Supplemental Digital Content is available in the text
Abstract
Liver stiffness (LS), assessed using transient elastography (TE), and (FIB-4) can both estimate the risk of developing hepatocellular carcinoma (HCC). We compared prognostic performances of LS and FIB-4 to predict HCC development in patients with chronic hepatitis B (CHB).
Data from 1308 patients with CHB, who underwent TE, were retrospectively analyzed. FIB-4 was calculated for all patients. The cumulative rate of HCC development was assessed using Kaplan–Meier curves. The predictive performances of LS and FIB-4 were evaluated using time-dependent receiver-operating characteristic (ROC) curves.
The mean age (883 men) was 50 years. During follow-up (median 6.1 years), 119 patients developed HCC. The areas under the ROC curves (AUROCs) predicting HCC risk at 3, 5, and 7 years were consistently greater for LS than for FIB-4 (0.791–0.807 vs 0.691–0.725; all P < 0.05). Similarly, when the respective AUROCs for LS and FIB-4 at every time point during the 7-year follow-up were plotted, LS also showed consistently better performance than FIB-4 after 1 year of enrollment. The combined use of LS and FIB-4 significantly enhanced the prognostic performance compared with the use of FIB-4 alone (P < 0.05), but the performance of the combined scores was statistically similar to that of LS alone (P > 0.05).
LS showed significantly better performance than FIB-4 in assessing the risk of HCC development, and the combined use of LS and FIB-4 did not provide additional benefit compared with the use of LS alone. Hence, LS assessed using TE might be helpful for optimizing HCC surveillance strategies.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a major cause of cirrhosis and hepatocellular carcinoma (HCC).1 Active antiviral treatment using potent antivirals has improved long-term prognoses by suppressing HBV replication, preventing liver damage, inducing fibrosis regression, and eventually reducing the risk of disease progression, including HCC development.2,3 Nevertheless, the risk of HCC remains, because advanced fibrosis or cirrhosis, which is the single most important risk factor for HCC, is not completely resolved by antiviral treatment.3–5 Hence, aside from the suppression of HBV replication using antivirals, it is of paramount importance to assess the degree of liver fibrosis and identify early compensated cirrhosis in order to stratify long-term prognoses, assess HCC risks, and optimize surveillance strategies for patients with chronic hepatitis B (CHB).
Although histological assessment is the gold standard for assessing the degree of liver fibrosis and cirrhosis, it is not feasible in clinical practice to use liver biopsy as a screening tool for patients with CHB. To date, several other noninvasive imaging modalities have been used to assess the degree of liver fibrosis.6–8 Among those, liver stiffness (LS) assessed using transient elastography (TE) was recently demonstrated to be a reliable and accurate noninvasive tool for assessing the degree of liver fibrosis.9,10 Recent large-scale longitudinal studies also showed a significant association between the LS value and the risk of HCC development in patients with CHB.11,12 In addition, various serologic biomarkers of liver fibrosis have been developed to stage the degree of liver fibrosis.8 Some biomarkers use the serum concentration of specific components related to fibrogenesis and fibrosis breakdown, whereas others are based on simple serological markers derived from blood tests in routine clinical practice reflecting liver function or portal hypertension.8 Of these serologic biomarkers, FIB-4,13 an index calculated from patient age, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count, has shown strong diagnostic performance in assessing the degree of liver fibrosis. FIB-4 has high clinical applicability, because it can easily be obtained by routine laboratory tests.14 In addition, similar to TE, FIB-4 has proven to be a significant prognostic predictor of HCC development in patients with CHB and other liver diseases.15,16
Because no comparative data have been available so far, we aimed to compare the prognostic performance of LS as measured by TE with that of FIB-4 in assessing the risk of HCC development in patients with CHB. We also investigated whether the prognostic performance could be enhanced when TE and FIB-4 are combined.
MATERIALS AND METHODS
Patients
The study population of this retrospective study was based upon that of our previous study, conducted to validate the prognostic performance of several risk-prediction models for HCC development.17 The inclusion criteria were as follows: (1) chronic HBV infection and (2) available TE data. The exclusion criteria were as follows: (1) unreliable LS values, (2) a history of HCC or liver decompensation, (3) coinfection with hepatitis C virus infection or other serious medical illness, and (4) an insufficient follow-up time or HCC development within 6 months since enrollment. Finally, a total of 1308 patients were enrolled for analysis.
This study was approved by the institutional review board of Severance Hospital.
Clinical Evaluation and Follow-up
Patients were followed-up every 3 to 6 months with routine laboratory tests. Furthermore, every 6 months, abdomen ultrasonography and serum α-fetoprotein were performed for HCC surveillance.
Diagnosis of Liver Cirrhosis and HCC
Diagnosis of liver cirrhosis was made by histological or clinical evaluation. In case that histological data were not available, liver cirrhosis was diagnosed clinically when one of the following criteria was fulfilled:18 (1) platelet count <100,000/μL and presence of ultrasonographic features such as a blunted, nodular liver edge accompanied by splenomegaly (>12 cm); or (2) evidence of portal hypertension.12 Diagnosis of HCC was made by either histological or radiological evaluation on the basis of the guidelines of the American Association for the Study of Liver Diseases.19
LS and FIB-4 Assessments
LS was measured using TE (FibroScan, EchoSens, Paris, France), performed by a single experienced technician blinded to the clinical and biochemical data of the patients. Fibroscan 502 with M probe had been applied until March 2013, and thereafter, Fibroscan 502 Touch with M ± probe was used. The principles of LS measurement were described previously.20 Only LS values with ≥10 valid measurements, a success rate ≥60%, and an interquartile range (IQR)-to-median ratio <30% were considered reliable. The LS was expressed in kilopascals (kPa). FIB-4 was assessed based on the laboratory parameters at the time of enrollment as follows: FIB-4 = age (years) × AST (U/L) / (platelets [109/L] × [ALT {U/L}]1/2).13
Statistical Analyses
When HCC occurred during the follow-up, the time to HCC development was calculated as the interval between the date of study entry and the date of HCC diagnosis. When HCC did not occur during the follow-up, it was calculated as the interval between the date of study entry and the date of last follow-up. Patients with follow-up duration of >7 years were censored at 7 years. The cumulative rate of HCC development was analyzed using the Kaplan–Meier method with comparisons by the log-rank test.
To evaluate the predictive values of FIB-4 and LS across the entire follow-up period of 7 years, we applied a time-dependent receiver-operating characteristic (ROC) curve method for censored survival data. Then, we compared the global concordance probability (expressed as the area under the ROC curve [AUROC]) of the models using LS and FIB-4, respectively. A greater AUROC indicates better predictive performance. The differences in the AUROC between the LS and FIB-4 models were tested using a bootstrap resampling method.21 In addition, we stratified the study population using cutoff values of LS (<8, 8–13, 13–18, 18–23, and >23 kPa) and FIB-4 (<1.25, 1.25–1.70, 1.70–24.0, and >2.40) defined in previous studies12,15 to compare the prognostic performances of LS and FIB-4 in the setting of categorical stratification.
All statistical procedures were conducted using SAS software version 9.2 (SAS Institute) and R software version 3.1.1 (http://cran.r-project.org/). A P value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
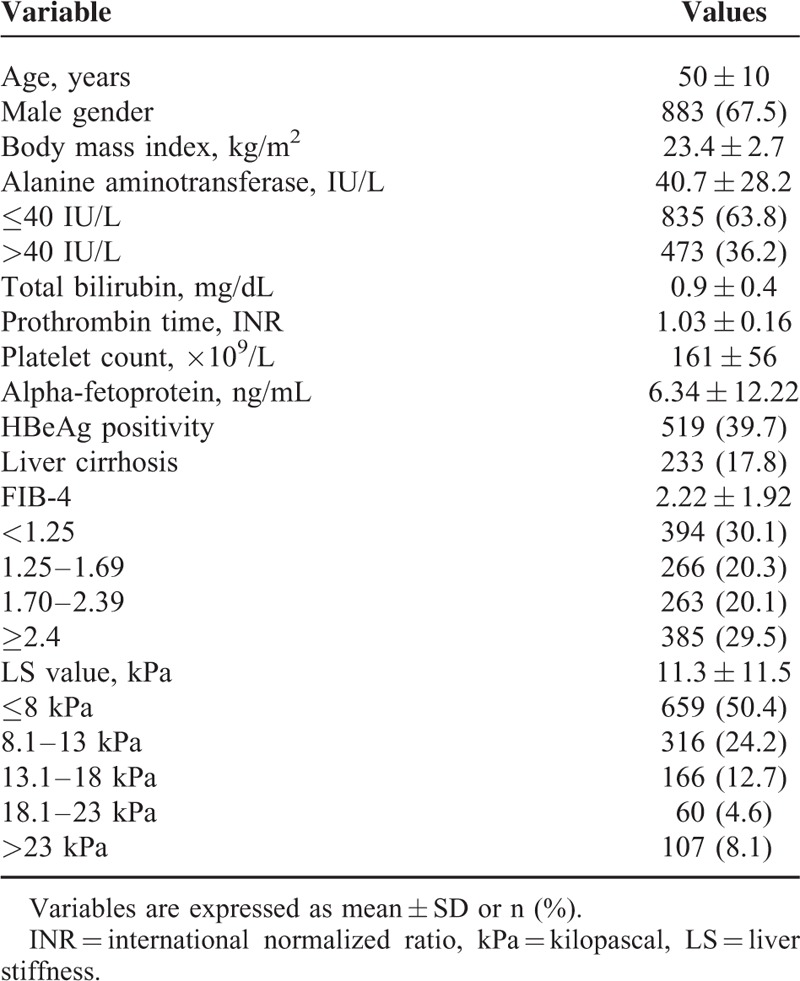
The baseline characteristics of the study population (n = 1308) are summarized in Table 1. The mean age of the entire study population (883 men and 425 women) was 50 years. Liver cirrhosis was identified in 233 (17.8%) patients. HBeAg was positive in 519 (39.7%) patients. Overall, 848 patients (64.8%) experienced antiviral therapy; 434 patients had previous or ongoing use of antiviral agents at enrollment and 414 received antiviral therapy starting after enrollment. The mean FIB-4 and LS values were 2.22 and 11.3 kPa, respectively.
TABLE 1.
Baseline Characteristics of the Study Population (n = 1308)
HCC Development During the 7-year Follow-Up
The median follow-up duration was 6.1 (IQR: 4.1–6.9) years. During the 7-year follow-up (the sum of the follow-up duration of each patient: 7068.74 person-years), 119 patients developed HCC. The cumulative incidence of HCC at 3, 5, and 7 years was 5.09%, 8.70%, and 11.03%, respectively. When the study population was stratified according to the presence of liver cirrhosis, the cumulative incidence of HCC at 3, 5, and 7 years was 3.68%, 6.33%, and 8.52%, respectively, in patients without liver cirrhosis and 11.90%, 20.25%, and 23.28%, respectively, in patients with liver cirrhosis.
Cumulative Incidence of HCC According to Stratified LS and FIB-4 Values
The cumulative incidence of HCC during the 7-year follow-up according to the stratified LS and FIB-4 values is depicted in Figure 1A and B. The respective cumulative incidences of HCC at 3, 5, and 7 years were 1.09%, 2.40%, and 4.03%, respectively, in patients with LS ≤8 kPa; 4.57%, 8.60%, and 12.72% in patients with 8 kPa<LS≤13 kPa; 7.93%, 15.05%, and 17.82% in patients with 13 kPa<LS≤18 kPa; 16.80%, 22.94%, and 22.94% in patients with 18 kPa<LS≤23 kPa; and 19.98%, 32.24%, and 34.95% in patients with LS>23 kPa. The cumulative incidence of HCC increased in a step-wise manner among the 5 groups (log-rank test, all P < 0.05 between the 2 adjacent curves; Figure 1A).
FIGURE 1.
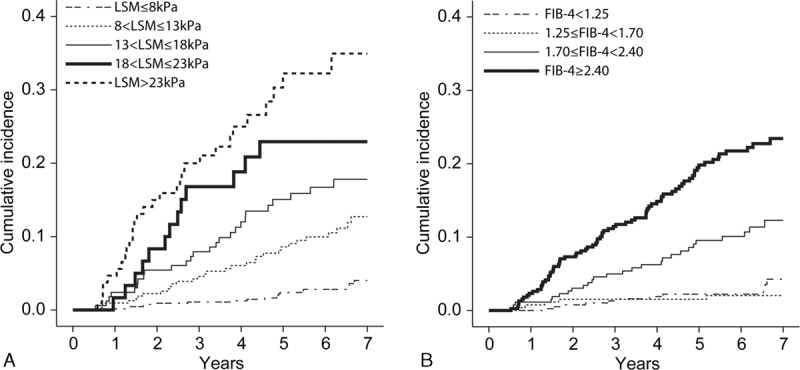
Cumulative development of HCC according to stratified LS (A) and FIB-4 (B) value. The cumulative rate of HCC development increased significantly in association with increased LS or FIB-4 values (log-rank test; all P < 0.05 between the 2 adjacent curves). However, the Kaplan–Meier survival curve of patients with FIB-4 <1.25 and those with FIB-4 ≥1.25 and <1.70 overlapped (log-rank test, P = 0.445). HCC = hepatocellular carcinoma, LS = liver stiffness.
The respective cumulative incidences of HCC at 3, 5, and 7 years were 1.31%, 2.23%, and 4.26%, respectively, in patients with FIB-4<1.25; 1.53%, 1.53%, and 2.03% in patients with 1.25≤ FIB-4<1.70; 4.98%, 9.55%, and 12.29% in patients with 1.70≤ FIB-4<2.40; and 11.45%, 19.82%, and 23.44% in patients with FIB-4≥2.40. The cumulative incidence of HCC increased in a step-wise manner among 3 of the groups (1.25≤FIB-4<1.70, 1.70≤FIB-4<2.40, and FIB-4≥2.40; log-rank test, all P<0.05 between the 2 adjacent curves). However, the Kaplan–Meier curves of the patients with FIB-4<1.25 and those with 1.25≤FIB-4<1.70 overlapped (log-rank test, P = 0.445; Figure 1B).
Comparison of the Prognostic Performances of LS and FIB-4 in Predicting HCC
The performances of LS and FIB-4 in predicting HCC during 3-year, 5-year, and 7-year follow-up, as well as the comparisons among them, are summarized in Table 2. During the 7-year follow-up period, the AUROCs of LS ranged from 0.795 to 0.807, whereas those of FIB-4 ranged from 0.742 to 0.749. When the AUROCs of LS and FIB-4 were compared, LS consistently showed significantly greater prognostic performance than FIB-4 (all P < 0.05). When the AUROCs of LS and FIB-4 at every time point during the 7-year follow-up were plotted, LS showed consistently better performance than FIB-4 after 1 year of enrollment (Figure 2).
TABLE 2.
Performances of LS and FIB-4 to Predict HCC Development During 7-Year Follow-Up and Their Comparisons
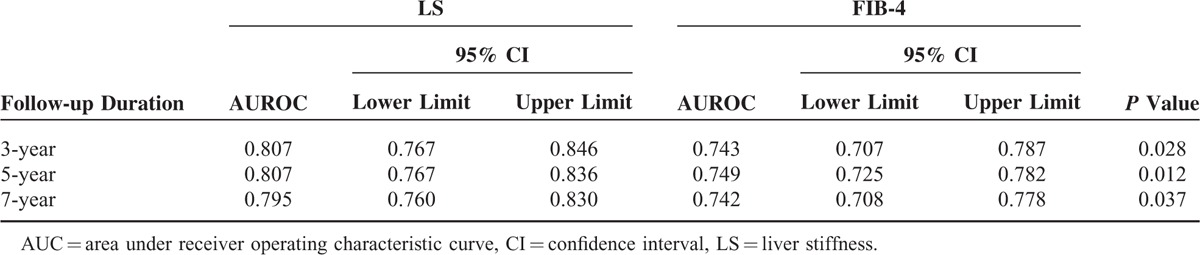
FIGURE 2.
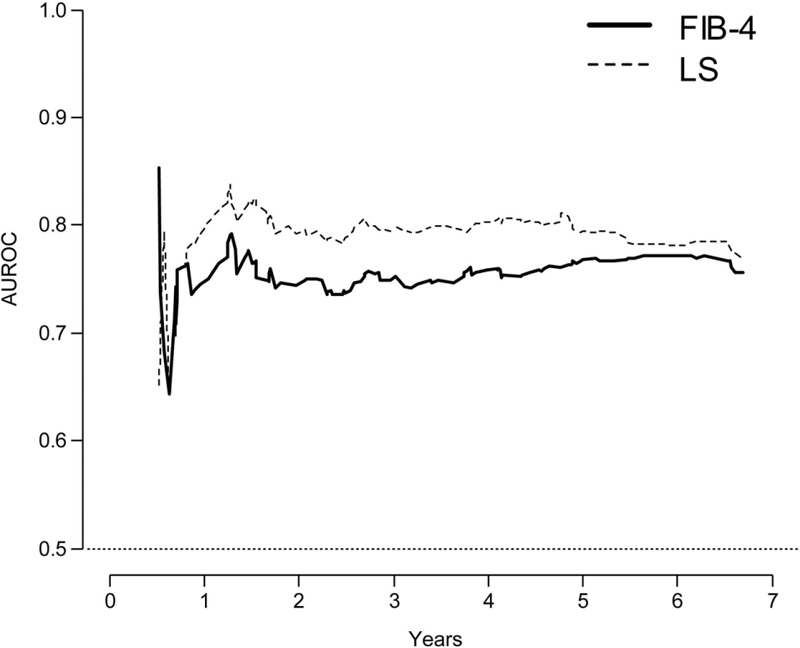
Time-dependent ROC curve analyses during 7-year follow-up. The AUROC of LS is consistently higher than that of FIB-4 after 1 year of enrollment. AUROC = area under receiver operating characteristic curve, LS = liver stiffness, ROC = receiver-operating characteristic.
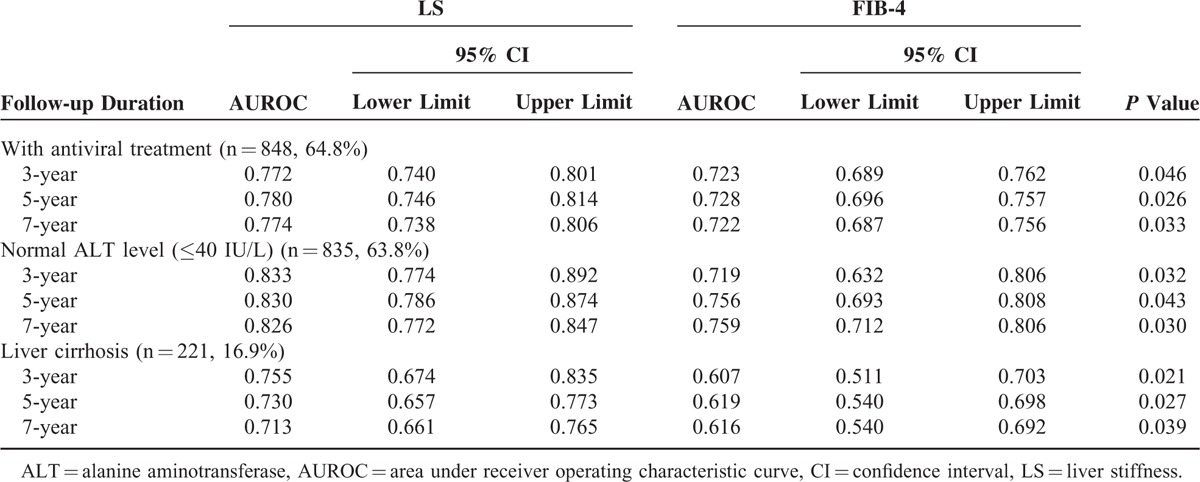
The prognostic performances of LS and FIB-4 were also compared among subgroups stratified according to antiviral treatment status, ALT level, and fibrotic burden, which can influence the accuracy of TE (Table 3). In the subgroups with antiviral treatment at enrollment (n = 848, 64.8%), normal ALT level (≤40 IU/L, n = 835, 63.8%), and liver cirrhosis (n = 221, 16.9%), LS showed significantly (P < 0.05) better performance than FIB-4 in predicting HCC development over 3 years (AUROC: 0.772 vs 0.723, 0.833 vs 0.719, and 0.755 vs 0.607, respectively), 5 years (AUROC: 0.780 vs 0.728, 0.830 vs 0.756, and 0.730 vs 0.619, respectively), and 7 years (AUROC: 0.774 vs 0.722, 0.826 vs 0.759, and 0.713 vs 0.616, respectively).
TABLE 3.
Subgroup Analysis According to Antiviral Treatment Status, ALT Level, and Fibrotic Burden
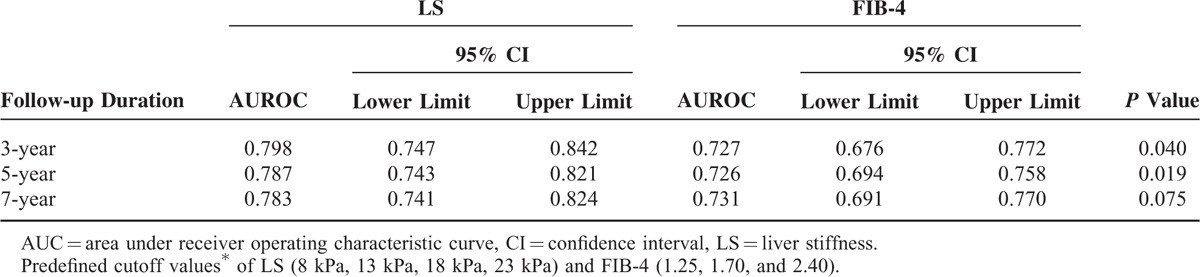
In addition, when LS and FIB-4 were processed as categorical scales using predefined cutoffs (<8 kPa, 8–13 kPa, 13–18 kPa, 18–23 kPa, and >23 kPa for LS12 and <1.25, 1.25–1.70, 1.70–24.0, and >2.40 for FIB-415), LS showed significantly better performance than FIB-4 in predicting HCC development within 3 years or 5 years (all P < 0.05). The difference was marginal, however, in terms of predicting HCC development within 7 years (P = 0.075; Table 4).
TABLE 4.
Performances of Stratified LS and FIB-4 Using Predefined Cutoff Values∗ to Predict HCC Development During 7-Year Follow-Up and Their Comparisons
The Prognostic Performance of the combined use of LS and FIB-4
Although LS was superior to FIB-4 in predicting HCC development, both tests showed acceptable performance (AUROC approximately ≥0.75). We investigated whether the prognostic performance could be enhanced when LS and FIB-4 were simultaneously entered into the equation. When LS and FIB-4 were simultaneously used in a continuous manner to predict HCC development during the 7-year follow-up, and when combined performance was compared with that of using LS or FIB-4 alone, the AUROC of the combined values was similar to that of LS (AUROC: 0.814 [95% CI: 0.777–0.850] vs 0.795 [95% CI: 0.760–0.830], P = 0.469), but significantly greater than that of FIB-4 (AUROC: 0.752 [95% CI: 0.718–0.788], P = 0.018; Supplementary Table 1). Similar results occurred when stratified LS and FIB-4 using previously defined cutoff values were entered into the equation (AUROC: 0.794 [95% CI: 0.754–0.837] for the combined stratified values vs 0.773 [95% CI: 0.731–0.814] for the stratified LS only [P = 0.481] and 0.731 [95% CI: 0.691–0.770] for the stratified FIB-4 only [P = 0.031]; Supplementary Table 1).
DISCUSSION
In this study, LS assessed using TE was consistently superior to FIB-4 in predicting HCC development at each time point during the 7-year follow-up period, although both tests could estimate the risk of developing HCC and stratify prognoses among subgroups of patients with CHB. We acknowledge that FIB-4 is calculated using age, AST, ALT, and platelet count, which are obtained during routine clinical practice; therefore, FIB-4 can be useful for assessing the risk of HCC because of its minimal cost, easy applicability, and considerable diagnostic performance, especially when TE is not available. Nevertheless, our results suggest that LS should be favored to improve prognostication in present HCC surveillance strategies for patients with CHB, as far as resources permit.12
Recently, several Asian studies have investigated the prognostic role of LS in the noninvasive assessment of the risk for CHB-related HCC development.12,22–24 In a large Korean prospective cohort study that enrolled 1130 patients with CHB, stratified LS was identified as 1 independent risk factor for HCC development, with a relative risk of 3.07, 4.68, 5.55, and 6.60 for LS of 8–13, 13–19, 18–23, and >23 kPa, respectively, compared with LS < 8 kPa as a reference.12 Another study from Hong Kong,22 in which 528 patients with HBeAg-negative CHB were followed up, showed that the cumulative incidence of HCC was higher in patients with LS ≥ 10 kPa compared with those with LS < 10 kPa (9% vs 0%, respectively; P < 0.001). A multivariate analysis showed that only LS was significantly associated with HCC development. Furthermore, it was reported that TE can identify patients with CHB and subclinical cirrhosis that are at increased risk for developing HCC.18 Based on the prognostic role of TE, the prognostic efficacies of conventional HCC prediction models, such as REACH-B and CU-HCC score, might be improved by incorporating the LS values to generate modified REACH-B11 and LSM-HCC models.25 Our results, along with those of numerous previous studies, all support the prognostic role of TE in predicting HCC development in patients with CHB, most likely because a wide, continuous, dynamic range of up to 75 kPa is applied to evaluate the degree of liver fibrosis. Consequently, more detailed prognostication is allowed not only for patients with clinical cirrhosis, but also for patients without overt clinical evidence of cirrhosis. Although use of LS has become more and more popular worldwide, the issue of its limited use due to the high cost of TE remains.
FIB-4, in conjunction with LS, might be regarded as one of acceptable noninvasive methods for evaluating the degree of fibrosis. However, in terms of the performance for noninvasive prediction of liver fibrosis, FIB-4 does not surpass LS.26,27 As a matter of fact, FIB-4 only provides the moderate degree of diagnostic value comparable to that of aspartate aminotransferase-to-platelet ratio index (APRI).28 Nevertheless, FIB-4 has important advantages of good availability and cost-effectiveness as APRI which has recently been recommended in resource-limited settings as a screening tool for the underlying fibrotic burden in patients with CHB according to the WHO guideline.29 Furthermore, similar to LS, FIB-4 has been evaluated with regard to its longitudinal perspective to predict the development of HCC and other prognostic outcomes.15,30–32 In our study, stratification by LS was more appropriate for detailed prognostication than stratification by FIB-4, which resulted in the overlap of the Kaplan–Meier curves for the subgroups with FIB-4 < 1.25 and 1.25 ≤ FIB-4<1.70, respectively. That phenomenon was also observed in an investigation by Suh et al,15 suggesting that FIB-4 has the limited capability in differentiating the risk of HCC development among patients in the early stages of disease in contrast to LS. In addition, LS was consistently superior to FIB-4 in predicting HCC development at each time point during the 7-year follow-up period. We can, therefore, cautiously speculate that TE might have significant clinical merit in stratifying patients with CHB into those who would or would not benefit from regular HCC surveillance, and in designing more individualized surveillance strategies. When LS and FIB-4 were simultaneously used to predict prognosis, no additional benefit was observed compared with the use of LS alone.
To the best of our knowledge, this is the first study to directly compare the prognostic performances of LS and FIB-4 in predicting HCC development in patients with CHB. If the prognostic performance of FIB-4 was comparable to that of LS, the rationale to use TE for the aim of predicting HCC could be challenged, especially in terms of cost-effectiveness. Because LS has a more reliable predictive capacity compared with FIB-4 based on our study, it is reasonable to argue that LS should be recommended as the primary tool for first assessment of the fibrotic burden, and consequently for providing future prognosis in routine clinical practice. Thereafter, FIB-4 might be suggested as an alternative method especially when the resource is limited. Another strength of our study is that we consecutively enrolled a relatively large number of patients with CHB (n > 1300) from a single center with long-term follow-up (up to 7 years), providing statistical power and reliability.
In subgroups that included patients with antiviral therapy, as well as those with normal serum ALT levels, LS had significantly better prognostic performance compared with FIB-4. In the current era of potent antiviral therapies, most treated patients can eventually get positive virological and biochemical responses through the use of antiviral therapy.33–36 As necro-inflammation resolves either naturally or through antiviral therapy, the effect of serum AST or ALT levels on the disease progression also diminishes. That is one of the reasons why the prognostic performance of FIB-4, which includes AST and ALT as constituent variables, might be suboptimal in those 2 subgroups. In addition, in subgroups with liver cirrhosis, LS showed better performance than FIB-4, suggesting that LS, which has a wide, continuous dynamic range of up to 75 kPa, might have advantage over FIB-4 in predicting prognoses.
Several issues remain unresolved in our study. First, the study cohort consisted only of patients from a single tertiary center, which might have limited generalizability to the entire spectrum of patients with chronic HBV infection and to populations in other regions. Hence, further studies are required with a community-based cohort to resolve those issues, and external validation will be necessary to significantly enhance the value of our study. Second, if data from liver biopsy, the gold-standard test for liver fibrosis, had been available for all the patients, more comprehensive conclusions might have been drawn. However, liver biopsy for the sole purpose of estimating the fibrotic burden for screening, might often be unavailable, primarily because of its invasiveness.
In conclusion, LS assessed using TE showed significantly greater prognostic performance than FIB-4 in predicting the development of HBV-related HCC. The combined use of LS and FIB-4 did not provide additional benefit compared with the use of LS alone. Hence, LS assessment using TE might be favored for the improvement of current HCC surveillance strategies, as far as resources permit. Future studies of larger and diversified populations are required to validate our results.
Supplementary Material
Acknowledgments
The authors are grateful to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Republic of Korea) for his help with the figures.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, AUROCs = areas under the ROC curves, CHB = chronic hepatitis B, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, IQR = interquartile range, kPa = kilopascals, LS = liver stiffness, ROC = receiver-operating characteristic, TE = transient elastography.
Financial support: this study was in part supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1008585). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
SUK and BKK equally contributed to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008; 48:335–352. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS. Personalized treatment of hepatitis B. Clin Mol Hepatol 2015; 21:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papatheodoridis GV, Dalekos GN, Yurdaydin C, et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol 2015; 62:363–370. [DOI] [PubMed] [Google Scholar]
- 4.Papatheodoridis GV, Manolakopoulos S, Touloumi G, et al. Hepatocellular carcinoma risk in HBeAg-negative chronic hepatitis B patients with or without cirrhosis treated with entecavir: HepNet. Greece cohort. J Viral Hepat 2015; 22:120–127. [DOI] [PubMed] [Google Scholar]
- 5.Arends P, Sonneveld MJ, Zoutendijk R, et al. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut 2014. [DOI] [PubMed] [Google Scholar]
- 6.Wong GL, Espinosa WZ, Wong VW. Personalized management of cirrhosis by non-invasive tests of liver fibrosis. Clin Mol Hepatol 2015; 21:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin J, Khatri G, Gopal P, et al. Accuracy of ultrasound and noninvasive markers of fibrosis to identify patients with cirrhosis. Dig Dis Sci 2015; 60:1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez SM, Crespo G, Navasa M, et al. Noninvasive assessment of liver fibrosis. Hepatology 2011; 53:325–335. [DOI] [PubMed] [Google Scholar]
- 9.Kim do Y, Kim SU, Ahn SH, et al. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci 2009; 54:1758–1763. [DOI] [PubMed] [Google Scholar]
- 10.Castera L. Noninvasive assessment of liver fibrosis. Dig Dis 2015; 33:498–503. [DOI] [PubMed] [Google Scholar]
- 11.Lee HW, Yoo EJ, Kim BK, et al. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am J Gastroenterol 2014; 109:1241–1249. [DOI] [PubMed] [Google Scholar]
- 12.Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011; 53:885–894. [DOI] [PubMed] [Google Scholar]
- 13.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 14.Kim BK, Kim do Y, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010; 30:546–553. [DOI] [PubMed] [Google Scholar]
- 15.Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 2015; 61:1261–1268. [DOI] [PubMed] [Google Scholar]
- 16.Tamaki N, Kurosaki M, Matsuda S, et al. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J Gastroenterol 2014; 49:1495–1503. [DOI] [PubMed] [Google Scholar]
- 17.Jung KS, Kim SU, Song K, et al. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015; 62:1757–1766. [DOI] [PubMed] [Google Scholar]
- 18.Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology 2015; 61:1851–1859. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 21.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005; 61:92–105. [DOI] [PubMed] [Google Scholar]
- 22.Fung J, Lai CL, Seto WK, et al. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat 2011; 18:738–744. [DOI] [PubMed] [Google Scholar]
- 23.Kim BK, Fung J, Yuen MF, et al. Clinical application of liver stiffness measurement using transient elastography in chronic liver disease from longitudinal perspectives. World J Gastroenterol 2013; 19:1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao JH. Risk stratification of HBV infection in Asia-Pacific region. Clin Mol Hepatol 2014; 20:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong GL, Chan HL, Wong CK, et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol 2014; 60:339–345. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez M, Trepo E, Degre D, et al. Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease. Eur J Gastroenterol Hepatol 2015; 27:1074–1079. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Wang LC, Chen EQ, et al. Prospective evaluation of FibroScan for the diagnosis of hepatic fibrosis compared with liver biopsy/AST platelet ratio index and FIB-4 in patients with chronic HBV infection. Dig Dis Sci 2011; 56:2742–2749. [DOI] [PubMed] [Google Scholar]
- 28.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015; 61:292–302. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Guidelines for the prevention, care, and treatment of persons with chronic hepatitis B infection. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 30.Pang Q, Xu XS, Zhang JY, et al. FIB-4 as a prognostic model for patients with hepatitis B-associated hepatocellular carcinoma. Hepatology 2015; 62:1325–1326. [DOI] [PubMed] [Google Scholar]
- 31.Toyoda H, Kumada T, Tada T, et al. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery 2015; 157:699–707. [DOI] [PubMed] [Google Scholar]
- 32.Berenguer J, Zamora FX, Aldamiz-Echevarria T, et al. Comparison of the prognostic value of liver biopsy and FIB-4 index in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis 2015; 60:950–958. [DOI] [PubMed] [Google Scholar]
- 33.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455. [DOI] [PubMed] [Google Scholar]
- 34.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009; 50:661–662. [DOI] [PubMed] [Google Scholar]
- 35.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006; 354:1011–1020. [DOI] [PubMed] [Google Scholar]
- 36.European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


