Abstract
Breast cancer, the most common cancer in women, is a serious public health issue. Triple-negative breast cancer (TNBC), which lacks expression of the estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2, accounts for ∼15% of breast cancer cases. Treatment of TNBC patients has proven difficult because of the lack of expression of hormone receptors. We conducted a retrospective study to investigate the prognostic impact of histone methyltransferase, hSETD1A, on overall survival in TNBC cases after surgery. In total, 159 TNBC cases were enrolled and clinicopathological characteristics were obtained from medical records. hSETD1A status of each subject was determined using immunohistochemistry. The chi-squared test was used to compare 5-year overall survival rates of all subjects according to clinical characteristics, and both univariate and multivariate analyses were conducted to calculate the hazard ratios and 95% confidence intervals. Advanced tumor-node-metastasis stage stage, larger tumor size, vascular invasion, metastasis in the initial diagnosis, and hSETD1A expression were correlated with worse outcome. Among all factors identified, metastasis in the initial diagnosis had the greatest impact on survival. The results indicated that hSETD1A positivity was correlated with shorter survival among TNBC cases, suggesting it may serve as a prognostic biomarker for patients with TNBC.
INTRODUCTION
Breast cancer is the most common cancer in women; therefore, it is increasingly recognized as a serious, worldwide public health concern. Breast cancer is more prevalent in developed countries. More developed countries account for ∼50% of all breast cancer cases and 38% of deaths caused by breast cancer.1 In contrast, the incidence of breast cancer is relatively low in Africa and Asia when compared with Western Europe and North America. However, the incidence has been rising in some low prevalence countries, including China. The reason behind such a phenomenon has not yet been completely identified, but may be attributed partly to changing reproductive patterns, lack of physical exercise, and the prevalence of obesity among the female population.2 Moreover, the availability of screening, such as mammography, could also contribute to the elevated incidence due to an increase in detected cases. Breast cancer-related mortality has been attenuated in developed countries because of effective treatment and early detection because of effective screening programs.3 Unlike in developed countries, limited access to effective treatment and delayed introduction of screening programs has resulted in much higher breast cancer mortality rates in developing countries.4
The prognosis of breast cancer can be decided by various factors, for example, stage of breast cancer, which is an important prognostic factor when compared with others. In addition, advanced grade and age <40 or >80 years have been associated with poorer prognosis in daily practice.5 With the application of hormone therapy, it provides a solution for treating inoperable cases and can also serve as adjuvant therapy to inhibit cancer growth after surgery.6 Although hormone therapy does have its unique advantages when compared with other therapies, its effectiveness largely depends on the receptor status of breast cancer patients. Triple-negative breast cancer (TNBC), which lacks expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), is totally unresponsive to hormone therapy.7 Roughly 15% of breast cancer cases are identified as TNBC.8 Currently, the information on prognosis of TNBC patients is still conflicted; pooled data, however, revealed that 20-year survival rates with optimal treatment of TNBC are very close to those of hormone-positive breast cancer cases.9
Histone methyltransferase hSETD1A is a member of the trithorax family, which is involved with the methylation of histone H3 lysine 4 (H3K4), and H3K4 is associated with transcriptional activation. The alternation of methylation of H3K4 has been found in several human cancers, and a recent study revealed that hSETD1A is capable of regulating metastasis in breast cancer.10 However, previous studies have not been able to establish a link between hSETD1A and prognosis in TNBC. Therefore, we conducted a retrospective study to investigate the association between hSETD1A and overall survival in TNBC.
METHODS
Study Participants
The medical records of all female breast cancer cases that had received surgery at the Department of Surgery, Fuzhou General Hospital between January 2008 and December 2014 were intensively reviewed. In total, 982 cases with breast cancer were identified and enrolled in the first stage of our study. Based on the pathology reports, we further selected 159 TNBC cases that were reported to lack the expression of ER, PR, and HER2 as assessed using immunohistochemistry (IHC). All enrolled cases that had received breast-conserving surgery subsequently received postoperative radiotherapy. Systemic adjuvant chemotherapy was administered to all the patients, and none of them were treated with hormonal therapy or HER2-targeted therapy.
Clinicopathological characteristics of TNBC cases, including age, histological grade, menopausal status, tumor-node-metastasis stage (TNM) stage, vascular invasion, and lymphatic metastasis, were retrieved from the hospital's medical record database. The survival status of cases was obtained via multiple channels, including medical records, telephone interviews, and the Index System of Social Security Death. The duration of follow-up was estimated from the day of surgery to the end point of follow-up observation (November 2015). The duration of observation ranged from 150 to 2610 days with a median of 1020 days.
Overall survival refers to the percentage of TNBC cases that are alive from surgery to the date of observation. The endpoint event of our study was defined as deceased because of TNBC or its progression, and alive on the date of observation, or deceased because of other causes than TNBC, and its complications were defined as censoring. The Ethics Committee of Fuzhou General Hospital approved this study, and all procedures of this study were conducted under the guidance of the ethical principles contained in the Declaration of Helsinki.
Immunohistochemical Determination of hSETD1A
IHC was conducted to evaluate the hSETD1A expression in tissue samples acquired from 159 TNBC cases. Briefly, tissue samples were fixed in 10% buffered formalin and embedded in paraffin for a routine histologic examination. Tissue sections were cut from a paraffin block of each specimen and applied to slides for IHC. Slides were stained with hematoxylin and eosin with additional immunostains for ER, PR, HER2, and hSETD1A. Primary antibodies (rabbit monoclonal antibody; 1:100 dilution; ab70378, Abcam, MA) were used to evaluate hSETD1A. Staining was performed using goat anti-rabbit biotinylated IgG (Abcam, MA) and incubating in phosphate-buffered saline containing 1% bovine serum albumin for 30 min at ambient temperature, followed by incubation with the ABC reagent (Vectorlabs, CA) for an additional 30 minutes. Immunostaining was visualized using 3,30-diaminobenzidine (Sigma-Aldrich, Darmstadt, Germany).
Two pathologists evaluated all histologic and IHC tumor slides separately for nuclear grade. HER2 status was evaluated using IHC, and ER and PR were considered positive if there were at least 1% positive invasive tumor nuclei in the sample. HSETD1A was considered positive if 10% or more of tumor cells showed positive membrane expression (see Figure 1).
FIGURE 1.
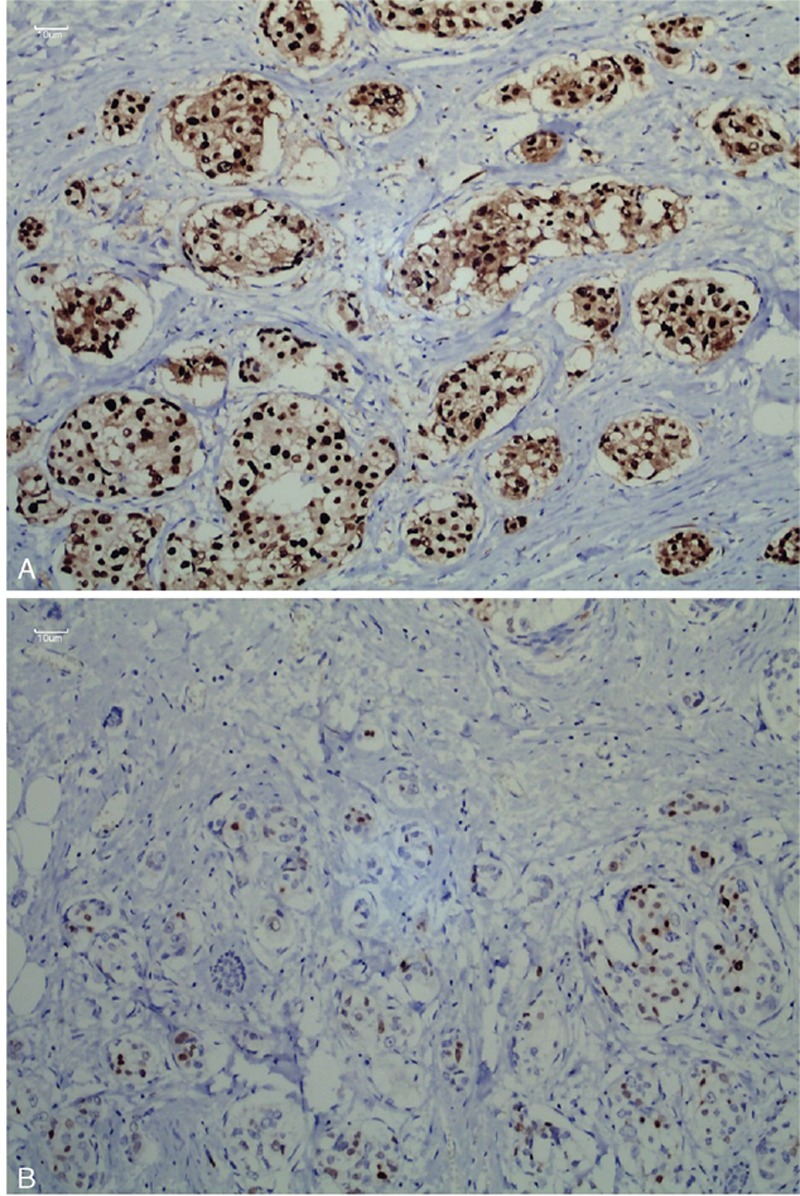
Immunohistochemical analysis of hSETD1A expression: (A) hSETD1A-positive and (B) hSETD1A-negative.
Quantitative Determination of hSETD1A Expression Using qRT-PCR
Samples from 30 TNBC cases that were still alive were subjected to qRT-PCR to compare hSETD1A expression in TNBC with corresponding adjacent noncancerous tissues. Samples were stored at −80 °C prior to RNA extraction. Total RNA was extracted from tissues according to instructions included with the High Pure RNA Isolation Kit (Omega, GA). Single-stranded cDNA was synthesized using a high-capacity cDNA reverse transcription kit (Omega, GA). Forward and reverse primer sequences were as follows: 5′-TGACTGGCTCAACGACACTC-3′ and 5′-TGATGGGTAGTAGCCACG-3′, respectively. The following thermal cycling conditions were used: 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 10 seconds, 60 °C for 30 seconds, and 72 °C for 10 seconds. Expression levels of mRNA were first normalized to GAPDH mRNA and then expressed as fold of normal using the 2-ΔΔCt method according to Liu et al.11
Statistical Analysis
The 5-year overall survival rate of all subjects according to clinical characteristics was calculated using the Kaplan–Meier method, and the statistical comparison was performed using the log-rank test. The comparison of age, survival, histological grade, menopausal status, TNM stage, tumor size, vascular invasion, and metastasis between hSETD1A-positive and hSETD1A-negative TNBC cases was also conducted using the chi-squared test. The above-mentioned clinical characteristics were included in the survival analysis, and the Cox proportional hazards regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). The number at risk of all subjects in regular interval was also demonstrated in results, in detail; it was calculated by reducing the number of deaths plus individuals censored at the end of previous period. Factors identified as statistically significant (P < 0.05) were further subjected to multivariate analysis, and the forward Wald method was applied to select variables with an entry probability of 0.05 and 0.10 for removal. A matched T test was used to compare hSETD1A expression among 30 paired TNBC tissue and adjacent noncancerous tissue samples determined using qRT-PCR. All statistical analyses were 2-sided and were performed using STATA version 12.0 (StataCorp LP, College Station, TX).
RESULTS
In total, 159 eligible TNBC cases were enrolled in our study and hSETD1A expression was determined using IHC. Clinical characteristics of enrolled cases were obtained by reviewing medical records and survival status was determined using multiple channels including medical records, telephone interviews, and the Index System of Social Security Death. Detailed characteristics of 159 TNBC cases are shown in Table 2. In total, 76 TNBC cases were deceased during our observation, and the cause of death (COD) of all deceased subjects was TNBC; no other COD was identified.
TABLE 2.
The Comparison of Characteristics Between hSETD1A-Positive and -Negative TNBC Cases

Comparison of 5-year Overall Survival Rates With Clinicopathologic Characteristics
Based on the results obtained from the log-rank test, we found that the 5-year overall survival rate was significantly higher in TNBC cases that were TNM stage I-II, had tumor sizes smaller than 2 cm, had an absence of vascular invasion and metastasis, and more importantly, that were hSETD1A-negative (P < 0.05). Therefore, hSETD1A may be a prognostic factor of overall survival among TNBC cases. No significant difference was observed when comparing overall survival with age, histological grade, or menopausal status (Table 1).
TABLE 1.
The Comparison of 5-Year Overall Survival by Characteristics Among TNBC Cases

Comparison of Clinicopathologic Characteristics With hSETD1A Status
Among the 159 TNBC cases we included in our study, 78 of them were identified as hSETD1A-negative, whereas the remaining 81 subjects were hSETD1A-positive. We further investigated the distribution of the above-mentioned characteristics among hSETD1A-positive and hSETD1A-negative TNBC cases using the chi-squared test. Among all factors, we found that the distribution of histological grade, TNM stage, vascular invasion, and metastasis were significantly different among the 2 groups with different hSETD1A expression status (P < 0.05). However, no significant difference was obtained when comparing the distribution of age, survival, menopausal status, or tumor size (P > 0.05) (Table 2).
Overall Survival Analysis of TNBC Cases
HRs and 95% confidence intervals of all analyzed factors are presented in Table 3. We found that TNM stage, tumor size, vascular invasion, metastasis, and hSETD1A status (see Figure 2) were significantly associated with overall survival in univariate analysis. The HR for hSETD1A was 4.16 with a 95% CI of 2.53 to 6.85. Based on the results of univariate analysis, we further conducted a multivariate analysis among the significant factors to address the potential confounding effects. After variable selection with an entry criterion of 0.05 and removal criterion of 0.10, metastasis and hSETD1A remained in the final equation. The HRs for metastasis and hSETD1A positivity were 3.10 (95% CI: 1.67–5.80) and 2.61 (95% CI: 1.50–4.57), respectively.
TABLE 3.
The Overall Survival Analysis on the TNBC Cases After Surgery

FIGURE 2.
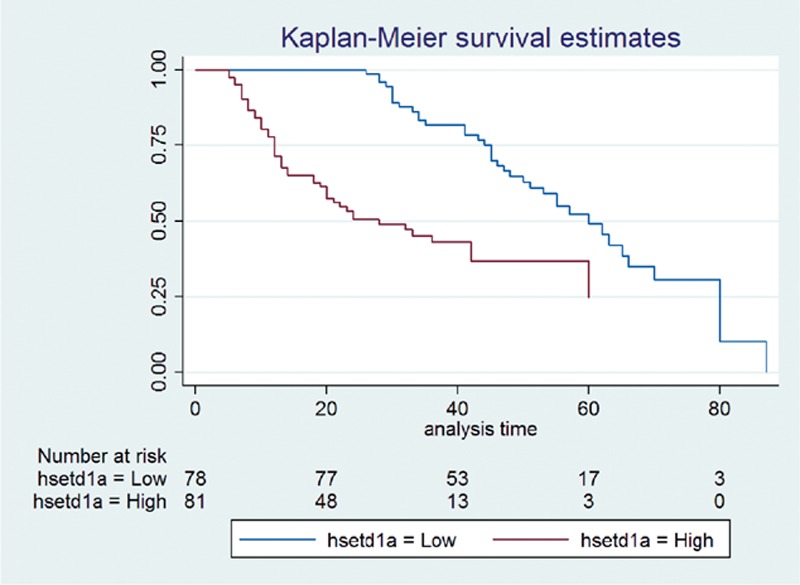
Kaplan–Meier analysis of overall survival in 159 TNBC cases in relation to the hSETD1A status. TNBC = triple-negative breast cancer.
Comparison of Quantitated hSETD1A Expression Among 30 Paired Tissues
We used qRT-PCR to examine the hSETD1A transcript level in 30 paired TNBC samples and noncancerous samples. The expression level of hSETD1A is significantly upregulated in the TNBC samples when comparing with the paired normal tissues. The mean and standard deviation for TNBC tissue and noncancerous tissue were 2.06 ± 0.83 and 0.97 ± 0.39, respectively. The mean and standard deviation of paired differences between the 2 groups were −1.09 and 1.03, respectively. With the degree of freedom of 29, and T statistics of −5.79, the P value was 0.000, suggesting that the difference was statistically significant (Figure 3).
FIGURE 3.
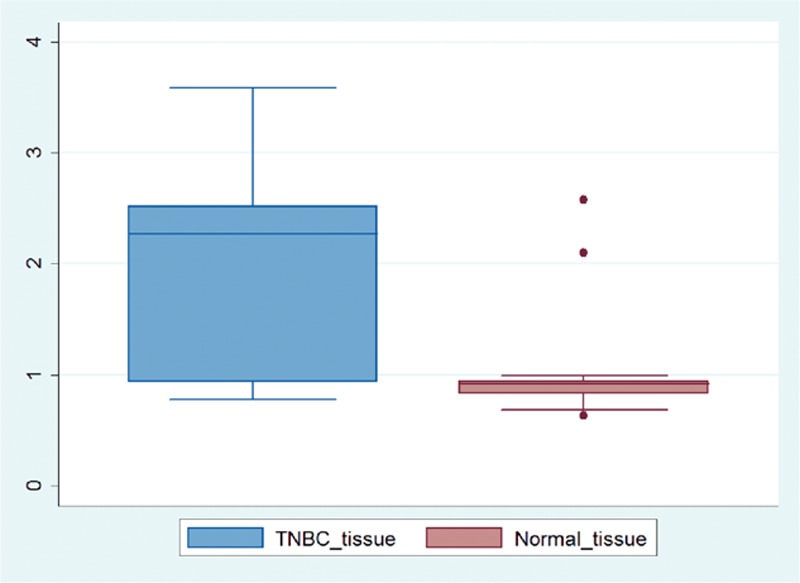
The quantitated hSETD1A expression between 30 paired TNBC and noncancerous samples. TNBC = triple-negative breast cancer.
DISCUSSION
We have performed a retrospective study on 159 TNBC cases after surgery with a median observation of 1020 days, and the association between certain clinical characteristics and TNBC overall survival was investigated. Moreover, we have performed IHC to determine the hSETD1A status of all enrolled cases and the impact of hSETD1A expression on the prognosis of TNBC.
We first sought to compare 5-year overall survival rates between different clinical characteristics. The TNM stage is a type of classification that involves local tumor growth, regional lymph node, and distant metastasis, and is widely applied in clinical practice to evaluate the progress of cancer globally. Generally speaking, overall survival of breast cancer is inversely associated with TNM stage, and a prospective study that enrolled 441 Korean breast cancer patients revealed that the advanced TNM stage was not only associated with poorer outcomes but also increased the probability of recurrence within 2 years after surgery.12 Consistent with these previous findings, we found that overall survival rates were significantly reduced among TNBC cases with TNM stage III–IV using the log-rank test. Additionally, the Kaplan–Meier method revealed that TNM stage III–IV was associated with shorter overall survival with an HR of 2.97 (95% CI: 1.86–4.76). The 5-year overall survival rate was also significantly lower between TNBC cases with tumor sizes >2 cm (16.2% vs. 44.8%) compared with tumor sizes ≤2 cm.
A strong and quantitative correlation between tumor size and breast cancer survival has been reported in the literature. The equation deduced from 2223 breast cancer cases suggested that each millimeter of tumor diameter was associated with an additional ∼1% chance of mortality.13 In addition, a cohort study with a large sample size further confirmed the impact of tumor size among breast cancer patients, and the impact of tumor size on survival was stronger among the lymph node positive cases when compared with the negative cases.14 Univariate analysis also suggested that a tumor size >2 cm was associated with worse outcomes with an HR of 2.20 (95% CI: 1.29–3.74). Comparison of the findings with those of other studies confirmed that tumor size was an independent prognostic factor in TNBC cases.
In this study, vascular invasion and metastasis were found to cause shorter survival among TNBC cases, with the most striking finding being that the 5-year overall survival rate reached 0% in TNBC cases in which metastasis was present in the first diagnosis. By definition, vascular invasion refers to tumor emboli present within a definite endothelial-lined space in the breast surrounding the invasive carcinoma,15 and vascular invasion has been recognized as a crucial step in the complicated process of metastasis as well as an important indicator in the decision-making process.
Currently, existing research has acknowledged the critical importance of its impact on survival among lymph node-negative breast cancer patients, with a mortality rate of 53% in patients with vascular invasion and 29% in those without it.16 Despite this, the role of vascular invasion in lymph node-positive breast cancer is still controversial. Our findings were consistent with a follow-up study conducted in 967 lymph node-positive breast cancer patients, which indicated that vascular invasion was also associated with poorer survival.17
Owing to limitation of sample size and concern regarding statistical power, we were not able to further divide our cases according to lymph node status; however, the results did support the prognostic value of vascular invasion in TNBC, both in overall survival rate comparisons and Kaplan–Meier analysis. As stated previously, metastasis in the first diagnosis has a great impact on overall survival in TNBC cases; for those without metastasis, the 5-year survival rate was 37.1%, but 0% in the presence of metastasis.
For many years, metastasis has been directly associated with poorer survival in breast cancer cases, and significant differences between types of metastasis has also been identified. Generally speaking, patients with liver, lung, or brain metastases have a poorer outcome when compared with patients with local metastases, such as the breast, chest wall, or armpit. Moreover, the survival of cases with bone metastasis has been classified as an intermediate prognosis. A cohort study performed among the Danish population showed that the 5-year survival rate for patients with bone metastasis was 9.5% (95% CI: 6.9–12.6).18 Although we have not calculated the survival rate by the type of metastasis because of insufficient data, strong evidence of worse outcome caused by metastasis has been observed both in the comparison of survival rates and univariate analysis. Furthermore, metastasis has also been included in the final equation calculated using multivariate analysis with an HR of 3.10 (95% CI: 1.67–5.80). Based on the evidence obtained, we can conclude that metastasis in the initial diagnosis is the major risk factor related to poorer survival in TNBC cases.
The main purpose of this study was to assess the impact of hSETD1A on overall survival among TNBC cases after surgery, and based on the results of IHC and statistical analysis, we found a positive correlation between them. The 5-year overall survival rate among hSETD1A-negative cases was 39.6%, which dropped to 3.5% among cases that were hSETD1A-positive. We further investigated the association between hSETD1A expression and prognosis in TNBC using univariate and multivariate analyses, and the results confirmed the positive association. The HR of hSETD1A in univariate and multivariate analyses were 4.16 (95% CI: 2.53–6.85) and 2.61 (95% CI: 1.50–4.57), respectively.
In recent years, epigenetics has become a hot topic in cancer development, and a great deal has been published in the scientific literature. Histone modification has been accepted as one of the key epigenetic regulatory mechanisms that plays a vital role in various biological processes. The methylation of H3K4 was found to be completed by a Histone-lysine N-methyltransferase 2 (KMT2) family of proteins, as well as hSETD1A.19 The most interesting part of this process was that ablation of hETD1A, but not other genes in the KMT2 family, had a dramatic impact on global H3K4me3 enrichment and gene expression.20 Previous animal experiments demonstrated that knockout of the MLL1 SET domain would result in phenotypic defects among rats, revealing the great importance of H3K4 methylation in regulating genetics and phenotypes.21–24 Moreover, elevation in H3K4me3 levels was observed during epithelial–mesenchymal transition, which is a process characterized by the loss of cell adhesion and increased cell mobility.25 Therefore, it is reasonable to assume that hSETD1A was involved in breast cancer development by promoting the epithelial–mesenchymal transition process.
In a study investigating the function of hSETD1A in colorectal cancer cells, researchers found that expression levels of hSETD1A were positively correlated with H3K4me3 enrichment at the promoters of Wnt/β-catenin target genes and the aberrant activation of these genes in human colorectal cancer.26 So far, little is known about the involvement of hSETD1A in carcinogenesis, and the previous cellular study suggested that it may work via the Wnt signaling pathway. As for breast cancer cell lines, hSETD1A expression appears to be positively associated with metastasis, and ablation of hSETD1A would suppress the migration and invasion in vitro and decrease metastasis in vivo.10 Furthermore, the comparison between hSETD1A quantitated expression among 30 paired TNBC and adjacent noncancerous tissues revealed that even in individual TNBC cases, expression of hSETD1A was significantly elevated in cancer tissues.
Owing to ethical concern and study design, we did not collect samples from all 159 TNBC cases and only 30 survival cases were included in the qRT-PCR. However, the significant difference obtained with the matched T test was sufficient to demonstrate the important role of hSETD1A in TNBC development and prognosis. Thus far, there is no population-based study regarding the association between hSETD1A status and prognosis in TNBC that has been reported, and the prognostic value of hSETD1A in TNBC has not been carefully assessed. Our study involved 159 TNBC cases and provided quantitative effects of hSETD1A on overall survival.
In summary, we conducted a retrospective study to identify prognostic factors in TNBC and to evaluate the impact of hSETD1A expression. According to our analysis, advanced TNM stage, larger tumor size, vascular invasion, metastasis in the initial diagnosis, and hSETD1A positivity were correlated with worse outcome. It has been generally realized that TNBC has its unique recurrence and developmental pattern and requires systemic therapy when compared with hormone-positive breast cancer. The results of our analysis revealed that hSETD1A expression may lead to shorter survival in TNBC cases, but because of limitation of sample size, further investigation with larger cohorts and longer observation times is still needed.
Footnotes
Abbreviations: ER = estrogen receptor, H3K4 = histone H3 Lysine 4, HER2 = human epidermal growth factor receptor 2, IHC = immunohistochemistry, MLL = mixed lineage leukemia, PR = progesterone receptor, TNBC = triple-negative breast cancer.
YYZ and KB equally contributed to this study.
Funding: the present study was supported by grants from the Committee of Jinshan District of Shanghai Science and Technology of Innovation Fund (2013-3-15) to Yanyan Zhu.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Sellers TA, Trapido E. Epidemiology—identifying the causes and preventability of cancer? Nat Rev Cancer 2006; 6:75–83. [DOI] [PubMed] [Google Scholar]
- 3.Youlden DR, Cramb SM, Dunn NA, et al. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012; 36:237–248. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010; 19:1893–1907. [DOI] [PubMed] [Google Scholar]
- 5.Brandt J, Garne JP, Tengrup I, et al. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol 2015; 13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaby V, Tawk R, Sanogo V, et al. A review of systematic reviews of the cost-effectiveness of hormone therapy, chemotherapy, and targeted therapy for breast cancer. Breast Cancer Res Treat 2015; 151:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulkes W, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363:1938–1948. [DOI] [PubMed] [Google Scholar]
- 8.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist 2011; 16 suppl 1:1–11. [DOI] [PubMed] [Google Scholar]
- 10.Salz T, Deng C, Pampo C, et al. Histone methyltransferase hSETD1A is a novel regulator of metastasis in breast cancer. Mol Cancer Res 2015; 13:461–469. [DOI] [PubMed] [Google Scholar]
- 11.Liu TC, Lin SF, Chang JG, et al. Epigenetic alteration of the SOCS1 gene in chronic myeloid leukaemia. Br J Haematol 2003; 123:654–661. [DOI] [PubMed] [Google Scholar]
- 12.O JH, Choi WH, Han EJ, et al. The prognostic value of (18)F-FDG PET/CT for early recurrence in operable breast cancer: comparison with TNM stage. Nucl Med Mol Imaging 2013; 47:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaelson JS, Silverstein M, Sgroi D, et al. The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer 2003; 98:2133–2143. [DOI] [PubMed] [Google Scholar]
- 14.Narod SA. Tumour size predicts long-term survival among women with lymph node-positive breast cancer. Curr Oncol 2012; 19:249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mascarel I, MacGrogan G, Debled M, et al. D2-40 in breast cancer: should we detect more vascular emboli? Mod Pathol 2009; 22:216–222. [DOI] [PubMed] [Google Scholar]
- 16.Lee AH, Pinder SE, Macmillan RD, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 2006; 42:357–362. [DOI] [PubMed] [Google Scholar]
- 17.Song YJ, Shin SH, Cho JS, et al. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011; 14:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cetin K, Christiansen CF, Sværke C, et al. Survival in patients with breast cancer with bone metastasis: a Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open 2015; 5:e007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser S, Schaft J, Lubitz S, et al. Multiple epigenetic maintenance factors implicated by the loss of Mll2 in mouse development. Development 2006; 133:1423–1432. [DOI] [PubMed] [Google Scholar]
- 20.Deng C, Li Y, Liang S, et al. USF1 and hSET1A mediated epigenetic modifications regulate lineage differentiation and HoxB4 transcription. PLoS Genet 2013; 9:e1003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terranova R, Agherbi H, Boned A, et al. Histone and DNA methylation defects at Hox genes in mice expressing a SET domain-truncated form of Mll. Proc Natl Acad Sci U S A 2006; 103:6629–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefevre GM, Patel SR, Kim D, et al. Altering a histone H3K4 methylation pathway in glomerular podocytes promotes a chronic disease phenotype. PLoS Genet 2010; 6:e1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Liu N, Yin Y, et al. Histone H3K4 methylation regulates hyphal growth, secondary metabolism and multiple stress responses in Fusarium graminearum. Environ Microbiol 2015; 17:4615–4630. [DOI] [PubMed] [Google Scholar]
- 24.Stein AB, Jones TA, Herron TJ, et al. Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. J Clin Invest 2011; 121:2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald OG, Wu H, Timp W, et al. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol 2011; 18:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salz T, Li G, Kaye F, et al. hSETD1A regulates Wnt target genes and controls tumor growth of colorectal cancer cells. Cancer Res 2014; 74:775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]


