Abstract
The association between psoriasis and carotid intima-media thickness (CIMT) or impaired flow-mediated dilation (FMD) remains controversial. We aimed to evaluate the extent of subclinical atherosclerosis as measured by CIMT and FMD in patients with psoriasis by conducting a meta-analysis.
A systematic literature search was performed using PubMed, Embase, Cochrane databases, China National Knowledge Infrastructure, and VIP databases up to February 2015. Observational studies investigating CIMT or FMD in patients with psoriasis and controls were eligible. Psoriatic patients and controls were at least age- and sex-matched. Random-effects analysis was used to estimate the weighted mean difference (WMD) and 95% confidence interval (CI) between psoriatic patients and controls.
A total of 20 studies were identified and analyzed. Meta-analysis showed that psoriatic patients had a significantly thicker CIMT (WMD 0.11 mm; 95% CI 0.08–0.15) and lower FMD (WMD −2.79%; −4.14% to −1.43%) than those in controls. Subgroup analysis indicated that psoriatic arthritis appeared to have less impaired FMD (WMD −2.45%) and thinner CIMT (WMD 0.10 mm). Psoriatic patients with mean age >45 years had much thicker CIMT (WMD 0.13 mm). The impaired FMD (WMD −3.99%) seemed more pronounced in psoriatic patients with mean age <45 years.
This meta-analysis suggests that patients with psoriasis are associated with excessive risk of subclinical atherosclerosis. Screening and monitoring CIMT and brachial artery FMD may be recommended to identify a subgroup of psoriatic patients at higher risk for cardiovascular events.
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease characterized by relapsing thick scaling plaques.1 The prevalence ranged from 0.91% to 8.5% in the adult population.2 Among psoriatic patients, approximately 6% to 42% of the whites3 and 1% to 9% Asian patients4 were reported to have psoriatic arthritis. Psoriatic arthritis is defined as inflammatory arthritis associated with psoriasis. Psoriasis not only negatively affects the quality of life, but also increases risk of cardiovascular events5 and cardiovascular mortality.6 Therefore, early detection of subclinical atherosclerosis in psoriatic patients would help to reduce cardiovascular morbidity and mortality.
Endothelial function7 and carotid intima-media thickness (CIMT)8 have been suggested to be an important marker of subclinical atherosclerosis. Assessment of flow-mediated dilation (FMD) with high-resolution ultrasound in the brachial artery is a widely used method to evaluate the endothelial function.9 CIMT is usually determined by using B-mode ultrasound technique in the common carotid artery. Determination of FMD and CIMT is widely used in clinical practice because of their noninvasive technique. Most studies have shown evidence of subclinical atherosclerosis in psoriatic patients as indicated by increased CIMT10–23 or impaired FMD14,15,17,19,24–26 than the matched controls. However, conflict findings regarding the relationship between psoriasis and subclinical atherosclerosis risk still exist.27–30 These conflicting results might be correlated with the severity or duration of psoriasis and population studied.
This meta-analysis aims to quantitatively estimate the association between psoriasis and subclinical atherosclerosis as measured by CIMT and FMD in patients with psoriasis by conducting a meta-analysis.
METHODS
Search Strategy
This study was conducted according to the recommendations of the Meta-Analysis of Observational Studies in Epidemiology.31 This meta-analysis was not based on the individual participant data; ethical approval was not applicable. A systematic search of studies published before February 2015 was conducted through PubMed, Embase, Cochrane databases, China National Knowledge Infrastructure, and VIP databases. The following medical subject headings terms were used for the literature search: “psoriasis” OR “psoriatic arthritis” AND “carotid intima-media thickness” OR “carotid atherosclerosis” AND “endothelial function” OR “flow-mediated dilation” AND “subclinical atherosclerosis”. Only fully published articles in peer-reviewed journals were included. The references of retrieved articles were also reviewed to identify any relevant study.
Inclusion and Exclusion Criteria
Inclusion criteria were: observational studies investigating the relationship between psoriasis with or without psoriasis arthritis and endothelial function (determination by FMD of the brachial artery using ultrasound technique) or mean CIMT; reporting CIMT or FMD as continuous data for patients with psoriasis and controls; psoriatic patients and controls were at least age- and sex-matched. Exclusion criteria were: lack of an eligible control group; evaluating endothelial function except for FMD; studies did not provide CIMT or FMD as mean values and standard deviation (SD) or standard error.
Data Extraction and Quality Assessment
The following data were extracted from each included study: first author's name, publication year, geographic region, study design, type of psoriasis, psoriasis severity (Psoriasis Area and Severity Index score), characteristics of participants (number, age, gender), CIMT (mean and SD), FMD (mean and SD), matched factor, whether exclusion of cardiovascular risk factors in participant selection. The methodological quality of the selected studies was assessed by using the Newcastle–Ottawa Scale (NOS)32 with the following 3 items: selection of the study groups, between-group comparability, and the ascertainment of either the exposure or the outcome. Study with NOS score ≥5 was judged to be of higher quality.
Statistical Analyses
CIMT and FMD were expressed as continuous data. The pooled effect size was calculated as the weighted mean difference (WMD) with 95% confidence interval (CI) by the inverse variance optimal approach between the psoriatic patients and control group. The degree of heterogeneity across studies was tested by using the I2 statistic and Cochran Q statistic. A I2 statistic value <50% or Cochran Q value of P < 0.05 was considered substantial heterogeneity. Random-effects analysis was used to estimate the effect size because of the anticipated clinical heterogeneity among included studies. The presence of publication bias was investigated by the Egger regression33 and Begg correlation test34 with a P value <0.01, which is statistically significant. Subgroups analyses were performed according to the type of disease (psoriasis or psoriatic arthritis), study design (case-control or cross-sectional), matched factor (matched BMI or not), mean age of psoriatic patients (>45 years or <45 years), and whether participants with cardiovascular risk factors were excluded. All statistical analyses were performed with Stata software 12.0 (Statacorp, College Station, TX).
RESULTS
Study Selection
The initial literature search yielded a total of 766 potentially relevant articles. After screening on the basis of abstracts or titles, 711 articles were excluded. After full manuscripts assessed for eligibility, 26 articles appeared to satisfy the inclusion criteria. Six articles were excluded for the following reasons: one study35 did not provide SD of the outcome; 2 studies36,37 did not provide average intima-media thickness values; and participants’ selection did not match with age or sex in 2 studies.38,39 One study40 was further excluded because the reported FMD value was particularly high in both psoriatic patients and controls than other studies. Finally, only 20 studies10–28,30 were included in the quantitative synthesis. The flowchart of the studies’ selection process is outlined in Figure 1.
FIGURE 1.
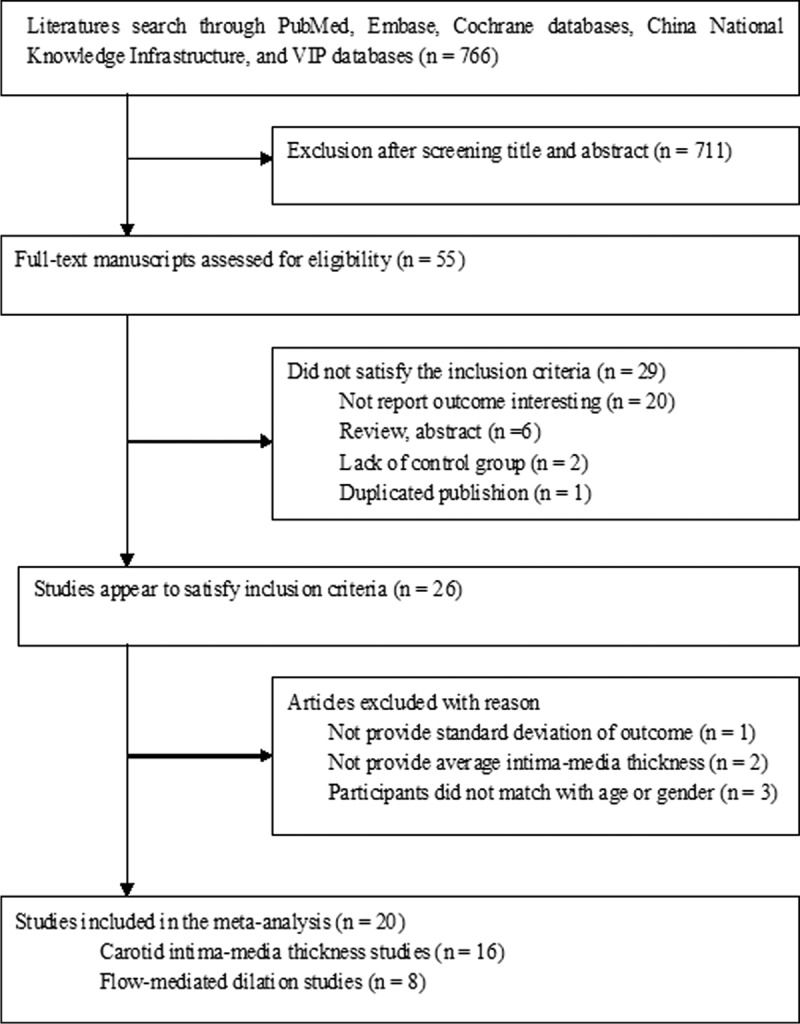
Flowchart of the study selection.
Study Characteristics
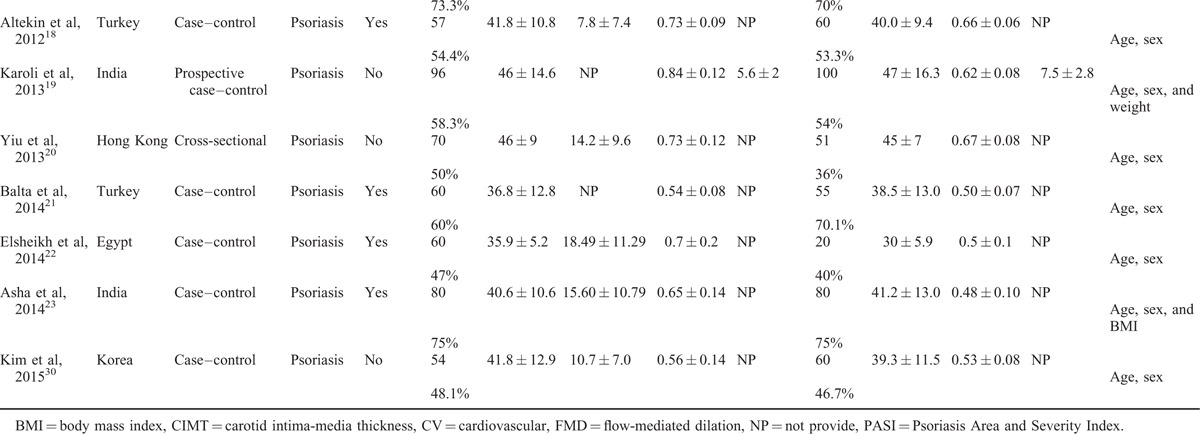
Twenty studies10–28,30 comprising 1066 psoriatic patients and 962 controls were included in the meta-analysis. The sample size of individual study ranged from 54 to 196. Of the 20 studies, 1610,11,13–19,21–23,25,27,28,30 were case–control studies, 312,20,24 were cross-sectional studies, and 126 was cross-sectional plus case–control design. All the studies were published from 2007 to 2014. All studies clearly reported matching of patients and controls by sex and age design. Ten studies excluded participants with preexisting cardiovascular risk factors. The studies enrolled participants with a mean age range from 30 to 57.85 years. The characteristics of the included studies are shown in Table 1 .10–28,30 All quality scores of the included studies were >5 according to the NOS. NOS scores of the included studies are presented in Table 2.10–28,30
TABLE 1.
Baseline Characteristics of Studies Included in the Meta-Analysis

TABLE 1 (Continued).
Baseline Characteristics of Studies Included in the Meta-Analysis
Psoriasis and CIMT
Sixteen studies10–23,27,30 assessed the CIMT difference in 934 psoriatic patients and 866 controls. As shown in Figure 2, the pooled random-effect difference in CIMT indicated that psoriatic patients had a significant increase in CIMT compared with controls (WMD 0.11 mm; 95% CI 0.08–0.15). Substantial heterogeneity was observed (I2 = 90.7%; P < 0.001). Neither the Begg rank correlation test (P = 0.137) nor the Egger linear regression test (P = 0.634) showed evidence of publication bias.
FIGURE 2.

Forest plots showing the weighted mean difference of carotid intima-media thickness between psoriatic patients and controls in a random-effects model.
Psoriasis and FMD
Eight studies14,15,17,19,24–26,28 assessed the FMD difference in 424 psoriatic patients and 400 controls. As shown in Figure 3, the heterogeneity across the included trials was significant (I2 = 97%; P < 0.001), the pooled random-effect difference in FMD showed that psoriatic patients had a significant decrease in FMD than controls (WMD −2.79%; 95% CI −4.14% to −1.43%). Neither the Begg rank correlation test (P = 0.348) nor the Egger linear regression test (P = 0.149) showed evidence of publication bias.
FIGURE 3.
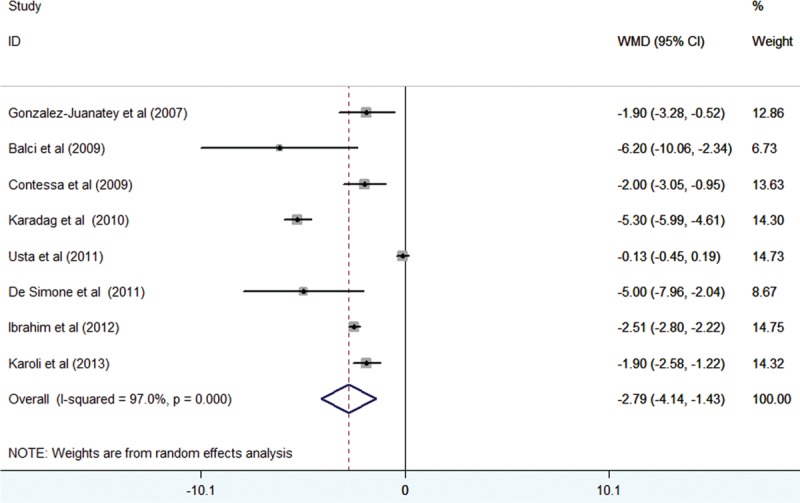
Forest plots showing the weighted mean difference of brachial artery flow-mediated dilation psoriatic patients and controls in a random-effects model.
Subgroup and Sensitivity Analyses
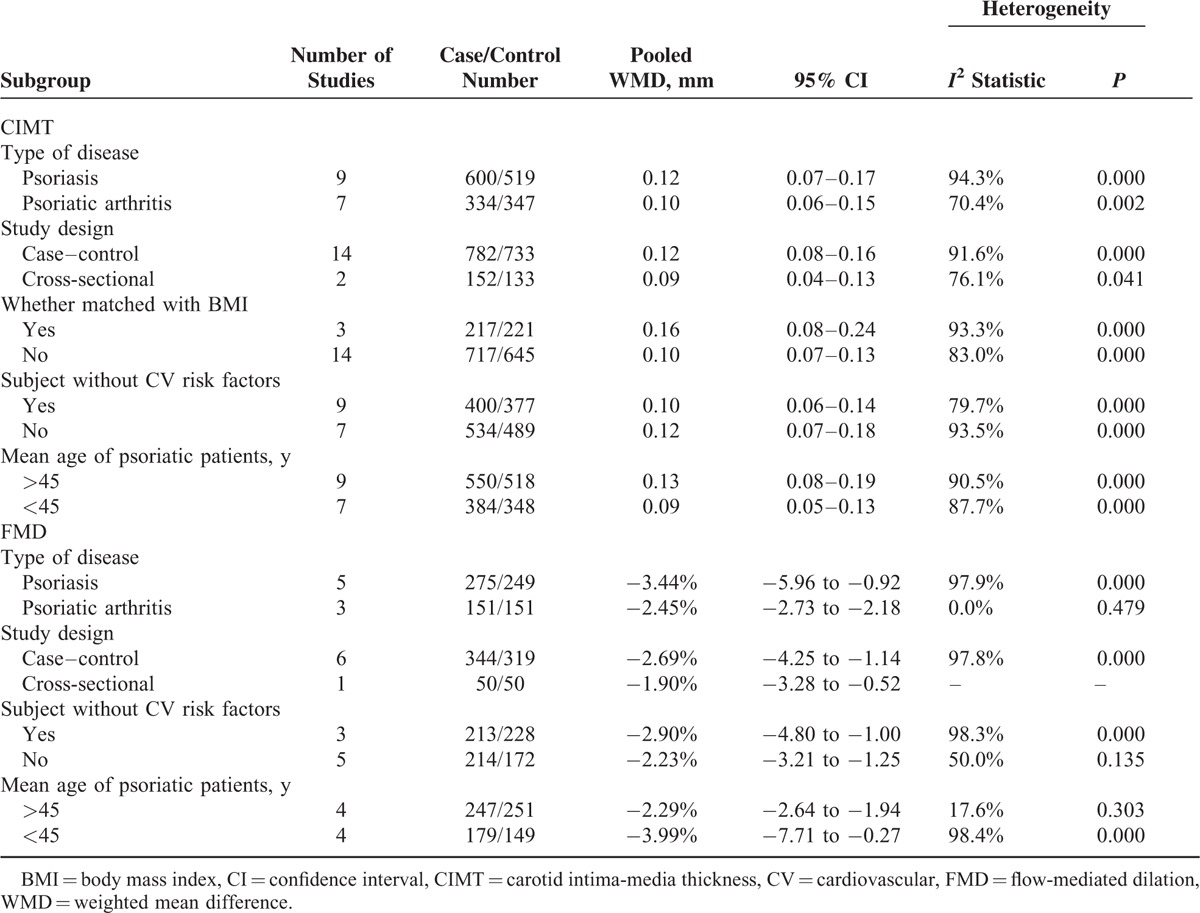
Subgroup analyses showed that psoriatic arthritis alone appeared to have less CIMT (WMD 0.10 mm; 95% CI 0.06–0.15) and less impaired FMD (WMD −2.45%; 95% CI −2.73 to −2.18). Cardiovascular risk factors, age, and BMI of participants could influence the results. The detailed results of the subgroup analyses are shown in Table 3. Sensitivity analyses indicated that the pooled effect sizes of WMD for CIMT (Figure 4A) or FMD (Figure 4B) changed very little by sequential omission of individual trials.
TABLE 2.
Quality Assessment of Studies Included in Meta-Analysis
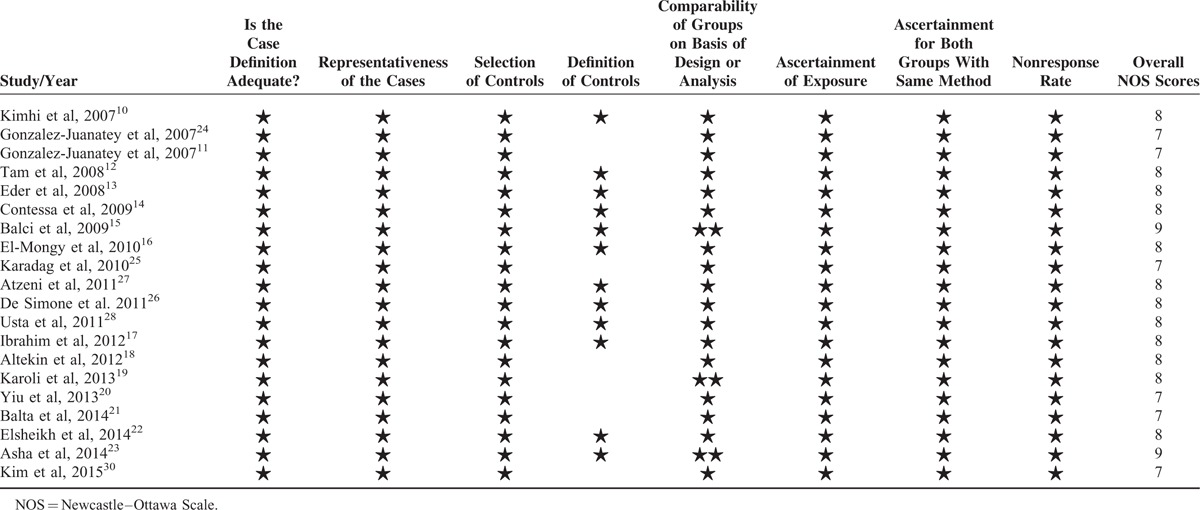
FIGURE 4.

Sensitivity analyses on carotid intima-media thickness (A) and brachial artery flow-mediated dilation (B).
TABLE 3.
Subgroup Analyses of CIMT and FMD
DISCUSSION
This meta-analysis suggests that patients with psoriasis are associated with an increased CIMT and impaired brachial artery FMD than the healthy controls, which reflects the subclinical atherosclerosis. Compared with controls, psoriatic patients had 2.79% reduction in brachial artery FMD and 0.11 mm thicker CIMT. Particularly, psoriatic arthritis had less impaired brachial artery FMD and thinner CIMT than total psoriatic patients.
When we investigated the relationship between psoriasis and subclinical atherosclerosis, the impact of type of disease, BMI, concomitant cardiovascular risk factors, and age should be considered. Subgroup analysis showed that patients with psoriatic arthritis appeared to have less impaired brachial artery FMD and thinner CIMT than the total psoriatic patients. CIMT may be affected by other cardiovascular risk factors. However, WMD of impaired FMD or increased CIMT remained statistically significant for participants without clinical evidence of atherosclerotic risk factors. These findings implied that the clustering of cardiovascular risk factors in psoriatic patients may amplify the effect of psoriasis on subclinical atherosclerosis. Psoriatic patients had a higher prevalence and incidence of obesity.41 Subgroup analysis indicated that the pooled WMD of CIMT was increased when we pooled studies of matching factor with BMI. Together these findings, psoriatic patients were associated with accelerated subclinical atherosclerosis and might be independent of the classical atherosclerotic risk factors.
Elder people are usually associated with accelerated atherosclerosis. In this study, psoriatic patients with mean age >45 years appeared to have greater thicker CIMT than those younger than 45 years. On the contrary, the impaired brachial artery FMD was more pronounced in patients with mean age <45 years. Impaired endothelial function might precede any change in CIMT. These findings revealed that determination of FMD may be recommended for psoriatic patients with mean age <45 years, whereas measurement of CIMT might be suitable for the older patients.
The association between psoriasis and risk of cardiovascular disease is controversial. A well-designed meta-analysis suggested that psoriasis was associated with ischemic heart disease but not cerebrovascular disease and cardiovascular mortality.42 Our meta-analyses indicated that patients with psoriasis were associated with excessive risk of subclinical atherosclerosis. Accordingly, a systematic review summarized that patients with psoriasis and psoriatic arthritis had impaired endothelial function compared with the general population, as measured by pulse wave velocity and aortic stiffness parameters.43 A more recent published meta-analysis44 suggested that patients with psoriatic arthritis appeared significantly associated with markers of subclinical atherosclerosis. However, this meta-analysis mainly focused on patients with psoriatic arthritis but not address total psoriatic patients. These meta-analyses supported that patients with psoriasis may increase future cardiovascular morbidity and mortality.
The exact mechanisms of psoriasis in promoting atherosclerosis remain unclear. Psoriasis is considered a systemic inflammatory condition. The chronic systemic inflammatory state has been linked to an acceleration of the atherosclerotic lesions. Chronic systemic inflammation induces endothelial dysfunction, altered glucose metabolism, and insulin resistance that play a significant role in the progress of atherosclerosis.45,46 Moreover, many immunological factors involved in psoriasis, such as C-reactive protein and tumor necrosis factor-α, also contribute to atherosclerosis.47
Several limitations should be considered. First, the causal association between subclinical atherosclerosis and psoriasis could not be defined because of the case–control or cross-sectional nature of the included studies. Second, studies using other techniques to evaluate subclinical atherosclerosis were not included in this meta-analysis. Third, as for the included studies did not provide data about the severity or duration of psoriasis on the subclinical atherosclerosis, so we could not determine whether the severity of psoriasis or longer duration of the disease increased the extent of subclinical atherosclerosis. Fourth, significant heterogeneity in pooled CIMT (I2 = 90.7%) and FMD (I2 = 97%) was observed. The sources of heterogeneity might be correlated with the study design, age of the participant, presence or absence of psoriatic arthritis, and with or without atherosclerotic risk factors. Finally, we were unable to determine the effects of pharmacologic therapy on the progression of atherosclerosis in psoriatic patients.
CONCLUSIONS
This meta-analysis suggests that psoriatic patients are associated with excessive risk of subclinical atherosclerosis compared with the healthy controls. Assessment of CIMT and FMD of the brachial artery may be recommended to identify a subgroup of patients at higher risk for cardiovascular events in psoriatic patients. Psoriatic patients with mean age >45 years appeared to have greater thicker CIMTA and need frequent follow-up. Moreover, further studies are needed on whether the treatment of psoriasis will reverse subclinical atherosclerosis.
Footnotes
Abbreviations: CIMT = carotid intima-media thickness, FMD = impaired flow-mediated dilation, WMD = weighted mean difference, CI = confidence interval, SD = standard deviation.
The authors report no conflicts of interest.
REFERENCES
- 1.Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol 2015; 172:1665–1668. [DOI] [PubMed] [Google Scholar]
- 2.Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133:377–385. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD. Psoriatic arthritis. Dermatol Ther 2009; 22:40–55. [DOI] [PubMed] [Google Scholar]
- 4.Tam LS, Leung YY, Li EK. Psoriatic arthritis in Asia. Rheumatology (Oxford) 2009; 48:1473–1477. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc 2013; 2:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol 2013; 27 suppl 3:12–29. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero D, Paolillo S, Ratta GD, et al. Endothelial function as a marker of pre-clinical atherosclerosis: assessment techniques and clinical implications. Monaldi Arch Chest Dis 2013; 80:106–110. [DOI] [PubMed] [Google Scholar]
- 8.Bauer M, Caviezel S, Teynor A, et al. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med Wkly 2012; 142:w13705. [DOI] [PubMed] [Google Scholar]
- 9.Stout M. Flow-mediated dilatation: a review of techniques and applications. Echocardiography 2009; 26:832–841. [DOI] [PubMed] [Google Scholar]
- 10.Kimhi O, Caspi D, Bornstein NM, et al. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum 2007; 36:203–209. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, et al. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum 2007; 57:1074–1080. [DOI] [PubMed] [Google Scholar]
- 12.Tam LS, Shang Q, Li EK, et al. Subclinical carotid atherosclerosis in patients with psoriatic arthritis. Arthritis Rheum 2008; 59:1322–1331. [DOI] [PubMed] [Google Scholar]
- 13.Eder L, Zisman D, Barzilai M, et al. Subclinical atherosclerosis in psoriatic arthritis: a case-control study. J Rheumatol 2008; 35:877–882. [PubMed] [Google Scholar]
- 14.Contessa C, Ramonda R, Lo Nigro A, et al. Subclinical atherosclerosis in patients with psoriatic arthritis: a case-control study. Preliminary data Reumatismo 2009; 61:298–305. [DOI] [PubMed] [Google Scholar]
- 15.Balci DD, Balci A, Karazincir S, et al. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol 2009; 23:1–6. [DOI] [PubMed] [Google Scholar]
- 16.El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol 2010; 24:661–666. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim SE, Helmi A, Yousef TM, et al. Association of asymptomatic hyperuricemia and endothelial dysfunction in psoriatic arthritis. Egyptian Rheumatol 2012; 34:83–89. [Google Scholar]
- 18.Altekin ER, Koc S, Karakas MS, et al. Determination of subclinical atherosclerosis in plaque type psoriasis patients without traditional risk factors for atherosclerosis. Turk Kardiyol Dern Ars 2012; 40:574–580. [DOI] [PubMed] [Google Scholar]
- 19.Karoli R, Fatima J, Shukla V, et al. A study of cardio-metabolic risk profile in patients with psoriasis. J Assoc Physicians India 2013; 61:798–803. [PubMed] [Google Scholar]
- 20.Yiu KH, Yeung CK, Zhao CT, et al. Prevalence and extent of subclinical atherosclerosis in patients with psoriasis. J Intern Med 2013; 273:273–282. [DOI] [PubMed] [Google Scholar]
- 21.Balta S, Balta I, Mikhailidis DP, et al. Bilirubin levels and their association with carotid intima media thickness and high-sensitivity c-reactive protein in patients with psoriasis vulgaris. Am J Clin Dermatol 2014; 15:137–142. [DOI] [PubMed] [Google Scholar]
- 22.Elsheikh RG, Amin Tel S, El-Ashmawy AA, et al. Evaluation of subclinical atherosclerosis in egyptian psoriatic patients. J Saudi Heart Assoc 2014; 26:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asha K, Sharma SB, Singal A, et al. Association of carotid intima-media thickness with leptin and apoliprotein b/apoliprotein a-i ratio reveals imminent predictors of subclinical atherosclerosis in psoriasis patients. Acta Medica (Hradec Kralove) 2014; 57:21–27. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Juanatey C, Llorca J, Miranda-Filloy JA, et al. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum 2007; 57:287–293. [DOI] [PubMed] [Google Scholar]
- 25.Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? Increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol 2010; 49:642–646. [DOI] [PubMed] [Google Scholar]
- 26.De Simone C, Di Giorgio A, Sisto T, et al. Endothelial dysfunction in psoriasis patients: cross-sectional case-control study. Eur J Dermatol 2011; 21:510–514. [DOI] [PubMed] [Google Scholar]
- 27.Atzeni F, Sarzi-Puttini P, Sitia S, et al. Coronary flow reserve and asymmetric dimethylarginine levels: new measurements for identifying subclinical atherosclerosis in patients with psoriatic arthritis. J Rheumatol 2011; 38:1661–1664. [DOI] [PubMed] [Google Scholar]
- 28.Usta M, Yurdakul S, Aral H, et al. Vascular endothelial function assessed by a noninvasive ultrasound method and serum asymmetric dimethylarginine concentrations in mild-to-moderate plaque-type psoriatic patients. Clin Biochem 2011; 44:1080–1084. [DOI] [PubMed] [Google Scholar]
- 29.Dowlatshahi EA, Kavousi M, Nijsten T, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the rotterdam study. J Invest Dermatol 2013; 133:2347–2354. [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Yang HS, Lee YW, et al. Evaluation of the beta stiffness index and carotid intima-media thickness in Asian patients with psoriasis. Angiology 2015; 66:889–895. [DOI] [PubMed] [Google Scholar]
- 31.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. Jama 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 32.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (nos) for assessing the quality if nonrandomized studies in meta-analyses. http://www.Ohri.Ca/programs/clinical_epidemiology/oxford.Asp Assessed February 10, 2016. [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 35.Robati RM, Partovi-Kia M, Haghighatkhah HR, et al. Increased serum leptin and resistin levels and increased carotid intima-media wall thickness in patients with psoriasis: is psoriasis associated with atherosclerosis? J Am Acad Dermatol 2014; 71:642–648. [DOI] [PubMed] [Google Scholar]
- 36.Orgaz-Molina J, Magro-Checa C, Rosales-Alexander JL, et al. Vitamin d insufficiency is associated with higher carotid intima-media thickness in psoriatic patients. Eur J Dermatol 2014; 24:53–62. [DOI] [PubMed] [Google Scholar]
- 37.Enany B, El Zohiery AK, Elhilaly R, et al. Carotid intima-media thickness and serum leptin in psoriasis. Herz 2012; 37:527–533. [DOI] [PubMed] [Google Scholar]
- 38.Martyn-Simmons CL, Ranawaka RR, Chowienczyk P, et al. A prospective case-controlled cohort study of endothelial function in patients with moderate to severe psoriasis. Br J Dermatol 2011; 164:26–32. [DOI] [PubMed] [Google Scholar]
- 39.Evensen K, Slevolden E, Skagen K, et al. Increased subclinical atherosclerosis in patients with chronic plaque psoriasis. Atherosclerosis 2014; 237:499–503. [DOI] [PubMed] [Google Scholar]
- 40.Ulusoy RE, Karabudak O, Yokusoglu M, et al. Noninvasive assessment of impaired endothelial function in psoriasis. Rheumatol Int 2010; 30:479–483. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes 2012; 2:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol 2013; 69:1014–1024. [DOI] [PubMed] [Google Scholar]
- 43.Brezinski EA, Follansbee MR, Armstrong EJ, et al. Endothelial dysfunction and the effects of tnf inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des 2014; 20:513–528. [DOI] [PubMed] [Google Scholar]
- 44.Di Minno MN, Ambrosino P, Lupoli R, et al. Cardiovascular risk markers in patients with psoriatic arthritis: a meta-analysis of literature studies. Ann Med 2015; 47:346–353. [DOI] [PubMed] [Google Scholar]
- 45.Mehlis SL, Gordon KB. The immunology of psoriasis and biologic immunotherapy. J Am Acad Dermatol 2003; 49:S44–S50. [DOI] [PubMed] [Google Scholar]
- 46.Ghazizadeh R, Shimizu H, Tosa M, et al. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci 2010; 7:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramonda R, Lo Nigro A, Modesti V, et al. Atherosclerosis in psoriatic arthritis. Autoimmun Rev 2011; 10:773–778. [DOI] [PubMed] [Google Scholar]


