heitmann A, et al. Short-term heart rate variability—influence of gender and age in healthy subjects. PloS one 2015; 10:e0118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laversuch CJ, Seo H, Modarres H, et al. Reduction in heart rate variability in patients with systemic lupus erythematosus. J Rheumatol 1997; 24:1540–1544. [PubMed] [Google Scholar]
- 22.Toussirot E, Serratrice G, Valentin P. Autonomic nervous system involvement in rheumatoid arthritis. 50 cases. J Rheumatol 1993; 20:1508–1514. [PubMed] [Google Scholar]
- 23.Gunes Y, Tuncer M, Guntekin U, et al. Effects of ankylosing spondylitis on the heart. Acta Cardiol 2009; 64:385–392. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Shaffer ML, Rodriguez-Colon S, et al. The circadian pattern of cardiac autonomic modulation in a middle-aged population. Clin Auton Res 2011; 21:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev 2012; 16:47–66. [DOI] [PubMed] [Google Scholar]
- 26.Rosenwinkel ET, Bloomfield DM, Arwady MA, et al. Exercise and autonomic function in health and cardiovascular disease. Cardiol Clin 2001; 19:369–387. [DOI] [PubMed] [Google Scholar]


