Abstract
The main objective of this study is to evaluate general anesthesia or propofol-based sedation methods at gastric endoscopic submucosal dissection (ESD) procedures.
The anesthetic method administered to cases undergoing upper gastrointestinal ESD between 2013 and 2015 was retrospectively investigated. Procedure time, lesion size, dissection speed, anesthesia time, adverse effects such as gag reflex, nausea, vomiting, cough, number of desaturation episodes (SpO2 < 90%), oropharyngeal suctioning requirements, hemorrhage, perforation, and amount of anesthetic medications were recorded.
There were 54 and 37 patients who were administered sedation (group S) and general anesthesia (group G), respectively. The demographics of the groups were similar. The calculated dissection speed was significantly high in group G (36.02 ± 20.96 mm2/min) compared with group S (26.04 ± 17.56 mm2/min; P = 0.010). The incidence of nausea, cough, number of oropharyngeal suctioning, and desaturation episodes were significantly high in group S compared with that in group G (P < 0.5). While there was no difference between the groups in terms of hemodynamic parameters, in group S the use of propofol and in group G the use of midazolam and fentanyl were significantly higher (P < 0.05). Anesthesia time, postoperative anesthesia care unit, and hospital stay durations were not significantly different between the groups.
General anesthesia increased dissection speed and enhanced endoscopist performance when compared with propofol-based sedation technique.
INTRODUCTION
Upper gastrointestinal tumors constitute an important fraction of all gastrointestinal tumors. Endoscopic submusal dissection (ESD) is a technique that became increasingly popular since it enables en bloc resection of the lesion. Curative resection rates have been increased with the utilization of ESD in comparison with mucosal resection.1,2 However, ESD procedures require long durations for complete removal of the lesion and a patient with little or no movement during that time period. Interruptions of the procedure for safety concerns and patient movement further increase the procedural duration and sedation requirement. In theory, complication rates may increase with increased operative and anesthesia duration.3 There is no consensus in the literature regarding the technique of anesthesia/sedation method for ESD procedures. General anesthesia was offered as an anesthetic technique for ESD procedures in 1 study; however, some other studies have described conventional endoscopist-controlled or anesthesist-maintained sedation protocols.4–7 We used both methods at our institution for ESD. For the purpose of clarity, we have compared anesthesia and sedation methods utilized at our institution in terms of operative durations, interruption, and complication rates.
METHODS
This study was approved by the local ethics committee of Katip Celebi University (Date: 12.11.2015, No: 218). All the patients who had undergone ESD by a single expert (FA) on ESD at the Department of Gastroenterology, Ataturk Training and Research Hospital, Katip Celebi University, Turkey, between the years 2013 and 2015, were included in this study. Data of 163 upper gastrointestinal ESD patients were prospectively recorded, and anesthesia management was retrospectively investigated. Thirty-two cases with lesions located in the esophagus and duodenum were excluded from the study due to manipulation difficulties, causing a longer intervention duration and possibility of developing different complications. The first 40 cases undergoing ESD were excluded from the evaluation considering the effect of the learning process8 (Figure 1).
FIGURE 1.
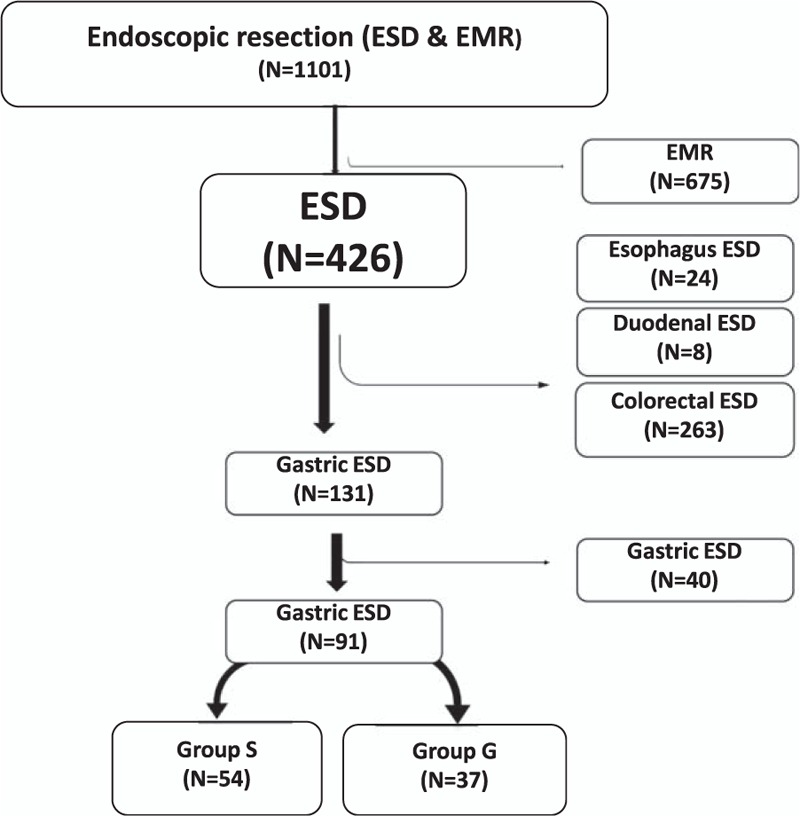
Flow chart showing the distribution of cases, which are applied with endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR), according to localization.
Anesthetic Management
Before the procedure, all patients had their body mass index recorded. Patients were evaluated according to the American Society of Anesthesiology (ASA) risk score, and those in ASA 1 to 3 were included in the study. Patients were informed about the risks of sedation and general anesthesia administration, and written consent was obtained from all. During the development of our endoscopy unit, when there was no anesthesia machine available, all patients were administered propofol-based sedation. After the availability of anesthesia machine, all the patients were administered general anesthesia with endotracheal intubation. Patients who were administered sedation were classified under group S, whereas who had general anesthesia were assigned to group G. In group S, after induction with midazolam (0.03 mg/kg) and fentanyl (1–2 mcg/kg), propofol (0.5–3 mg/kg) was administered with a sedation depth sensor, so that bispectral index (BIS) was between 60 and 80.9 In group G, after induction with midazolam (0.03 mg/kg), fentanyl (1–2 mcg/kg), propofol (1–2.5 mg/kg), and rocuronium (0.6 mg/kg), endotracheal intubation was performed. For maintenance, fentanyl, rocuronium, and sevoflurane were administered in O2/air targeting BIS values were in between 40 and 60 (10). Patients’ heart rate (HR), mean arterial blood pressure (MAP), and peripheral oxygen saturation (SpO2) values were recorded at 5-minute intervals during the procedure. The difference between the lowest and highest HR and MAP was recorded to evaluate hemodynamic instability.
Duration of Anesthesia
The time elapsed from the patient preparation for anesthesia until patient's awakening was accepted as the anesthesia duration, and total administered medication amounts were determined from the anesthesia record forms.
Adverse Events
Adverse events such as gag reflex, nausea, vomiting, cough, number of desaturation episodes and oropharyngeal suctioning requirements (defined as peripheral oxygen saturation falling below 90%), and complications of hemorrhage, perforation, and pneumonia were recorded.
Postanesthesia Discharge Unit and Discharge Times
Duration of patient stay at the postanesthetic care unit until patient's Modified Aldrete Score was >9 was recorded.10,11 The number of days patients remained in hospital after the procedure was also determined from the files.
ESD Technique
Before the procedure, patients were asked about accompanied diseases, medications used, and diagnostic or therapeutic interventions (endoscopic mucosal resection, polypectomy, biopsy, etc) for the endoscopically determined lesions. During the procedure, devices and equipment, total duration of the procedure, and adverse effects were all recorded in an electronic form.
Single-channel endoscope was used with a water jet system (GIF-190, Olympus, Tokyo, Japan) with a transparent hood attached (D-20111804 Olympus, Tokyo, Japan). The circumferences of lesions were marked with a Dual Knife (KD-650U, Olympus, Tokyo, Japan). Submucosal dissection was carried out with a Dual/Hook Knife or IT-Knife2 until complete removal of the lesion.
Examination of the Lesion and Measurement of its Size
Excised lesions were fixed and transported to the laboratory in formaldehyde. The size of the lesions was measured by Vernier Caliper as the longest dimension of the tissue, the longest lesion length, the largest tissue width, and the largest lesion width, and the results were recorded.
Duration of Procedure and Dissection Speed
The time between the initial incision and separation of the lesion from normal tissue was recorded as duration of the procedure (minute). The dissection speed was calculated as was in the previous studies.12,13 It was calculated by dividing the duration of procedure into the area of the resected specimen (mm2/min). Briefly, the area of the resected specimen was calculated as follows: 3.14 × 0.25 × long axis × minor axis.
Statistical Analysis
Differences in the categorical variables were analyzed with the Fisher exact test or a chi-square test. For comparisons of continuous variables, a Student t test or the Mann–Whitney U test was used, as appropriate. A 2-tailed P value <0.05 was considered statistically significant.
RESULTS
While there were 54 patients administered sedation in group S, 37 patients had general anesthesia, constituting group G. There was no difference between the patients in terms of demographic data, ASA classification, sample size, and lesion size (Table 1). In group G, the dissection speed was significantly shorter than in group S (P = 0.010). In terms of anesthesia duration, postanesthesia care unit (PACU) time, and hospital stay, there was no significant difference between the groups (data is shown at Table 2). Whereas there was no significant difference between the groups for the lowest and highest MAP and HR values, there were significant differences between the groups in terms of anesthetic medications used (P > 0.05). Total amount of administered propofol was higher in group S, whereas more midazolam and fentanyl were used in group G (Table 3). Nausea, cough, and number of desaturations were significantly higher in group S compared with that in group G (Table 4). In group S, 46.3% of patients needed oropharyngeal suctioning, whereas in group G, 2.7% of patients needed it, and this difference was statistically significant (P < 0.01). In group S, 1 patient (1.8%) developed clinically and radiologically confirmed aspiration pneumonia and his treatment was successfully completed with antibiotherapy. Tissue diagnosis of the removed lesions was similar between the groups (Figure 2).
TABLE 1.
Demographic Data of Patients

TABLE 2.
Procedure Time, Anesthesia Time, Dissection Speed, PACU Time and Discharge Time in the Groups
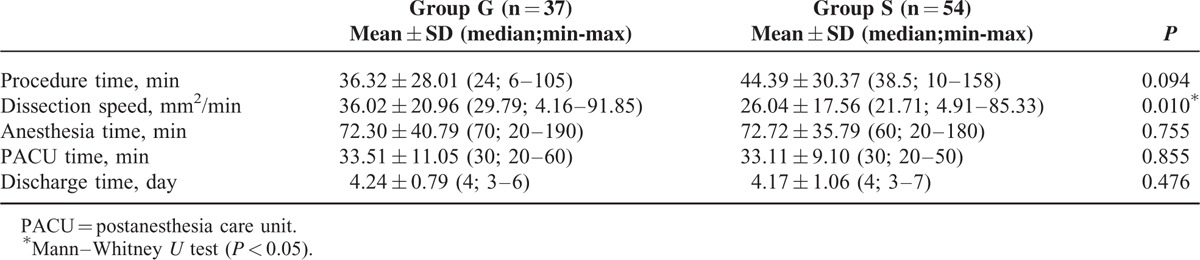
TABLE 3.
Amount of Anesthetic Agents, Mean Arterial Pressure and Heart Rate in the Groups

TABLE 4.
Perioperative Complications
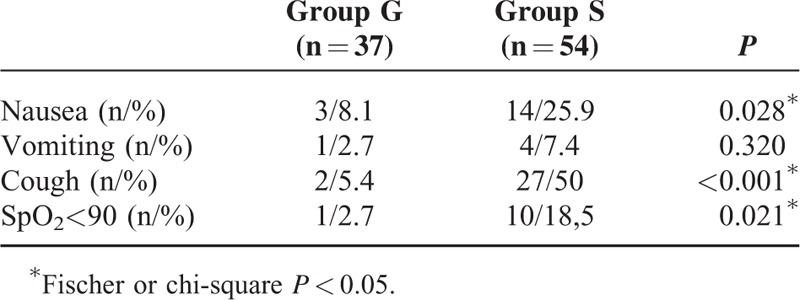
FIGURE 2.
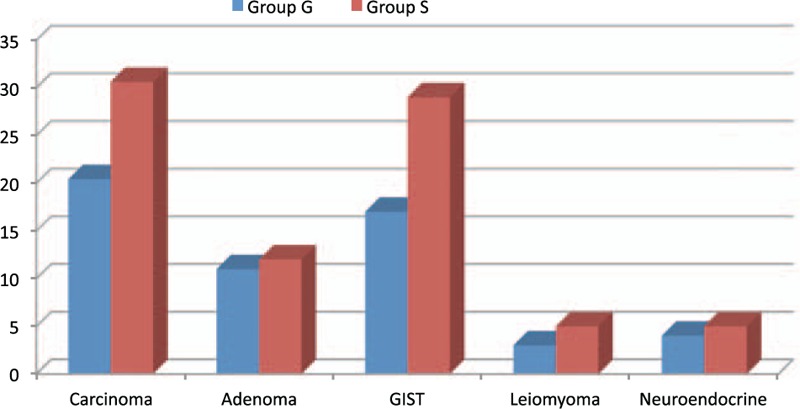
Pathology diagnosis.
DISCUSSION
The research demonstrated that the size of dissection per unit time was higher in group G compared with group S. Additionally, perioperative nausea, cough, and respiratory events were identified to be lower in group G. The PACU and hospital stay of patients were determined to be similar between the groups.
For ESD procedure, generally benzodiazepine and propofol-based sedation methods are chosen. Especially, in the upper gastrointestinal system, lesion location, manipulation difficulties, and patient movement may lengthen the duration of the procedure. Lengthy procedures increase the patients’ feeling of discomfort and increase the number of interventions by the anesthesiologist to ensure a reliable safe airway.5 Selecting the dissection speed as an evaluation parameter eliminates the effect of long procedural durations related to lesion size, with a consideration of reaching an objective criteria. An anesthesia method which diminishes the number of interruptions to maintain a reliable airway can increase the speed of endoscopic dissection, thus finally decreasing the overall procedure time. Sedation method frequently requires additional interventions during the procedure. In a research, it was observed that in 137 gastric ESD cases with propofol-based deep sedation with anesthesiologist control, 30% required third-person intervention for patient immobilization, whereas in 533 cases under endoscopist control, 72% required a third person and the difference was significant.5 In the same study, it was found that though the SpO2 rates in anaesthesiologist-controlled propofol-based sedation were low, no difference was identified in complication rates and hospital stay time. In our research, the lack of difference between the general anesthesia and sedation groups in terms of PACU stay and hospital stay is similar with the findings in this study. On the other hand, the general anesthesia group does not require interruptions. Rong et al4 researched the effect of general anesthesia administration on the reliability and procedure duration for ESD. This research found that procedure duration for esophageal and gastric tumors was shorter in the group given general anesthesia compared with the group given sedation. In our research, different to this research, cases only with gastric localization were chosen and dissection speed was evaluated as the size of tissue removed in unit time. Removing more tissue per unit time means that especially for ESD applications with large mass dissection, shorter times are required for the procedure under general anesthetic. Our research showing no difference between the groups in terms of anesthesia and postoperative observation duration indicates that general anesthetic administration did not require more time.
The study by Rong et al found no difference between the groups in terms of major complications like gastric perforation and postprocedure hemorrhage, but 80% of general anesthesia patients were satisfied, whereas this rate remained at 20% in the group administered sedation.4 In our analysis, there was no difference for complications like perforation and hemorrhage, though the incidence of interventions for cough, desaturation, and oropharyngeal suctioning requirements was higher in group S. It is thought that development of desaturation episodes and frequent oropharyngeal suctioning requirements led to breaks in the procedure or slowing of dissection speed, contributing to the longer procedure duration in group S. Additionally, the increase in number of desaturation episodes and oropharyngeal suctioning requirements coincidentally occured with aspiration pneumonia complication in 1 patient after the procedure. In 1 gastric ESD sedation case in our research, with frequent low SpO2 development and as a result requiring more aspiration interventions, aspiration pneumonia was diagnosed on later lung X-rays and treatment began. Due to limited number of patients with this rare type of complication, it is difficult to find a research including these patients. However, in research on 2 different sedation protocols including 293 patients, it was confirmed that aspiration pneumonia was encountered in the patient groups for both sedation protocols.6 This research used propofol and remifentanil infusions; however, the doses were chosen differently to bring sedation or analgesia to the forefront. In the group with moderate sedation target, 8 cases (5.2%) and in the group with analgesia target, 2 (1.4%) cases were encountered with aspiration pneumonia. Though no statistical difference was found between the groups, the researchers reported they changed the protocol to reduce the incidence of aspiration pneumonia. In other researches, the incidence of aspiration pneumonia in sedation-administered gastric ESD cases varied from 1.6% to 6.6%, showing parallels with the increase in procedure duration, patient age, and comorbid diseases.14,15
Park et al7 compared 2 different sedation methods administered for upper gastrointestinal ESD. This research compared 2 different sedation methods of maintenance of sedation with midazolam/propofol injection given by the endoscopist, with continuous propofol infusion given by an anesthesiologist in terms of patient and endoscopist satisfaction, hemodynamic and respiratory side effects, unwanted deep sedation level, frequency of events requiring intervention, full dissection, and complications. According to the results of the research endoscopist, satisfaction was higher for anesthesiologist control; however, there was no difference determined in terms of hemodynamic–respiratory side effects and minor problems. Within the total of 154 cases participating in the research, there was no case of aspiration and linked pneumonia treatment reported. The frequency of unwanted deep sedation episodes was 17.1% under endoscopist control and 5.1% in the anaesthesiologist-controlled sedation group. Additionally, in both groups, respiratory events occurred at a level reaching 15%, and a total of 3 patients (1.9%) were reported to require respiratory support with mask ventilation. According to the latest definition by the ASA, deep sedation requires the patient make a “purposeful” response to repeated or painful stimuli, whereas the general anesthesia definition criterion is a patient not responsive to painful stimuli, requiring frequent airway interventions.16 As a result, a case requiring airway intervention and mask ventilation may be considered to have entered general anesthesia without endotracheal intubation for a time. In our research, in group S, despite the frequency of desaturation development, we did not encounter any case requiring mask ventilation, but aspiration pneumonia did occur. In theory, incidence of such a complication is expected to increase linearly with increasing respiratory events, unwanted deep sedation episodes, and mask ventilation need during an upper gastrointestinal procedure.
In the scope of our research, whereas there was no statistically significant difference between the groups in terms of the MAP and HR measured at certain intervals during the procedure, in group G, there was less hemodynamic instability found. This situation may lead to the consideration that while under general anesthetic, interventions to patients’ MAP and HR were more easily performed.
Our study has some unique aspects compared with previous studies. The first is that this study shows that endoscopic treatment which is common in far eastern countries like Japan and Korea can be successfully performed by endoscopists and anesthesiologists with western education in endoscopy units with the necessary equipment. The second is that this is the first study to compare general anesthesia and sedation performed completely under anesthesiologist observation. The third is that as our study was performed by the same endoscopist and the same anesthesia expert, and confounding factors such as the learning process and procedure experience were reduced to a minimum. The fourth is that because we only included ESD cases with gastric localization, the results of our research were more homogeneous.
However, there are some limiting factors to our study. The first is that compared with the far east, our gastric case numbers were low. The second is the high incidence of respiratory events observed in the sedation group may not be fully explained since it may have multifactorial reasons. However, large prospective studies with standardized sedation protocols may reveal the presence of serious respiratory complications secondary to aspiration.
In conclusion, general anesthesia administration may prevent an increase in procedure time due to frequent breaks caused by gag reflex, cough, mobilization, and oropharyngeal suctioning needs of the patient, and thus reduce the dissection time. Finally, ensuring the reliability of the airway with endotracheal intubation increases the comfort of the endoscopist, in addition to preventing respiratory problems for the anesthesiologist, creating a safe reliable alternative to sedation methods for gastric ESD procedures.
Footnotes
Abbreviations: ASA = American Society of Anesthesiology, BIS = bispectral index, ESD = endoscopic submucosal dissection, HR = heart rate, MAP = mean arterial blood pressure = SpO2 , peripheral oxygen saturation.
A part of this study presented at Turkish Gastroenterology Congress 2015, Antalya, Turkey.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Othman MO, Wallace MB. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) in 2011, a Western perspective. Clin Res Hepatol Gastroenterol 2011; 35:288–294. [DOI] [PubMed] [Google Scholar]
- 2.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006; 64:877–883. [DOI] [PubMed] [Google Scholar]
- 3.Lin JP, Zhang YP, Xue M, et al. Endoscopic submucosal dissection for early gastric cancer in elderly patients: a meta-analysis. World J Surg Oncol 2015; 6:293.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rong QH, Zhao GL, Xie JP, et al. Feasibility and safety of endoscopic submucosal dissection of esophageal or gastric carcinomas under general anesthesia. Med Princ Pract 2013; 22:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nonaka S, Kawaguchi Y, Oda I, et al. Safety and effectiveness of propofol-based monitored anesthesia care without intubation during endoscopic submucosal dissection for early gastric and esophageal cancers. Dig Endosc 2015; 27:665–673. [DOI] [PubMed] [Google Scholar]
- 6.Yoo YC, Park CH, Shin S, et al. A comparison of sedation protocols for gastric endoscopic submucosal dissection: moderate sedation with analgesic supplementation vs analgesia targeted light sedation. Br J Anaesth 2015; 115:84–88. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Shin S, Lee SK, et al. Assessing the stability and safety of procedure during endoscopic submucosal dissection according to sedation methods: a randomized trial. PLoS One 2015; 24:e0120529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto T, Saito Y, Fukunaga S, et al. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312. [DOI] [PubMed] [Google Scholar]
- 9.Rosow C, Manberg PJ. Bispectral index monitoring. Anesthesiol Clin N Am 2001; 19:947–966. [DOI] [PubMed] [Google Scholar]
- 10.Alderette JA, Kroulik DA. Postanesthetic recovery score. Anesth Analg 1970; 51:543–546. [PubMed] [Google Scholar]
- 11.Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs 1998; 13:148–155. [DOI] [PubMed] [Google Scholar]
- 12.Aslan F, Alper E, Cekic C, et al. Endoscopic submucosal dissection in gastric lesions: the 100 cases experience from a tertiary reference center in West. Scand J Gastroenterol 2015; 50:368–375. [DOI] [PubMed] [Google Scholar]
- 13.Toyonaga T, Man-i M, Chinzei R, et al. Endoscopic treatment for early stage colorectal tumors: the comparison between EMR with small incision, simplified ESD, and ESD using the standard flush knife and the ball tipped flush knife. Acta Chir Iugosl 2010; 57:41–46. [DOI] [PubMed] [Google Scholar]
- 14.Akasaka T, Nishida T, Tsutsui S, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by Osaka University ESD Study Group. Dig Endosc 2011; 23:73–77. [DOI] [PubMed] [Google Scholar]
- 15.Watari J, Tomita T, Toyoshima F, et al. Miwa H The incidence of “silent” free air and aspiration pneumonia detected by CT after gastric endoscopic submucosal dissection. Gastrointest Endosc 2012; 76:1116–1123. [DOI] [PubMed] [Google Scholar]
- 16.Continuum of depth of sedation: definition of general anesthesia and levels of sedation/analgesia Available at: http://www.asahq.org. Standards and Guidelines Accessed February 5, 2016. [Google Scholar]


