Abstract
To compare the remission of type 2 diabetes mellitus (T2DM) through treatment with laparoscopic sleeve gastrectomy (LSG) or laparoscopic Roux-en-Y gastric bypass (LRYGB), and to analyze the cost-effectiveness of medical treatment, LSG, and LRYGB in T2DM patients (BMI ≥ 28).
A 2-group randomized controlled trial was conducted at Diabetes Surgery Centre, Beijing Shijitan Hospital in Beijing, China. Subjects were 80 patients ages 16 to 65 years with a body mass index of 28 kg/m2 or more and duration of T2DM no more than 15 years. Subjects were randomly assigned (1:1) to undergo either LSG (n = 40) or LRYGB (n = 40) between February 3, 2011 and October 31, 2013. Of those patients, 72 (90%) were available at follow-up at 2 years. These patients included 34 (85%) who underwent LSG and 38 (95%) who underwent LRYGB. This study presents the follow-up data at 2 years, which compared LSG and LRYGB in T2DM patients. Partial remission and complete remission were determined, and weight loss, BMI, changes in abdominal circumference, cholesterol, and triglycerides were measured. The cost-effectiveness of each type of bariatric surgery was analyzed with a Markov simulation model that yielded quality-adjusted life-years (QALYs) and costs.
From our analysis results, LSG and LRYGB are both have taken a great effect on the reduction of fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), and bodyweight in patients with T2DM. The cost-effectiveness ratios of medical treatment, LSG, and LRYGB respectively are 1589.02, 1028.97, and 1197.44 dollars per QALY.
Our analysis indicates that LSG appear to provide a cost-effective method of T2DM treatment for the patients.
INTRODUCTION
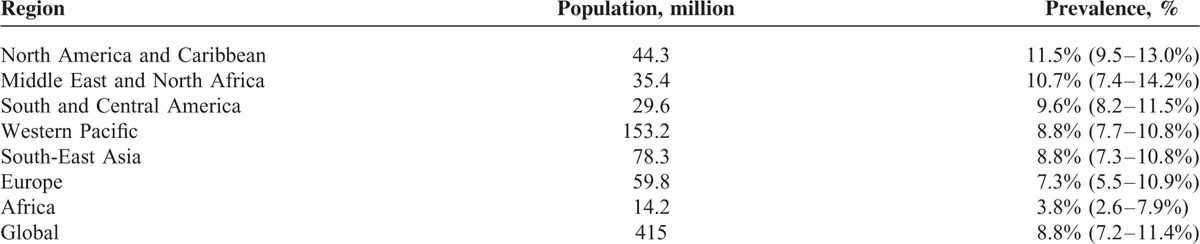
As society develops and lifestyles change, diabetes mellitus has become prevalent, threatening to reduce life expectancy for humans around the global.1–3 Globally, there were a total of 415 million patients aged 20 to 79 with diabetes in 2015, and 90% of those patients had type 2 diabetes mellitus (T2DM).4 As well as, recent studies have found that there were 109.6 million persons with diabetes in China in 2015, which have topped the world, following by India (69.2 million), U.S. (29.3 million), Brazil (14.3 million), and Russian Federation (12.1 million).5–8
In addition, prevalence rate of diabetes was 8.8% around the world in 2015 (Table 1).8 Diabetes appears to be increasing rapidly in China. The overall prevalence of diabetes was estimated to be 11.6% in the Chinese adult population in 2010, which is considerably higher than its prevalence of less than 1% in 1980.9
TABLE 1.
Different Regions Ranked by Age-Adjusted Prevalence (%) of Diabetes (20–79 years) 2015
At this point in time, T2DM is mainly treated medically, including diet restrictions, strengthening exercises, oral hypoglycemic drugs, and insulin injections.10–13 However, there is no way to completely control the disease and its complications, and lifelong use of medications and insulin injections result in poor compliance among patients.14–17
A follow-up study by Mingrone et al18 in the Lancet found that surgery is more effective than medical treatment for the long-term control of T2DM in obese patients. Their study involved a randomized controlled trial that compared conventional medical treatment with laparoscopic Roux-en-Y gastric bypass (LRYGB) or biliopancreatic diversion with duodenal switch in terms of their outcomes at 5 years in 60 obese patients with T2DM. Their results indicated that bariatric surgery was a viable option since surgery achieved a partial remission in 19 (50%) of the 38 patients at 5 years while medical treatment failed to achieve a partial remission in any of the 15 patients who were treated medically. Mingrone et al noted that neither treatment achieved a complete remission in either group at 5 years according to the American Diabetes Association (ADA) definition. Hyperglycemia recurred in 15 (44%) of the 34 patients who underwent surgery that achieved a remission at 2 years.
Our study presents follow-up data at 2 years to compare 80 patients with T2DM who underwent laparoscopic sleeve gastrectomy (LSG) or LRYGB. Partial remission and complete remission were determined, weight loss, the body mass index (BMI), changes in abdominal circumference, cholesterol, and triglycerides were measured, and diabetes-related complications were assessed. On the basis of clinical observations, the cost-effectiveness of medical treatment, LSG, and LRYGB for patients with T2DM was estimated with a Markov simulation model.
METHODS
Study Design and Patients
A 2-group randomized controlled trial was conducted at Diabetes Surgery Centre, Beijing Shijitan Hospital in Beijing, China. Inclusion criteria were: the patients ages ≤65 years; BMI ≥ 28 kg/m2; duration of T2DM ≤ 15 years, in accordance with the ADA definition for T2DM: fasting plasma glucose (FPG) 7.0 mmol/L or greater, diagnosis, or use of a glucose-lowering drug;19 and ability to understand and comply with the study protocol.
In addition, the cost-effectiveness of each type of treatment was analyzed with a Markov simulation model. The Markov model has been used in health fields since the 1980s to depict the development of chronic disease. The model divides a disease into a number of different Markov states depending on their relationship to health, and it then simulates the development of that disease governed by the probability of a transition between states at a certain time. The model uses loop computations to estimate the outcomes and gains or losses of disease development. Details of the model and its validation can be found in the literature.20,21 Markov states of T2DM in the current study were Well, T2DM, and Death.
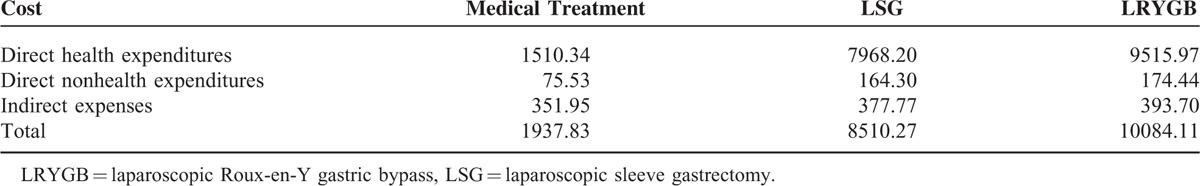
The mortality rate (used in Well state), prevalence rate, case fatality rate (used in T2DM state), and relevant parameters of T2DM are collected in China Health and Family Planning Statistical Year-Book, 2013 to 2015, and the remission rates for the 2 surgeries are based on the aforementioned data from research results. The total costs include direct health expenditures (outpatient costs, hospitalization costs, surgical fees, and drug costs), direct nonhealth expenditures (hiring caregivers, commercial medical insurance, board expenses, transportation, and health care products), and indirect expenses with data based on theoretical values and prices at Beijing Shijitan Hospital (Table 2).22
TABLE 2.
The Costs of Type 2 Diabetes Mellitus Used in the Markov Model in China ($)
Randomization
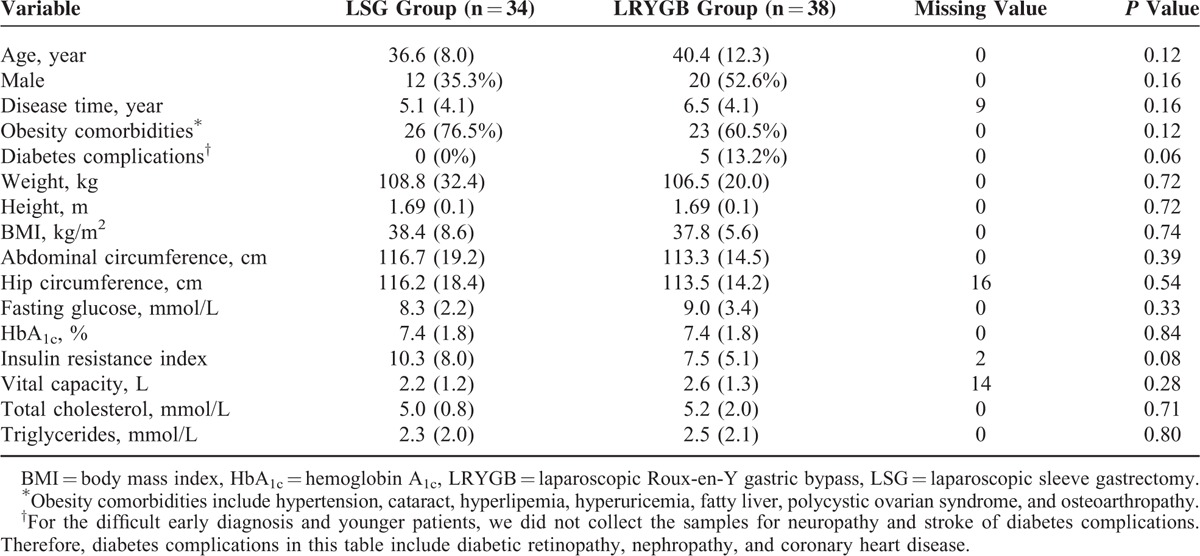
Eighty patients were randomly assigned (1:1) to undergo either LSG (n = 40) or LRYGB (n = 40) between February 3, 2011 and October 31, 2013. Of those patients, 72 (90%) were available at follow-up at 2 years. These patients included 34 (85%) who underwent LSG and 38 (95%) who underwent LRYGB (Figure 1). Study investigators were aware of treatment allocation from the point of randomization. There were no statistically significant differences in baseline values of the LSG group and the LRYGB group (Table 3).
FIGURE 1.
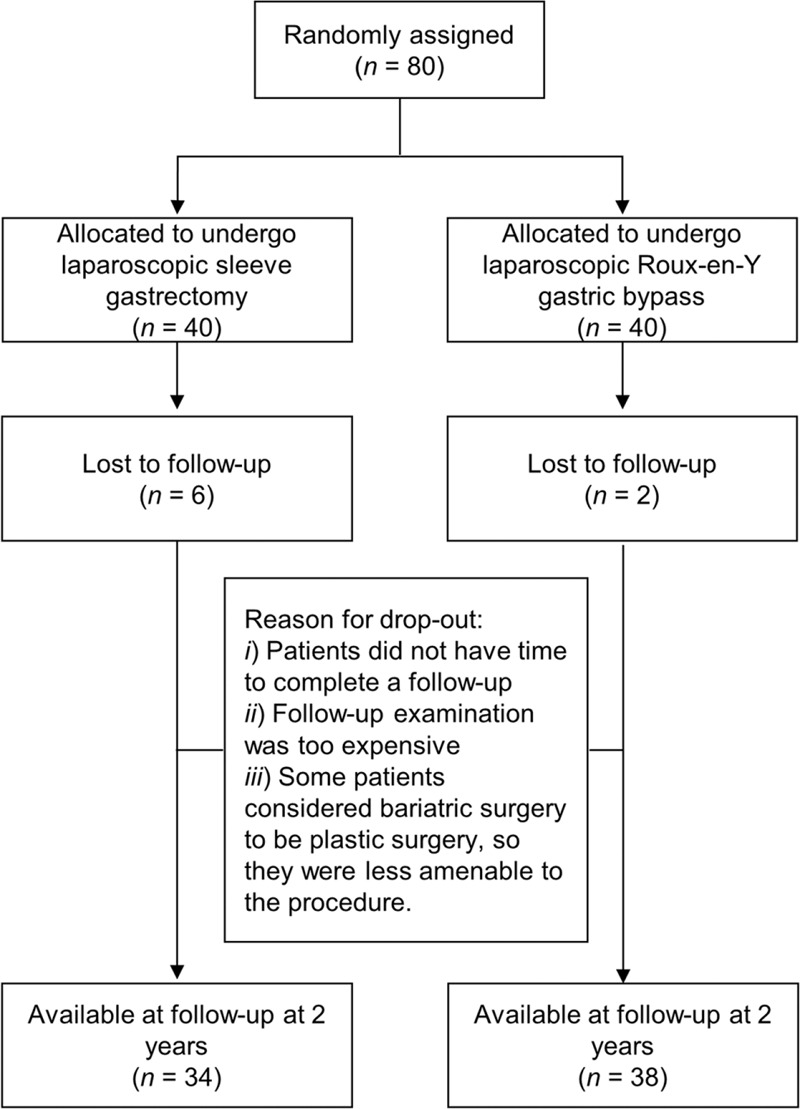
Trial profile. Eighty patients were randomly assigned (1:1) to undergo either LSG (n = 40) or LRYGB (n = 40) between February 3, 2011 and October 31, 2013. Of those patients, 72 (90%) were available for follow-up at 2 years. These patients included 34 (85%) who underwent LSG and 38 (95%) who underwent LRYGB. LRYGB = laparoscopic Roux-en-Y gastric bypass, LSG = laparoscopic sleeve gastrectomy.
TABLE 3.
Baseline Values for Patients in the LSG Group and the LRYGB Group
Outcomes
The primary endpoint was the rate of partial remission and complete remission by T2DM at 2 years. According to the criteria recommended by the ADA: partial remission is defined as an FPG concentration of 6.9 mmol/L or less and a hemoglobin A1c (HbA1c) concentration of 6.5% or less (≤47.5 mmol/mol) without active pharmacological treatment for at least 1 year while complete remission is defined as HbA1c < 6.0% and FPG < 5.6 mmol/L for at least 1 year without any medical or surgical treatment.23,24 The following variables were assessed as secondary outcomes: FPG, HbA1c, changes in bodyweight, BMI, abdominal circumference, plasma total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. Markov model outcomes included quality-adjusted life-years (QALYs) and costs.
Statistical Analysis
A χ2 test was used to compare the remission of T2DM in the LSG group and the LRYGB group. All of the secondary outcomes, which were expressed in absolute values and in proportion to baseline values, were tested with a t test. All of the outcomes were analyzed with SPSS (version 16.0), and P < 0.05 was considered statistically significant for all tests. A Markov model was built and analyzed with Treeage Pro (version 2011) to compare the cost-effectiveness of treatments.
RESULTS
Eighty patients were randomly assigned (1:1) to undergo either LSG (n = 40) or LRYGB (n = 40) between February 3, 2011 and October 31, 2013. Of those patients, 72 (90%) were available at follow-up at 2 years. These patients included 34 (85%) who underwent LSG and 38 (95%) who underwent LRYGB.
At 2 years, a partial remission was achieved in 26 (76.5%) in the LSG group and 22 (57.9%) in the LRYGB group. A total of 17 (50.0%) in the LSG group and 14 (36.8%) in the LRYGB group had met the standard of HbA1c < 6.0% and FPG < 5.6 mmol/L without glucose-lowering drugs, which indicates a complete remission. Similarly, 29 (85.3%) in the LSG group and 27 (71.1%) in the LRYGB group had HbA1c ≤ 6.5% with or without glucose-lowering drugs (Table 4). The 2 surgeries had a positive effect on the remission of T2DM. There were no significant differences in the results of the 2 groups (P > 0.05), which indicates that the 2 surgeries did not differ in terms of the remission of T2DM and glycemic control.
TABLE 4.
Type 2 Diabetes Mellitus Remission and Glycemic Control at the Follow-Up at 2 Years
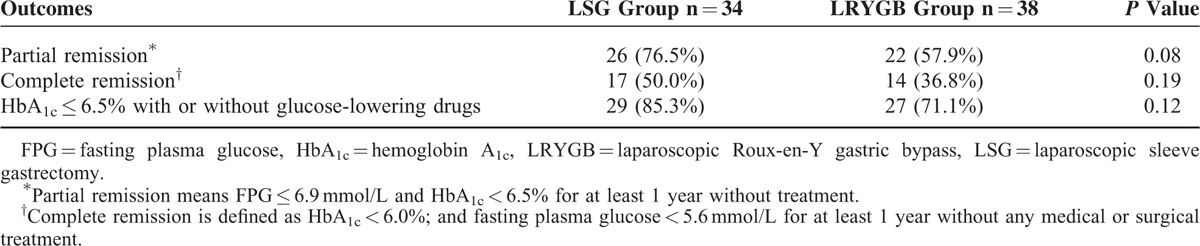
Secondary outcome measures included FPG, HbA1c, changes in bodyweight, BMI, abdominal circumference, plasma total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. Data on these measures are presented as the absolute change and percent change at 2 years from the baseline (Table 5). Mean FPG and HbA1c concentrations at 2 years were slightly lower in the LSG group than in the LRYGB group. The 2 surgeries both caused weight loss and changes in BMI and abdominal circumference, and these measures did not differ significantly between the 2 groups. The absolute change and percent change in these secondary outcome measures at 2 years did not differ significantly between the 2 groups. The 2 surgeries both had a substantial effect on the reduction of FPG, HbA1c, and bodyweight in patients with T2DM. Moreover, outcomes of obesity comorbidities and diabetes complications for the LSG group and LRYGB group also be revealed (Table 6).
TABLE 5.
Secondary Outcome Measures for the LSG Group and the LRYGB Group
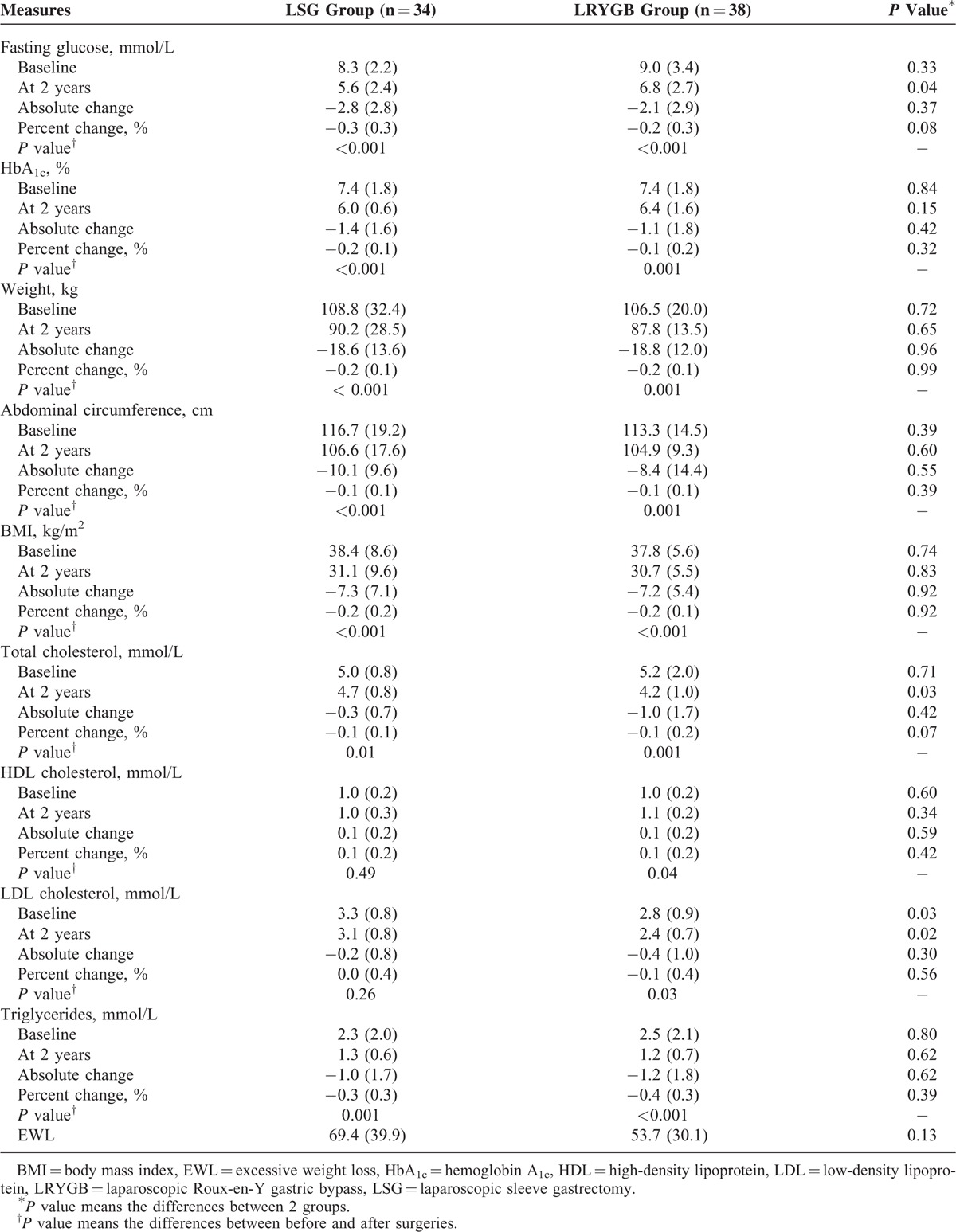
TABLE 6.
Outcomes of Obesity Comorbidities∗ and Diabetes Complications† for the LSG Group and LRYGB Group
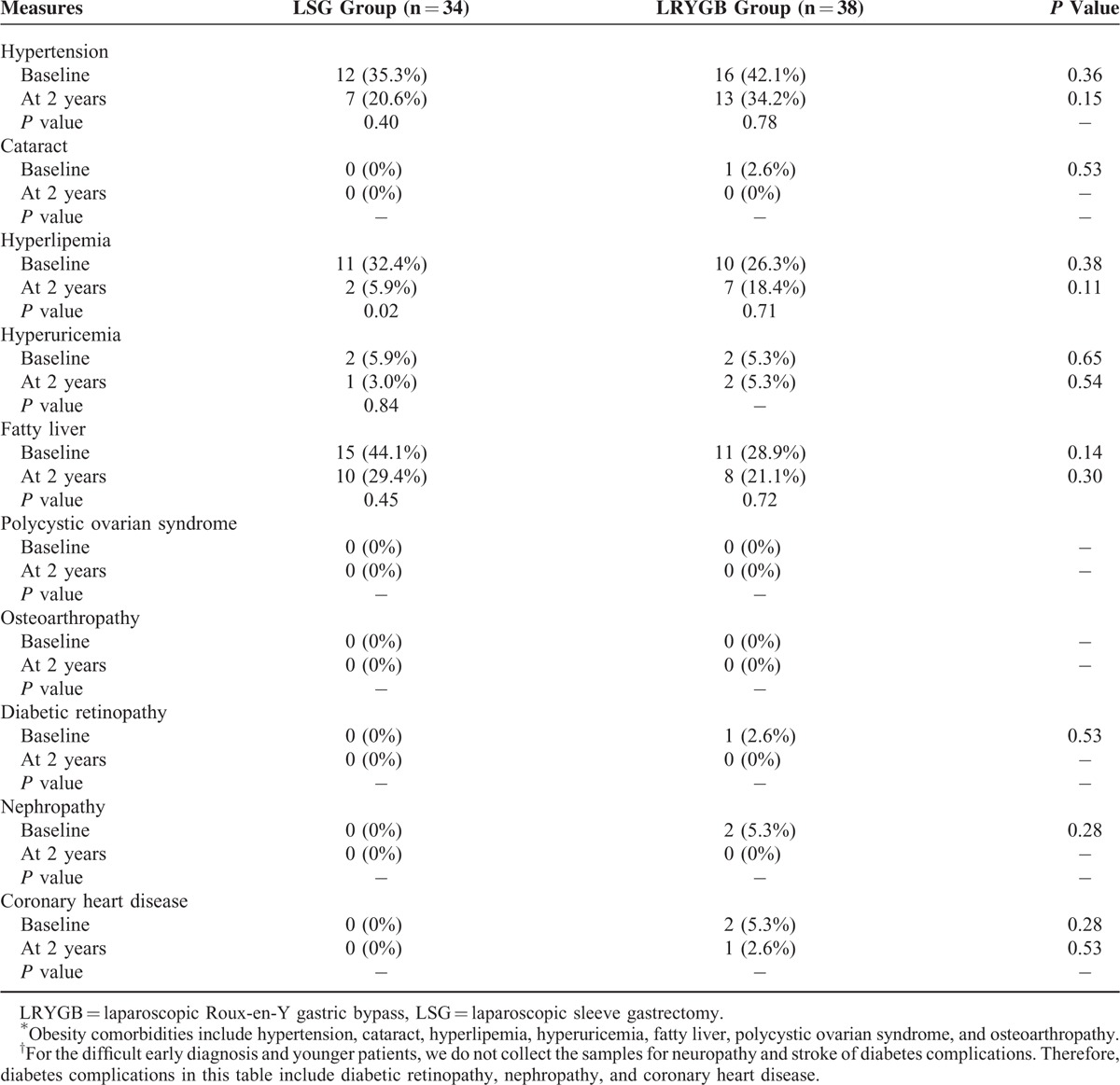
Given the similar outcomes of the surgeries, the question is then which is more cost-effective. The cost-effectiveness ratio was measured for the 3 different treatments. Costs and QALYs were discounted at an annual rate of 5% in accordance with China's consumer price index. A tree diagram is depicted in Figure 2. There were 3 Markov states of T2DM: Well, T2DM, and Death. Each state has different parameters and a probability of a transition between the states for the 3 treatments. The total costs per capita for patients with T2DM include direct health expenditures, direct nonhealth expenditures, and indirect expenses, and the total cost of medical treatment was 1937.83 dollars per year, that of LSG was 8510.27 dollars, and that of LRYGB was 10,084.11 dollars. Clearly, the lowest cost treatment is medical treatment since surgical fees are a one-time fee option, and surgery is more expensive than basic medicines such as acarbose tablets, gliquidone tablets, and Novolin. Therefore, patients undergoing medical treatment pay the least in the short term. The relative cost of medical treatment was 37,183 dollars, that of LSG was 42,795 dollars, and that of LRYGB was 49,646 dollars. The QALYs were 23.4 years for medical treatment, 41.59 years for LSG, and 41.46 years for LRYGB. Medical treatment had the lowest relative cost and the shortest QALYs. The cost-effectiveness ratio was 1589.02 dollars per QALY for medical treatment, 1028.97 dollars per QALY for LSG, and 1197.44 dollars per QALY for LRYGB (Table 7). Accordingly, LSG yielded the greatest benefit at the lowest cost for patients with T2DM (Figure 3).
FIGURE 2.
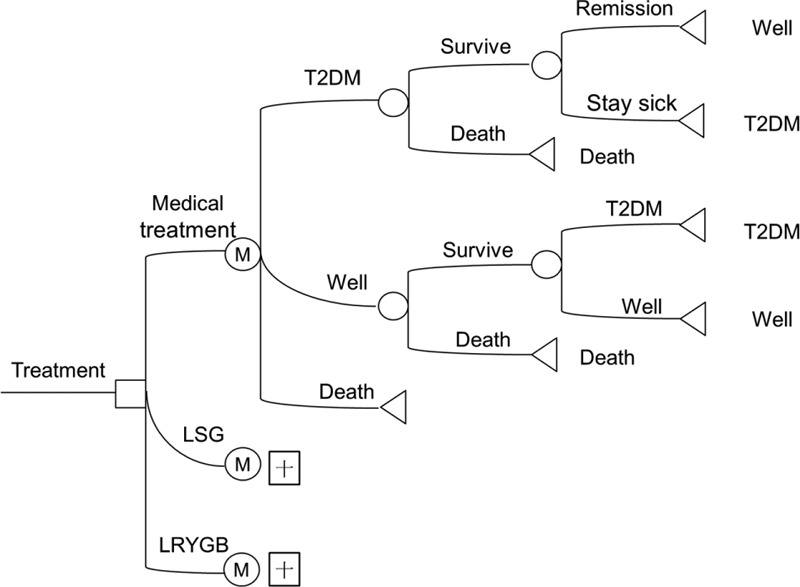
Tree diagram of a Markov model. Three Markov states are shown: Well, DM2, and Death. □ indicates a decision node, ○ indicates a chance node, Δ indicates a terminal node, and “M” indicates a Markov node.
TABLE 7.
Life-Years Gained and Cost-Effectiveness Ratios for Medical Treatment and Two Surgical Procedures

FIGURE 3.
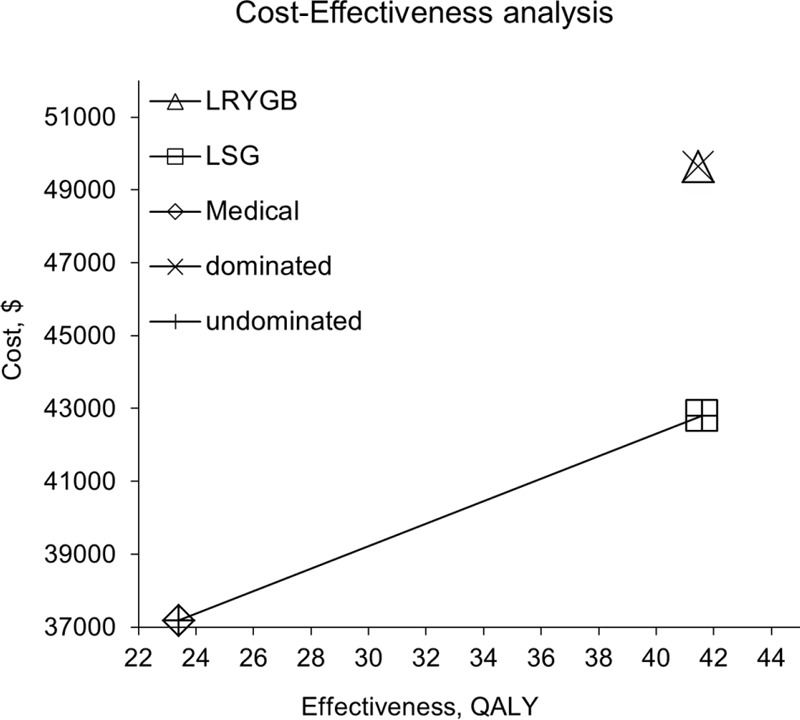
Cost-effectiveness analysis of Medical treatment, LSG, and LRYGB. The CEF is shown. × means the dominated strategy; + means the undominated strategy. LSG is the predominant strategy. CEF = cost-effective frontier, LRYGB = laparoscopic Roux-en-Y gastric bypass, LSG = laparoscopic sleeve gastrectomy.
Sensitivity analysis was performed and tornado diagrams were constructed to determine the robustness of the current model and to identify key factors affecting the cost-effectiveness ratio. The 95% confidence interval of the remission rate was used to determine lower and upper values to enter into the model, and the cost values were halved or doubled since confidence intervals were unavailable.25Figure 4 shows the effect of varying each parameter on the cost-effectiveness ratio in tornado analyses. Cost was found to be a key factor. Doubling the cost leads to a lower cost-effectiveness ratio, and halving the cost leads to a higher cost-effectiveness ratio. Moreover, LSG remained the predominant strategy regardless of changes in variables.
FIGURE 4.
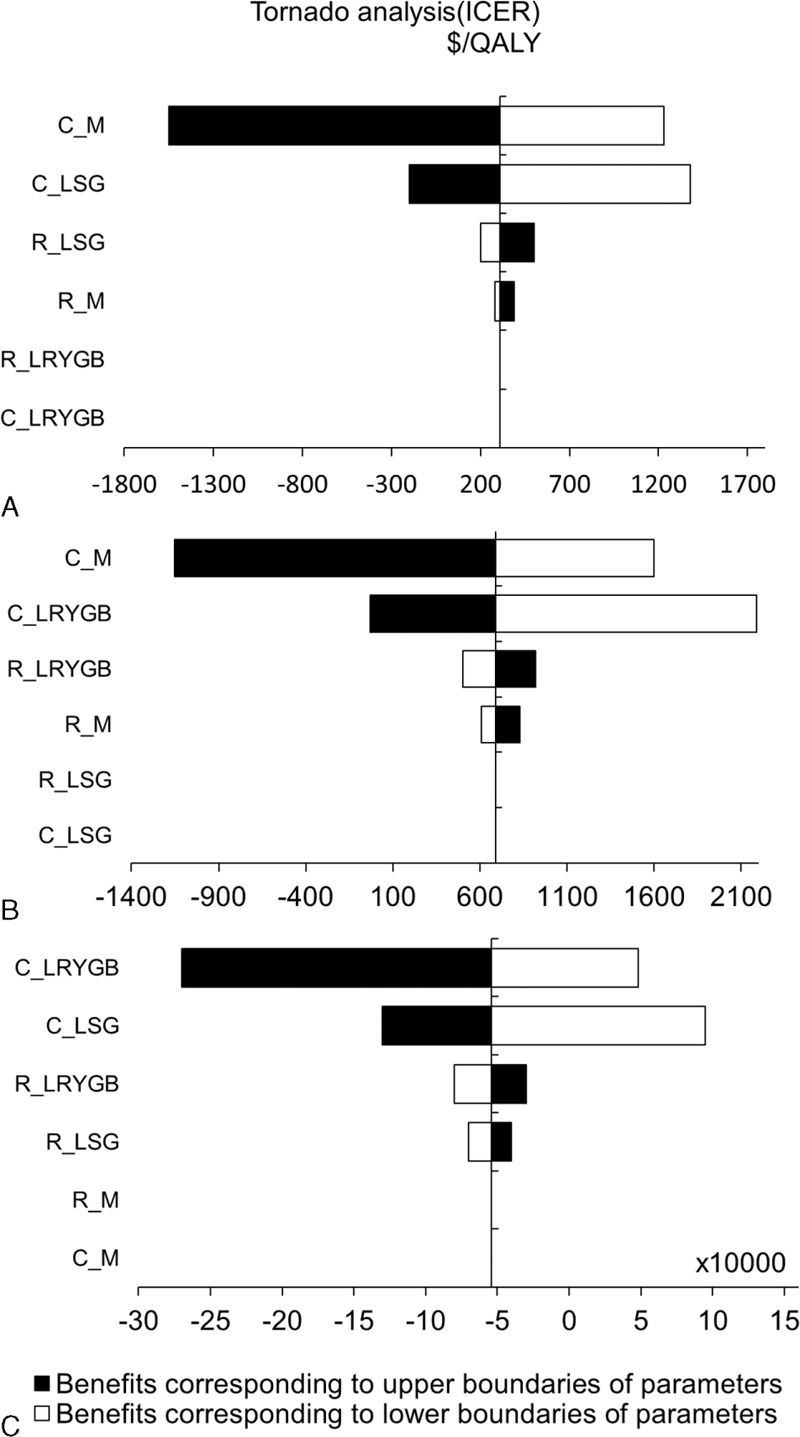
Tornado diagrams of each treatment: (A) medical treatment versus LSG; (B) medical treatment versus LRYGB; (C) LSG versus LRYGB. C_M means the cost of medical treatment, C_LSG means the cost of LSG; C_LRYGB means the cost of LRYGB, R_M means the remission rate for medical treatment, R_LSG means the remission rate for LSG, and R_ LRYGB means the remission rate for LRYGB. When, as an example, C_M in (A) is halved, an increased cost leads to a lower cost-effectiveness ratio. When C_M in (A) is doubled, a reduced cost leads to a higher cost-effectiveness ratio. LRYGB = laparoscopic Roux-en-Y gastric bypass, LSG = laparoscopic sleeve gastrectomy.
DISCUSSION
At the current time, there were few studies about the therapeutic effect and cost-effectiveness of bariatric surgery in Asia. Bariatric surgeries were performed in China but only in clinical trials until 2012, when the country began to recognize the appropriateness and validity of bariatric surgery. The Chinese Society for Metabolic & Bariatric Surgery was established in 2012. Japan has a similar society, the Japanese Society for the Surgery of Obesity and Metabolic Disorders, but bariatric surgeries were still seldom performed in Japan.26 As well, the economic aspects of conventional treatment and surgery have seldom been investigated in China. Western studies have found that surgery leads to greater gains in QALYs and is less costly than medical treatment for patients with T2DM over the long term.25,27,28 However, these studies mostly focused on LRYGB and biliopancreatic diversion with duodenal switch and did not include a comparison to LSG. Moreover, the homeostasis model assessment of insulin resistance differs in populations in different countries.29–34 Given China's diabetic population and the particularities of the Asian physique, this study will facilitate and guide cost-effective bariatric surgery for China specifically but for the rest of Asia as well.
On the baseline data of this study, the enrollment rate in the current trial was 100% because of the strict inclusion criteria. The follow-up rate was 90% and the reasons for drop-outs were: patients did not have time to complete a follow-up; follow-up examination was too expensive; and some patients viewed bariatric surgery as plastic surgery, so they were less amenable to the procedure.
In addition, the study population was relatively young and the age variation was minimal which was mainly from the small sample size. However, Annual Report (2014) and Diabetes Atlas (2015) of International Diabetes Federation both indicated that nearly half diabetes patients aged between 40 and 59 years in the world, with the Chinese patients being younger.8,35 Besides, for duration of T2DM ≤15 years of the inclusion criteria in the study, the younger patients were more acceptable for the surgery, while elder patients tend to maintain the medical control, which was similar with several studies.36,37
Moreover, the standard deviations of BMIs were relatively low, which was also mainly due to the small sample size. However, based on the different inclusion criteria of BMI between the guidelines of China and ADA, we can find diagnostic criteria of obesity also different in China, Europe, and America. The boundary of adult overweight is 24 of BMI, and adult obesity is 28 of BMI in China, so that the inclusion criteria were adjusted by changing a BMI ≥ 35 to a BMI ≥ 28, which better accommodated the requirements of this study. And there are several studies that conduct the comparison between the LSG and LRYGB group with BMI measured by 40 or over and low standard deviations.38,39
The current results indicated that LSG and LRYGB were both remarkably effective at long-term control of hyperglycaemia and improving the metabolic profile, allowing significant reductions in medication use and use of glucose-lowing drugs shown in Table 4. This study used the standard defined by ADA, which is stricter than general standards, so the partial remission and complete remission that were achieved are relatively ideal. A point worth noting is that no deaths occurred after either surgery. The results of this study basically agree with those of other studies, that is to say the surgeries are reasonable approaches to treating diabetes.40
Although, as a trend to more diabetes remission in the LRYGB group, there was still no statistical difference between the 2 groups with the partial remission and complete remission, which was similar with western studies. This is because the baseline data in our study show the mean of BMI in LSG group is larger than the one in LRYGB group, which means that the prognosis effect of LSG is better because the curable effect of the patients were better for obese patients. Certainly, the results are mainly caused by the relatively small sample size.
Besides, LRYGB appears to have a better effect on LDL and total cholesterol while HDL and triglycerids are not different, which also mainly because of the mean of weight and BMI in LRYGB group are smaller than the one in LSG group, and obese patients are more suitable for LSG surgery. Several studies show that a few secondary outcomes, such as LDL cholesterol, HDL cholesterol, or triglycerids, have statistical differences between the 2 groups.18 However, it is inadequate to prove the difference of clinical effect between the 2 groups only based on this individual or exceptional index, which may because of the small sample.
Several studies commonly used the excessive weight loss to assess the clinical effect of surgery, which was calculated as the difference between the weight at the time of implantation and the ideal body weight corresponding to a BMI of 25 kg/m2, reaching the value of 50% means the effective results of weight loss at 1 year after surgery. The result of our study achieved the 61.1%, which is similar with other Asian research (Table 5).41,42
Furthermore, there were only 4 cases occurred the mild postoperative complication which were cured after conservative treatment, which manifested as mild nausea, pain, emesis, and bleeding. This result was also similar with other research results.41
Determining which surgery is more cost-effective is another important issue in this study. First, what warrants special attention is whether different medications will affect the total costs for the medically treated group. Medications of T2DM patients in China according to the guideline are basically metformin, sulfonylurea, and insulin. This is the first one of the considered 4 conventional treatment strategies by Yuanhui Zhang et al,43 which includes metformin, sulfonylurea, and insulin; metformin, a dipeptidyl peptidase-4 inhibitor, and insulin; metformin, a glucagon-like peptide-1 agonist, and insulin; and metformin and insulin, proving different ways of drug use little impact on the cost. Therefore, different medications have no effect on our cost-effectiveness analysis.
According to the model, LSG and LRYGB appear to be relatively cost-effective treatments for diabetics, with cost-effectiveness ratios ranging from $1028.97 to $1197.44/QALY, as compared to $1589.02/QALY for medical treatment. Therefore, the one-off charge of surgeries is high, but they lead to greater gains in QALYs and are less costly than medical treatment for patients with T2DM over the long term. Relatively, LSG is as effective as LRYGB and is slightly more cost-effective than LRYGB, so that LSG therapy appears to be the cost-effective option for managing patients with T2DM.
The current study has several limitations. The first stems mainly from its relatively small sample size. Second, the current authors are cognizant of the lack of data on medical treatment in contrast to data on surgery. However, the patients who included in this study were all under medication more than 2 years with no remission and the primary endpoint of our study was conducted as 2 years, so that they accepted surgical treatment and had drug withdrawal after surgery. Third, limited data on the long-term effects of bariatric surgery are available, which means the follow-up in this study was only 2 years, so a consistent remission as defined by the ADA was not evident.
In conclusion, bariatric surgery is not a cost-saving way to control T2DM since the one-time fee is prohibitive for some patients, but the increased costs of that surgery come with greater benefits. From an economic perspective, LSG is a cost-effective intervention for managing T2DM. The hope is that estimates of the cost-effectiveness of bariatric surgery will become more systematic, helping to facilitate and guide future policy decisions regarding the treatment of diabetes.
Acknowledgements
The authors thank the Beijing Municipal Administration of Hospitals Special Fund to Support the Development of Clinical Medicine (grant no.Z131107002213168) for funding. The authors also thank all the patients who participated in this trial.
Footnotes
Abbreviations: ADA = American Diabetes Association, BMI = body mass index, FPG = fasting plasma glucose, HbA1c = hemoglobin A1c, LRYGB = laparoscopic Roux-en-Y gastric bypass, LSG = laparoscopic sleeve gastrectomy, QALY = quality-adjusted life-years, T2DM = type 2 diabetes mellitus.
QT and ZS contributed equally to this work.
WT designed the research; ZS and GX collected materials and data; QT performed statistical analysis and produced the initial draft; NZ, PS, and LX revised the manuscript; All authors were involved in the interpretation of the analyses and writing of the paper and approved the final version of the paper and take responsibility for its content.
Ethics, consent, and permissions: The ethics committee of Beijing Shijitan Hospital approved the study protocol (No: 8861). Patient records were anonymized and de-identified prior to analysis. All of the patients signed a consent form before surgery. All of the participants consented to publication of the study's results.
This research was funded by the Beijing Municipal Administration of Hospitals Special Fund to Support the Development of Clinical Medicine (grant no. Z131107002213168).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Park CH, Noh JS, Fujii H, et al. Oligonol, a low-molecular-weight polyphenol derived from lychee fruit, attenuates gluco-lipotoxicity-mediated renal disorder in type 2 diabetic db/db mice. Drug Discov Ther 2015; 9:13–22. [DOI] [PubMed] [Google Scholar]
- 2.Shi WC, Qiu W, Wang WH, et al. Osteoprotegerin is up-regulated in pancreatic cancers and correlates with cancer-associated new-onset diabetes. Biosci Trends 2014; 8:322–326. [DOI] [PubMed] [Google Scholar]
- 3.Zhao EF, Zhang YF, Zeng XL, et al. Association between maternal diabetes mellitus and the risk of congenital malformations: a meta-analysis of cohort studies. Drug Discov Ther 2015; 9:274–281. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet 2014; 383:1947–1948. [DOI] [PubMed] [Google Scholar]
- 5.Pan XR, Yang WY, Li GW, et al. Prevalence of diabetes and its risk factors in China, 1994. Diabetes Care 1997; 20:1664–1669. [DOI] [PubMed] [Google Scholar]
- 6.Gu D, Reynolds K, Duan X, et al. Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Diabetologia 2003; 46:1190–1198. [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362:1090–1101. [DOI] [PubMed] [Google Scholar]
- 8.International Diabetes Federation. Diabetes Atlas. Seventh Edition. 2015. Available at http://www.diabetesatlas.org/resources/2015-atlas.html [Accessed February 9, 2016]. [Google Scholar]
- 9.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310:948–959. [DOI] [PubMed] [Google Scholar]
- 10.Hirotani Y, Okumura K, Yoko U, et al. Effects of Gosha-jinki-gan (Chinese herbal medicine: Niu-Che-Sen-Qi-Wan) on hyperinsulinemia and hypertriglyceridemia in prediabetic Zucker fatty rats. Drug Discov Ther 2013; 7:105–108. [PubMed] [Google Scholar]
- 11.Kotani K, Imazato T, Anzai K. Kyushu Diabetes Testing Study Group. Expected role of medical technologists in diabetes mellitus education teams. Biosci Trends 2015; 9:205–206. [DOI] [PubMed] [Google Scholar]
- 12.Masood T, Kushwaha RS, Singh R, et al. Circadian rhythm of serum 25 (OH) vitamin D, calcium and phosphorus levels in the treatment and management of type-2 diabetic patients. Drug Discov Ther 2015; 9:70–74. [DOI] [PubMed] [Google Scholar]
- 13.Yin YH, Qi FH, Song ZH, et al. Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats. Biosci Trends 2014; 8:217–226. [DOI] [PubMed] [Google Scholar]
- 14.Loney-Hutchinson LM, Provilus AD, Jean-Louis G, et al. Group visits in the management of diabetes and hypertension: effect on glycemic and blood pressure control. Curr Diab Rep 2009; 9:238–242. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CC, Almulaifi A, Chen JC, et al. Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35: five-year outcomes. JAMA Surg 2015; 16:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Kotani K, Shuumarjav U, Taniguchi N, et al. Possible relationship between the heart rates and serum amyloid A in a hyperglycemic population. Biosci Trends 2015; 9:79–81. [DOI] [PubMed] [Google Scholar]
- 17.Khan IA, Jahan P, Hasan Q, et al. Validation of the association of TCF7L2 and SLC30A8 gene polymorphisms with post-transplant diabetes mellitus in Asian Indian population. Intractable Rare Dis Res 2015; 4:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015; 386:964–973. [DOI] [PubMed] [Google Scholar]
- 19.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26:S5–S20. [DOI] [PubMed] [Google Scholar]
- 20.Herman WH, Hoerger TJ, Brandle M, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005; 142:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scanlon PH, Aldington SJ, Leal J, et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess 2015; 19:1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZW. Study on the burden of diabetes in China. Fudan University. 2007. Available at http://cdmd.cnki.com.cn/Article/CDMD-10246-2007168675.htm [Accessed November 2, 2015]. [Google Scholar]
- 23.American Diabetes Association. Standards of medical care in diabete-2013. Diabetes Care 2013; 36:S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care 2009; 32:2133–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henteleff HJ, Birch DW, Hallowell PT, et al. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Can J Surg 2013; 56:353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki A, Wakabayashi G, Yonei Y. Current status of bariatric surgery in Japan and effectiveness in obesity and diabetes. J Gastroenterol 2014; 49:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikramuddin S, Klingman D, Swan T, et al. Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am J Manag Care 2009; 15:607–615. [PubMed] [Google Scholar]
- 28.Keating CL, Dixon JB, Moodie ML, et al. Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modeled lifetime analysis. Diabetes Care 2009; 32:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013; 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto M, Hayata K, Chiba J, et al. Multiplex cytokine analysis of Werner syndrome. Intractable Rare Dis Res 2015; 4:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan P, Grbovic E, Inci S, et al. Azathioprine-induced atrial fibrillation. Intractable Rare Dis Res 2015; 4:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Q, Li XQ, Song PP, et al. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov Ther 2015; 9:380–385. [DOI] [PubMed] [Google Scholar]
- 33.Khan IA, Vattam KK, Jahan P, et al. Importance of glucokinase -258G/A polymorphism in Asian Indians with post-transplant and type 2 diabetes mellitus. Intractable Rare Dis Res 2016; 5:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meneghello C, Segat D, Fortunati E. Insulin-driven translational capacity is impaired in primary fibroblasts of Prader Willi. Intractable Rare Dis Res 2016; 5:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Diabetes Federation. Annual Report (2014). 2014. Available at http://www.idf.org/annual-report-2014 [Accessed February 13, 2016]. [Google Scholar]
- 36.Kehagias I, Karamanakos SN, Argentou M, et al. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg 2011; 21:1650–1656. [DOI] [PubMed] [Google Scholar]
- 37.Boza C, Gamboa C, Salinas J, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. Surg Obes Relat Dis 2012; 8:243–249. [DOI] [PubMed] [Google Scholar]
- 38.Carlin AM, Zeni TM, English WJ, et al. The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 2013; 257:791–797. [DOI] [PubMed] [Google Scholar]
- 39.Benaiges D, Flores Le-Roux JA, Pedro-Botet J, et al. Sleeve gastrectomy and Roux-en-Y gastric bypass are equally effective in correcting insulin resistance. Int J Surg 2013; 11:309–313. [DOI] [PubMed] [Google Scholar]
- 40.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 41.Park JY, Kim YJ. Laparoscopic gastric bypass vs sleeve gastrectomy in obese Korean patients. World J Gastroenterol 2015; 21:12612–12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano OK, Zhang Y, Cumella L, et al. Excess weight loss and cardiometabolic parameter reduction diminished among hispanics undergoing bariatric surgery: outcomes in more than 2000 consecutive hispanic patients at a single institution. J Am Coll Surg 2016; 222:166–173. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, McCoy RG, Mason JE, et al. Second-line agents for glycemic control for type 2 diabetes: are newer agents better? Diabetes Care 2014; 37:1338–1345. [DOI] [PubMed] [Google Scholar]


