Abstract
Osteoarthritis (OA) is the most common form of arthritis in older individuals and is among the most prevalent and disabling chronic conditions worldwide.
We conducted a meta-analysis to determine the efficacy and safety of celecoxib, a cyclooxygenase-2 (COX-2) inhibitor in the treatment of osteoarthritis. Studies were pooled, and mean difference (MD), relative risk (RR), and its corresponding 95% confidence interval (CI) were calculated. Fifteen relevant articles were included for this meta-analysis study.
We observed that osteoarthritis total score (MD = −4.41, 95% CI −7.27 to −1.55), pain subscale score (MD = −0.86, 95% CI −1.10 to −0.62), and function subscale score (MD = −2.90, 95% CI −5.12 to −0.67) in OA patients treatment with celecoxib was significantly improved than that with placebo. There was no significant difference in the incidence of adverse events (AEs), SAEs, and discontinuations due to AEs; however, the incidence of gastrointestinal AEs in OA patients treatment with celecoxib is significantly higher than that with placebo. For AE, the incidence of abdominal pain in OA patients with celecoxib was significantly higher than that with placebo (RR = 2.24, 95% CI: 1.40–3.58; P = 0.839, I2 = 0%). There was no significant difference in diarrhea, dyspepsia, headache, and nausea.
This meta-analysis indicated that celecoxib treatment (200 mg orally once daily) led to significant improvement in the pain and function of osteoarthritis. However, compared with placebo control, celecoxib resulted in greater gastrointestinal AEs, especially abdominal pain after approximately 10 to 13 weeks of treatment. The current study, therefore, provides valuable information to help physicians make treatment decisions for their patients with OA.
INTRODUCTION
Osteoarthritis (OA) is a type of joint disease, destruction and loss of articular cartilage, subchondral bone changes, and synovitis. These physical changes leading to chronic pain, stiffness, and disability, which are those suffering from this condition, a marked adverse impact on quality of life of patients.1,2 OA is one of the leading causes of disability in the world, with an aging population and an increase in obesity rates, and its prevalence is increasing in Asia.3
Pain relief is the main indication for drug treatment in patients with OA, most OA patients visit a doctor for their pain treatment.4,5 Clinical trial data show that the traditional nonsteroidal antiinflammatory drugs (NSAIDs) are more effective than acetaminophen in the treatment of patients with symptoms and signs of OA.6,7 However, NSAIDs possess significant limitations for serious adverse events (SAEs), including upper gastrointestinal (GI) ulceration and hemorrhage, that is associated with their use.8,9 In addition, older persons, so most of the traditional NSAID-related adverse events (AEs) are at particularly high risk for severe upper GI tract in patients with OA.10,11 The traditional NSAIDs are nonspecific inhibitors of the enzyme cyclooxygenase 2 isoforms (COX-1 and COX-2), prostaglandin synthesis of arachidonic acid catalyzed by 2 key steps.12,13 As a result, the traditional NSAIDs produce mechanism-based GI toxicity by inhibiting platelet COX-1.
The selective COX-2 inhibitor, celecoxib (Celebrex), in contrast, spares COX-1 in therapeutic and supra-therapeutic dosages14,15 to provide effective anti-inflammatory and analgesic effect,16 there is no increased risk with use of NSAIDs properties of GI and hematologic AEs, even though the long-term management.17,18 In order to determine the efficacy, GI safety, and tolerability of celecoxib (a COX-2 inhibitor) for the treatment of OA, we conducted this meta-analysis.
MATERIALS AND METHODS
Search Strategy
We are looking for relevant research to August, 2015 with the following terms and their combinations through PubMed and EMBASE databases: “celecoxib” and “osteoarthritis.” All scan summary, research, and references were reviewed. Only English-language publications include. In addition, reference is also retrieved the manuscript is manually search for further relevant publications. Ethical review is not required for the meta-analysis study.
Selection Criteria
Controlled clinical trials to assess the efficacy and safety of celecoxib in OA were included if they meet the following criteria: eligibility is limited to randomized controlled trials of OA; study compared the efficacy and safety of celecoxib in treatment of OA pain; research report specific data related pain intensity and decrease AEs; and only placebo randomized controlled trials published in English may be included, both blinded and nonblinded trials were included.
Data Extraction
All the available data were extracted from each study by 2 investigators independently according to the inclusion criteria listed above. The efficacy outcomes were: OA total score; OA pain subscale score; and OA function subscale score. The safety outcomes included: AEs, SAEs, GI AEs, discontinuations due to AEs, abdominal pain, diarrhea, dyspepsia, headache, and nausea.
Statistical Analysis
All results summarized using STATA Software (version 12, StataCorp, College Station, TX). We calculated the mean difference (MD) and 95% confidence intervals (CI) for the continuous data, and calculate the risk ratio (RR) and 95% CIs for dichotomous data. Preliminary analysis using a fixed effect model (Mantel–Haenszel method), if there are study heterogeneity (P < 0.1), using a random effects model. By Begg funnel plot and Egger test to assess publication bias visually evaluated symmetry (P < 0.05 was considered statistically significant).
RESULTS
Characteristics of the Studies
There were 258 papers relevant to the search words. Subsequently, 233 irrelevant articles were excluded. The remaining articles were systematically reviewed, and all 25 articles qualified for full-text reading. After full-text reading, 13 articles were deemed unsuitable and were therefore excluded, and 12 articles were identified to be included for qualitative analysis. Finally, 12 articles including 15 studies16,19–29 were incorporated into the current meta-analysis (Table 1). The flow-chart of selection of studies and reasons for exclusion is presented in Figure 1. The pharmacokinetic and metabolic characteristics of celecoxib are summarized in (Table 2).
TABLE 1.
Characteristics of Randomised Controlled Trials Included in This Meta-Analysis

FIGURE 1.

Flow diagram of studies identification.
TABLE 2.
Pharmacokinetic characteristics of Celecoxib30
Quantitative Synthesis
All 15 studies including 7868 patients explored the efficacy and safety of celecoxib for the treatment of OA patients.
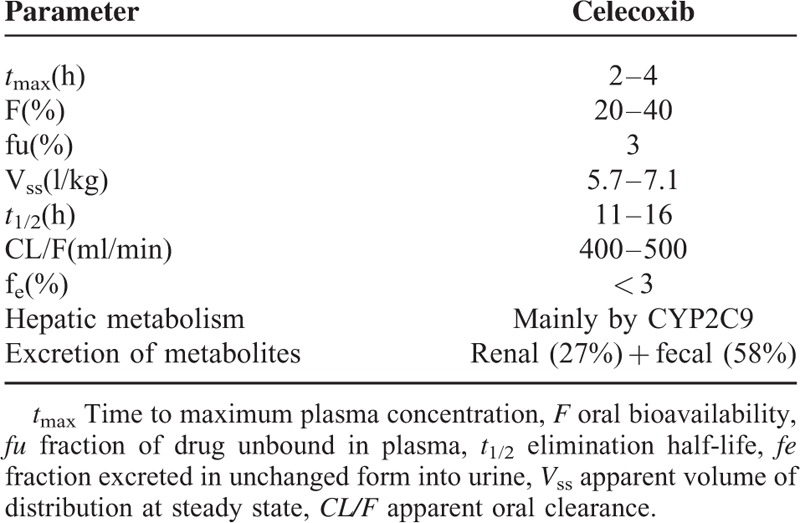
Osteoarthritis Total Score
This outcome was reported in 6 trials, all comparing celecoxib to placebo. There were 4155 cases of patients, 2426 cases in treatment group, and 1729 cases in control group. The heterogeneity was statistically significant (P < 0.001, I2 = 98.7%), the random effect model was used. The difference in the OA total score was significant (MD = −4.41, 95% CI −7.27 to −1.55), as shown in Figure 2A.
FIGURE 2.

Efficacy outcomes at 10 to 13 weeks in randomised controlled trials of celecoxib versus placebo. (A) Osteoarthritis total score, (B) osteoarthritis pain subscale score, and (C) osteoarthritis function subscale score.
Osteoarthritis Pain Subscale Score
This outcome was reported in 5 trials, all comparing celecoxib to placebo. There were 3750 cases of patients, 2224 cases in treatment group, and 1526 cases in control group, the heterogeneity was not statistically significant, the fixed effect model was used (P = 0.486, I2 = 0%). The difference in the OA pain subscale score was significant (MD = −0.86, 95% CI −1.10 to −0.62), as shown in Figure 2B.
Osteoarthritis Function Subscale Score
This outcome was reported in 5 trials, all comparing celecoxib to placebo. There were 3750 cases of patients, 2224 cases in treatment group, and 1526 cases in control group, the heterogeneity was statistically significant, the random effect model was used (P < 0.001, I2 = 93%). The difference in the OA function subscale score was significant (MD = −2.90, 95% CI −5.12 to −0.67), as shown in Figure 2C.
Adverse Events (AEs)
This outcome was reported in 14 trials, all comparing celecoxib to placebo. A total of 7136 patients were enrolled, 4036 patients in the treatment group and 3100 cases in the control group, there was no heterogeneity between the study (P = 0.624, I2 = 0%), the fixed effect model was used. There was no significant difference in the incidence of AEs (RR = 1.04, 95% CI: 0.99–1.09), as shown in Figure 3A.
FIGURE 3.
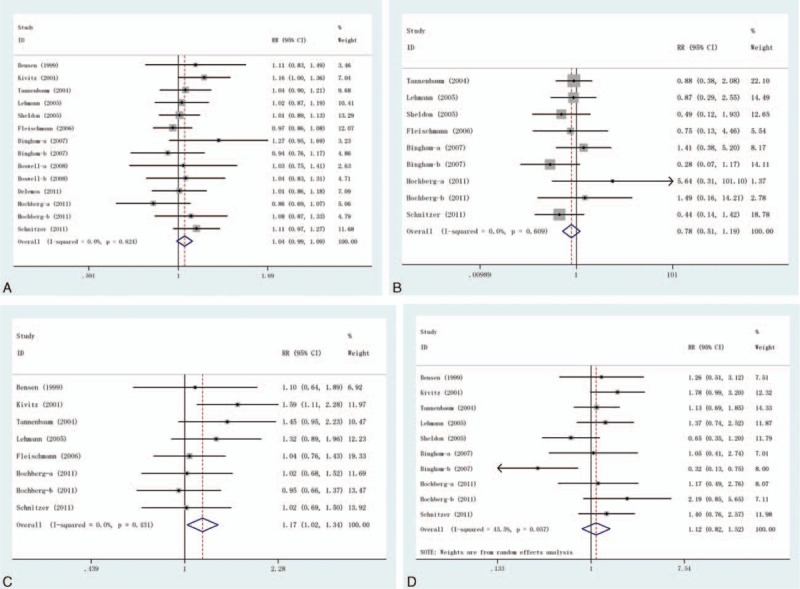
Adverse effects of treatment in randomised controlled trials of celecoxib versus placebo at 10 to 13 weeks. (A) AEs, (B) SAEs, (C)GI AEs, and (D) discontinuations due to AEs. AE = adverse event, GI = gastrointestinal, SAE = serious adverse event.
Serious Adverse Events (SAEs)
This outcome was reported in 9 trials, all comparing celecoxib to placebo. A total of 5317 patients were enrolled, 3131 patients in the treatment group and 2186 cases in the control group, there was no heterogeneity between the study (P = 0.609, I2 = 0%), the fixed effect model was used. There was no significant difference in the incidence of SAEs (RR = 0.78, 95% CI: 0.51–1.19), as shown in Figure 3B.
Gastrointestinal AEs
This outcome was reported in 8 trials, all comparing celecoxib to placebo. A total of 4640 patients were enrolled, 2659 patients in the treatment group and 1981 cases in the control group, there was no heterogeneity between the study (P = 0.431, I2 = 0%), the fixed effect model was used. However, there was significant difference in the incidence of GI AEs (RR = 1.17, 95% CI: 1.02–1.34), as shown in Figure 3C.
Discontinuations Due to AEs
This outcome was reported in 10 trials, all comparing celecoxib to placebo. A total of 5472 patients were enrolled, 3096 patients in the treatment group and 2376 cases in the control group, the heterogeneity was statistically significant (P = 0.057, I2 = 45.5%), the random effect model was used. However, there was no significant difference in the incidence of GI AEs (RR = 1.12, 95% CI: 0.82–1.52), as shown in Figure 3D.
Abdominal Pain
This outcome was reported in 6 trials, all comparing celecoxib to placebo. A total of 2985 patients were enrolled, 1604 patients in the treatment group and 1381 cases in the control group, there was no heterogeneity between the study (P = 0.839, I2 = 0%), the fixed effect model was used. However, there was significant difference in the incidence of abdominal pain (RR = 2.24, 95% CI: 1.40–3.58), as shown in Figure 4A.
FIGURE 4.

Forest plot of the RR and 95% CIs of studies on the incidence of AEs in patients with osteoarthritis treatment with celecoxib compared with placebo. (A) Abdominal pain, (B) diarrhea; (C) dyspepsia, (D) headache, and (E) nausea. AE = adverse event, CI = confidence interval, RR = relative risk.
Diarrhea
This outcome was reported in 11 trials, all comparing celecoxib to placebo. There were 5729 cases of patients, 3104 cases in treatment group and 2625 cases in control group, the heterogeneity was not statistically significant, the fixed effect model was used (P = 0.675, I2 = 0%). But there was no significant difference in the incidence of diarrhea (RR = 0.99, 95% CI: 0.76–1.30), as shown in Figure 4B.
Dyspepsia
This outcome was reported in 9 trials, all comparing celecoxib to placebo. There were 4552 cases of patients, 2509 cases in treatment group and 2043 cases in control group, the heterogeneity was not statistically significant, the fixed effect model was used (P = 0.751, I2 = 0%). But there was no significant difference in the incidence of dyspepsia (RR = 1.13, 95% CI: 0.87–1.46), see Figure 4C.
Headache
This outcome was reported in 11 trials, all comparing celecoxib to placebo. There were 5729 cases of patients, 3104 cases in treatment group and 2625 cases in control group, the heterogeneity was not statistically significant, the fixed effect model was used (P = 0.481, I2 = 0%). But the difference in the incidence of headache was not significant (RR = 0.94, 95% CI: 0.79–1.10), as shown in Figure 4D.
Nausea
This outcome was reported in 6 trials, all comparing celecoxib to placebo. There were 3093 cases of patients, 1542 cases in treatment group and 1551 cases in control group, the heterogeneity was not statistically significant, the fixed effect model was used (P = 0.674, I2 = 0%). However, there was no significant difference in the incidence of nausea (RR = 1.17, 95% CI: 0.80–1.69), as shown in Figure 4E.
Publication Bias
Finally, the Egger regression test showed no evidence of asymmetrical distribution in the funnel plot in incidence of AEs (Begg test P = 0.661; Egger test P = 0.560) and incidence of GI AEs (Begg test P = 0.386; Egger test P = 0.847) (Figure 5).
FIGURE 5.

Begg funnel plot for publication bias test. Each point represents a separate study for the indicated association. (A) Incidence of adverse events, (B) incidence of GI AEs. AE = adverse event, GI = gastrointestinal.
DISCUSSION
NSAIDs produce analgesic and antiinflammatory effects through inhibition of COX activity in the arachidonic acid cascade, and thereby blocking prostaglandin (PG) synthesis.30 In the 1990s, 2 COX isoenzymes – COX-1 and COX-2 – were identified.31,32 As we all know, under normal physiological circumstances, COX-1 is constitutively expressed in most tissues for participation and PG synthesis, PG protects the stomach mucosa and platelet function and maintains kidney function.33,34 Instead, COX-2 has been proven to be produced in inflammatory tissues and participation in the synthesis of PG correlated with inflammation.35,36 Therefore, conventional NSAIDs block the COX-1 and COX-2 to act a therapeutic part role, in varying degrees, through inhibition of COX-2. Side effects of NSAIDs, especially in the upper GI tract, is believed to be caused mainly by inhibition of COX-1 activity, which is involved in the protection of stomach mucosa.37,38 Therefore, to minimize the adverse effects, NSAIDs that target COX-2, while keeping its antiinflammatory and analgesic action. In 1998, celecoxib was approved as the 1st selective COX-2 inhibitors.38 Celecoxib is a member of the coxib family, which included in the anatomical therapeutic chemical classification system; it is made of a 3-dimensional structure design of molecules with COX-2 selectivity.39 Celecoxib is a sulfonamide and can produce severe hypersensitivity reactions in patients with sulfonamide allergies, resulting in AEs that include asthma, nasal polyps, and rhinitis.40
In this study, we conducted a meta-analysis to determine the efficacy and safety of celecoxib for the treatment of OA patients. Fifteen relevant studies including 7868 patients were included for this meta-analysis study. We observed that OA total score (MD = −4.41, 95% CI −7.27 to −1.55), pain subscale score (MD = −0.86, 95% CI −1.10 to −0.62), and function subscale score (MD = −2.90, 95% CI −5.12 to −0.67) in OA patients treatment with celecoxib was significantly improved than that with placebo. There was no significant difference in the incidence of AEs, SAEs, and discontinuations due to AEs; however, the incidence of GI AEs in OA patients treatment with celecoxib is significantly higher than that with placebo. For AE, the incidence of abdominal pain in OA patients with celecoxib was significantly higher than that with placebo (RR = 2.24, 95% CI: 1.40–3.58; P = 0.839, I2 = 0%). There was no significant difference in diarrhea, dyspepsia, headache, and nausea. The current study, therefore, provides valuable information to help physicians make treatment decisions for their patients with OA.
As already been noted, COX-2 expression in the kidney constitutively, which is adjusted in response to changes in intravascular volume.41 The formation of COX-2-dependent prostaglandin is necessary for normal kidney function. COX-2 inhibitors may temporarily reduce urinary sodium excretion and may cause mild to moderately elevated blood pressure.42,43 For kidney damage, coxibs and tNSAIDs have similar outcomes. They can cause renal insufficiency, hypertension, sodium retention and peripheral edema, hyperkalemia, and papillary necrosis.42 An observational study has shown that rofecoxib but not celecoxib or tNSAIDs was correlated with an increased risk of the elevation of blood pressure and edema than nonusers of any drug.44 Recently, in a large meta-analysis of 114 studies including 116,094 patients, rofecoxib was correlated with an increased risk of peripheral hypertension, edema, and kidney dysfunction. By contrast, celecoxib was correlated with the risk of the control group.45
There are several limitations in this meta-analysis that should be addressed. First, our study may be impaired to extract the raw data from including research. Second, in this study, there is potential for publication bias, because we do not take some unpublished papers and abstracts, and consider their data are not available to us. A third of a potential limitation is that language can also introduce a bias. Specifically, we select only the English language, the exclusion of other qualified researchers. Despite these limitations, this is the 1st example of a meta-analysis on the efficacy and safety of celecoxib for the treatment of OA patients. Application of statistical methods to the results of several studies with our meta-analysis, and to achieve strong objectivity, all the research methods were strict inclusion and exclusion criteria to demonstrate the effectiveness and significance of our conclusions.
In conclusion, this analysis suggests celecoxib (200 mg orally once daily), compared with placebo control, resulted in a greater reduction in pain and improved function, and acceptable adverse effects for the treatment of OA pain after approximately 10 to 13 weeks of treatment. Further studies with larger data set and well-designed models are required to validate our findings.
Footnotes
Abbreviations: AE = adverse event, CI = confidence interval, COX = cyclooxygenase, GI = gastrointestinal, MD = mean difference, NSAID = nonsteroidal antiinflammatory drug, OA = osteoarthritis, RR = relative risk, SAE = serious adverse event, UGI = ulceration and hemorrhage.
CX and KG contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Breedveld FC. Osteoarthritis-the impact of a serious disease. Rheumatology (Oxford) 2004; 43 suppl 1:i4–i8. [DOI] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 1994; 84:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransen M, Bridgett L, March L, et al. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis 2011; 14:113–121. [DOI] [PubMed] [Google Scholar]
- 4.Creamer P, Hochberg MC. Osteoarthritis. Lancet 1997; 350:503–509. [DOI] [PubMed] [Google Scholar]
- 5.Schnitzer TJ. Osteoarthritis treatment update. Minimizing pain while limiting patient risk. Postgrad Med 1993; 93:89–92. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Zhao S, Lane N. Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum 2000; 43:378–385. [DOI] [PubMed] [Google Scholar]
- 7.Pincus T, Swearingen C, Cummins P, et al. Preference for nonsteroidal antiinflammatory drugs versus acetaminophen and concomitant use of both types of drugs in patients with osteoarthritis. J Rheumatol 2000; 27:1020–1027. [PubMed] [Google Scholar]
- 8.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 1999; 240:188–199. [DOI] [PubMed] [Google Scholar]
- 9.Garciá-Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with non-steroidal anti-inflammatory drugs. Lancet 1994; 343:769–772. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 1995; 38:1500–1505. [DOI] [PubMed] [Google Scholar]
- 11.Tenenbaum J. The epidemiology of nonsteroidal anti-inflammatory drugs. Can J Gastroenterol 1999; 13:119–122. [DOI] [PubMed] [Google Scholar]
- 12.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J 1998; 12:1063–1073. [PubMed] [Google Scholar]
- 13.Smith WL, De Witt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol 1996; 62:167–215. [DOI] [PubMed] [Google Scholar]
- 14.Leese PT, Hubbard RC, Karim A, et al. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000; 40:124–132. [DOI] [PubMed] [Google Scholar]
- 15.Whelton A. Cox-1 sparing and cox-2 inhibitory drugs: the renal and hepatic safety and tolerability profiles of celecoxib. Am J Ther 2000; 7:151–152. [PubMed] [Google Scholar]
- 16.Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999; 74:1095–1105. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein FE, Faich G, Goldstein JL, et al. The celecoxib longterm arthritis safety study (CLASS). JAMA 2000; 284:1247–1255. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan-Machlis B, Klostermeyer BS. The cyclooxygenase-2 inhibitors: safety and effectiveness. Ann Pharmacother 1999; 33:979–988. [DOI] [PubMed] [Google Scholar]
- 19.Kivitz AJ, Moskowitz RW, Woods E, et al. Comparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hip. J Int Med Res 2001; 29:467–479. [DOI] [PubMed] [Google Scholar]
- 20.Tannenbaum H, Berenbaum F, Reginster JY, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a 13 week, randomised, double blind study versus placebo and celecoxib. Ann Rheum Dis 2004; 63:1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann R, Brzosko M, Kopsa P, et al. Efficacy and tolerability of lumiracoxib 100 mg once daily in knee osteoarthritis: a 13-week, randomized, double-blind study vs. placebo and celecoxib. Curr Med Res Opin 2005; 21:517–526. [DOI] [PubMed] [Google Scholar]
- 22.Sheldon E, Beaulieu A, Paster Z, et al. Efficacy and tolerability of lumiracoxib in the treatmentof osteoarthritis of the knee: a 13-week, randomized, double-blind comparison with celecoxib and placebo. Clin Ther 2005; 27:64–77. [DOI] [PubMed] [Google Scholar]
- 23.Fleischmann R, Sheldon E, Maldonado-Cocco J, et al. Lumiracoxib is effective in the treatment of osteoarthritis of the knee: a prospective randomized 13-week study versus placebo and celecoxib. Clin Rheumatol 2006; 25:42–53. [DOI] [PubMed] [Google Scholar]
- 24.Bingham CO, Sebba AI, Rubin BR, et al. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford) 2007; 46:496–507. [DOI] [PubMed] [Google Scholar]
- 25.Boswell DJ, Ostergaard K, Philipson RS, et al. Evaluation of GW406381 for treatment of osteoarthritis of the knee: two randomized, controlled studies. Medscape J Med 2008; 10:259. [PMC free article] [PubMed] [Google Scholar]
- 26.DeLemos BP, Jim X, Carmela B, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther 2011; 18:216–226. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg MC, Fort JG, Svensson O, et al. Fixed-dose combination of enteric-coated naproxen and immediate-release esomeprazole has comparable efficacy to celecoxib for knee osteoarthritis: two randomized trials. Curr Med Res Opin 2011; 27:1243–1253. [DOI] [PubMed] [Google Scholar]
- 28.Schnitzer TJ, Dattani ID, Seriolo B, et al. A 13-week, multicenter, randomized, double-blind study of lumiracoxib in hip osteoarthritis. Clin Rheumatol 2011; 30:1433–1446. [DOI] [PubMed] [Google Scholar]
- 29.Holt RJ, Fort JG, Grahn AY, et al. Onset and durability of pain relief in knee osteoarthritis: pooled results from two placebo trials of naproxen/esomeprazole combination and celecoxib. Phys Sportsmed 2015; 43:200–212. [DOI] [PubMed] [Google Scholar]
- 30.FitzGerald GA. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov 2003; 2:879–890. [DOI] [PubMed] [Google Scholar]
- 31.DeWitt DL, Smith WL. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci USA 1988; 85:1412–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie WL, Chipman JG, Robertson DL, et al. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA 1991; 88:2692–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seibert K, Zhang Y, Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA 1994; 91:12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999; 106:13S–24S. [DOI] [PubMed] [Google Scholar]
- 35.O’Banion MK, Winn VD, Young DA. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci USA 1992; 89:4888–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hempel SL, Monick MM, Hunninghake GW. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest 1994; 93:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masferrer JL, Zweifel BS, Manning PT, et al. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA 1994; 91:3228–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iseki S. Immunocytochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the rat stomach. Histochem J 1995; 27:323–328. [DOI] [PubMed] [Google Scholar]
- 39.Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996; 384:644–648. [DOI] [PubMed] [Google Scholar]
- 40.Barkin RL, Beckerman M, Blum SL, et al. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging 2010; 27:775–789. [DOI] [PubMed] [Google Scholar]
- 41.de Leval X, Hanson J, David JL, et al. New developments on thromboxane and prostacyclin modulators. Part II: prostacyclin modulators. Curr Med Chem 2004; 11:1243–1252. [DOI] [PubMed] [Google Scholar]
- 42.Brater DC, Harris C, Redfern JS, et al. Renal effects of COX-2-selective inhibitors. Am J Nephrol 2001; 21:1–15. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz JI, Vandormael K, Malice MP, et al. Comparison of rofecoxib, celecoxib, and naproxen on renal function in elderly subjects receiving a normal-salt diet. Clin Pharmacol Ther 2002; 72:50–61. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe F, Zhao S, Pettitt D. Blood pressure destabilization and edema among 8538 users of celecoxib, rofecoxib, and nonselective nonsteroidal antiinflammatory drugs (NSAID) and nonusers of NSAID receiving ordinary clinical care. J Rheumatol 2004; 31:1143–1151. [PubMed] [Google Scholar]
- 45.Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: metaanalysis of randomized trials. JAMA 2006; 296:1619–1632. [DOI] [PubMed] [Google Scholar]


