Abstract
Few studies were conducted to explore the prognostic factors for nonendemic nasopharyngeal carcinoma (NPC) in the era of 3-dimensional conformal radiation therapy (3DCRT)/intensity-modulated radiation therapy (IMRT). The aim of this study was to evaluate the potential prognostic factors for nonendemic NPC.
Between January 2004 and December 2011, a total of 393 nonendemic NPC patients receiving 3DCRT/IMRT were reviewed according to the inclusion and exclusion criteria. The prognostic factors we analyzed included age, T stage, N stage, lymph node diameter, primary tumor volume, WHO histology types, and cranial nerve related symptoms. All patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) system. The factors found to be associated with the endpoints by univariate analyses were then entered into multivariate Cox proportional hazards regression analysis.
The median follow-up time was 61.4 months (range: 4–130 months). The 5-year local recurrent-free survival (LRFS), nodal relapse-free survival (NRFS), distant metastasis free survival (DMFS), and disease-specific survival (DSS) for all patients were 89.3%, 96.4%, 73.5%, and 74.3%, respectively. Multivariate analysis indicated that N stage (N2–3), WHO pathologic type II, and primary tumor volume (>23 mL) were 3 independent prognostic factors for DSS and DMFS. According to the number of prognostic factors, patients were divided into 3 risk groups: low-risk group (patients without any risk factors); intermediate-risk group (patients with only 1 risk factor); and high-risk group (patients with more than 2 risk factors). The 5-year DSS for low, intermediate, and high-risk groups were 91.5%, 75.2%, and 49.3%, respectively (P < 0.001). The 5-year DMFS for low, intermediate, and high-risk groups were 89.4%, 77.9%, and 49.4%, respectively (P < 0.001).
Advanced N stage (N2–3), larger tumor volume (>23 mL), and histological WHO type II are independently prognostic factors for nonendemic NPC patients in China.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is typically endemic in southern China and Southeast Asia where undifferentiated NPCs occur more frequently.1 In the era of 3-dimensional conformal radiation therapy (3DCRT)/intensity-modulated radiation therapy (IMRT) combined with chemotherapy, the NPC patients’ survivals have improved significantly. Especially for the patients from endemic area, the 5-year overall survival (OS), local recurrent-free survival (LRFS), and distant metastasis free survival (DMFS) have achieved approximately 84%, 92%, and 84%, respectively.1,2 However, the survival outcomes are slightly lower for nonendemic area NPC patients receiving 3DCRT/IMRT, and the 5-year OS, LRFS, and DMFS are approximate 72%, 83%, and 73%, respectively.3–6 The reasons of survival difference between endemic and nonendemic NPC remain unknown. May be there are potential tumor intrinsic prognostic factors that impact on slightly inferior survival outcomes for nonendemic NPC patients.
Besides TNM stages, which is the most common index to predict the NPC patients’ prognosis, there are also some potential prognostic factors evaluated by many studies, such as primary tumor volume, tumor diameter, WHO histological types, C-reaction protein, serum lactic dehydrogenase, body mass index, and serum Epstein-Barr virus (EBV) DNA.2,7–12 The problem is that most of these studies are from endemic NPC areas; it is unknown whether these prognostic factors from endemic studies could directly apply to nonendemic NPC patients. Until now, few studies were conducted to explore the prognostic factors for nonendemic NPC because the incidence of this disease is lower. The aim of this study was to explore the specific prognostic factors for nonendemic NPC.
MATERIALS AND METHODS
Patients’ Selection
Between January 2004 and December 2011, a total of 423 patients with nonmetastatic and histologically proven NPC were identified. All pathological slices were reviewed by 3 experienced pathologists. The inclusion criteria were as follows: histologically confirmed nasopharyngeal squamous cell carcinoma by biopsy; AJCC stage I-IVB without distant metastasis; no previous treatment for NPC; no history of previous head neck malignant disease; patients’ primary residences limited to the northwest of China, which is a nonendemic area for NPC; receiving 3DCRT/IMRT as initial treatment; the Karnofsky performance score ≥70. The exclusion criteria included nonsquamous cell carcinoma; long-term resident history in endemic area; and received 2-dimension radiation therapy. Of 30 patients excluded in this study, 15 patients had nonsquamous cell carcinoma or nonundifferentiated carcinoma, 11 patients had long-term living history in endemic area, and 4 patients received 2-dimension radiation therapy. This study has been approved by ethnic committee.
Clinical Staging
All patients had complete history and physical examinations, blood work, imaged by computed tomography (CT) and magnetic resonance imaging (MRI) of head and neck, and chest images, abdominal sonography, and whole body bone scan. The positron emission tomography (PET)-CT was performed on 54 of 393 patients (13.7%). Patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) system. Two radiologists reviewed all the imaging records and disagreements were resolved by consensus.
Treatment Methods
Radiation Therapy
All patients were immobilized in the supine position with head, neck, and shoulder thermoplastic mask. A contrasting CT image was obtained from the simulator for treatment planning. All patients were scanned with serial 3 mm slices from vertex to 5 cm below clavicles. MRI was the most common image to accurately delineate the target for most of patients. Of 54 patients who received PET/CT, 47 of their images were fused into the treatment planning system for target delineation.
The treatment planning approaches have been described by previous studies.7,13 The gross tumor volume (GTV) includes the nasopharyngeal GTV (GTVnx) and involved lymph nodes volume (GTVnd) as demonstrated by imaging and physical examinations. The high-risk clinical tumor volume of nasopharynx (CTVnx) included GTVnx and 5 mm margin and encompasses the entire nasopharyngeal mucosa. The CTV1 included CTVnx and the area with high-risk tumor invasion and lymphatic levels. The CTV2 covered the lower lymphatic levels. The planning target volume (PTV) was created on the basis of the CTVs and 3 mm margin. The prescribed radiation dose was defined as follows: a total dose of 72.6 Gy in 33 fractions at 2.2 Gy/fraction to the PTV of GTVnx, 66 to 72.6 Gy to positive lymph nodes, 66 Gy to PTV of CTVnx, 60 to 63 Gy to PTV of CTV1, and 50.4 to 56 Gy to PTV of CTV2. All patients were treated with 1 fraction daily for 5 days per week. The dose received by each organ at risk (OAR) should be no more than its tolerance.14
Chemotherapy
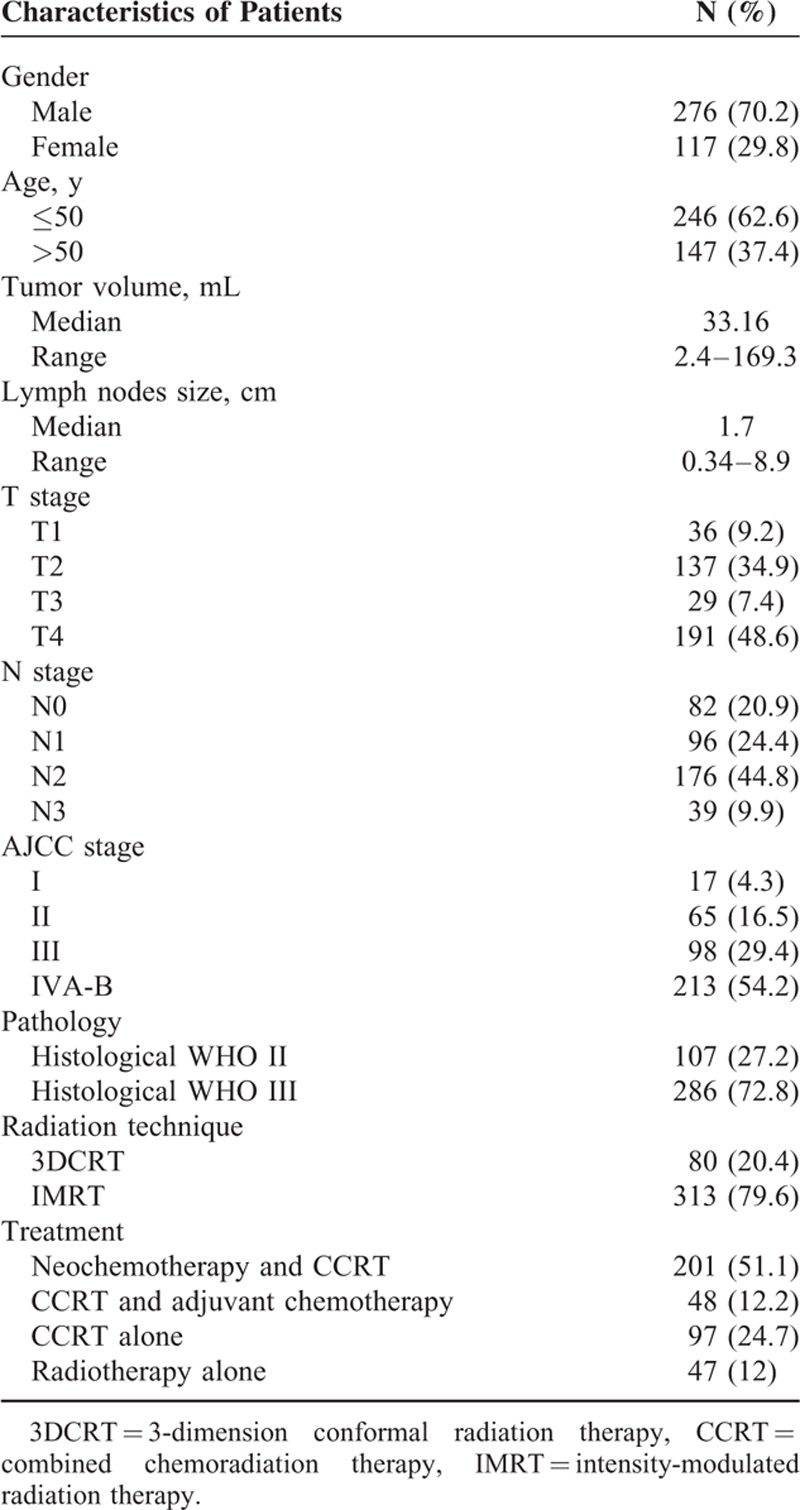
During the study period, chemotherapy was not recommended to the patients with stage I and contraindications for its use. A total of 346 patients received chemotherapy combined with radiation therapy. The neoadjuvant chemotherapy consisted of 2 to 3 cycles of TP regimen (docetaxel 75 mg/m2 intravenous injection in d1, cisplatin 30 mg/m2/d IV for 3 days) or PF regimen (cisplatin 30 mg/m2/d IV for 3 days, 5-FU 800–1000 mg/m2/d IV in d1–d5) or GP regimen (gemcitabine 1000 mg/m2/d1, 8 IV, cisplatin 30 mg/m2/d IV for 3 days) at a 2 weeks’ interval before the initial radiotherapy. Concurrent chemotherapy was only consisted of cisplatin (100 mg/m2 every 3 weeks or 40 mg/m2 weekly). If adjuvant chemotherapy was performed, the chemotherapy would be administrated at a 3 weeks interval after the initial radiotherapy. The regimens of adjuvant chemotherapy consisted of 2 to 3 cycles of TP or PF or GP, which dosages were same as the neoadjuvant chemotherapy above mentioned. For all patients, 201 patients (51.1%) received neoadjuvant chemotherapy and chemoradiation therapy, 48 patients (12.2%) patients received chemoradiation therapy and adjuvant chemotherapy, 97 patients (24.7%) received chemoradiation therapy, and 47 patients (12%) received radiation therapy alone (Table 1).
TABLE 1.
Patients’ Characteristics
Statistical Analysis
Disease-specific survival (DSS) was measured from the date of diagnosis to death or at the last follow-up. LRFS and nodal relapse-free survival (NRFS) were measured from the date of diagnosis to the date of the first observation of local and nodal recurrence. DMFS was measured from the date of diagnosis to the date of the first observation of distant metastasis. The Kaplan-Meier method was used to calculate the accurate rate of these endpoints. The prognostic factors included in analysis were age, gender, T stage, N stage, lymph node diameter, primary tumor volume, WHO histology types, and cranial nerve involvement. T stage and N stage were verified according to MRI by 2 radiologists. Lymph node diameter was defined as the largest diameter of lymph node according to MRI. Two pathologists who specialized in NPC verified the WHO histological types according to the following criteria: undifferentiated subtype was defined as syncytial sheets of large tumor cell without distinct border, vesicular nuclei, and large central nucleoli; differentiate subtype was defined as cellular stratification, pavementing, and well-defined cell distinct (Figure 1). Primary tumor volume was contoured on the planning system according to MRI by 1 radiation oncologist, and then verified by another radiation oncologist. The cut-off value of primary tumor volume was identified by receiver operating characteristic (ROC) curves and then analyzed. Factors were found to be associated with the endpoints by univariate analyses and then entered into multivariate Cox proportional hazards regression analysis. The hazard ratio (HR) and its 95% confidence interval (95% CI) were used to indicate the prognostic value of risk factors. A 2-sided P value of less than 0.05 was considered significant. The statistical package for social science, version 16.0 (SPSS, Chicago, IL) was used for the statistical analysis.
FIGURE 1.
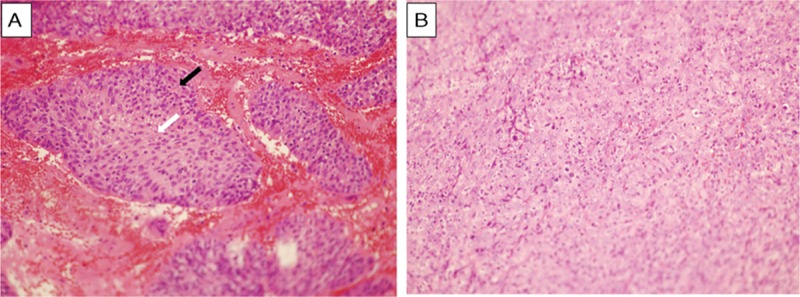
Microscopy morphology of nasopharyngeal nonkeratinizing carcinoma was shown here. (A) Differentiated subtype (40×) showing cellular stratification (black arrow), pavementing, and well-defined cell distinct (white arrow). (B) Undifferentiated subtype (40×) showing syncytial sheets of large tumor cell without distinct border, vesicular nuclei, and large central nucleoli.
RESULTS
Patients’ Characteristics
Patients’ characteristics are presented in Table 1. The male/female ratio was 2.4:1. The median age was 48 years (range, 13–78). According to the cut-off point of primary tumor volume by ROC analysis, all patients were divided into high primary tumor volume group (>23 mL) or low primary tumor volume group (≤23 mL). The median of lymph nodes size was 1.7 cm in the longest diameter. AJCC stage III-IV (83.6%) was the most common stage in this study. The most common histological type was WHO III (72.8%), whereas 27.2% had WHO II disease. Most patients received IMRT (79.6%), and radiotherapy combined with chemotherapy was the most common treatment modality in this study.
Treatment Outcomes
With a median follow-up of 61.4 months (range, 4–169), a total of 42 of 393 (10.7%) patients developed local recurrence, 14 of 393 (3.6%) had nodal relapse, 104 of 393 (26.5%) developed distant metastasis, and 101 of 393 (25.7%) patients developed cancer-specific death. LRFS, NRFS, DMFS, and DSS at 5-year were 89.3%, 96.4%, 73.5%, and 74.3%, respectively.
Identification of Primary Tumor Volume
The primary tumor volume cut-off points for DSS and DMFS were 23.4 mL [sensitivity 80.2%, specificity 34.9%; AUC (area under the ROC curve) 0.62; P < 0.001] and 23.1 mL (sensitivity 81.7%, specificity 33.6%; AUC 0.64; P < 0.001). Therefore, 23 mL was considered as the cut-off point for the primary tumor volume. The cut-off point of lymph node for OS could not be identified because the AUC failed to achieve a significant difference. The lymph node cut-off point for DMFS was 1.25 cm (sensitivity 80%, specificity 36.7%; AUC 0.61; P = 0.001). Therefore, 1.25 cm was considered as the cut-off point for lymph node size.
Univariate Analysis of Potential Prognostic Factors
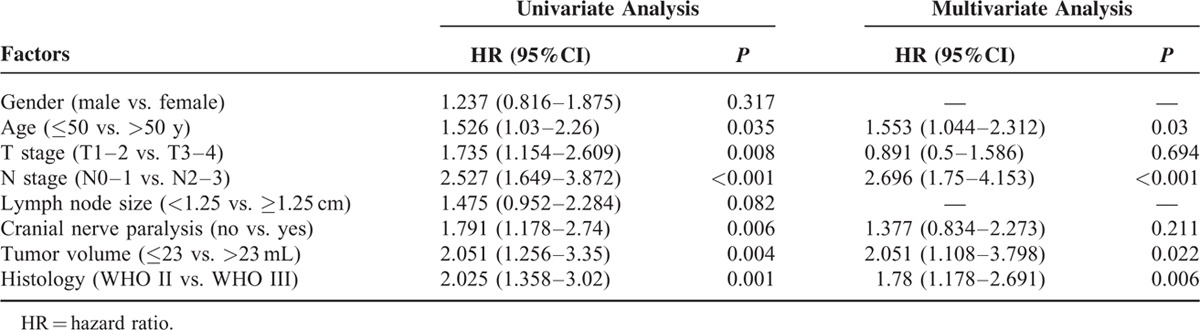
Tables 2 and 3 show the outcomes of univariate analyses for death and distant metastases. On univariate analysis, T stage (T2–3), N stage (N2–3), lymph node size (≥1.25 cm), cranial nerve involvement, histological WHO types (WHO type II), and primary tumor volume (>23 mL) were associated with unfavorable DSS and DMFS, with significant differences (all P < 0.05). Patients with cranial nerve involvement had lower local regional control rate than patients without cranial nerve involvement (5-year LRFS, 81.6% vs. 91.2%, P = 0.009, data were not shown). There were no significant differences between 2 radiation techniques for every endpoint (all P > 0.05, data were not shown). Gender was also not associated with any endpoints on this study (all P > 0.05). Elder patients (≥50 years) had significantly worse DSS (5-year DSS for age groups ≥50 vs. <50, 63.7% vs. 76.8%, P = 0.033). However, there were no significant differences regarding the 5-year DMFS and LRFS between the 2 age groups (all P > 0.05).
TABLE 2.
Prognostic Factors for Distant Metastasis
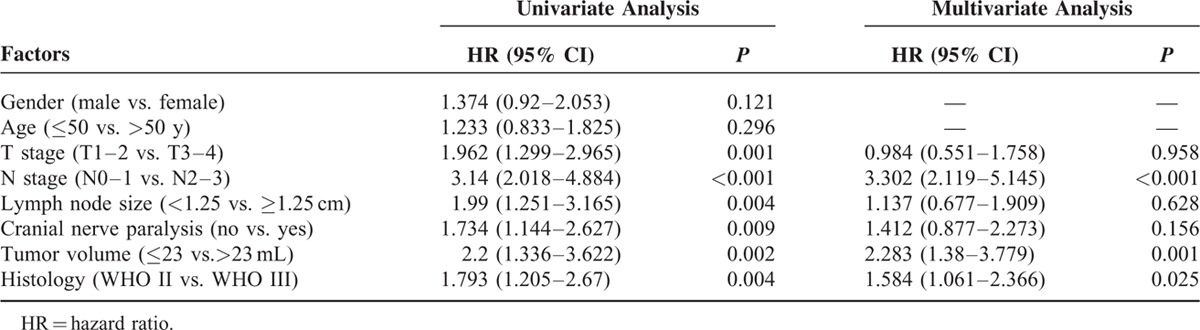
TABLE 3.
Prognostic Factors for Overall Survival
Independent Prognostic Factors for DSS and DMFS
The backward method of Cox regression model was used to identify the independent prognostic factors for DSS and DMFS (Tables 2 and 3). The independent prognostic factors for death included N stage (N2–3), histological WHO type II, tumor volume (>23 mL), and age (≥50 years), with significant differences (all P < 0.05). For distant metastases, the independent prognostic factors included N stage (N2–3), histological WHO type II, and tumor volume (>23 mL), with significant differences (all P < 0.05). Therefore, the crossover-independent prognostic factors for death and distant metastases included N stage (N2–3), histological WHO type II, and tumor volume (>23 mL).
Prognostic Model
As the N stage (N2–3), histological WHO type II, and tumor volume (>23 mL) were independent prognostic risk factors for DSS and DMFS, a prognostic model for nonendemic NPC in China was constructed: low-risk group (patients without any risk factors); intermediate-risk group (patients with only 1 risk factor); and high-risk group (patients with more than 2 risk factors).
Of 101 patients who died from cancer during follow-up time, 7 of 87 (8%) were in low-risk group, 35 of 167 (21%) in intermediate-risk group, and 59 of 139 (42.4%) in high-risk group. The 5-year DSS for 3 risk groups were 91.5%, 75.2%, and 49.3%, respectively (P < 0.001). The HRs of intermediate and high-risk groups were 3.372 and 8.591 for DSS when compared with low-risk group, respectively (P < 0.05). Of 104 patients who developed distant metastasis, 9 of 87 (10.3%) patients were in in low-risk group, 32 of 167 (19.2%) in intermediate-risk group, and 63 of 139 (45.3%) in high-risk group. The 5-year DMFS for 3 risk groups were 89.4%, 77.9%, and 49.4%, respectively (P = 0.000). The HRs of intermediate and high-risk group were 2.253 and 6.786 for DMFS when compared with low-risk group, respectively (P < 0.05) (Figure 2).
FIGURE 2.
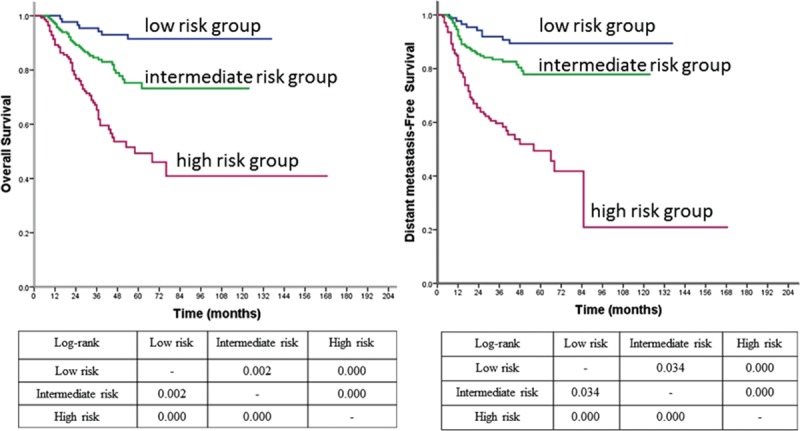
Survival curves of OS and DMFS according to the prognostic model in all patients. DMFS =distant metastasis free survival, OS = overall survival.
Model Predictions in Advanced Stage Patients
Subgroup analyses were conducted to evaluate the prognostic model for patients with stage III-IV NPC. The low, intermediate, and high-risk groups DSS at 5 years were 96%, 74.8%, and 49.5%, respectively (P = 0.000). The HRs of intermediate and high-risk group were 7.377 and 18.255 for DSS when compared with low-risk group, respectively (P < 0.05). The 5-year DMFS of low, intermediate, and high-risk groups were 88.6%, 75.7%, and 49.2%, respectively (P = 0.000). Compared with low and intermediate groups, patients in high-risk group had a high probability to develop distant metastasis (P < 0.05). However, the prognostic model failed to detect a significant difference between low-risk group and intermediate-risk group for DMFS (P = 0.164) (Figure 3).
FIGURE 3.
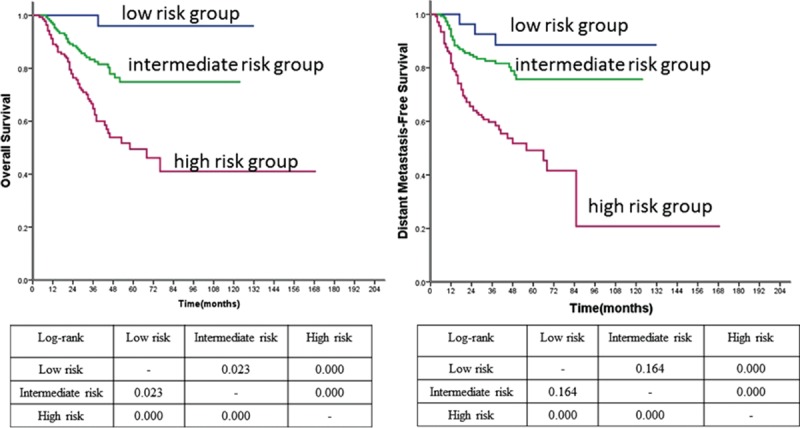
Survival curves of OS and DMFS according to the prognostic model in stage III-IVB patients. DMFS =distant metastasis free survival, OS = overall survival.
DISSCUSSION
To our knowledge, this is the first study to detect the potential prognostic factors and establish a prognostic model for death and distant metastasis for nonendemic NPC patients who were treated with 3DCRT/IMRT with systemic chemotherapy. Our data showed that AJCC stage N2 to 3, histological WHO type II, and tumor volume >23 mL were independent prognostic factors for DSS and DMFS in nonendemic NPC patients. The specific prognostic model, which was established according to the number of risk factors, predicts the risks of death and distant metastasis in nonendemic NPC patients. According to the model, the more are the numbers of prognostic factors, the higher is the incidence of death and distant metastasis. Patients in low-risk group had low incidence of death (5-year DSS, 91.5%) and distant metastasis (5-year DMFS, 89.4%) than intermediate and high-risk group. Therefore, this model may be helpful to predict patients’ prognoses that therefore could stratify patients and design an individualized treatment regimen to avoid either over or under the treatment. Further study needs to be designed to estimate the prognostic role of model.
Advanced N stage and tumor volume have been identified as important prognostic factors for NPC patients by many published data from endemic areas.2,15–21 There is a general consensus that patients with advanced N stage had a trend to develop distant metastases and further adversely effect on OS. In our study, advanced N stage was significantly associated with death and distant metastasis. This finding is consistent with the previous studies.22 The primary tumor volume was fund to be a valuable prognostic factor for nonendemic NPC patients in our previous study.7 A smaller primary tumor volume (≤23 mL) contributed to better survival and less incidence rate of distant metastasis. The theory of this could be that a large volume of tumor might harbor a large number of clonogenic tumor cells, which might express many adverse biological factors, such as radioresistance and altered levels of intercellular communication factors.23 Therefore, primary tumor volume should be considered as an important supplementary risk factor to predict patients’ prognoses.
The predicting value of histological WHO type II remains unclear in NPC patients because of the low proportion of this type. The incidence rate of this type is less than 5% in endemic NPC patients.24–26 However, our study reported a 27.2% of histological WHO type II, which has a much higher proportion than endemic areas. Several studies showed that WHO II histological type might be a special prognostic factor for nonendemic NPC, but the results were still controversial.7,27 Several studies and our previous data demonstrated that WHO type II was a poor prognostic factor for survival and local control in NPC patients.7,25 However, some reports also noted no differences in survival and distant metastases among histological types.27,28 In our study, WHO type II was a basic prognostic factor to predict distant metastasis (HR, 1.584) and poor DSS (HR, 1.854) for nonendemic NPC patients. This suggested that besides T and N stage, WHO type II histology should be considered as one of prognostic factors applying for systemic chemotherapy; in the meanwhile, the prognostic value of histological WHO type II should be further investigated by large cohort studies.
Although IMRT, which has the capacity to provide excellent conformal and precise coverage with sharp dose gradient, has been accepted as the standard treatment for NPC, treatment of advanced T stage NPC still remains challenging because of the proximity of the tumor to the skull base and central nerve system.29 Especially for the T4 stage NPC, with large GTV and base of skull extension, it is likely to associate to harbor a large amount of hypoxia tumor cells. Some reports demonstrated that patients with T4 stage had a trend to develop distant metastasis because lymphatics were easily invaded by tumor cells.30,31 This study reported that advanced T stage was associated with poor survival and distant metastasis. However, multivariate analysis failed to identify the advanced T stage as an independent predicting factor for DSS and DMFS. This may be explained that the predicting role was compromised by N stage and tumor volume in this cohort.
Some studies have reported that MRI evidence of cranial nerve involvement was an unfavorable prognostic factor in endemic NPC, but the prognostic impact was still controversial.32–34 The fact is that the MRI findings are not always consistent with clinical symptoms of cranial paralysis.32 In this study, the cranial nerve involvement was defined as patients with evidence of nerve paralysis. Although the cranial nerve impairment was detected to be associated with DSS and DMFS, we failed to identify it as an independently prognostic factor for death and distant metastasis. Therefore, the MRI findings of cranial nerve involvement remain unclear for a prognostic role in nonendemic NPC, and further studies are needed for further investigation.
It was reported that nonkeratinizing carcinoma was always associated with high plasma EBV DNA load that was related to poor prognosis.35–37 However, we excluded EBV DNA load as a risk factor from the analysis because we only had less than 5% patients presenting with significant high EBV DNA load in this study. Therefore, there was less power to yield valuable outcomes in this situation. The reason may be explained as follows: low incidence of elevated EBV DNA load in nonendemic NPC; and lack of unified standard to detect the virus DNA.
The limitations of this study included, first, a retrospective without larger sample size was conducted to detect the prognostic factors and establish a prognostic model, and second, other potential prognostic factors were excluded from this study, such as C-reaction protein, serum lactic dehydrogenase, body mass index, and serum EBV DNA copies. Therefore, cohort studies with large sample size and prospective studies need to be conducted to further explore the potential prognostic factors and evaluate the prognostic model for nonendemic NPC patients.
CONCLUSION
Advanced N stage (N2–3), larger primary tumor volume (>23 mL), and histological WHO type II are independently prognostic factors for nonendemic NPC patients of China.
Footnotes
Abbreviations: 3DCRT = 3-Dimensional Conformal Radiation Therapy, CTV = clinical target volume, DMFS = distant metastasis free survival, DSS = disease-specific survival, GTV = gross tumor volume, IMRT = Intensity-Modulated Radiation Therapy, LRFS = local recurrent-free survival, NPC = nasopharyngeal carcinoma, NRFS = nodal relapse-free survival, PTV = planning target volume.
JZ, CL, and L-NZ contribute equally to this study.
The authors declare that they have no competing interests.
REFERENCES
- 1.Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014; 110:398–403. [DOI] [PubMed] [Google Scholar]
- 2.Guo R, Sun Y, Yu X-L, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 2012; 104:294–299. [DOI] [PubMed] [Google Scholar]
- 3.Airoldi M, Gabriele AM, Garzaro M, et al. Induction chemotherapy with cisplatin and epirubicin followed by radiotherapy and concurrent cisplatin in locally advanced nasopharyngeal carcinoma observed in a non-endemic population. Radiother Oncol 2009; 92:105–110. [DOI] [PubMed] [Google Scholar]
- 4.Demirci S, Kamer S, Kara G, et al. Does the prognosis of nasopharyngeal cancer differ among endemic and non-endemic regions? Acta Otolaryngol 2011; 131:852–860. [DOI] [PubMed] [Google Scholar]
- 5.Boscolo-Rizzo P, Tirelli G, Mantovani M, et al. Non-endemic locoregionally advanced nasopharyngeal carcinoma: long-term outcome after induction plus concurrent chemoradiotherapy in everyday clinical practice. Eur Arch Otorhinolaryngol 2015; 272:3491–3498. [DOI] [PubMed] [Google Scholar]
- 6.Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys 2014; 88:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L-N, Zhou B, Shi M, et al. Clinical outcome for nasopharyngeal carcinoma with predominantly WHO II histology treated with intensity-modulated radiation therapy in non-endemic region of China. Oral Oncol 2012; 48:864–869. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Liang S, Deng Y, et al. Prognostic significance of maximum primary tumor diameter in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2014; 90:S521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia W-X, Zhang H-B, Shi J-L, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification. Eur J Cancer 2013; 49:2152–2160. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer 2013; 49:1619–1626. [DOI] [PubMed] [Google Scholar]
- 11.Huang P-Y, Wang C-T, Cao K-J, et al. Pretreatment body mass index as an independent prognostic factor in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy: findings from a randomised trial. Eur J Cancer 2013; 49:1923–1931. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Chen Q, Guo S, et al. The impact of plasma Epstein–Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer 2014; 111:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Shi M, Hsia Y, et al. Failure patterns and survival in patients with nasopharyngeal carcinoma treated with intensity modulated radiation in Northwest China: a pilot study. Radiat Oncol 2012; 7:717X–727X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009; 27:3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu S-T, Wu P-H, Hou Y-Y, et al. Primary tumor volume of nasopharyngeal carcinoma: significance for recurrence and survival. J Chinese Med Assoc 2008; 71:461–466. [DOI] [PubMed] [Google Scholar]
- 16.Chua DT, Sham JS, Kwong DL, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys 1997; 39:711–719. [DOI] [PubMed] [Google Scholar]
- 17.Lee C-C, Ho H-C, Lee M-S, et al. Primary tumor volume of nasopharyngeal carcinoma: significance for survival. Auris Nasus Larynx 2008; 35:376–380. [DOI] [PubMed] [Google Scholar]
- 18.Sze W-M, Lee AW, Yau T-K, et al. Primary tumor volume of nasopharyngeal carcinoma: prognostic significance for local control. Int J Radiat Oncol Biol Phys 2004; 59:21–27. [DOI] [PubMed] [Google Scholar]
- 19.Ho FC, Tham IW, Earnest A, et al. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. Bmc Cancer 2012; 12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King AD, Ahuja AT, Leung SF, et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 2000; 22:275–281. [DOI] [PubMed] [Google Scholar]
- 21.Sakata K-i, Hareyama M, Tamakawa M, et al. Prognostic factors of nasopharynx tumors investigated by MR imaging and the value of MR imaging in the newly published TNM staging. Int J Radiat Oncol Biol Phys 1999; 43:273–278. [DOI] [PubMed] [Google Scholar]
- 22.Huang PY, Zeng Q, Cao KJ, et al. Ten-year outcomes of a randomised trial for locoregionally advanced nasopharyngeal carcinoma: a single-institution experience from an endemic area. Eur J Cancer 2015; 51:1760–1770. [DOI] [PubMed] [Google Scholar]
- 23.Johnson CR, Thames HD, Huang DT, et al. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys 1995; 33:281–287. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys 2008; 71:1356–1364. [DOI] [PubMed] [Google Scholar]
- 25.Cheung F, Chan O, Ng WT, et al. The prognostic value of histological typing in nasopharyngeal carcinoma. Oral Oncol 2012; 48:429–433. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer 2013; 119:2230–2238. [DOI] [PubMed] [Google Scholar]
- 27.Luo S, Zhao L, Wang J, et al. Clinical outcomes for early - stage nasopharyngeal carcinoma with predominantly WHO II histology treated by intensity - modulated radiation therapy with or without chemotherapy in nonendemic region of China. Head Neck 2014; 36:841–847. [DOI] [PubMed] [Google Scholar]
- 28.Perez CA, Devineni VR, Marcial-Vega V, et al. Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys 1992; 23:271–280. [DOI] [PubMed] [Google Scholar]
- 29.Takiar V, Ma D, Garden AS, et al. Disease control and toxicity outcomes for T4 carcinoma of the nasopharynx treated with intensity-modulated radiotherapy. Head Neck 2016; 38 suppl 1:E925–E933. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Liu LZ, Chen M, et al. Prognostic value of subclassification using MRI in the t4 classification nasopharyngeal carcinoma intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys 2012; 84:196–202. [DOI] [PubMed] [Google Scholar]
- 31.Cao CN, Luo JW, Gao L, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for T4 nasopharyngeal carcinoma. Oral Oncol 2013; 49:175–181. [DOI] [PubMed] [Google Scholar]
- 32.Zong J, Lin S, Chen Y, et al. Does MRI-detected cranial nerve involvement affect the prognosis of locally advanced nasopharyngeal carcinoma treated with intensity modulated radiotherapy? PLoS One 2014; 9:e100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Liu LZ, Mao YP, et al. Prognostic value of magnetic resonance imaging-detected cranial nerve invasion in nasopharyngeal carcinoma. Br J Cancer 2014; 110:1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Liang S, Li L, et al. Prognostic impact of magnetic resonance imaging-detected cranial nerve involvement in nasopharyngeal carcinoma. Cancer 2009; 115:1995–2003. [DOI] [PubMed] [Google Scholar]
- 35.Tang LQ, Li CF, Chen QY, et al. High-sensitivity C-reactive protein complements plasma Epstein-Barr virus deoxyribonucleic acid prognostication in nasopharyngeal carcinoma: a large-scale retrospective and prospective cohort study. Int J Radiat Oncol Biol Phys 2015; 91:325–336. [DOI] [PubMed] [Google Scholar]
- 36.Shen T, Tang LQ, Luo DH, et al. Different prognostic values of plasma Epstein-Barr virus DNA and maximal standardized uptake value of 18F-FDG PET/CT for nasopharyngeal carcinoma patients with recurrence. PLoS One 2015; 10:e0122756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hui EP, Ma BB, Chan KC, et al. Clinical utility of plasma Epstein-Barr virus DNA and ERCC1 single nucleotide polymorphism in nasopharyngeal carcinoma. Cancer 2015; 121:2720–2729. [DOI] [PubMed] [Google Scholar]


