Abstract
Vascular inflammation is present in a subset of patients with cerebral amyloid angiopathy (CAA) and has a major influence in determining the disease manifestations. Radiological characterization of this subset is particularly important to achieve early recognition and treatment. We conducted this study to investigate the role of imaging in differentiating CAA with and without inflammation. We reviewed neuroimaging findings for 54 patients seen at Mayo Clinic over 25 years with pathological evidence of CAA and with available neuroimaging at the time of diagnosis. Clinical data were also recorded. Patients were grouped into CAA alone (no vascular inflammation), Aβ-related angiitis or ABRA (angiodestructive inflammation), and CAA-related inflammation or CAA-RI (perivascular inflammation). Imaging findings at presentation were compared among patient subgroups. Radiological features supporting a diagnosis of ABRA or CAA-RI were identified. Radiologic findings at diagnosis were available in 27 patients with CAA without inflammation, 22 with ABRA, and 5 with CAA-RI. On MRI, leptomeningeal disease alone or with infiltrative white matter was significantly more frequent at presentation in patients with ABRA or CAA-RI compared with those with CAA (29.6% vs. 3.7%, P = 0.02; and 40.7% vs. 3.7%, P = 0.002, respectively), whereas lobar hemorrhage was more frequent in patients with CAA (62.3% vs. 7.4%, P = 0.0001). Overall, leptomeningeal involvement at presentation was present in 70.4% of patients with ABRA or CAA-RI and in only 7.4% of patients with CAA (P = 0.0001). The sensitivity and specificity of leptomeningeal enhancement to identify patients with ABRA or CAA-RI were 70.4% and 92.6%, respectively, whereas the positive likelihood ratio (LR) was 9.5. The sensitivity and specificity of intracerebral hemorrhage to identify patients with CAA were 62.9% and 92.6%, respectively, whereas the positive LR was 8.5. Microbleeds were found in 70.4% of patients with inflammatory CAA at presentation. In conclusion, leptomeningeal enhancement and lobar hemorrhage at presentation may enable differentiation between CAA with and without inflammation. The identification at initial MRI of diffuse cortical-subcortical microbleeds in elderly patients presenting with infiltrative white matter process or prominent leptomeningeal enhancement is highly suggestive of vascular inflammatory CAA.
INTRODUCTION
Cerebrovascular deposition of amyloid (sporadic cerebral amyloid angiopathy or CAA) is characterized by deposition of amyloid-β in the media and adventitia of cortical and leptomeningeal vessels.1–4 Vascular inflammation may also be present in amyloid-affected vessels. Two types of inflammatory response have been reported.1,6–8 Aβ-related angiitis or ABRA is an angiodestructive process characterized by a vasculitic transmural, often granulomatous, inflammatory infiltration. CAA-related inflammation (CAA-RI) is characterized by an inflammatory reaction that surrounds amyloid-laden vessels, without angiodestructive features. Prevalence of CAA increases with increasing age, but often remains clinically occult. When clinically evident, CAA most commonly presents as a lobar intracerebral hemorrhage in an elderly normotensive patient.2,3 In patients with associated vascular inflammation, intracranial hemorrhage is less frequent and such patients often present with findings of subacute cognitive decline, seizures, and headaches and respond to immunosuppressive therapy.1,6–8
CAA, CAA-RI, and ABRA all have varied neuroradiographic appearances including intraparenchymal lobar hemorrhage, microhemorrhages centered at the gray white junction, white matter infiltrative nonenhancing T2 hyperintense mass-like lesions, leptomeningeal disease, or a combination of any of the above. Few series have focused on the radiological-pathology correlative findings, which may guide the clinical and surgical decision-making process.9–14 Radiological characterization of CAA patients with vascular inflammation is particularly important to achieve early recognition and treatment, avoid nondiagnostic biopsies, improve outcomes, and minimize the risk of unnecessary treatment with glucocorticoids and/or cyclophosphamide. The purposes of this study were to review our experience with imaging findings at presentation of pathology-proven CAA and to identify radiological features supporting a diagnosis of ABRA or CAA-RI.
PATIENTS AND METHODS
Identification of the Patients
In this study, we included all patients seen at Mayo Clinic in Rochester, MN, between January 1987 and December 2011 diagnosed on pathology specimen with CAA and had available neuroimaging at presentation.1 All pathological specimens were reviewed by one neuropathologist (CG) to exclude other vascular pathologies, to evaluate the presence of concomitant neurodegenerative changes of Alzheimer type, and to evaluate the presence and distribution of inflammation associated with amyloid-β. A detailed description of the pathologic and classification procedures including imaging categorization was reported in previous studies.1,6,15–18 Patients were grouped into 3 groups: CAA alone (no vascular inflammation), ABRA (angiodestructive inflammation), and CAA-RI (perivascular inflammation without angiodestructive process).1 The review of all available neuroimaging findings was performed by an experienced CAQ neuroroadiologist (JMM), who had no access to the clinical data or knowledge of the pathology report.
Imaging modalities reviewed included computed tomography (CT), magnetic resonance imaging (MRI), CT-angiography (CTA), MR-angiography (MRA), and catheter angiography. All these imaging modalities were performed and interpreted by defined clinical protocols used by the Division of Neuroradiology at the Mayo Clinic.
Neuroimaging was reviewed for the location and presence of the following findings: lobar hemorrhages, microhemorrhages at the gray white junction on gradient-echo (GRE) or susceptibility-weighted imaging (SWI), associated vasogenic edema in the underlying white matter, leptomeningeal or pachymeningeal involvement, enhancing mass-like lesions, cerebral infarcts, attenuation of these changes on CT, postgadolinium enhancement characteristics, or any combination of above.
Medical history and clinical and laboratory findings at presentation and during the follow-up as well as response to treatment, relapse occurrence, follow-up functional status, and cause of death were recorded.
Standard Protocol Approvals, Registrations, and Patients Consents
The Mayo Institutional Review Board approved this study. The IRB number was 11–003530.
Statistical Analysis
Numeric parameters were compared by using a 2-sided 2-sample t test or a Wilcoxon rank-sum test when the distributions were skewed. Comparisons of categorical variables were performed using the χ2 or Fischer exact test when cell counts were <5. We determined sensitivities (% of patients with a disease who have a positive test), specificities (% of patients without the condition who have a negative test), predictive values (positive predictive value [PPV] = number of true positives/number of true positives + number of false positives, negative predictive value [NPV] = number of true negatives/number of true negatives + number of false negatives), and likelihood ratios (LRs) (LR+ = sensitivity/1-specificity, LR− = 1−sensitivity/specificity) with 95% confidence intervals (CIs).19 Significance was defined at P < 0.05. The statistical analysis was performed using SAS version 8 (SAS Institute, Cary, NC).
RESULTS
Seventy-eight consecutive patients with cerebral vascular Aβ deposition were identified.1 Neuroimaging findings at presentation were available for review in 54 cases (27 CAA, 22 ABRA, and 5 CAA-RI), who represent the study population. Ten cases were evaluated with CT alone (ABRA = 1, CAA = 9), 16 cases were evaluated with MRI alone (ABRA = 11, CAA-RI = 3, CAA = 2), and 28 cases were evaluated with CT and MRI (ABRA = 10, CAA-RI = 2, CAA = 16). CTA, MRA, and conventional angiography were additionally performed in 21 cases (ABRA = 6, CAA-RI = 3, CAA = 12). No angiographic evidence of vasculitis (smooth-wall segmental narrowing, dilatation, or occlusion affecting multiple cerebral arteries)15 was observed in the 3 groups of patients. Twenty-nine cases had either a GRE or SWI sequence performed.
Location of Imaging Abnormalities
In 18 cases, imaging abnormalities involved 1 lobe (ABRA = 3, CAA = 15). One case of CAA involved a cerebellar hemisphere. In 35 cases, abnormalities were multilobar (ABRA = 19, CAA-RI = 5, CAA = 11). Twenty-five cases were bilateral (ABRA = 14, CAA-RI = 3, CAA = 8). Patients with ABRA and CAA-RI had a significantly higher frequency of multilobar abnormalities compared to those with CAA (62.9% vs. 29.6%, P = 0.028). The frontal lobe was the most frequently involved (ABRA = 22, CAA-RI = 3, CAA = 16).
Enhancement
Twenty-three cases demonstrated some form of postcontrast enhancement. Twenty-one cases had leptomeningeal enhancement (ABRA = 15, CAA-RI = 4, CAA = 2), and 2 cases of CAA had mass-like enhancement.
Vasogenic Edema/Mass Effect/Previous hemorrhage
Thirty-nine cases showed evidence of vasogenic edema (ABRA = 16, CAA-RI = 4, CAA = 23). The mass effect from lobar hemorrhage resulted in hydrocephalus or herniation respectively in 5 and 6 cases of CAA. Evidence of previous microhemorrhage (foci of hemosiderin) was seen in 29 cases by utilization of GRE and/or SWI sequences (ABRA = 15, CAA-RI = 4, CAA = 10). Table 1 shows these radiological findings at presentation.
TABLE 1.
Radiological (MRI and/or CT) Findings at Presentation
CAA radiological Findings at Presentation and During Follow-Up
Lobar hemorrhage was the most frequent presentation, occurring in 17 of 27 (63%) of those with CAA. A single lobar hemorrhage was present in 15, whereas 2 other patients had multiple intraparenchymal hemorrhages of different ages. A leptomeningeal process was the only MRI finding in 1 patient (3.7%). Another had a leptomeningeal process with associated vasogenic edema underlying this region (3.7%). An infiltrative white matter process mimicking a low-grade glioma was present in 4 others (14.8%) (Figure 1). Four patients had other findings at presentation: 2 had a mass-like lesion, 1 subarachnoid hemorrhage, and 1 multiple cortical infarctions and intraparenchymal hemorrhages of different ages. Three patients had multiple modalities of presentation. Follow-up images were available in 3 patients with CAA. All 3 patients had 1 relapse. Two relapses were characterized by the presence of a leptomeningeal process with infiltrative white matter, occurring 9 months and 8 years, respectively, after the initial diagnosis. The third patient had a lobar hemorrhage 2 years after the first episode characterized by a multilobar infiltrative white matter process mimicking low-grade glioma.
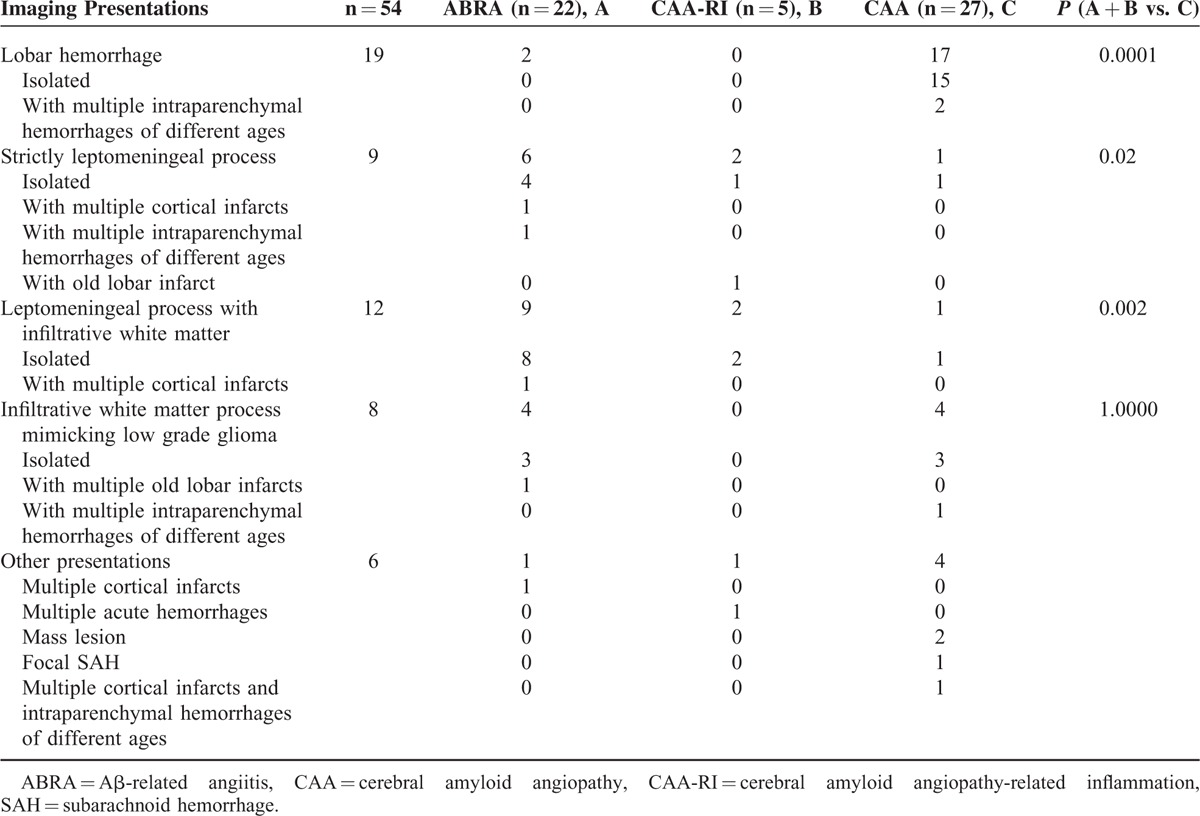
FIGURE 1.
Magnetic resonance imaging of a patient with CAA showing an infiltrative white matter process mimicking low grade infiltrating glioma. A, Fluid Attenuation Inversion Recovery (FLAIR) MRI images show a T2 hyperintense infiltrative white matter process in the left temporal/parietal lobes with associated mass effect; mild contralateral infiltrative T2 hyperintensity is also present. There was no associated enhancement. B, Axial susceptibility weighted imaging (SWI) shows multiple bilateral microhemorrhages centered at the grey white junction in both cerebral hemispheres, particularly evident in the area of mass-like T2 hyperintensity.
Illustrative Cases of CAA
Case 1
A 62-year-old woman presented with recurrent multifocal bilateral paraesthesias. Brain MRI showed a leptomeningeal process and infiltrative white matter abnormalities in right parietal lobe. Cerebral biopsy showed CAA with involvement of leptomeningeal and superficial cortical vessels. The patient was not treated and 4 months later at MRI, there was an almost complete resolution of the lesions. Five months later, she developed aphasia with MRI evidence of a recurrent leptomeningeal process with infiltrative white matter abnormalites in the left temporal lobe. She was treated with only three 1 g/pulses of methylprednisolone (oral glucocorticoids did not follow pulse therapy) with significant improvement of the language (80% of normal) in the following 10 days. Seven months later, brain MRI had markedly improved showing only few tiny foci of hemosiderin on GRE and language was normal.
Case 2
A 61-year-old woman presented with a right frontal lobar hemorrhage. Medical history was negative. Brain biopsy performed during hematoma evacuation showed CAA. Eight years later, she developed seizures and brain MRI showed infiltrative T2 signal in the white matter with leptomeningeal enhancement in the right temporal lobe which spontaneously resolved. Three years later, microhemorrhages were observed on follow-up brain MRI.
Case 3
A 47-year-old man developed progressive cognitive decline, personality change, confusion, spells, and right hemiparesis. Brain MRI showed an infiltrative white matter process mimicking low-grade glioma in the parietal lobe bilaterally and in the right frontal lobe; in addition, there were multiple intraparenchymal hemorrhages of different ages. A brain biopsy showed CAA. His neurological condition and brain MRI gradually improved without therapy. Two years later, his neurologic status suddenly deteriorated and brain MRI showed a new right frontal lobe hemorrhage.
ABRA Radiological Findings at Presentation and During Follow-Up
Leptomeningeal disease only (Figure 2) and with infiltrative white matter abnormalities (Figure 3) were the 2 most frequent radiological findings at presentation and were observed in 6 (27.3%) and 9 (40.9%) of the 22 patients respectively, followed by an infiltrative white matter process mimicking low-grade glioma alone in 4 patients (18.2%) (Table 1). Multiple cortical infarcts were present in 3 patients (14%). In 1 patient, infarcts were the only finding at presentation, whereas in the other 2, additional lesions developed. Multiple old lobar infarcts were observed in another patient associated with an infiltrative white matter process mimicking low-grade glioma. Lobar hemorrhage was observed in only 2 patients.
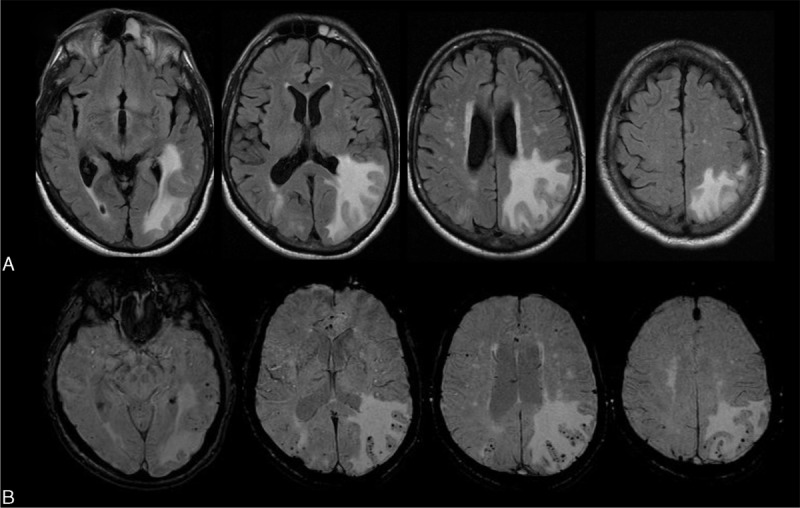
FIGURE 2.
Magnetic resonance imaging of a patient with ABRA showing a strictly leptomeningeal process. A, Fluid Attenuation Inversion Recovery (FLAIR) sequence shows non nulling of the subarachnoid signal involving the left temporal lobe consistent with a leptomeningeal process. No underlying white matter abnormalities. B, Post Gadolinium T1-weighted images shows avid leptomeningeal enhancement. C, Axial susceptibility weighted imaging (SWI) shows multiple bilateral cortical-subcortical microhemorrhages throughout cerebral hemispheres.
FIGURE 3.
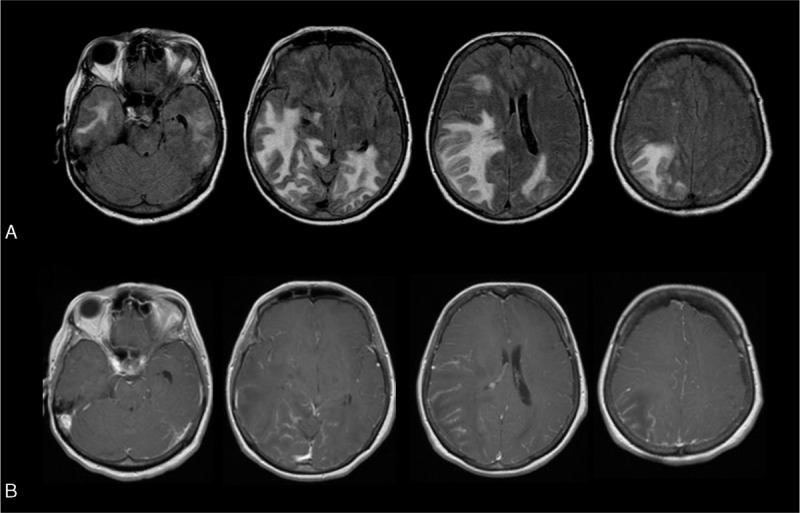
Magnetic resonance imaging of a patient with ABRA showing a leptomeningeal process with infiltrative white matter abnormalities as well. Without GRE or SWI imaging, this can be mistaken for an infiltrative low grade glioma. A, Fluid Attenuation Inversion Recovery (FLAIR)-weighted MRI images show multilobar infiltrative subcortical white matter T2 hyperintensities, with associated mass effect. B, Post Gadolinium T1-weighted MRI images show avid leptomeningeal enhancement without parenchymal enhancement.
Follow-up images were available in 4 patients with ABRA. Three of the 4 had clinical relapses. One patient had 3 relapses, one had 2, and 1 had 1 relapse. A new lobar hemorrhage was observed in all 3 relapses. Lobar hemorrhage was associated with subdural and subarachnoid hemorrhage in 1, recurrence of leptomenigeal enhancement in 1, and leptomeningeal process with infiltrative white matter process in 1.
Illustrative Cases of ABRA
Case 1
A 69-year-old woman presented with a 3-week history of spells of numbness moving up her left arm and in her left leg to the knee. The episodes lasted about 15 minutes, and as a second area became affected, the previous area improved. Brain MRI showed leptomeningeal enhancement in frontal and parietal lobes. Two months later, her neurological status deteriorated and a follow-up MRI showed a right frontal lobar hemorrhage. Brain biopsy showed necrotizing vasculitis and CAA. Prednisone 60 mg daily was started and she improved. Two months later, MRI showed improvement of leptomeningeal enhancement, but also revealed a new small hemorrhage in the left posterior temporal lobe. Mycophenolate mofetil was started and she improved further. Eight months later, the follow-up MRI showed a complete resolution of leptomeningeal enhancement. Medications were then discontinued. However, during subsequent follow-up, the patient had 3 clinical recurrences, 12 months, 29 months, and 35 months later. In the first, recurrence MRI showed a new left temporal lobe lobar hemorrhage, in the second, recurrence of leptomeningeal enhancement, and in the third, a right frontal lobar hemorrhage with subdural and subarachnoid hemorrhage.
Case 2
A 65-year-old man developed a right frontal hemorrhage that was evacuated. One month later, he noted headache, confusion, seizures, aphasia, and rapid cognitive decline. Brain MRI showed leptomeningeal enhancement involving the right frontal and temporal lobes and multiple infarcts in the overlying cortex. Cerebral biopsy showed ABRA. The patients were treated with methylprednisolone pulse therapy (1 g/day for 3 days) followed by oral prednisone 60 mg/daily. The patient radiographically and clinically improved, and 18 months later, he was able to stop prednisone without recurrence.
Case 3
A 67-year-old woman developed confusion, aphasia, and spells, lasting 5 to 10 minutes, with numbness in her arm and trunk. On several occasions, her face was drooped on the left and her speech was slurred. MRI showed leptomeningeal enhancement with T2 signal hyperintensity involving cortex and adjacent white matter in the right frontal lobe and left parietal and frontal lobes. Brain biopsy showed ABRA. The patient was not treated and there was a progressive resolution of neurological manifestations and MRI findings. Seven months later, the patient was asymptomatic and follow-up MRI demonstrated minimal persisting leptomeningeal enhancement in the left parietal area and a marked reduction in the amount of T2 signal changes. Five years later, the patient had a recurrence characterized by spells of aphasia and MRI evidence of leptomeningeal enhancement and T2 signal hyperintensity in subcortical white matter with extension to the cortex in the right occipital and temporal lobes. The neurological symptoms resolved in the following month without therapy and a control MRI, 5 months later, showed resolution of leptomeningeal enhancement and marked reduction of white matter T2 abnormalities. SWIs showed a few punctate areas of hemosiderin deposition in the same regions where there were T2 abnormalities. Three years later, the patient was asymptomatic and brain MRI unchanged.
Case 4
A 62-year-old woman presented with a 2-month history of episodes of paresthesias marching up the left arm from the fingers to the left side of the neck and face. MRI showed right temporal and bilateral frontal leptomeningeal enhancement and an old right frontal hemorrhage. The patient was not treated and the spells and MRI leptomeningeal enhancement improved spontaneously. Five months later, she complained of headache and MRI showed a new left posterior frontal hemorrhage and 2 months later, brain MRI showed a new left anterior frontal hemorrhage. Brain biopsy was performed with evidence of ABRA.
CAA-RI Radiological Findings at Presentation and During Follow-Up
Only 5 patients were in this group. Isolated leptomeningeal enhancement or a leptomeningeal process with infiltrative white matter was observed in 4 of 5 patients (80%). No patients had a lobar hemorrhage. One patient presented with multiple acute hemorrhages alone. One patient with leptomeningeal disease alone also had evidence of an old frontal infarct. Thus, the group appeared to resemble ABRA more than CAA.
Comparisons Between CAA and ABRA + CAA-RI
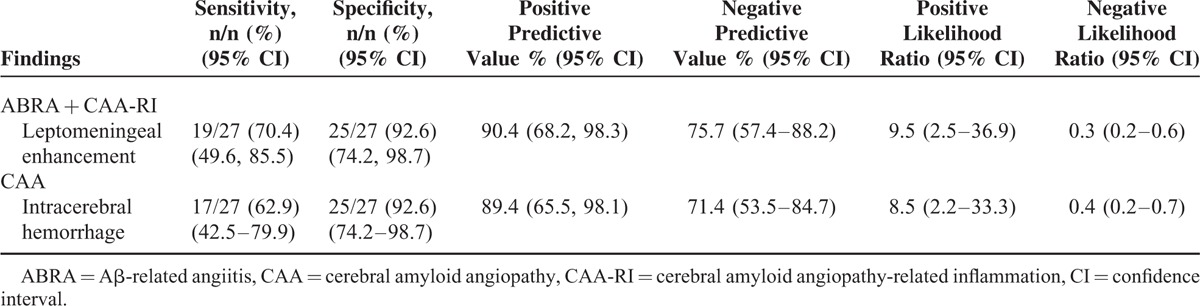
Leptomeningeal disease alone or with an infiltrative underlying white matter process was significantly more frequent in patients with ABRA and CAA-RI compared with those with CAA (8/27, 29.6% vs. 1/27, 3.7%, P = 0.02; and 11/27, 40.7% vs. 1/27, 3.7%, P = 0.002, respectively), whereas lobar hemorrhage was more frequent in patients with CAA (17/27, 62.3% vs. 2/27, 7.4%, P = 0.0001) (Table 1). Overall, leptomeningeal involvement at presentation was present in 70.4% (19/27) of patients with ABRA or CAA-RI and in only 7.4% (2/27) of patients with CAA (P = 0.0001). Table 2 shows the sensitivities, specificities, and LRs (with 95% CIs) of imaging findings that help differentiate patients with CAA from those with ABRA + CAA-RI. The sensitivity and specificity of leptomeningeal enhancement to identify patients with ABRA + CAA-RI were 70.4% and 92.6%, respectively, whereas the positive LR was 9.5. The positive predictive value was 90.4%. The sensitivity and specificity of intracerebral hemorrhage to identify patients with CAA were 62.9% and 92.6%, respectively, whereas the positive LR was 8.5. The positive predictive value was 89.4%.
TABLE 2.
Imaging Findings for the Diagnosis of Cerebral Amyloid Angiopathy With and Without Inflammation
DISCUSSION
The primary objective of this study was to assess imaging findings at diagnosis in a population of 54 patients with ABRA and CAA-RI or CAA. We found that leptomeningeal disease by itself or accompanied by infiltrative white matter process was significantly more frequent in patients with ABRA and CAA-RI than those with CAA, whereas lobar hemorrhage was more frequent in patients with CAA. Overall, leptomeningeal involvement at presentation was observed in 70% of the patients with vascular inflammation, but in only 7% with CAA. In addition to the sensitivity of 70%, MRI evidence of leptomeningeal enhancement had a specificity of 93% for the presence of vascular inflammation. The positive LR for this MRI finding was 9.5, indicating that leptomeningeal enhancement is almost 10 times more likely to be present in a patient with ABRA or CAA-RI inflammation than in a patient with CAA. We also observed that patients with intracerebral hemorrhage on imaging have an increased likelihood of CAA without inflammation (LR, 8.5). However, the absence of intracerebral hemorrhage decreased the probability of CAA without vascular inflammation. The negative LR for intracerebral hemorrhage was 0.4. The specificity of intracranial hemorrhage for CAA without inflammation was high (93%), whereas the sensitivity was relatively low (63%). Therefore, the presence of leptomeningeal enhancement in the absence of intracerebral hemorrhage increases the probability that a patient with CAA will have vascular inflammation.
We observed that ABRA and CAA-RI were more closely related to primary CNS vasculitis than to CAA without inflammatory infiltrates and that vascular inflammation in ABRA and CAA-RI more than Aβ deposition alone has a major influence in determining disease manifestations.1,5,6 In particular, the neurological manifestations of CAA with inflammatory vascular or perivascular infiltration respond to immunosuppressive treatment. Therefore, when neurologic and neuroradiologic findings are consistent with ABRA or CAA-RI, the physician should consider starting an appropriate immunosuppressive treatment also in absence of pathologic confirmation. If a biopsy is requested, the surgeon should direct the biopsy to the leptomeningeal and gray white junction.
Given the varied radiological presentations of these patients, GRE and/or SWI are essential in narrowing the differential diagnosis triaging the patients toward appropriate therapy.9–12 CAA inflammatory variants may present as an infiltrative, poorly marginated, diffuse lobar or multilobar T2-hyperintense process with associated mass effect and variable leptomeningeal enhancement mimicking CNS neoplasm or as strictly leptomeningeal process in the absence of lobar hemorrhage. Standard MRI sequences are not sensitive for detecting the microhemorrhages, which are also associated with these inflammatory CAA forms. In cases who present with a nonenhancing infiltrative white matter process and in which SWI or GRE imaging is not done, it is not uncommon that only the white matter is targeted in the biopsy resulting in a nondiagnostic biopsy and delayed treatment.11 The presence of multiple, cortical-subcortical microbleeds detected by GRE or by more sensitive sequences such as SWI is a suggestive radiological sign of vascular amyloid.9–11 We found microbleeds, using GRE and/or SWI sequences, in 19 of 27 (70.4%) patients with inflammatory CAA at presentation. The identification at initial MRI of diffuse cortical and subcortical microhemorrhages in a distribution typical of CAA in elderly patients presenting with infiltrative white matter process or prominent leptomeningeal enhancement is highly suggestive of vascular inflammatory CAA. These findings suggest that in the proper setting, ABRA and CAA-RI might be neuroradiologically diagnosed potentially avoiding therapeutic delays also in the absence of cerebral biopsy. Amyloid imaging with amyloid-binding positron emission tomography ligands, such as Pittsburgh Compound B, may also be useful in detecting CAA, although they cannot discriminate vascular from parenchymal amyloid deposits.20
Follow-up data provided some additional information on the course of CAA, in particular on the inflammatory lesions. Patients with CAA may have different clinical or neuroradiologic manifestations at different times. The presenting manifestation may suggest a non-inflammatory event such as lobar hemorrhage, but years later, an inflammatory appearance may develop (CAA case 2). In other patients, the presenting manifestation suggests inflammation and the relapse are similar (ABRA case 3) or noninflammatory (ABRA case 4). However, recurrent inflammatory lesions in a patient tended to occur in different cerebral lobes with a migratory pattern. In some, the clinical and radiologic alterations had a self-limited course. This migratory and reversible course with resolution without immunosuppressive therapy was previously described in some case reports.12,14,21 In these cases, as in some of our patients (particularly ABRA cases 3 and 4), the clinical symptoms and MRI findings recovered spontaneously in few months. These observations raise questions about whether some cases of ABRA can be observed without treatment.
Strengths of our study include the following: the relative large number of consecutive patients with CAA defined by uniform pathology criteria with cerebral imaging available at diagnosis, radiologic images that were performed by a defined clinical protocol, and reviewed for this study by a single experienced neuroradiologist. Limitations include the relative small number of patients with follow-up images available. Limitations also include the retrospective nature of the study, possible referral bias of cases, and sampling error of the biopsy specimens because of the patchy nature of the inflammatory response.
In conclusion, the clinician should be aware of the varied imaging appearance of ABRA, CAA-RI, and CAA. Diffuse lobar vasogenic edema or mass-like nonenhancing white matter T2 hyperdensities may mimic low-grade infiltrative glioma or gliomatosis cerebri. In these cases, SWI or GRE image aids in determining the correct diagnosis, also in absence of cerebral biopsy. If biopsy is obtained, it should be targeted at the leptomeningeal or gray white junction and not only the white matter.
Footnotes
Abbreviations: ABRA = Aβ-related angiitis, CAA = cerebral amyloid angiopathy, CAA-RI = CAA-related inflammation, CIs = confidence intervals, CT = computed tomography, CTA = CT-angiography, GRE = gradient-echo, LRs = likelihood ratios, MRA = MR-angiography, MRI = magnetic resonance imaging, NPV = negative predictive value, PPV = positive predictive value, SWI = susceptibility-weighted imaging.
JMM and CS contributed equally to the project.
CS is the visiting clinician at the Department of Neurology, Mayo Clinic, Rochester, MN.
There was no financial support for this work or other financial interests.
The authors report no potential conflicts of interest.
REFERENCES
- 1.Salvarani C, Hunder GG, Morris JM, et al. Aβ-related angiitis: comparison with CAA without inflammation and primary CNS vasculitis. Neurology 2013; 81:1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg S. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology 1998; 51:690–694. [DOI] [PubMed] [Google Scholar]
- 3.Vinters HV. Cerebral amyloid angiopathy: a critical review. Stroke 1987; 18:311–324. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SM, Vonsattel JPG, Stakes JW, et al. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology 1993; 43:2073–2079. [DOI] [PubMed] [Google Scholar]
- 5.Scolding NJ, Joseph F, Kirby PA, et al. Abeta-related angiitis: primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005; 128:500–515. [DOI] [PubMed] [Google Scholar]
- 6.Salvarani C, Brown RD, Jr, Calamia KT, 3rd, et al. Primary central nervous system vasculitis: comparison of patients with and without cerebral amyloid angiopathy. Rheumatology (Oxford) 2008; 47:1671–1677. [DOI] [PubMed] [Google Scholar]
- 7.Eng JA, Frosch MP, Choi K, et al. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol 2004; 55:250–256. [DOI] [PubMed] [Google Scholar]
- 8.Kinnecom C, Lev MH, Wendell L, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology 2007; 68:1411–1416. [DOI] [PubMed] [Google Scholar]
- 9.Chao CP, Kotsenas AL, Broderick DF. Cerebral amyloid angiopathy: CT and MR imaging findings. RadioGraphics 2006; 26:1517–1531. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai K, Tokumaru AM, Nakatsuka T, et al. Imaging spectrum of sporadic cerebral amyloid angiopathy: multifaceted features of a single pathological condition. Insights Imag 2014; 5:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotsenas AL, Morris JM, Wald JT, et al. Tumefactive cerebral amyloid angiopathy mimicking CNS neoplasm. AJR 2013; 200:50–56. [DOI] [PubMed] [Google Scholar]
- 12.Martucci M, Sarria S, Toledo M, et al. Cerebral amyloid angiopathy-related inflammation: imaging findings and clinical outcome. Neuroradiology 2014; 56:283–289. [DOI] [PubMed] [Google Scholar]
- 13.Kloppenborg RP, Richard E, Sprengers ME, et al. Steroid responsive encephalopathy in cerebral amyloid angiopathy: a case report and review of evidence for immunosuppressive treatment. J Neuroinflammation 2010; 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarazin M, Amarenco P, Mikol J, et al. Reversible leukoencephalopathy in cerebral amyloid angiopathy presenting as subacute dementia. Eur J Neurol 2002; 9:353–358. [DOI] [PubMed] [Google Scholar]
- 15.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol 2007; 62:442–451. [DOI] [PubMed] [Google Scholar]
- 16.Salvarani C, Brown RD, Jr, Calamia KT, 3rd, et al. Angiography-negative primary central nervous system vasculitis: a syndrome involving small cerebral vessels. Medicine (Baltimore) 2008; 87:264–271. [DOI] [PubMed] [Google Scholar]
- 17.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis with prominent leptomeningeal enhancement: a subset with a benign outcome. Arthritis Rheum 2008; 58:595–603. [DOI] [PubMed] [Google Scholar]
- 18.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet 2012; 380:767–777. [DOI] [PubMed] [Google Scholar]
- 19.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 1991; 44:763–770. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke 2015; 17:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh U, Gupta R, Krakauer JW, et al. Reversible leukoencephalopathy associated with cerebral amyloid angiopathy. Neurology 2004; 62:494–497. [DOI] [PubMed] [Google Scholar]


