Abstract
Chronic inflammation may promote development of coronary heart disease. Studies on the relationship between chronic pancreatitis (CP) and cardiovascular diseases are scant.
We conducted a nationwide retrospective cohort study to determine the risk of acute coronary syndrome (ACS) in patients with CP.
We randomly selected a comparison cohort of individuals without CP from the Taiwan National Health Insurance Research Database (N = 23.74 million) and frequency-matched them with patients with CP from 2000 to 2010 in a 1:4 ratio according to age, sex, and index year. The follow-up period lasted from the index date of the new CP diagnosis to the date of ACS diagnosis, censoring, or the end of 2011. We analyzed the risk of ACS by using Cox proportional-hazard models.
In total, 17,405 patients with CP and 69,620 individuals without CP were followed for 84,430 and 417,426 person-years. Most patients with CP were men, and the mean age of the patients was 48.3 ± 15.0 years. The overall ACS incidence was 2.15-fold higher in the CP cohort than in the non-CP cohort (4.89 vs 2.28 per 10,000 person-years) with an adjusted hazard ratio (aHR) of 1.40 (95% confidence interval [CI] 1.20–1.64). Compared with individuals without CP, patients with CP aged ≤39 years exhibited the highest risk of ACS (aHR 2.14, 95% CI 1.13–4.02), followed by those aged 40 to 54 years (aHR 1.66, 95% CI 1.23–2.24) and those aged 55 to 69 years (aHR 1.53, 95% CI 1.15–2.03).
CP may become an independent risk factor for ACS.
INTRODUCTION
Chronic pancreatitis (CP) is defined as chronic inflammation and fibrosis of the pancreas, resulting in irreversible morphological changes and functional abnormalities.1 The worldwide increase in the prevalence of CP is attributable to the increase in alcohol consumption and the increased availability of high-quality diagnostic modalities.2–5 Patients with CP may experience unremitting abdominal pain, chronic diarrhea, maldigestion, glucose intolerance, and weight loss, all of which substantially impair their quality of life.6 Moreover, CP requires repeated medical interventions and hospitalization, and increases the burden on medical resources.7–9
Heavy drinking increases the risk of high blood pressure, heart failure, and stroke.10–12 Alcohol abuse is a prominent cause of CP.2,13 Evidence reveals that mild-to-moderate alcohol consumption exerts a protective effect against coronary heart disease.14,15 However, chronic inflammation in CP can activate immune cells to promote atherosclerotic lesions, subsequently leading to acute coronary syndrome (ACS).16 Unstable angina and myocardial infarction constitute ACS, causing a sudden decrease in blood flow in the coronary arteries. ACS can cause ventricular arrhythmia, cardiovascular collapse, and death despite advanced treatment options. Although hypertension, diabetes, and hyperlipidemia are well-established risk factors for ACS, approximately half of the patients with ACS do not exhibit these risk factors.17 Most studies on the CP focused on treatment and the risk of pancreatic neoplasm.18–20 Data on patients with CP and ACS prevalence are scant. Therefore, we conducted a nationwide longitudinal cohort study to evaluate the incidence and risk of ACS in patients with CP.
METHODS
Data Source
In March 1995, the Ministry of Health and Welfare in Taiwan integrated 13 health insurance agencies into a nationwide, universal National Health Insurance (NHI) program. The NHI program covers over 99% of the 23.74 million residents of Taiwan (http://www.nhi.gov.tw). The National Health Research Institutes (NHRI) maintains the claims data of beneficiaries enrolled in the NHI program. The NHRI has established and released the National Health Insurance Research Database (NHIRD) annually to the public for research; all data related to personal identification are encrypted by the National Health Insurance Administration (NHIA) before release. In this study, we used a subset of the NHIRD containing healthcare data, such as inpatient claims and the registry of beneficiaries. All clinical diagnoses were recorded using the “International Classification of Diseases, Ninth Revision, Clinical Modification” (ICD-9-CM) codes.21 The study was exempted from a full review by the institutional research ethic committee (CMUH-104-REC2–115). The reliability and validity of this NHIRD database have been published.22,23
Study Design
The study design is a nationwide retrospective cohort study.
Sampled Participants
From the inpatient claims, we selected all adult patients with a first-time diagnosis of CP (ICD-9-CM 577.1) between 2000 and 2010 as the CP cohort. The date of CP diagnosis were defined as the index date. The recurrence rate of ACS remains high.24 Pancreatic cancer has a low survival rate in 1 year.25 Therefore, we excluded those with a history of ACS (ICD-9-CM 410, 411.1, and 411.8) or pancreatic cancer (ICD-9-CM 157) at the baseline. We also excluded patients aged <20 years, those with incomplete age or sex information. A non-CP comparison cohort was randomly selected from the NHI comprising beneficiaries aged ≥20 years and frequency-matched with the CP cohort in a 4:1 ratio according to age (every 5 years), sex, and the year of index date, with the same exclusion criteria as that of the CP cohort.
Exposure Variables
In Taiwan, the diagnosis of CP is made by physicians based on the clinical presentation and imaging studies, namely contrast-enhanced computer tomography, ultrasonography, magnetic resonance image, or endoscopic retrograde cholangiopancreatography.
Outcome and Comorbidities
The outcome of interest was new ACS diagnosis between 2000 and 2011. The baseline comorbidities were hypertension (ICD-9-CM 401–405), diabetes (ICD-9-CM 250), hyperlipidemia (ICD-9-CM 272), cerebrovascular accident (CVA; ICD-9-CM 430–438), atrial fibrillation (ICD-9-CM 427.31), heart failure (ICD-9-CM 428), chronic obstructive pulmonary disease (COPD; ICD-9-CM 491, 492, and 496), chronic kidney disease (CKD; ICD-9-CM 580–589), and acute pancreatitis (ICD-9-CM 577.0), all of which were identified from their diagnoses in the hospitalization records before the index date.
Statistical Analysis
Demographic characteristics and the prevalence of comorbidities in the CP and non-CP cohorts were compared using a chi-square test for categorical variables and a t test for continuous variables. The incidence densities of ACS were calculated according to sex, age, and the presence of comorbidities for each cohort. Univariable and multivariable Cox proportion-hazard regression models were used to examine the effect of CP on the risk of ACS and were expressed using hazard ratios (HRs) with a 95% confidence interval (CI). The multivariable model was simultaneously adjusted for age, sex, and comorbidities of hypertension, diabetes, hyperlipidemia, CVA, atrial fibrillation, heart failure, COPD, CKD, and acute pancreatitis. Additional data analysis was performed to evaluate the joint effect between CP and the ACS-associated risk factors on ACS. The Kaplan–Meier method was used for estimating the cumulative incidence of ACS between the CP and non-CP cohorts, and the differences were assessed using a log-rank test. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). P < 0.05 was considered significant.
RESULTS
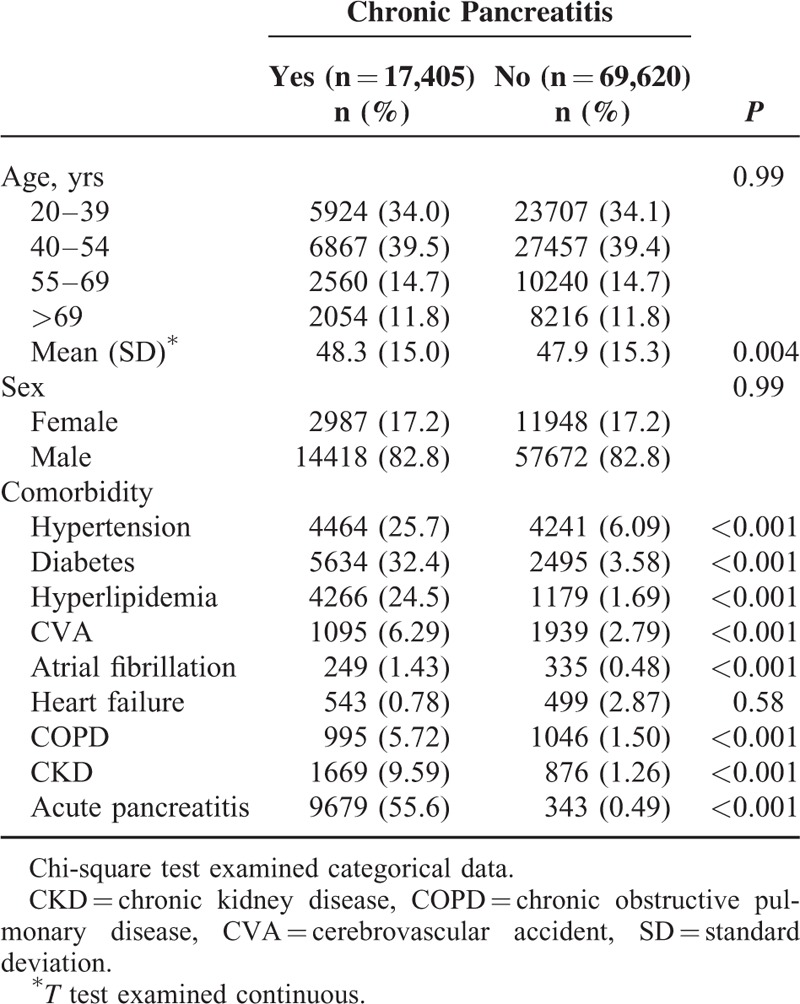
The average annual incidence rate of CP between 2000 and 2010 were 9.50 per 100,000 persons (data not shown). A flow diagram of the study participants is depicted in Figure 1. We identified 17,405 patients with CP and matched them with 69,620 participants without CP. The baseline characteristics of patients in the 2 cohorts are presented in Table 1. Approximately 73.5% of patients were aged <54 years, and most patients were men (82.8% vs 17.2%). The mean ages of the participants were 48.3 ± 15.0 and 47.9 ± 15.3 years in the CP and non-CP cohorts, respectively. Patients with CP had a higher prevalence of comorbidities than those without CP (all P < 0.001).
FIGURE 1.
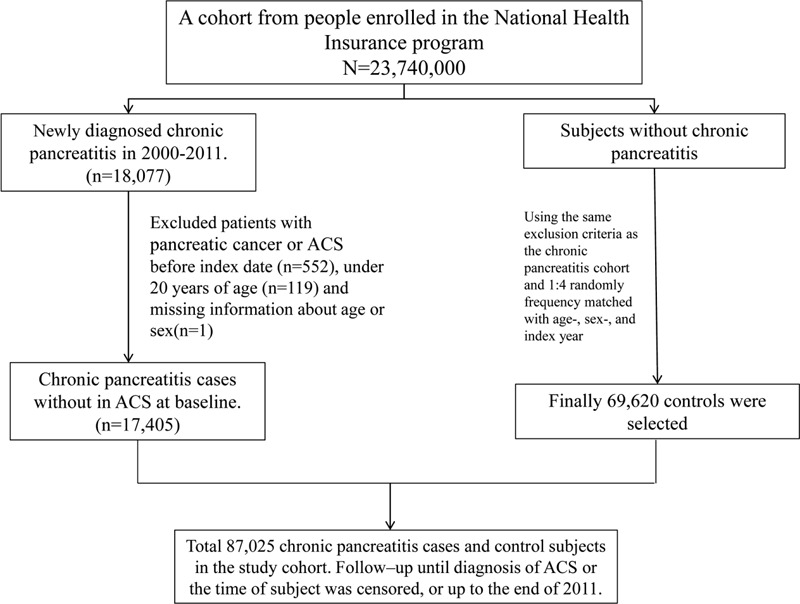
A flow diagram of the study participants.
TABLE 1.
Comparison of Demographics and Comorbidity Between Chronic Pancreatitis Patients and Controls
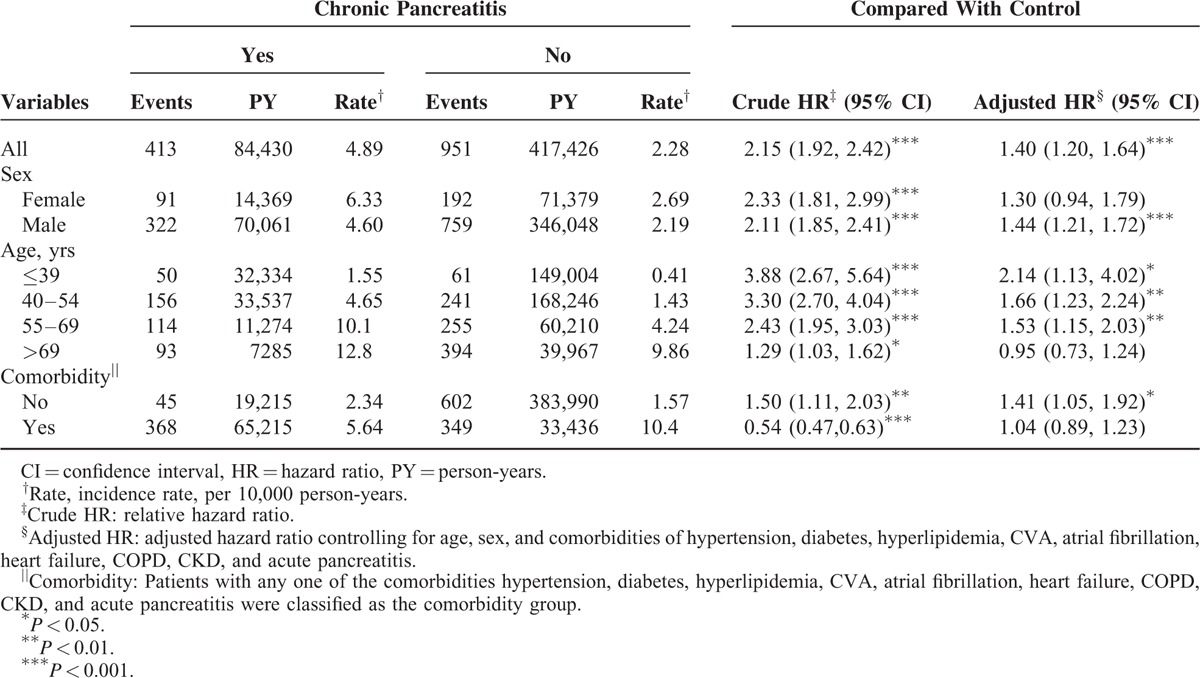
During the mean follow-up period of 4.85 and 6.00 years for the CP and non-CP cohorts, respectively, the cumulative incidence of ACS was significantly higher for patients in the CP cohort than in the non-CP cohort (log-rank test P < 0.001; Figure 2). The overall incidence of ACS was 2.15-fold higher in the CP cohort than in the non-CP cohort (4.89 vs 2.28 per 10,000 person-years), with an adjusted HR (aHR) of 1.40 (95% CI 1.20–1.64; Table 2). The incidence of ACS was 6.33 and 4.60 per 10,000 person-years for men and women, respectively, in the CP cohort. The sex-specific relative risk of ACS was significantly higher for men in the CP cohort than in the non-CP cohort (aHR 1.44, 95% CI 1.21–1.72). Compared with individuals without CP, patients with CP aged ≤39 years exhibited the highest risk of ACS (aHR 2.14, 95% CI 1.13–4.02), followed by those aged 40 to 54 years (aHR 1.66, 95% CI 1.23–2.24) and those aged 55 to 69 years (aHR 1.53, 95% CI 1.15–2.03). The incidence of ACS was higher in patients with comorbidities in both cohorts. In patients without comorbidities, the risk of ACS was consistently higher in the CP cohort than in the non-CP cohort (aHR 1.41, 95% CI 1.05–1.92).
FIGURE 2.
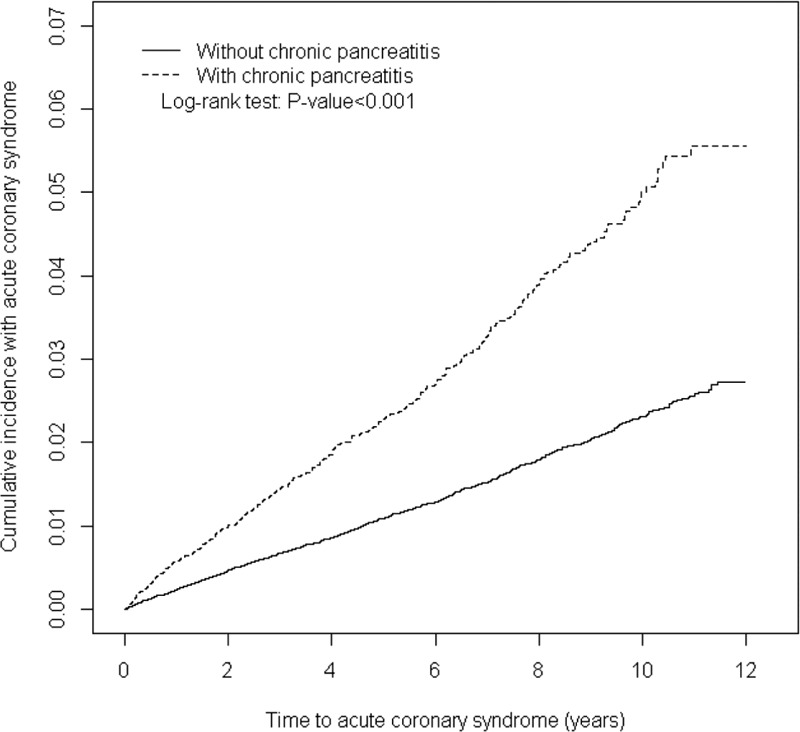
Cummulative incidence of ACS in patients with chronic pancreatitis and comparison patients. ACS = acute coronary syndrome.
TABLE 2.
Comparison of Incidence and Adjusted Hazard Ratio of ACS by Sex, Age and Comorbidity Between Chronic Pancreatitis Patients and Controls
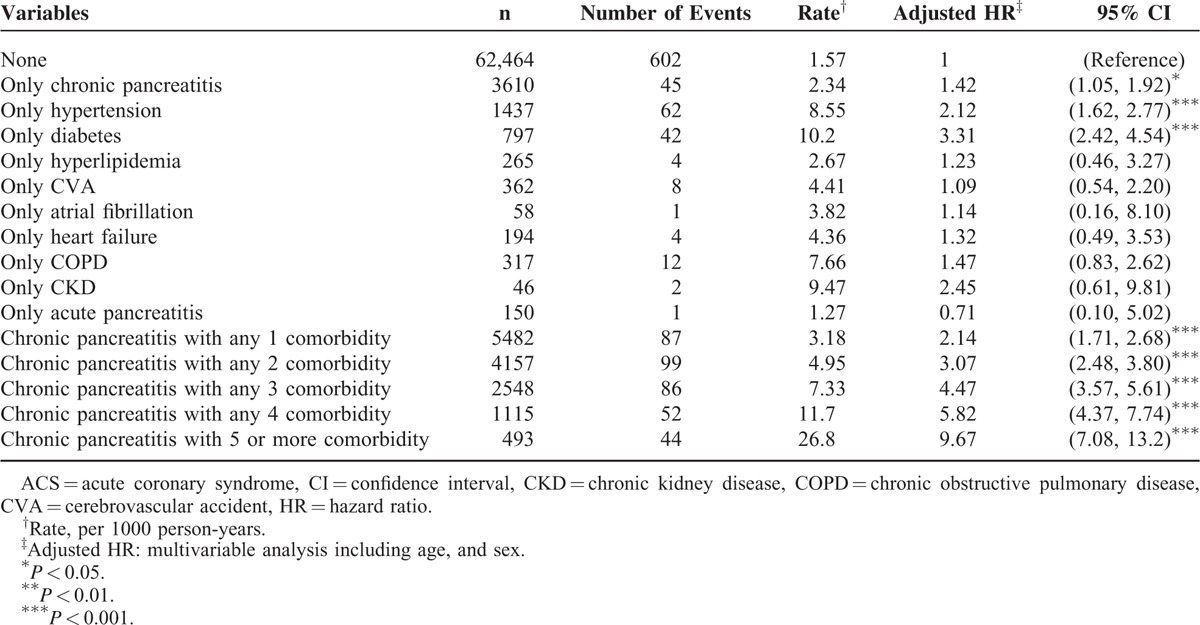
Compared with individuals without CP and lacking comorbidities, those with only diabetes (aHR 3.31, 95% CI 2.42–4.54) and those with only hypertension (aHR 2.12, 95% CI 1.62–2.77) had a higher risk of ACS. Moreover, compared with individuals without CP lacking comorbidities, patients with CP with 5 or more comorbidities were at a significantly higher risk of ACS (aHR 9.67, 95% CI 7.08–13.2), followed by those with 4 comorbidities (aHR 5.82, 95% CI 4.37–7.74), those with 3 comorbidities (aHR 4.47, 95% CI 3.57–5.61), those with 2 comorbidities (aHR 3.07, 95% CI 2.48–3.80), and those with 1 comorbidity (aHR 2.14, 95% CI 1.71–2.68; Table 3).
TABLE 3.
Joint Effects for ACS Between Chronic Pancreatitis and ACS-associated Risk Factors
DISCUSSION
The current study demonstrated that the overall incidence of ACS was 2.15-fold higher in the CP cohort than in the comparison cohort (4.89 vs 2.28 per 10,000 person-years). Patients in the CP cohort exhibited a higher proportion of comorbidities, except heart failure, than those in the comparison cohort did. After adjustment for covariates, patients with CP were at a 1.40-fold increased risk of ACS (aHR 1.40, 95% CI 1.20–1.64) compared with those without CP.
Endothelium is considered a regulator for blood flow and vascular homeostasis. Inflammation may cause endothelial dysfunction, which contributes to the initiation and progression of atherosclerotic diseases.26–28 Infiltration of macrophages and T lymphocytes in the pancreas has been established in CP.29–31 These inflammatory cells once enhance inflammation and tend to precipitate unstable or ruptured atherosclerotic plaques in the coronary arteries.32 In addition, the macrophages may produce prothrombotic and procoagulant factors enhancing thrombus formation at the site of plaque rupture.16
The activated pancreatic stellate cells (PSCs) induce fibrosis in CP.33 Several inflammatory cytokines, namely tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, and IL-6, can activate the PSCs. IL-6 presents at the atherosclerotic plaques and may enhance plaque instability.34 Secretion of TNF-α, a proinflammatory cytokine, is high in patients with acute myocardial ischemia and recurrent myocardial infarction, and may result in cardiac death.35
Most patients with CP in the current study were men, and their mean age was 48.3 ± 15.0 years. The findings of this study are consistent with those of previous reports.36 Although the incidence of ACS was higher in patients with CP than in those without CP for both sexes, men with CP were at a significantly higher risk of ACS than those without CP (aHR 1.44, 95% CI 1.21–1.72). Patients with CP aged ≤69 years were at a significantly increased risk of subsequent ACS compared with their counterparts without CP. Studies have reported that proinflammatory cytokines and coagulation factor production increase with age and cause frailty.37,38 Moreover, the prevalence of comorbidities is high in older adults, which may dilute the effect of CP.39 Therefore, clinicians should proactively examine men and adults aged ≤69 years with CP to prevent ACS.
Our study has several strengths. We determined that patients with CP are at an increased risk of subsequent ACS by using a nationwide epidemiologic design. We enrolled a large sample. Moreover, the NHI is mandatory in Taiwan, and the NHI beneficiaries are assigned unique personal identification numbers, enabling researchers to trace them throughout the follow-up period. Thus, our findings can be generalized to the entire population of Taiwan.
Several limitations must be considered while interpreting these findings. First, the NHIRD does not provide detailed personal information, such as smoking habits, body mass index, and physical activity levels, all of which are potential confounding factors in this study. Hypertension, COPD, and CVA are well-established comorbidities related to tobacco smoking and obesity.40–43 We adjusted the model for hypertension, COPD, and CVA to mediate the influence of smoking and obesity. Second, the lack of drug-treatment data, for example, those on statin therapy and using anticoagulants and antiplatelet drugs, is another limitation of this study. Finally, the potential misclassification bias of interest outcomes may exist in the healthcare claims data; however, the auditing mechanism of the NHIA can facilitate minimizing diagnostic uncertainty and misclassification. Although our meticulous study design adjusts for confounders, a key limitation of this study lies the potential for bias caused by possible unmeasured or unknown confounders.
In conclusion, this nationwide cohort study of 17,405 patients with CP, with 85,000 person-years of follow-up, demonstrated that patients with CP are at a 1.40-fold increased risk of ACS compared with the general population. Men with CP were at a 1.44-fold higher risk of ACS than those without CP. Patients with CP aged ≤69 years were at a significantly increased risk of subsequent ACS compared with their counterparts without CP. Clinicians should be aware of ACS risks in patients with CP and provide appropriate cardiovascular care in addition to CP treatment. However, future prospective studies are warranted to affirm the findings because of the retrospective observational design employed in this study.
Acknowledgments
This work was partially supported by grants from Taiwan Ministry of Health and Welfare Clinical Trial and Research Center for Excellence (DOH102-TD-B-111-004) and Chia-Yi Christian Hospital. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Abbreviations: ACS = acute coronary syndrome, aHR = adjusted hazard ratio, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, CP = chronic pancreatitis, CVA = cerebrovascular accident, IL = interleukin, NHI = National Health Insurance, NHIA = National Health Insurance Administration, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, PSCs = pancreatic stellate cells, TNF-α = tumor necrosis factor-alpha.
All authors have contributed significantly, and all authors are in agreement with the content of the manuscript. Conception/design: W-SC, M-TH; collection and/or assembly of data: W-SC, CLL; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors.
This work was partially supported by grants from Taiwan Ministry of Health and Welfare Clinical Trial and Research Center for Excellence (DOH102-TD-B-111–004) and Chia-Yi Christian Hospital.
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors state that they have no conflicts of interest.
REFERENCES
- 1.Ketwaroo GA, Freedman SD, Sheth SG. Approach to patients with suspected chronic pancreatitis: a comprehensive review. Pancreas 2015; 44:173–180. [DOI] [PubMed] [Google Scholar]
- 2.Herreros-Villanueva M, Hijona E, Banales JM, et al. Alcohol consumption on pancreatic diseases. World J Gastroenterol 2013; 19:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global Status Report on Alcohol and Health 2014. World Health Organization; 2014. Accessed July 6, 2015. [Google Scholar]
- 4.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jupp J, Fine D, Johnson CD. The epidemiology and socioeconomic impact of chronic pancreatitis. Best Pract Res Clin Gastroenterol 2010; 24:219–231. [DOI] [PubMed] [Google Scholar]
- 6.Gupta V, Toskes PP. Diagnosis and management of chronic pancreatitis. Postgrad Med J 2005; 81:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet 2011; 377:1184–1197. [DOI] [PubMed] [Google Scholar]
- 8.Spanier BW, Dijkgraaf MG, Bruno MJ. Trends and forecasts of hospital admissions for acute and chronic pancreatitis in the Netherlands. Eur J Gastroenterol Hepatol 2008; 20:653–658. [DOI] [PubMed] [Google Scholar]
- 9.Mirtallo JM, Forbes A, McClave SA, et al. International consensus guidelines for nutrition therapy in pancreatitis. JPEN J Parenter Enteral Nutr 2012; 36:284–291. [DOI] [PubMed] [Google Scholar]
- 10.Saunders JB, Beevers DG, Paton A. Alcohol-induced hypertension. Lancet 1981; 2:653–656. [DOI] [PubMed] [Google Scholar]
- 11.Lazarevic AM, Nakatani S, Neskovic AN, et al. Early changes in left ventricular function in chronic asymptomatic alcoholics: relation to the duration of heavy drinking. J Am Coll Cardiol 2000; 35:1599–1606. [DOI] [PubMed] [Google Scholar]
- 12.Gorelick PB. Alcohol and stroke. Stroke 1987; 18:268–271. [DOI] [PubMed] [Google Scholar]
- 13.Pezzilli R. Alcohol abuse and pancreatic diseases: an overview. Recent Pat Inflamm Allergy Drug Discov 2015; 9:102–106. [DOI] [PubMed] [Google Scholar]
- 14.Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011; 342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes MV, Dale CE, Zuccolo L, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 2014; 349:g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 17.Koenig W. Inflammation and coronary heart disease: an overview. Cardiol Rev 2001; 9:31–35. [DOI] [PubMed] [Google Scholar]
- 18.Rammohan A, Sathyanesan J, Rajendran K, et al. Synbiotics in surgery for chronic pancreatitis: are they truly effective? A single-blind prospective randomized control trial. Ann Surg 2015; 262:31–37. [DOI] [PubMed] [Google Scholar]
- 19.Ford K, Paul A, Harrison P, et al. Surgical success in chronic pancreatitis: sequential endoscopic retrograde cholangiopancreatography and surgical longitudinal pancreatojejunostomy (Puestow procedure). Eur J Pediatr Surg 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Howes N, Neoptolemos JP. Risk of pancreatic ductal adenocarcinoma in chronic pancreatitis. Gut 2002; 51:765–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004; 148:99–104. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CL, Lee CH, Chen PS, et al. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol 2014; 24:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung WS, Chu YH, Lin CL, et al. Increased risk of acute coronary syndrome among leptospirosis patients: a nationwide cohort analysis. Int J Cardiol 2015; 184:576–580. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 25.Office for National Cancer Statistics Bulletin. Cancer survival rates, Cancer Survival in England: Patients Diagnosed 2008-2012 and Followed up to 2013. Available at: http://www.ons.gov.uk/ons/rel/cancer-unit/cancer-survival-in-england--adults-diagnosed/2008-to-2012--followed-up-to-2013/stb-cancer-survival.html Accessed Febuary 12, 2016. [Google Scholar]
- 26.Esper RJ, Nordaby RA, Vilarino JO, et al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol 2006; 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pate M, Damarla V, Chi DS, et al. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem 2010; 52:109–130. [PubMed] [Google Scholar]
- 28.Kinlay S, Ganz P. Relation between endothelial dysfunction and the acute coronary syndrome: implications for therapy. Am J Cardiol 2000; 86:10J–13J. [DOI] [PubMed] [Google Scholar]
- 29.Goecke H, Forssmann U, Uguccioni M, et al. Macrophages infiltrating the tissue in chronic pancreatitis express the chemokine receptor CCR5. Surgery 2000; 128:806–814. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz-Winnenthal H, Pietsch DH, Schimmack S, et al. Chronic pancreatitis is associated with disease-specific regulatory T-cell responses. Gastroenterology 2010; 138:1178–1188. [DOI] [PubMed] [Google Scholar]
- 31.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 2015; 31:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulvihill NT, Foley JB. Inflammation in acute coronary syndromes. Heart 2002; 87:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol 2008; 23:34–41. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation 2006; 113:e72–e75. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000; 101:2149–2153. [DOI] [PubMed] [Google Scholar]
- 36.Banks PA, Conwell DL, Toskes PP. The management of acute and chronic pancreatitis. Gastroenterol Hepatol (N Y) 2010; 6 (2 suppl 3):1–16. [PMC free article] [PubMed] [Google Scholar]
- 37.Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med 2009; 122:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topinkova E. Aging, disability and frailty. Ann Nutr Metab 2008; 52 suppl 1:6–11. [DOI] [PubMed] [Google Scholar]
- 39.Karlamangla A, Tinetti M, Guralnik J, et al. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci 2007; 62:296–300. [DOI] [PubMed] [Google Scholar]
- 40.Yun M, Li S, Sun D, et al. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: the Bogalusa Heart Study. J Hypertens 2015; 33:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther 2010; 8:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathew B, Patel SB, Reams GP, et al. Obesity-hypertension: emerging concepts in pathophysiology and treatment. Am J Med Sci 2007; 334:23–30. [DOI] [PubMed] [Google Scholar]
- 43.Winter Y, Rohrmann S, Linseisen J, et al. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke 2008; 39:3145–3151. [DOI] [PubMed] [Google Scholar]


