Abstract
Programmed death-1 (PD-1) is crucial in cancer and is well characterized as a negative T-cell regulator that functions by delivering inhibitory signals. We aimed to evaluate the relationship between PD-1 polymorphisms (rs10204525, rs2227982, and rs7421861) and breast cancer risk.
We selected 560 breast cancer patients and 583 age-, sex-, and ethnicity-matched healthy controls from Northwest China. The PD-1 polymorphisms were genotyped by using Sequenom MassARRAY. Associations were estimated with odds ratios (ORs) and 95% confidence intervals (95% CIs).
For the rs10204525 and rs7421861 polymorphisms, no differences in breast cancer risk were found in any of the genetic models. For the rs2227982 polymorphism, the variant genotypes were significantly associated with decreased breast cancer risk (CT vs CC: OR = 0.68, 95% CI = 0.52–0.91; CT + TT vs CC: OR = 0.69, 95% CI = 0.53–0.90). In analyses stratified by age, the decreased risk was observed among the younger subjects (OR = 0.68, 95% CI = 0.47–0.97). We found that the decreased risk observed for the variant genotypes of rs2227982 was associated with the Her-2 status (CT vs CC: OR = 0.55, 95% CI = 0.37–0.84; CT + TT vs CC: OR = 0.56, 95% CI = 0.38–0.82). The haplotype analysis showed that the Ars10204525 Trs2227982 Crs7421861 haplotype was associated with a significantly decreased risk of breast cancer (OR = 0.50, 95% CI = 0.34–0.75).
Our findings support an association between the PD-1 rs2227982 polymorphism and decreased breast cancer risk, especially in Her-2 positive breast cancer patients in the Chinese population.
INTRODUCTION
Breast cancer is the 2nd cause of cancer death among females in more developed countries and remains the leading cause of cancer death among females in less developed countries.1 Furthermore, the incidence of breast cancer is increasing in developing countries. Although early diagnosis has contributed to the success of therapy, breast cancer remains a major women's health problem.2 The etiology of breast cancer is complicated and still unclear. Genetic mutations and environmental factors play an important role in the development and progression of breast cancer.3 The APE1 656 T>G polymorphism is reported to have a protective effect against breast cancer.4 In Caucasians, the CC homozygote of rs1800872 polymorphism in IL-10 gene has a 25% decreased risk of breast cancer compared to patients with the AA and AC genotypes.5 An increasing number of studies have shown that the immune system plays an important role in resisting and eliminating cancer cells, and can influence the occurrence of breast cancer.6,7 T cells have been shown to play the major role in the antitumor immune response.8
Programmed death-1 (PD-1, also called CD279), a 55-kDa type I trans-membrane glycoprotein and a member of the immunoglobulin superfamily, has been well characterized as a negative regulator of T cells and functions by delivering inhibitory signals. In a subgroup of thymic T-lymphocytes, PD-1 is produced in a way of constitutively expression, with up-regulated expression found in activated T-cells, B-cells, and myeloid cells.9,10 Through interactions between PD-1 and its ligands PD-L1 (B7-H1; CD274) or PD-L2 (B7-DC; CD273), PD-1 strongly inhibits the proliferation of CD4 and CD8 T lymphocytes and their cytokine production.11–13 Previous studies have emphasized the significant role of PD-1 in human disease. PD-1 deficiency results in the development of a lupus-like disease or a dilated cardiomyopathy in animal models.14,15 Accumulating evidence has shown that PD-1 is also crucial in human cancer. Up-regulated expression of PD-1 by cancer-specific T16–20 and the expression of PD-L1 by epithelial cancers21–23 suggested that PD-1/PD-L1 signaling pathway could maintain an immunosuppressive tumor microenvironment for tumors to evade immunity. Previous studies have shown that blocking the PD-1/PD-L1 pathway can result in an efficient antitumor T-cell response and better control of tumor growth.24 Immunotherapy clinical trials using antibody-mediated PD-1 blockade as a strategy are in progress in patients with various cancers.25,26 Patients suffering from melanoma, renal cell carcinoma, or nonsmall cell lung cancer, showed objective responses (responses rates, 6–28%), with intravenous injection of these antibodies. Therefore, it is important to confirm the role of PD-1/PD-L1 signaling pathway in breast cancer, in order to find out whether antibody therapies targeting this pathway could be suitable for breast cancer patients.
Recently, 2 studies focused on the genetic variants of PD-1 to investigate the relationship between genetic polymorphisms in PD-1 and susceptibility to breast cancer.27,28 Hua et al28 found that rs2227982 and rs7421861, but not rs36084323 and rs2227981, may contribute to the risk and development of breast cancer. In the study by Haghshenas et al27 the results showed no association between rs11568821 and rs2227982 polymorphisms and susceptibility to breast cancer. In addition, previous reports showed that the PD-1 rs10204525 polymorphism was associated with the development of hepatocellular carcinoma29 and esophageal cancer30 in a Chinese population. To clarify these inconsistent conclusions, we conducted this case–control study to determine the association between the PD-1 gene polymorphisms (rs10204525 A>G, rs2227982 C>T, and rs7421861 T>C) with breast cancer susceptibility in the Chinese Han population.
METHODS
Ethics Statement
This study was approved by the ethics committee of the Second Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China). The research protocol was implemented in accordance with the approved guidelines.
Subjects
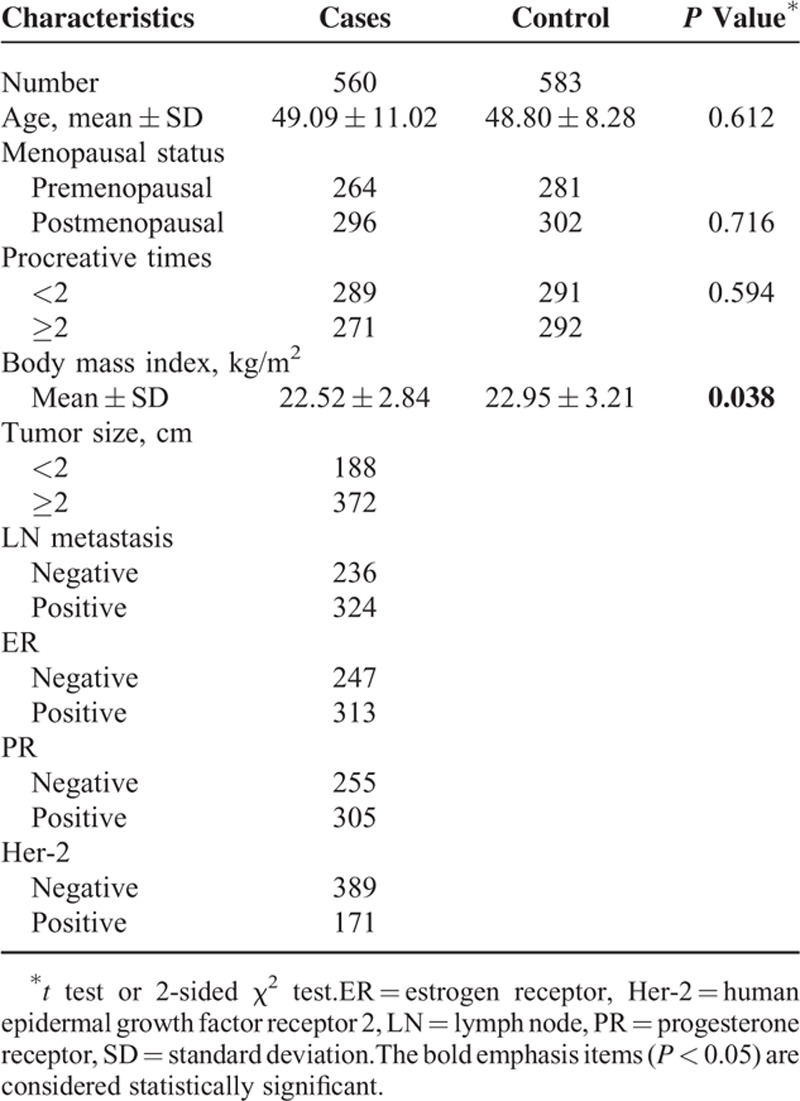
The cases included 560 Chinese women with sporadic breast cancer (mean age, 49.09 ± 11.02 years), whose blood samples were collected within 1 week after diagnosed. The control group was composed of 583 age- (mean age, 48.80 ± 8.28 years) and sex-matched healthy individuals (who were recruited volunteers) without any history of autoimmunity and malignancy (Table 1). All subjects were recruited from the Second Affiliated Hospital of Xi’an Jiaotong University (Shaanxi Province, China) between June 2010 and June 2014.31 And they were all of Han nationality from Northwest China. All breast cancer cases were confirmed by histological examination to be adenocarcinoma. Data outlining the clinicopathological characteristics of the patients, including tumor size, clinical stages, lymph node involvement, menopausal status, procreative times, estrogenic receptor (ER) status, progesterone receptor (PR) status, and human epidermal growth factor receptor type 2 (Her-2) status, were obtained from the patients’ medical records (Table 1). The methods were carried out in accordance with the approved guidelines.32 All participants were informed that their blood samples would be used for research projects, and their written consents were obtained. Following a self-administered, approximately 2 mL of venous blood sample was collected from each subject.
TABLE 1.
Characteristics of Breast Cancer Cases and Cancer-Free Controls
DNA Extraction and Genotyping
The blood samples were collected into tubes containing ethylene diaminetetra-acetic acid. The samples were then centrifuged at 8000g for 180 s at room temperature and stored at −80°C until analysis. Genomic DNA from the leukocytes of the peripheral blood was extracted by Universal Genomic DNA Extraction Kit Ver. 3.0 (TaKaRa, Akita, Japan). DNA concentration was measured by spectrometry (DU530 UV/VIS spectrophotometer, Beckman Instruments, Fullerton, CA). Three polymorphisms (rs10204525, rs2227982, and rs7421861) were selected for investigation in the present study. The Sequenom MassARRAY Assay Design 3.0 software was used to design the Multiplexed SNP MassEXTEND assay.33 Single nucleotide polymorphism (SNP) genotyping was performed by using the Sequenom MassARRAY RS1000 according to the standard protocol recommended by the manufacturer.34 The corresponding primers used for each SNP in the present study are listed in Table 2. The Sequenom Typer 4.0 software was used to perform data management and analyses.34
TABLE 2.
Primers Used for This Study
Statistical Analysis
In the overall population, the allelic frequencies of rs10204525, rs2227982, and rs7421861 are 0.352, 0.137, and 0.272, respectively. In the Chinese population, the allelic frequencies of the 3 polymorphisms are 0.302, 0.488, and 0.165, respectively.35 All statistical analyses were performed by using the SPSS software package (version 20.0; SPSS Inc., Chicago, IL). The Hardy–Weinberg equilibrium (HWE) was evaluated by comparing the expected and observed frequencies using algorithms in the Alrequin 3.1 program (L. Excoffier, CMPG, University of Berne, Switzerland). The observed genotype frequencies were compared with the expected values calculated from the HWE theory (p2 + 2pq + q2 = 1, where p is the frequency of the wild-type allele and q is the frequency of the variant allele) by using a χ2 test, with a degree of freedom equal to 1, among the cases and controls, respectively. The Pearson χ2 test was used to determine any significant differences in allele and genotype frequencies between the cases and the controls. The degree of risks associated with the alleles, genotypes, and haplotypes were estimated with an odds ratio (OR) and 95% confidence interval (CI). We evaluated the risks in the dominant (AA + Aa vs aa), recessive (aa vs Aa + AA), and allele (a vs A) models, where A is the major allele and a is the minor allele. For all of these tests, a 2-sided P < 0.05 was considered to be statistically significant.
RESULTS
Characteristics of the Study Population
The general characteristics of the subjects are summarized in Table 1. As expected, no significant differences in the distributions of age, menopausal status, and procreative times were found between the case and control groups (P > 0.05), which indicated that the cases and controls of this study were adequately matched for general characteristics. It is interesting that the body mass index (kg/m2) significantly differed between the case and control groups (P = 0.038), which confirmed that breast cancer is probably linked to weight in women. The genotypic frequencies for the PD-1 rs10204525, rs2227982, and rs7421861 polymorphisms among the controls were within the HWE (P = 0.8797, P = 0.5034, and P = 0.7456, respectively).
PD-1 Gene Polymorphisms and the Risk of Breast Cancer
The frequencies of the genotypes and alleles of the PD-1 gene polymorphisms in the breast cancer cases and healthy controls are shown in Tables 3–5, respectively. The frequencies of the genotypes AA, AG, and GG in the rs10204525 polymorphism were 46.0% (257/559), 44.4% (248/559), and 9.6% (54/559), respectively, in breast cancer patients and 50.0% (291/582), 41.2% (240/582), and 8.2% (51/582), respectively, in the control group. The frequencies of genotypes CC, CT, and TT in the rs2227982 polymorphism were 30.9% (172/557), 46.1% (257/557), and 23.0% (128/557), respectively, in the patients and 23.5% (137/582), 51.4% (299/582), and 25.1% (146/582), respectively, in the control group. The frequencies of genotypes TT, TC, and CC in the rs7421861 polymorphism were 60.9% (341/560), 35.0% (196/560), and 4.1% (23/560), respectively, in the patients and 59.8% (347/580), 35.3% (205/580), and 4.8% (28/580), respectively, in the control group.
TABLE 3.
Genotype Frequencies of PD-1 rs10204525 Polymorphism in Cases and Controls
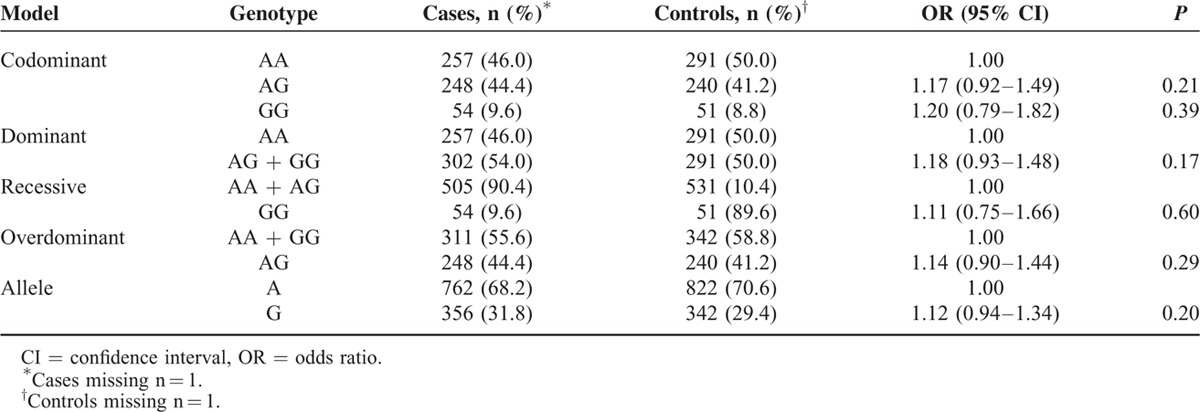
TABLE 5.
Genotype Frequencies of PD-1 rs7421861 Polymorphism in Cases and Controls
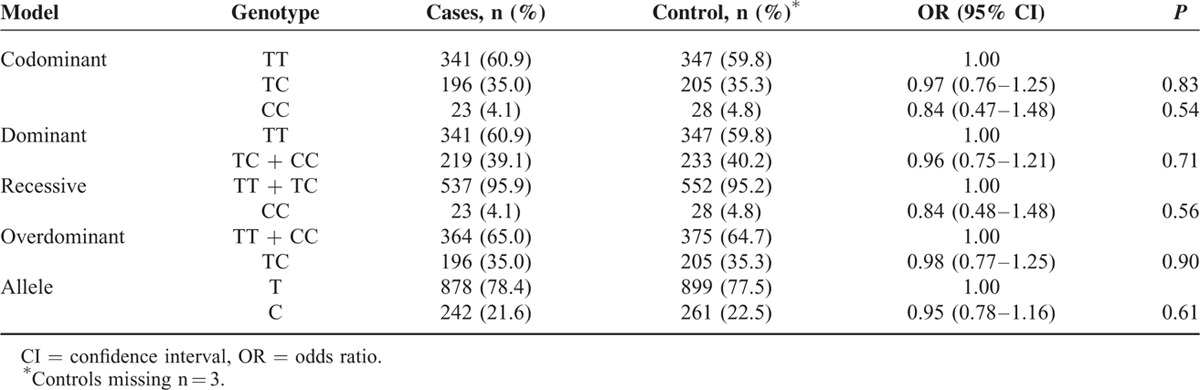
TABLE 4.
Genotype Frequencies of PD-1 rs2227982 Polymorphism in Cases and Controls
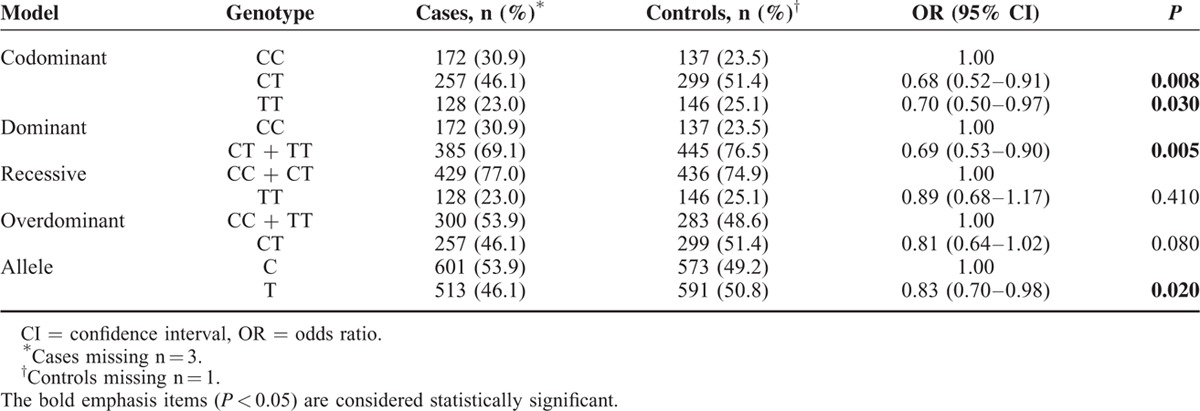
Statistical analysis revealed no significant association between the rs10204525 polymorphism and breast cancer risk in the dominant (AA + AG vs GG, OR = 1.18, 95% CI = 0.93–1.48, P = 0.17), recessive (GG vs AG + AA, OR = 1.11, 95% CI = 0.75–1.66, P = 0.60), and allele (G vs A, OR = 1.19, 95% CI = 0.90–1.58, P = 0.21) models. For rs7421861, there were also no significant associations in any of the genetic models (P > 0.05). For rs2227982, we obtained positive results. Both the CT and CC genotypes had lower frequencies in the breast cancer patients than in the controls (CT vs CC, OR = 0.68, 95% CI = 0.52–0.91, P = 0.008; TT vs CC, OR = 0.70, 95% CI = 0.50–0.97, P = 0.03). In addition, the results showed a significant association between the rs2227982 polymorphism and a decreased risk of breast cancer (the dominant model: CT + TT vs CC, OR = 0.69, 95% CI = 0.53–0.90, P = 0.005; the allele model: T vs C, OR = 0.83, 95% CI = 0.70–0.98, P = 0.02).
PD-1 Gene Polymorphisms and Clinicopathological Features
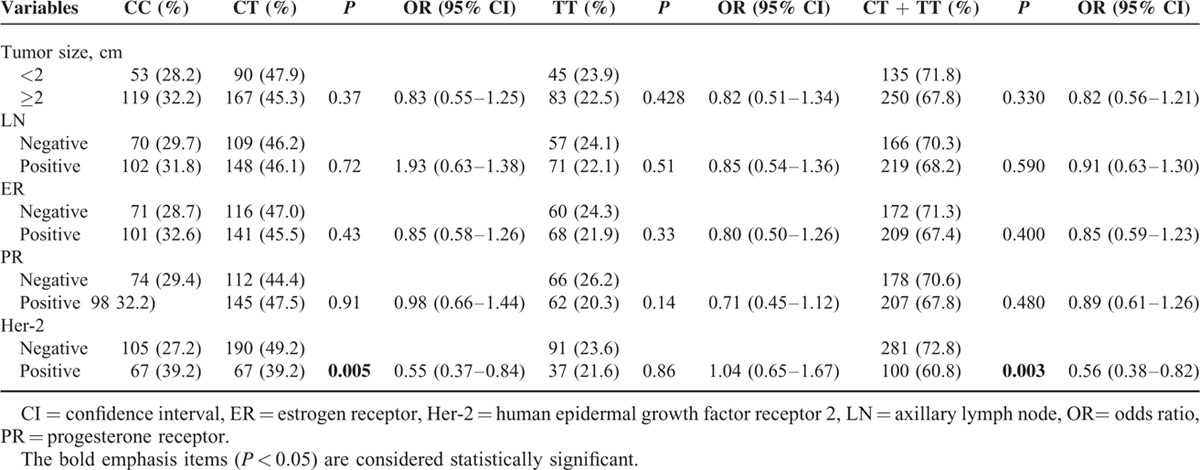
We also analyzed the association between the polymorphisms of the PD-1 gene and a series of clinicopathological features, including tumor size, lymph node metastasis, and the statuses of ER, PR, and Her-2. As shown in Table 6, the same analyses were also performed for the clinical features. When the CC genotype was used as the reference, we found that a decreased association between the variant genotypes of rs2227982 and Her-2 status (CT vs CC: OR = 0.55, 95% CI = 0.37–0.84, P = 0.005; CT + TT vs CC: OR = 0.56, 95% CI = 0.38–0.82, P = 0.003). However, no significant association was found between the rs2227982 polymorphism and the other clinical parameters of breast cancer patients. However, for rs10204525 and rs7421861 polymorphisms, we found no significant association between the 2 polymorphisms and the clinical parameters of the breast cancer patients (data not shown).
TABLE 6.
Associations Between the PD-1 rs2227982 Polymorphism and Clinical Characteristics of Patients With Breast Cancer
Stratified Analysis of PD-1 Polymorphisms and Breast Cancer Risk
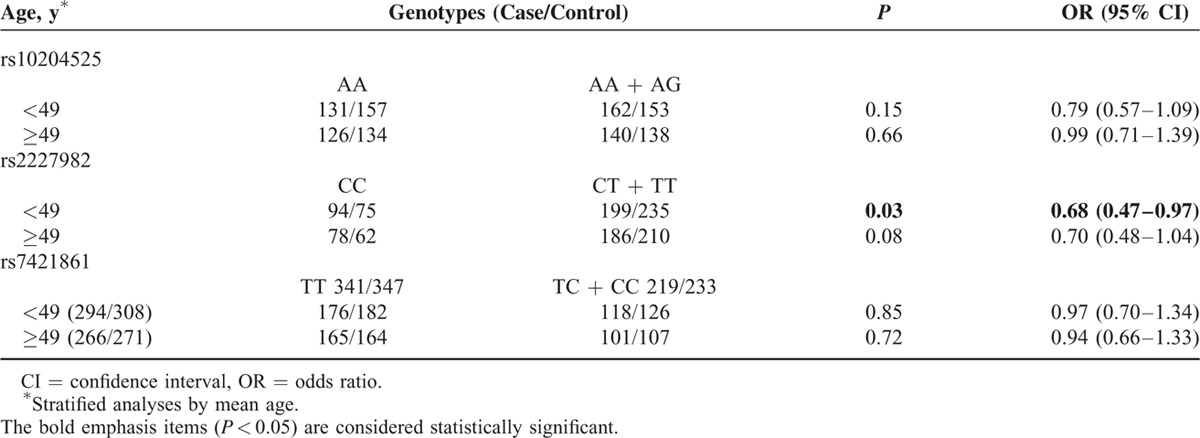
We then evaluated the effect of the PD-1 gene polymorphisms on breast cancer as stratified by age. As shown in Table 7, the risk effect of the rs2227982 variant genotypes (CT/TT) was more pronounced in younger subjects (OR = 0.68, 95% CI = 0.47–0.97, P = 0.03) rather than older subjects (OR = 0.70, 95% CI = 0.48–1.04, P = 0.08). The same analyses were also performed for the rs10204525 and rs7421861 polymorphisms; however, no positive results were observed.
TABLE 7.
Association Between the PD-1 Polymorphisms and Age of Patients With Breast Cancer
Association Between PD-1 Haplotypes and Breast Cancer Risk
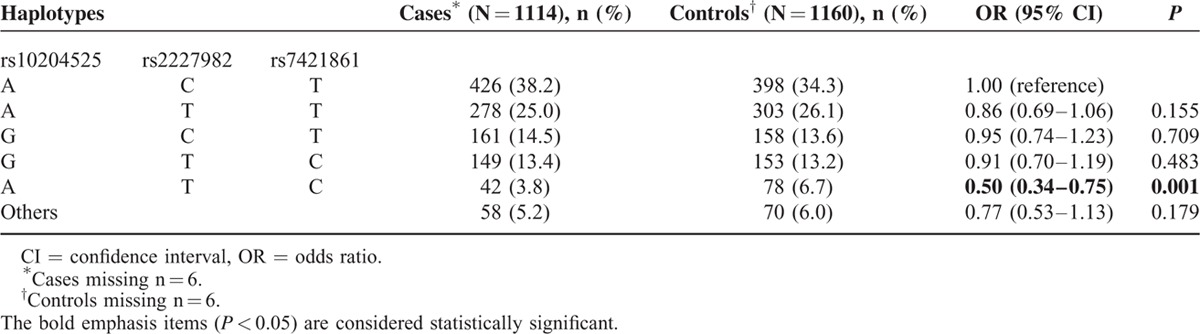
We further analyzed the association between haplotypes and the risk of breast cancer. In comparison to the most common haplotype Ars10204525 Crs2227982 Trs7421861, the Ars10204525 Trs2227982 Crs7421861 haplotype was associated with a significantly decreased risk of breast cancer (OR = 0.50, 95% CI = 0.34–0.75, P = 0.001, Table 8). We did not observe any associations with any other haplotypes in breast cancer.
TABLE 8.
Haplotype Frequencies of PD-1 Polymorphisms and Breast Cancer Risk
DISCUSSION
The immune system plays an important role in resisting and eliminating cancer cells and can influence the occurrence of cancer. As an immune gene with potent inhibitory effects on immune cells, PD-1 merits further investigations. The determination of genetic polymorphisms is a new route to investigate the etiology of such complex genetic diseases.36 The identification of SNPs that affect the PD-1 gene expression and contribute to cancer susceptibility is important, as it may help to predict at-risk individuals and clarify pathophysiological mechanisms relevant to cancer. PD-1 is an inhibitory receptor expressed on activated T and B cells whose activity may suppress antitumor immunity.37 Inhibitors of PD-1 may be promising in the treatment of triple-negative breast cancer by targeting the PD-1/PD-L1 immune checkpoint. Many studies have investigated the association of PD-1 gene variations with cancers. However, the results were inconsistent. Therefore, we chose the PD-1 polymorphisms rs10204525, rs2227982, and rs7421861, in order to investigate the association between the PD-1 gene and the risk of breast cancer in the Chinese population. In the present study, our data revealed that some of the alleles and genotypes of rs2227982, but not PD-1 rs10204525 and rs7421861, were associated with breast cancer risk.
In the PD-1 polymorphism rs10204525 A>G, we found that the frequencies of the rs10204525 genotypes AA, AG, and GG were not significantly different between the case and control groups (P > 0.05). Moreover, we analyzed the association between the rs10204525 polymorphism and breast cancer risk in the dominant and recessive models, but found no significant association between this SNP and breast cancer risk (P > 0.05). These results conflict with studies in hepatocellular carcinoma29 and esophageal cancer.30 These studies also found that the G allele of this SNP was likely to be associated with the decreased risk of hepatocellular carcinoma and esophageal squamous cell carcinoma, suggesting that the PD-1 rs10204525 G allele may be related to the increase in T-cell activity. PD-1 rs10204525, located in the 3′ untranslated region, may be involved in the modulation of the inflammatory cytokine levels via linkage disequilibrium with other nucleotide polymorphisms.38 However, in this study, we found no association between the PD-1 rs10204525 G allele and breast cancer risk.
For PD-1 rs2227982 C>T polymorphism, we found that the frequencies of the rs2227982 genotypes CC, CT, and TT were significantly different between the case and control groups (P < 0.05). Similarly, the results showed a significant association between PD-1 rs2227982 polymorphism and decreased breast cancer risk in the dominant and recessive models. The genetic variation in rs2227982 may result in an amino acid substitution from alanine to valine, which could lead to a different structure and different function for PD-1 and further influence the progression of different diseases.39,40 Therefore, whether this amino acid change alters the protein structure or affects its function needs to be further studied.
For the PD-1 polymorphism rs7421861, there were different results in other cancers. The rs7421861 CT genotype was significantly associated with the risk of colorectal cancer compared to the wild-type TT genotype.41 However, rs7421861 was found to have no association with gastric cardia adenocarcinoma.35 In the present study, there was also no significant association between the rs7421861 polymorphism and breast cancer risk in any of the genetic models.
In the analysis between PD-1 gene polymorphisms and clinical presentations, we found that the variant genotypes of rs2227982 had decreased associations with Her-2 status. However, the other polymorphisms were found to be irrelevant to clinical characteristics. As it is known, Her-2 has emerged as a well-known molecular biomarker in breast cancer of similar importance as ER. Her-2 is considered a prognostic factor for breast cancer.42 Our results therefore suggest that rs2227982 may play an important role in forecasting the prognosis of breast cancer.
It has been believed that haplotypes may have greater power in influencing a clinical response than any SNP analysis.43 We further analyzed the relationship between haplotypes of the PD-1 rs10204525, rs2227982, and rs7421861 polymorphisms and breast cancer risk. The results showed that the Ars10204525 Trs2227982 Crs7421861 haplotype was associated with a significantly decreased risk of breast cancer.
Some important limitations of this study should be addressed in this study. First, the single-center design may preclude extrapolation of our findings to other patient populations or ethnic groups. Second, our sample size was relatively small, which may limit the strength of our stratified analyses. Third, we used a hospital-based case–control design, which may involve selection bias. Fourth, we did not consider other important risk factors (e.g., lifestyle, environmental background, and other benign breast lesions), as we did not have access to the relevant data for the cases and controls. Therefore, further large-scale and well-designed studies regarding different ethnicities are still required to confirm our findings.
In conclusion, our results suggested that the PD-1 rs2227982 polymorphism is associated with a decreased risk of breast cancer, especially in Her-2 positive breast cancer patients in the Chinese Han population.
Acknowledgment
The authors would like to thank Editage for the language editing.
Footnotes
Abbreviations: BC = breast cancer, CI = confidence interval, ER = estrogen receptor, Her-2 = human epidermal growth factor receptor 2, HWE = Hardy–Weinberg equilibrium, LN = lymph node, OR = odds ratio, PD-1 = programmed death-1, PR = progesterone receptor, SNP = single nucleotide polymorphism.
HR and YL contributed equally to this work and share joint first authorship.
This study was supported by the National Natural Science Foundation, China (No. 81471670 and 81274136); the China Postdoctoral Science Foundation (No. 2014M560791 and 2015T81037); the Fundamental Research Funds for the Central Universities, China (No. 2014qngz-04); and the specialized Research Fund of the Second Affiliated Hospital of Xi’an Jiaotong University, China (RC [GG] 201203).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Maccio A, Madeddu C, Mantovani G. Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: new therapeutic perspectives. Obes Rev 2009; 10:660–670. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph A, Chang-Claude J, Schmidt MK. Gene-environment interaction and risk of breast cancer. Br J Cancer 2016; 114:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang H, Dai Z, Ma X, et al. A genetic variant in the promoter of APE1 gene (-656 T>G) is associated with breast cancer risk and progression in a Chinese population. Gene 2013; 531:97–100. [DOI] [PubMed] [Google Scholar]
- 5.Dai ZJ, Wang XJ, Zhao Y, et al. Effects of interleukin-10 polymorphisms (rs1800896, rs1800871, and rs1800872) on breast cancer risk: evidence from an updated meta-analysis. Genet Test Mol Biomarkers 2014; 18:439–445. [DOI] [PubMed] [Google Scholar]
- 6.Caras I, Grigorescu A, Stavaru C, et al. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother 2004; 53:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andre F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res 2013; 19:28–33. [DOI] [PubMed] [Google Scholar]
- 8.Mojtahedi Z, Mohmedi M, Rahimifar S, et al. Programmed death-1 gene polymorphism (PD-1.5 C/T) is associated with colon cancer. Gene 2012; 508:229–232. [DOI] [PubMed] [Google Scholar]
- 9.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2:261–268. [DOI] [PubMed] [Google Scholar]
- 10.Kitazawa Y, Fujino M, Wang Q, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation 2007; 83:774–782. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter L, Fouser LA, Jussif J, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol 2002; 32:634–643. [DOI] [PubMed] [Google Scholar]
- 13.Haspot F, Fehr T, Gibbons C, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood 2008; 112:2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11:141–151. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001; 291:319–322. [DOI] [PubMed] [Google Scholar]
- 16.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia (New York, NY) 2006; 8:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res 2007; 67:1842–1852. [DOI] [PubMed] [Google Scholar]
- 18.Ghebeh H, Barhoush E, Tulbah A, et al. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: implication for immunotherapy. BMC Cancer 2008; 8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sfanos KS, Bruno TC, Meeker AK, et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate 2009; 69:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793–800. [DOI] [PubMed] [Google Scholar]
- 22.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003; 170:1257–1266. [DOI] [PubMed] [Google Scholar]
- 23.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8:467–477. [DOI] [PubMed] [Google Scholar]
- 24.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haghshenas MR, Naeimi S, Talei A, et al. Program death 1 (PD1) haplotyping in patients with breast carcinoma. Mol Biol Rep 2011; 38:4205–4210. [DOI] [PubMed] [Google Scholar]
- 28.Hua Z, Li D, Xiang G, et al. PD-1 polymorphisms are associated with sporadic breast cancer in Chinese Han population of Northeast China. Breast Cancer Res Treat 2011; 129:195–201. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Li N, Zhu Q, et al. Genetic variations of PD1 and TIM3 are differentially and interactively associated with the development of cirrhosis and HCC in patients with chronic HBV infection. Infect Genet Evol 2013; 14:240–246. [DOI] [PubMed] [Google Scholar]
- 30.Qiu H, Zheng L, Tang W, et al. Programmed death-1 (PD-1) polymorphisms in Chinese patients with esophageal cancer. Clin Biochem 2014; 47:612–617. [DOI] [PubMed] [Google Scholar]
- 31.Dai ZJ, Liu XH, Ma YF, et al. Association between single nucleotide polymorphisms in DNA polymerase kappa gene and breast cancer risk in Chinese Han population: a STROBE-compliant observational study. Medicine (Baltimore) 2016; 95:e2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Kang HF, Ma XB, et al. Functional promoter -765 G C variant in COX-2 gene is associated with the susceptibility of breast cancer in Chinese Han women. Cancer Cell Int 2014; 14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007; 39:347–351. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Jin T, Zhang J, et al. Polymorphisms of TREH, IL4R and CCDC26 genes associated with risk of glioma. Cancer Epidemiol 2012; 36:283–287. [DOI] [PubMed] [Google Scholar]
- 35.Tang W, Chen Y, Chen S, et al. Programmed death-1 (PD-1) polymorphism is associated with gastric cardia adenocarcinoma. Int J Clin Exp Med 2015; 8:8086–8093. [PMC free article] [PubMed] [Google Scholar]
- 36.Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447:1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Criscitiello C, Curigliano G. Immunotherapy of breast cancer. Prog Tumor Res 2015; 42:30–43. [DOI] [PubMed] [Google Scholar]
- 38.Bennet AM, Alarcon-Riquelme M, Wiman B, et al. Decreased risk for myocardial infarction and lower tumor necrosis factor-alpha levels in carriers of variants of the PDCD1 gene. Hum Immunol 2006; 67:700–705. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Lee YA, Woo DH, et al. Association of the programmed cell death 1 (PDCD1) gene polymorphism with ankylosing spondylitis in the Korean population. Arthritis Res Ther 2006; 8:R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Hu LH, Li YR, et al. Programmed cell death 1 gene polymorphisms is associated with ankylosing spondylitis in Chinese Han population. Rheumatol Int 2011; 31:209–213. [DOI] [PubMed] [Google Scholar]
- 41.Ge J, Zhu L, Zhou J, et al. Association between co-inhibitory molecule gene tagging single nucleotide polymorphisms and the risk of colorectal cancer in Chinese. J Cancer Res Clin Oncol 2015; 141:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrup M, Stal O, Olsen K, et al. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat 2000; 63:23–29. [DOI] [PubMed] [Google Scholar]
- 43.Cheng XL, Ning T, Xu CQ, et al. Haplotype analysis of CTLA4 gene and risk of esophageal squamous cell carcinoma in Anyang area of China. Hepatogastroenterology 2011; 58:432–437. [PubMed] [Google Scholar]


