Abstract
Calcifying fibrous tumor (CFT) is a benign lesion characterized by its specific histological findings and is found as solitary or multiple lesions in several locations of the human body. The aim of the present systematic review is to give a detailed account of all reported cases of CFT in the literature and to analyze the available data, to completely characterize the entity from epidemiological, medical, and surgical aspects.
A bibliographic research was performed from 1988 until 2015. A database with the patients’ characteristics was made, including sex, age, location of the tumor, symptoms, symptoms duration, size of the tumor, diagnostic methods, treatment, metastasis, and follow-up.
A total of 104 articles were identified, reporting 157 cases of CFT. Mean age of patients was 33.58 years and the ratio between men and women was 1:1.27. The most common locations of CFT were stomach (18%), small intestine (8.7%), pleura (9.9%), mesentery (5%), and peritoneum (6.8%). Mean diameter of the tumor was estimated 4.6 cm. The correlations proceeded showed that as age increases, size decreases (P = 0.001) and that the tumor is larger in females (P = 0.027). Kruskal-Wallis test showed that the larger tumors appear in the neck and adrenal gland (P = 0.001). The percentage of asymptomatic patients was 30.57%. Computed tomography and biopsy were the most common tests for the diagnosis of CFT. Open surgical procedure was performed in the majority of cases. The median hospitalization was 6.06 days and the mean follow-up period was 29.97 months. Recurrences were mentioned in 10 of 96 patients with available data. No deaths owing to CFT were mentioned in the literature.
CFT should be included in the differential diagnosis of enlarging mass revealed by clinical or imaging examination either incidentally or after specific acute or chronic symptomatology.
INTRODUCTION
Calcifying fibrous tumor (CFT) is a rare benign lesion that is composed of abundant dense well-circumscribed hyalinized collagen with lymphoplasmatic infiltrate, spindle cells, lymphoid aggregates, and psammomatous or dystrophic calcifications. This tumor was first described in the deep soft tissue in children by Rosenthal and Abdul-Karim,1 but subsequent reports revealed the occurrence of this lesion also in adults.2–8 Common sites that CFT arises from are pleura, abdominal cavity, mediastinum, heart, lung, neck, mandible, oral, inguinal, paratesticular, intrascrotal, spine, back, arm, and thigh. The etiology and pathogenesis of the tumor are controversial.
The aim of the present systematic review is to give a detailed account of all reported cases of CFT in the literature and to analyze the available data, to completely characterize the entity from epidemiological, medical, and surgical aspects.
METHODS
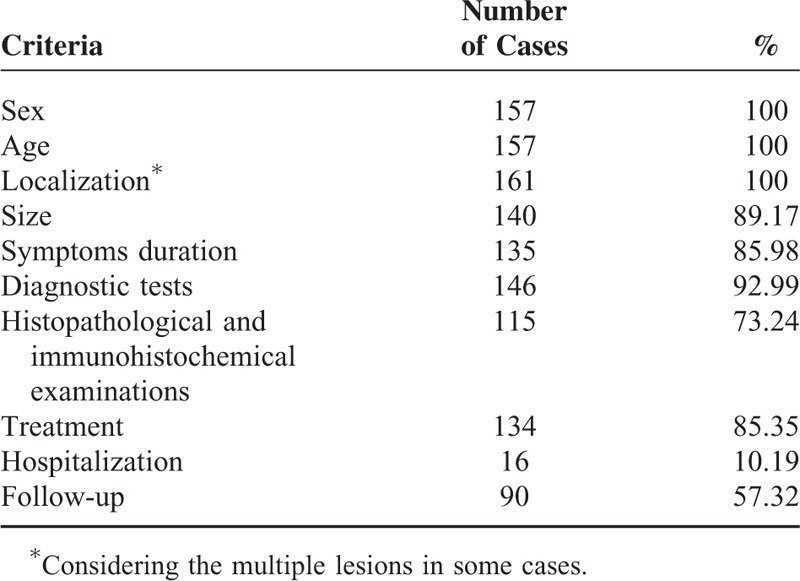
A bibliographic research was performed using PubMed, Scopus, and Embase. The search terms employed were “calcifying fibrous tumor” and “calcifying fibrous pseudotumor.” Since 1988, when the first description of CFT was made, until 2015, 104 articles were found in total (Figure 1). Among these, we spotted 157 well-documented cases of CFT. Ninety-nine articles were in English, 3 articles were in French, and 1 article was in Chinese. These articles were carefully studied and a database with the patients’ characteristics was made. The database included sex, age, location of the tumor, symptoms, symptoms duration, size of the tumor, diagnostic methods, treatment, metastasis, and follow-up. The cases that fulfilled at least 8 of these 10 criteria have been included in the statistical analysis. Table 1 displays the number of cases presenting the criteria. An ethical approval is not required because this study is a review of the existing international literature.
FIGURE 1.
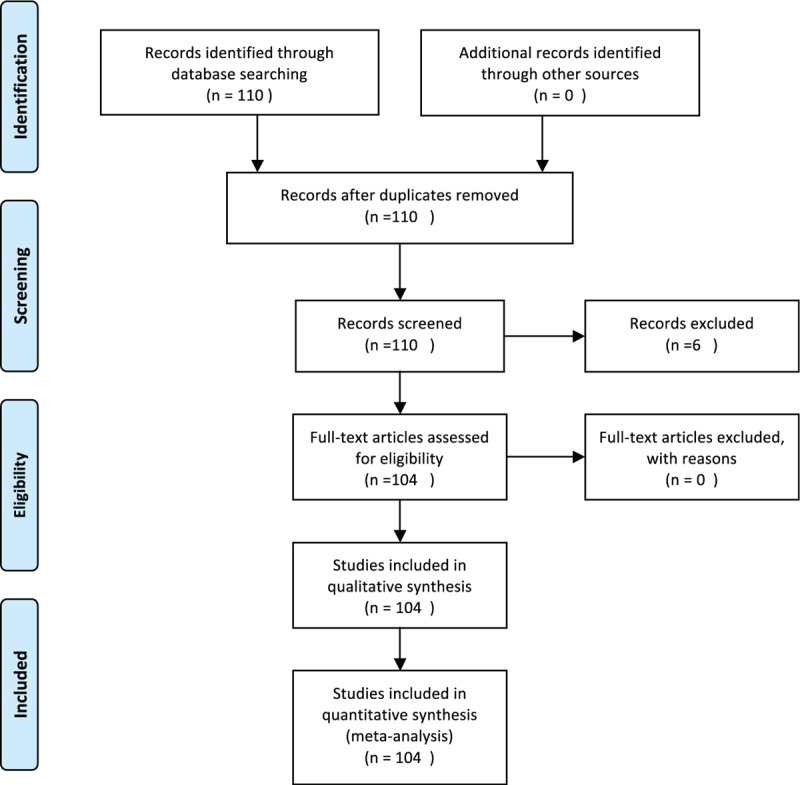
Flow Diagram according to PRISMA Statement 2009.
TABLE 1.
Cases of Calcifying Fibrous Tumor Fulfilling Criteria
To express results, descriptive statistics were used appropriately. Means, medians, and standard deviations were used for continuous variables and frequencies for categorical variables. Spearman test has been calculated for the correlation between size and age. Independent samples t test has been calculated for the distribution of age among males and females. We compared the size of the tumor of male versus female subjects, using Mann–Whitney U test. Mann–Whitney U test was also calculated for the distribution of size across categories of symptoms duration. As far as size and localization of CFT are concerned, Kruskall–Wallis test has been calculated for this correlation. In this test, locations of the tumor with a frequency <5 cases have been excepted, so that the statistical error is minimized. Statistical significant levels were those with P < 0.05.9 The statistical analysis was performed in SPSS version 23 (SPSS Inc, Chicago, IL).
RESULTS
Characteristics of CFTs were determined concerning sex, age, localization, size of the tumor, symptoms duration, diagnostic methods, histopathological and immunohistochemical examination, treatment, follow-up, recurrences, and metastasis.
Concerning sex, 43.94% of the patients were male (69 patients), whereas 56.05% were female (88 patients). The ratio between men and women was 1:1.27, suggesting a female predominance in the reported CFT population.
The age distribution is shown in Figure 2, from which it is concluded that CFT most frequently appeared in age ranges from 0 to 4, 25 to 29, and 30 to 34 years. Mean age of the 157 patients (100%) was 33.58 years, ranging from 5 weeks to 84 years. We compared the distribution of age among male and female patients and we found that the age at which tumor appeared does not differ statistically between males and females (35.11 ± 21.52 and 31.93 ± 18.76 years respectively, P = 0.321).
FIGURE 2.
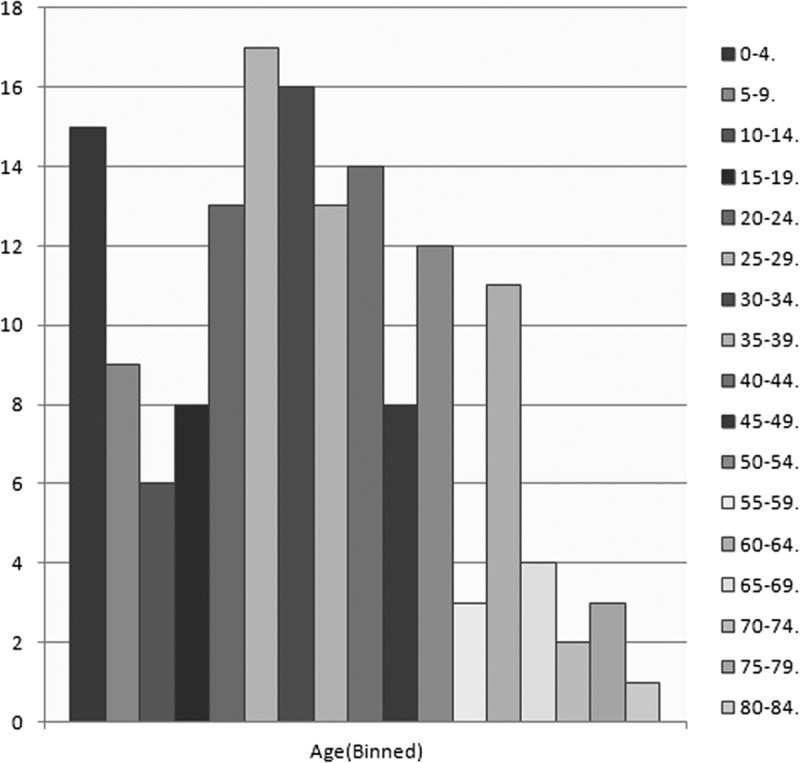
Age (Binned).
As far as the localization of the tumor is concerned, CFT can be found as solitary or multiple lesions. In our database, 9 of 157 patients (5.73%) had multiple lesions, which means that the total amount of cases calculated in the location section was 161. The most common locations of CFT are stomach (29 patients, 18%), small intestine (14 patients, 8.7%), pleura (16 patients, 9.9%), neck (10 patients, 6.2%), mesentery (8 patients, 5%), mediastinum (8 patients, 5%), and peritoneum (11 patients, 6.8%). Frequent sites that this tumor arises from are omentum (4 patients, 2.5%), thigh (5 patients, 3.1%), adrenal gland (5 patients, 3.1%), back (5 patients, 3.1%), lung (3 patients, 1.9%), and pericardium (3 patients, 1.9%), whereas rare locations are mandible (2 patients, 1.2%), spleen (2 patients, 1.2%), liver (2 patients, 1.2%), axilla (2 patients, 1.2%), rectum (2 patients, 1.2%), abdomen (2 patients, 1.2%), abdominal wall (2 patients, 1.2%), forearm (2 patients, 1.2%), lower leg (2 patients, 1.2%), intrascrotal (2 patients, 1.2%), and colon (2 patients, 1.2%). There are also cases reported in the literature, in which CFT occurred at some sites only once such as esophagus, spine, heart, retroperitoneal, breast, gallbladder, subscapular, upper arm, spermatic cord, mesocolon, tongue, oral, shoulder/wrist, paratesticular, lower leg, groin, fallopian tube, and nuchal. Figure 3 displays the localization of CFTs.
FIGURE 3.
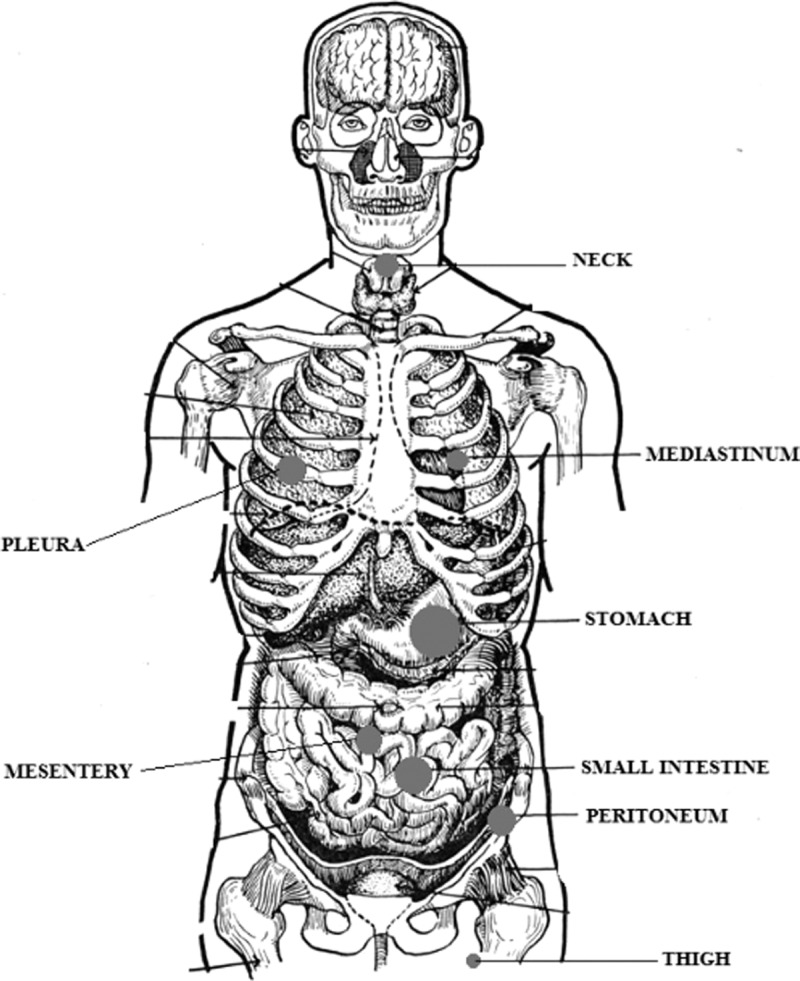
Schematic localization of most frequent sites of calcifying fibrous tumors. The diameter of the dot is proportional to the frequency of appearance.
Furthermore, 140 of 157 cases (89.17%) provided information about the size of the tumor. Mean diameter of the tumor was estimated 4.6 cm, ranging from 0.1 to 25 cm. Figure 4 displays graphically the tumors’ size. The statistical analysis showed a statistically significant correlation between size and age (P = 0.001, r = −0.285). As seen in Figure 5, the association between these 2 variables is negative; as age increases, size decreases. The comparison of the size of the tumor of male and female subjects proved that the tumor is larger in females (U = 1875.5, P = 0.027). Figure 6 shows the distribution of size across males and females. Moreover, the distribution of size across categories of symptoms’ duration has been proved to be the same (P = 0.485). As far as the localization of CFT is concerned, Kruskall–Wallis test has proved that the larger tumors appear in neck and adrenal gland, as shown in Figure 7 (P = 0.001).
FIGURE 4.
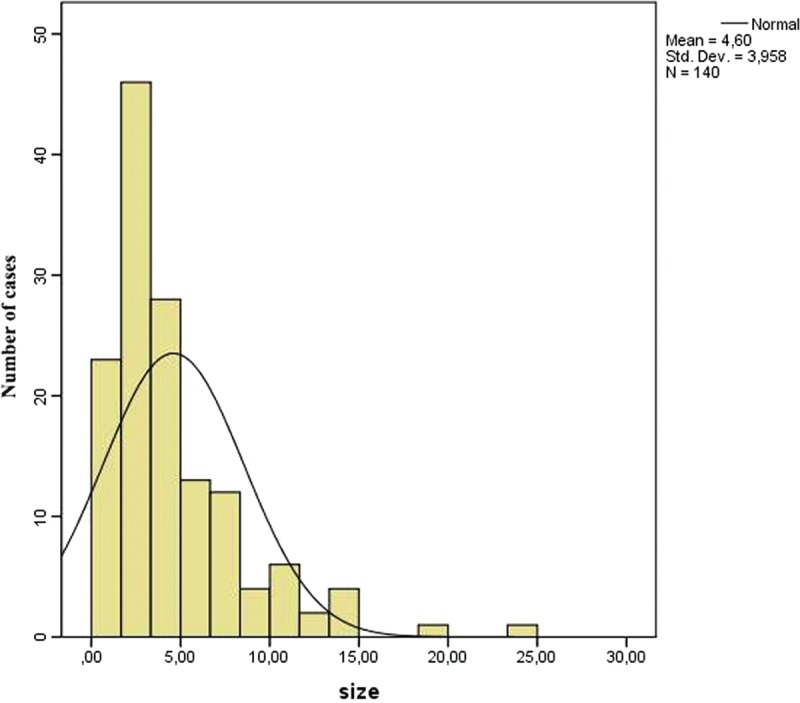
Distribution of diameter of calcifying fibrous tumor.
FIGURE 5.
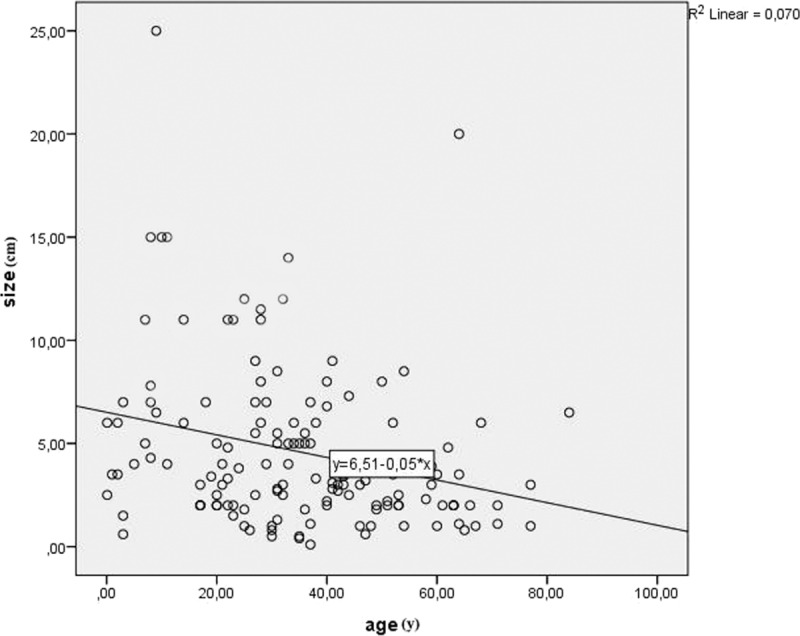
Correlation between size and age (P = 0.001, r = −0.285).
FIGURE 6.
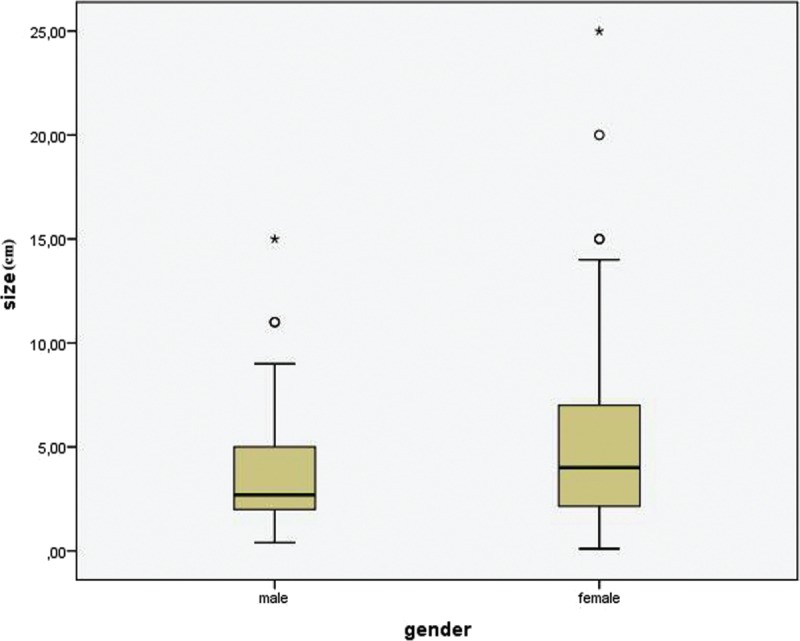
Box-plot demonstrating the size distribution among males and females (P = 0.027).
FIGURE 7.
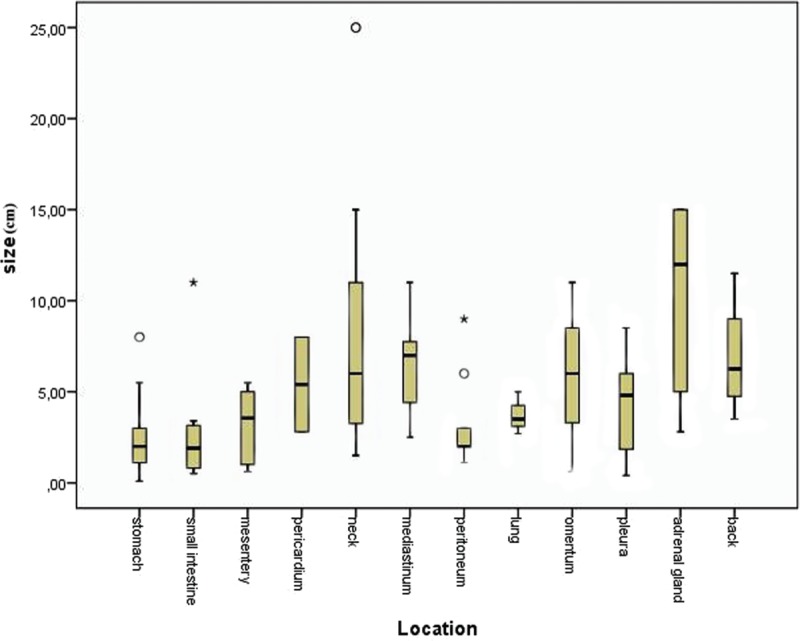
Box-plot demonstrating the size distribution among calcifying fibrous tumor locations (P = 0.001).
Symptomatology of the tumor could not be evaluated because of heterogeneity of the localization of CFT. However, we distinguished symptomatic from asymptomatic (48 patients, 30.57%) cases. The symptoms are characterized as nonspecific, including lack of appetite, fever, weight loss, fatigue, progressive weakness, chest or back pain, fever, dyspnea, shortness of breath or ecchymosis, and erythema, and as specific ones, referring to each organ system. The symptoms’ duration was estimated and separated into 2 main categories: acute and chronic. Among 135 (85.98%) cases with data on this section, 24 (17.77%) patients presented with acute symptoms and 111 (82.22%) had chronic symptomatology.
As far as the diagnostic tests required for the diagnosis of CFT are concerned, computed tomography (CT), laboratory test, and biopsy seemed to be the most common ways to diagnose CFT or to exclude other similar conditions. Data were available for 146 (92.99%) patients, from whom 63 (43.15%) got a CT scan, 35 (23.97%) laboratory test, and 44 (30.13%) underwent a biopsy. Helpful imaging examinations proved to be echo (28 patients, 19.17%), endoscopy (31 patients, 21.23%), magnetic resonance imaging (MRI) (25 patients, 17.12%), and radiographs (25 patients, 17.12%) according to the localization of the tumor. All preoperative imaging tests revealed the tumor, its size, and localization, but the final diagnosis was made with the histopathological examination.
For the histopathological and immunohistochemical examination of CFT, among 115 (73.24%) cases with available data, only 9 (7.82%) had positive lymph nodes. Calcifications-psammoma bodies, inflammatory cells, and dense hyalinized collagen were found at a rate of 100%, as expected by definition. Various markers are used to characterize CFTs. Figure 8 shows the distribution of positive and negative expression of immunohistochemical markers.
FIGURE 8.
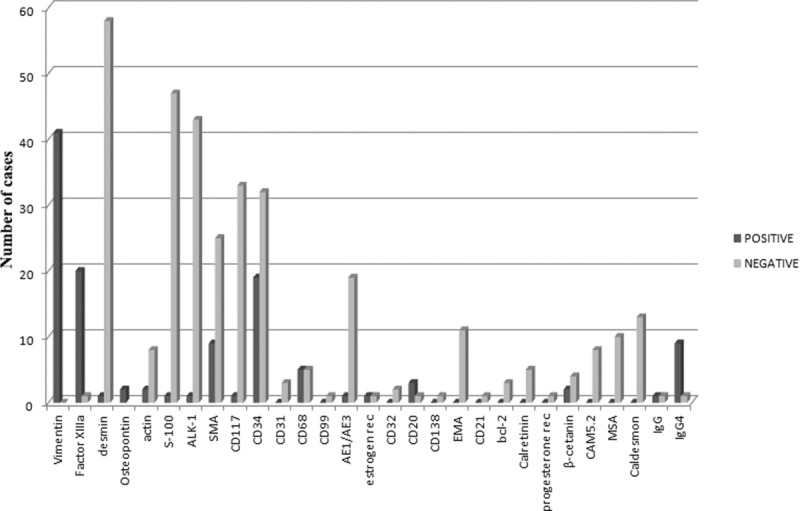
Distribution of expression of immunohistochemical markers.
Surgical excision is the main treatment for patients with CFT. In our database, open surgical procedure and endoscopic procedure are used for the excision of the tumor. Open surgical excision was performed in 116 of 134 (86.56%) cases with information provided, whereas the endoscopic one was performed in 18 (13.43%) cases.
Duration of hospitalization was provided only for 16 cases (10.19%), giving this analysis low credibility. However, the median hospitalization was 6.06 days, ranging from 1 to 18 days, depending on the location of the tumor.
Follow-up period was mentioned for 90 (57.32%) patients. The mean period was 29.97 months, ranging from 1 to 228 months. It was <60 months for 80 (88.88%), 60 to 120 months for 5 (5.55%), and >120 months for 5 (5.55%) patients. During the follow-up period, recurrence of the tumor was developed in 10 patients. Eight patients (8.33%) had 1 recurrence and 2 patients (2.08%) had 2 recurrences. Special note has to be made to 1 patient that had 1 possible recurrence that has not been confirmed because of discontinuance of follow-up.10 Only 1 patient with tumor recurrence had also positive lymph nodes detected in operation, but there are not so much data as needed to establish a correlation between recurrences and positive lymph nodes or inappropriate surgical technique.
No deaths owing to CFT were reported in the literature and as a result long-term survival was estimated at a rate of 100%.
DISCUSSION
History
CFTs are rare, benign lesions, which show a predilection for soft tissue. They are characterized by the presence of hypocellular, densely hyalinized collagen with psammomatous or dystrophic calcification and mononuclear inflammatory infiltrate. They were first described in 1988 by Rosenthal and Abdul Karim1 as a “childhood fibrous tumor with psammoma bodies” in 2- and 11-year-old girls in peripheral axial soft tissue. Fetch et al2 reported 10 cases of a distinctive benign fibrous lesion and first used the term “calcifying fibrous pseudotumor.” “Pseudotumor” was used to reflect the belief that the underlying process was most likely inflammatory.2 However, Nascimento et al11 suggested a true neoplasm with a tendency for local recurrence. The World Health Organization (WHO) established the name for this lesion in 2002 as “Calcifying Fibrous Tumors” in the newly published classification of tumors of soft tissue and bone.12 Furthermore, WHO classified the pseudotumor as a tumor of the soft tissue in 1999.13 In addition, CFT of the pleura is included in the neoplasms of the pleura by Hammar et al in 2008.14
Many scientists tried to sum up CFTs in large series. The first attempt was made by Jeong et al4 in 1996 who summarized 16 cases. Some years later, Hoffman et al15 reviewed 20 patients between 1988 and 1997, whereas Ben Izhak et al16 calculated 7 cases of CFTs in the abdominal cavity. Moreover, Elpek et al17 mentioned 34 cases of intra-abdominal CFTs that were referred in the literature until then. As far as CFT of the pleura is concerned, Shibata et al13 calculated 10 patients with CFT of the pleura, Isaka et al18 mentioned only 6 cases, and Ishida et al19 compiled only 7 cases of the tumor in this specific location. Last but not least, Im et al20 found 6 cases of intestinal CFT between 2008 and 2014. Searching carefully the English literature from 1993 to 2015, we found a total of 157 well-documented cases.
Epidemiology and Pathogenesis
Calcifying fibrous tumor seems to have a female predilection (ratio 1:1.27). Age distribution seems to be trimodal with one pick at 0 to 4 years, a second one in the mid-20s, and a third one in the mid-30s. This trimodal distribution may reflect different pathogenesis in the 3 different groups. Concerning the third spike (around thirties), there are many indications that CFT results as a late sclerosing stage of myofibroblastic tumor.5,21–23 Trauma may also be the basis of pathogenesis of this second spike.8,24 Concerning the early first spike, one can blame genetic and/or embryologic factors for CFT. Notably, Fukunaga et al7 found a diploid DNA content into CFT. Moreover, one cannot exclude embryonic ectopic remnants leading in the early years of life to CFTs.
CFTs are located in several organ systems, but mainly in the gastrointestinal track. Considering that the lumen of the gastrointestinal tract is actually external environment, the traumatic etiopathology of CFTs seems realistic. In those cases, the trauma results possibly by aggressive factors included “by accident” in food.
The frequency of the abdominal CFT was estimated to be 1 case per year worldwide based on histopathological archives, but a frequency for CFT as a whole does not exist yet. One retrospective study by Ogasawara et al25 has suggested that the incidence of gastric submucosal lesions is 0.36%, whereas Agaimy et al26 confirmed this suggested rarity of CFT of gastrointestinal track. Most lesions are generally solitary, but multiple CFTs are also described in the literature.5,21,27–29 In 9 cases, there were multiple foci of CFTs.
Clinical Features
The majority of the cases are asymptomatic and the tumor has been found incidentally on a routine imaging examination. When symptomatology is present, the clinical features of CFT are variable and could be categorized into 2 groups: acute or chronic findings. As a chronic condition, it can be seen as a visible, enlarging painless mass or as a facial growth, as far as the extremities are concerned.1,7,30–36 In a case report, the patient complained about a foreign body sensation in throat, whereas in another case of CFT of the spine, the patient noticed a flank pain in spine with worsening intensity.37,38 In the thoracic cavity, CFT may cause chest or back pain, fever, dyspnea, systolic heart murmur, shortness of breath or ecchymosis, and erythema.3,6,18,39–49 Intrabdominal CFTs present with a great variety of symptoms, both general and more specific.10,11,16,20,21,23,27,44,50–75 Lack of appetite, fever, weight loss, fatigue, and progressive weakness are examples of general symptoms. More specific ones are dyspepsia, flatulence, halitosis, nausea, vomiting, red blood per rectum, and altered bowel habits. The predominant symptom of intrabdominal CFT is pain. It is characterized as dull, intermittent, progressive, acute, sharp, crampy or not, episodic, local, radiating or not, not- related with the intake of food or epigastric pain after eating with early satiety. Moreover, 7 cases of gastric CFT with an ulcer on its surface have been reported.26,53,65,73,76–78 As an acute condition, it usually causes acute peritonitis or there are several case reports that state CFT in the intestine causes intussusception.8,23,54,79
Differential Diagnosis
The differential diagnosis of CFT depends on its localization. The major diagnostic problem is to determine the character of the tumor, which means that it is difficult to distinguish malignant from benign tumors in the preoperative stage. As far as the neck, the pleura, and the thoracic cavity are concerned, the differential diagnosis includes fibromatosis, solitary fibrous tumor (SFT), calcified granulomas, calcified pleural plaques, chronic fibrous pleuritis, intrapulmonary tumors, inflammatory myofibroblastic tumor (IMT), intermediate fibrous histiocytoma, rare dendrocytoma, desmoplastic mesothelioma, and amyloid tumor.4,13,15,28,42,80–85 Kawahara et al86 mentioned another tumor that should be taken in consideration in differential diagnosis: the monophasic synovial sarcoma. This type of tumor consists of spindle cells in dense cellular sheets or vague fascicles; hyalinization and calcifications can occasionally be seen and the cells are positive for cytokeratin and epithelial membrane antigen (EMA), which distinguish this tumor from CFT. Calcified granulomas contain residual histiocytes and multinuclear giant cells, whereas calcified pleural plaques and chronic fibrous pleuritis are characterized by diffuse pleural thickening. The cells in chronic fibrous pleuritis are more adjacent to the pleura and the capillaries are vertically aligned. Rare dendrocytoma stains for XIIIa factor consists of dermal dendrocytes and is characterized by a “donation.” Intrapulmonary tumors are most commonly childhood lung tumors and there is no specific method (laboratory check or imaging) in order that these tumors are distinguished from CFT. Malignant mesothelioma has got sarcomatous areas and a weak positive staining for osteopontin, but is strongly positive for cytokeratin.
Leiomyoma, desmoid fibromatosis, calcifying aponeurotic fibroma, and myositis ossificans circumscripta (MOC) must be considered in the differential diagnosis of tumors in the musculoskeletal system.31,39 MOC can be distinguished from CFT only by MRI or scintigraphy. Leiomyoma has undergone degenerative changes, has calcifications and ossifications, and its cells are positive or smooth muscle actin (SMA), desmin, caldesmon, and XIIIa factor.17 Calcifying aponeurotic fibroma belongs to the same category of tumors as CFT, but occurs typically in hand and feet, it is less circumscribed, and has metaplastic cartilage and multinuclear giant cells. Desmoid fibromatosis is a locally aggressive fibrogenic neoplasm, characterized by lack of psammoma bodies and by the positive expression of β-catanin.
Mangat et al43 mentioned 2 diseases that must be considered in the diagnosis of CFT of the breast. The first one is the idiopathic benign calcifications, which follow different patterns of calcium layering, and the second one refers to the diabetes mastopathy, caused by type I diabetes mellitus and characterized by a palpable mass with sclerosing lymphocytic lobulitis and no calcifications, which has mammographically increased density, and in the ultrasound scan has different acoustic shadowing.
As far as adrenal gland is concerned, Lau et al55 have included IMT, SFT, and peripheral nerve sheath tumors such as schwannoma and neurofibroma into the differential diagnosis of CFT of this anatomical site.
The principle consideration in differential diagnosis of intrabdominal CFT is gastrointestinal tumors (GISTs).20,51,54,71,81,87–89 GIST is much more cellular than CFT and does not consist of hyalinized collagen and calcifications and is not characterized by chronic inflammation. The mesenteric one has got a malignant behavior. GISTs are described as solid masses in CT scan.90 The cells of GIST are strongly positive for CD117 and CD34 and there are also some references in which it is mentioned that GIST is also positive for platelet-derived growth factor receptor.16,54 Other tumors that must be included in the differential diagnosis of intrabdominal CFT are solitary fibrous tumors, IMTs, carcinoid tumors, lipomas, reactive nodular fibrous tumors, leiomyoma, schwannoma, inflammatory fibrous polyps (IFPs), desmoid-type fibromatosis, retroperitoneal fibrosis, associated fibrous-inflammatory screlotizing infiltrations, calcifying aponeurotic fibroma, neuroendocrine tumors, myomas, cystic lesions, neurofibromas, small bowel desmoids, plexiform fibromyxoma, mesenteric fibromatosis, sclerosing mesenteritis, and adrenal neuroblastoma.91–97 Furthermore, heterotopic pancreas and enlarged lymph nodes should also be included.16,17,20,25,51,54,69,71,81,98 Plexiform fibromyxoma, mentioned by Jang et al,81 is larger (>5 cm) than CFT, is commonly a tumor of the antrum with capillary network, fibromyxoid stroma, hypercellularity, and positive staining for SMA. Sclerosing mesenteritis is characterized by inflammatory tissue and fat necrosis, which distinguish it from CFT. Mesenteric fibromatosis can infiltrate growth patterns and as far as desmoid type fibromatosis Im et al20 reported that except for the other above mentioned characteristics, it has also a negative staining for XIIIa factor and Farah et al69 added that it has also not got inflammatory aggregates, calcifications and has got thin-walled vessels. Schwannoma has peritumoral and intratumoral lymphoid, which is a common characteristic with CFT, but this one has no schwannoma tissue and a negative expression for S-100. Reactive nodular fibrous pseudotumor is characterized by overlying inflammatory process, such as an ulcer, a diverticula, a strangulated hernia, or a fistula, by the infiltration of small bowel, lack of calcifications, and by the positive staining for vimentin and cytokeratin. Amyloid tumors have got a giant cell response. IFPs consist of whorl-like arrangement of spindle cells around blood vessels and glands, eosinophilic infiltration, and CD34-positive staining. Solitary fibrous tumor has got hypercellular areas, patternless arrangement of spindle cells, and is positive for CD34, CD99, and bcl-2. In the case of gallbladder CFT, stromal tumors, IMT, and SFT must be considered.71 In the case of spleen CFT, hamartoma, littoral cell angioma, inflammatory pseudotumor, and follicular dendritic cell tumor must be included in the differential diagnosis.52 Littoral cell angioma is characterized by positive staining for XIIIa factor, CD31, CD34, CD68, KP-1, CD8, whereas follicular dendritic cell tumor is positive for CD21, CD35, and S-100. Hamartoma is an overgrowth of structures of red pulp of the spleen and is positive only for CD8, which is a pathognomonic sign of this tumor.
Nevertheless, the main consideration on the differential diagnosis of CFT is IMTs.11,15,20,25,28,51,54,55,70,71,79,81,89,99–102 IMTs tend to affect younger patients than CFT, they have symptoms and signs such as fever, pain, weight loss, malaise, anemia, thrombocytosis, increased sedimentation rate, and hypergammaglobulinemia, and can have multiple lesions, in contrast with CFT that can be asymptomatic and is rarely multifocal. Coffin et al,100 on the basis of 84 IMTs, found 3 basic histological patterns, in which there are similarities with CFT but in IMTs include acute inflammatory cells, plasma cells, lymphocytes, inflammatory infiltrate and proliferation leading to granulation tissue and fibromatosis.5,11,99 Immunohistochemically, IMT is strongly positive for SMA, actin, desmin, and anaplastic lymphoma kinase (ALK-1). The last one is a pathgnomonic sign of IMT. In addition to this, it has a focal staining for XIIIa factor. IMT may recur locally, but it rarely metastizes.11 Its prognostic significance is unclear, but it can progress and lead to death.99
Diagnosis
The diagnosis of CFT is based on the imaging findings and the pathological findings (histological appearance and immunohistochemical studies) owing to heterogeneity of symptoms. A variety of imaging techniques can be used to examine CFT. The radiographic appearance of CFT reflects its histological composition. Plain radiograph provides with the basic information needed to determine the exact anatomical site of the tumor.31 In a conventional radiograph, calcifications could also not be seen. Ultrasonographically, tumors are well-circumscribed masses with various echogenicity because of scattered calcifications. Furthermore, acoustic shadowing may also be noticed.31,38,103 CT scan is much more sensitive at detecting early mineralization.104 Calcifications demarcate the tumor from the surrounding tissue. The histological composition and the calcium layering create the pattern on CT scan. In the literature, there are variable descriptions of this pattern. Typical examples are “irregular,” “scattered,” “punctate,” “Thick and Band-like,” “clustered,” “amorphous.” Contrast-enhanced CT can also be used to achieve a better imaging result. Fan et al65 mentioned that CFT on a CT scan may be a round hyper- or hypodense mass. MRI has similar findings with CFT, but could also provide additional and important anatomical information about the tumor. CFT is a hypointense signal ass on T1-weighted and T2-weighted MRI and an isointense signal in gadolinium T1-weighted imaging.17,56,57,59,68,105 In the literature, it is also referred that on 3-dimensional reconstruction, CFT can be shown similar to fibromatosis.2,32 Endoscopic imaging may also be used to acquire more precise information about the nature of the tumor according to the anatomical site at which it is located. For example, on endoscopic ultrasound in a case of gastric CFT, the tumor was a hypoechoic mass with hyperechoic foci owing to calcifications.17,68 Laboratory examination proved not to be so useful in CFT diagnosis. The essential tool for certain diagnosis of CFT remains biopsy.
Pathology
Histological Appearance
CFT originates from the subcutaneous and the deep soft tissue and, macroscopically, it is a well-circumscribed, nonencapsulated mass, which has got a wide range of size and diameter (0.1–25 cm) and can infiltrate the surrounding tissues. Microscopically, it is characterized by the presence of hypocellular, densely hyalinized collagen with psammomatous or dystrophic calcifications, a proliferation of (myo)fibroblastic spindle cells, mononuclear inflammatory infiltrate, and lymphoid aggregates such as focal plasmocytes, eosinophils, neutrophils, and mast cell infiltrations. Sometimes, lymph nodes can be found enlarged and positive for CFT.
Immunohistochemical Studies
Immunohistochemical studies are of great significance for the differential diagnosis of CFT. Most scientists agree that fibroblasts in CFT do express vimentin and factor XIIIa, whereas, on the contrary, they do not express desmin, actin, S100, ALK-1, SMA, CD117, CD31, CD99, AE1/AE3, CD32, CD20, CD130, EMA, CD21, bcl-2, estrogen receptor, progesterone receptor, calretinin, β-catanin, CAM5.2, caldesmon, and osteopontin. Furthermore, there are several articles in the English medical literature in which it is reported that CD34, CD68, IgG, and IgG4 can be occasionally focally expressed in mast cells of CFT.99,106 Larson et al66 mentioned that CFT shares certainly some morphologic features with an IgG4-related disease, as they found increased IgG4 in serum of a patient with CFT. Based on the sex predominance of these tumors, we believe that further study should be done on sex hormone receptors.
Treatment
All scientists agree that CFT should be excised when diagnosed. Local excision is the preferred therapeutic approach in CFT. There are 2 types of excision that are recommended in the literature: the open surgical excision and the endoscopic one. It is found that the majority of cases are treated with the classic surgical excision, leading to the conclusion that open surgical excision has a predominant role in treating CFT until today. There are 2 references in the literature presenting endoscopic excision of CFT, including 5 cases of CFT of the stomach.25,71 Moreover, laparoscopic and minimally invasive surgery (video-assisted thoracoscopic surgery) are used in several cases for the excision of the tumor.13,57,59,67,80,86,107–109
Prognosis
In general, the prognosis is excellent in CFTs. No recurrences or metastasis are observed, except for 6 cases of patients who appeared with a recurrence within their follow-up period, including 2 cases of neck CFT in an infant, 2 cases of neck CFT in 3-year-old children, 1 case of small intestine CFT in 43-year-old man, who also had a metastasis, and 1 case of upper extremities CFT in an 1-year-old child.2,10,11,21,28,33,66,81 More than 88% of the patients are cured by the local surgical excision of the tumor. No deaths have been noted owing to CFT and long-term survival of patient seems to be certain.
CONCLUSION
In summary, we conclude that CFT is a benign lesion that is characterized by its wide variety of localizations and should be taken into consideration in the differential diagnosis of an enlarging mass revealed by clinical or imaging examinations either incidentally or after specific acute or chronic symptomatology. The final diagnosis of CFT is made among several similar entities only with histological and immunohistochemical studies, as the tumor is characterized by its specific unique findings in these examinations. Further dedicated studies should be performed for the identification of the exact pathogenesis of the tumor and the evaluation of the age distribution and female predominance of the tumor.
Acknowledgments
We like to express our sincere gratitude to Mr. Panteleimon Pantelidis for his notable contribution to the statistical analysis of this systematic review in SPSS.
Footnotes
Abbreviations: AE1/AE3 = cytokeratin, ALK-1 = anaplastic lymphoma kinase-1, CFT = calcifying fibrous tumor, CT = computed tomography, EMA = epithelial membrane antigen, GIST = gastrointestinal stromal tumor, IMT = inflammatory myofibroblastic tumor, MOC = myositis ossificans circumscripta, MRI = magnetic resonance imaging, MSA = muscle-specific actin, SFT = solitary fibrous tumor, SMA = smooth muscle actin.
The authors report no conflicts of interest.
REFERENCES
- 1.Rosenthal N, Abdul-Karim F. Childhood fibrous tumor with psammoma bodies. Clinicopathologic features in two cases. Arch Pathol Lab Med 1988; 112:798–800. [PubMed] [Google Scholar]
- 2.Fetsch J, Montgomery E, Meis J. Calcifying fibrous pseudotumor. Am J Surg Path 1993; 17:502–508. [DOI] [PubMed] [Google Scholar]
- 3.Pinkard N, Wilson R, Lawless N, et al. Calcifying fibrous pseudotumor of pleura. Am J Clin Pathol 1996; 150:189–194. [DOI] [PubMed] [Google Scholar]
- 4.Jeong HS, Lee GK, Sung R, et al. Calcifying fibrous pseudotumor of mediastinum–a case report. J Korean Med Sci 1997; 12:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dorpe J, Ectors N, Geboes K, et al. Is calcifying fibrous pseudotumor a late sclerosing stage of inflammatory myofibroblastic tumor? Am J Surg Pathol 1999; 23:329–335. [DOI] [PubMed] [Google Scholar]
- 6.Dumont P, de Muret A, Skrobala D, et al. Calcifying fibrous pseudotumor of the mediastinum. Ann Thorac Surg 1997; 63:543–544. [DOI] [PubMed] [Google Scholar]
- 7.Fukunaga M, Kikuchi Y, Endo Y, et al. Case report: calcifying fibrous pseudotumor. Pathol Int 1997; 47:60–63. [DOI] [PubMed] [Google Scholar]
- 8.Kocova I, Michal M, Sulc M, et al. Calcifying fibrous pseudotumour of visceral peritoneum. Histopathology 1997; 31:182–184.Doi:10.1046/j.1365-2559.1997.5860817.x. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell Publishing, Peat J, Barton B. Medical Statistics. A Guide to Data Analysis and Critical Appraisal. 2005; 24–241. [Google Scholar]
- 10.Tseng IT, Chen ST, Huang ZZ, et al. Multiple calcifying fibrous tumors in the small intestine and the mesentery. Formosan J Surg 2012; 45:33–36. [Google Scholar]
- 11.Nascimento AF, Ruiz R, Hornick JL, et al. Calcifying fibrous ’pseudotumor’: clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol 2002; 10:189–196. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery E, Rubin SC, Thomas GM. Fletcher CDM, Unni K, Mertens F, et al. Calcifying fibrous tumor. World Health Organization Classification of Tumours, pathology and genetics of tumours of soft tissue and bone. Lyon, France: IArC Press; 2002. 77–78. [Google Scholar]
- 13.Shibata K, Yuki D, Sakata K. Multiple calcifying fibrous pseudotumors disseminated in the pleura. Ann Thorac Surg 2008; 85:e3–e5. [DOI] [PubMed] [Google Scholar]
- 14.Hammar S, Henderson DW, Klebe S, et al. Dail and Hammar's pulmonary pathology. Neoplastic Lung Cancer 2008; II43:558–743. [Google Scholar]
- 15.Hoffmann H, Beaver ME, Maillard AA. Calcifying fibrous pseudotumor of the neck. Arch Pathol Lab Med 2000; 124:435–437. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Izhak O, Itin L, Feuchtwanger Z, et al. Calcifying fibrous pseudotumor of mesentery presenting with acute peritonitis. Case report with immunohistochemical study and review of literature. Int J Surg Pathol 2001; 9:249–253. [DOI] [PubMed] [Google Scholar]
- 17.Elpek G, Küpesiz G, Ögüs M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol Int 2006; 56:227–231. [DOI] [PubMed] [Google Scholar]
- 18.Isaka M, Nakagawa K, Maniwa T, et al. Disseminated calcifying tumor of the pleura: review of the literature and a case report with immunohistochemical study of its histogenesis. Gen Thorac Cardiovasc Surg 2011; 59:579–582. [DOI] [PubMed] [Google Scholar]
- 19.Ishida M, Okabe H. Disseminated calcifying tumor of the pleura. Pathol Int 2013; 63:333–335. [DOI] [PubMed] [Google Scholar]
- 20.Im S, Jung JH, Yoo C, et al. Calcifying fibrous tumor presenting as rectal submucosal tumor: first case reported in rectum. World J Surg Oncol 2014; 3:12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen KT. Familial peritoneal multifocal calcifying fibrous tumor. Am J Clin Pathol 2003; 119:811–815. [DOI] [PubMed] [Google Scholar]
- 22.Pomplun S, Goldstraw P, Davies SE, et al. Calcifying fibrous pseudotumour arising within an inflammatory pseudotumour: evidence of progression from one lesion to the other? Histopathology 2000; 37:380–382. [DOI] [PubMed] [Google Scholar]
- 23.Lee D, Suh YL, Lee SK. Calcifying fibrous pseudotumour arising in a gastric inflammatory myofibroblastic tumor. Pathology 2006; 38:588–591. [PubMed] [Google Scholar]
- 24.Valladolid G, Weisenberg E, Sundaresan R, et al. Calcifying fibrous tumor of the small intestine associated with Castleman-like lymphadenopathy. J Gastrointest Surg 2014; 18:1205–1208. [DOI] [PubMed] [Google Scholar]
- 25.Ogasawara N, Izawa S, Mizuno M, et al. Gastric calcifying fibrous tumor removed by endoscopic submucosal dissection. World J Gastrointest Endosc 2013; 5:457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agaimy A, Bihl M, Tornillo L, et al. Calcifying fibrous tumor of the stomach: clinicopathologic and molecular study of seven cases with literature review and reappraisal of histogenesis. Am J Surg Pathol 2010; 34:271–278. [DOI] [PubMed] [Google Scholar]
- 27.Azam F, Chatterjee M, Kelly S, et al. Multifocal calcifying fibrous tumor at six sites in one patient: a case report. World J Surg Oncol 2014; 12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill KA, Gonzalez-Crussi F, Omeroglu A, et al. Calcifying fibrous pseudotumor involving the neck of a five-week-old infant. Presence of factor XIIIa in the lesional cells. Pathol Res Pract 2000; 196:527–531. [DOI] [PubMed] [Google Scholar]
- 29.Gil-Albarova J1, Gómez-Palacio VE, Fuertes-Zarate A, et al. Benign calcifying fibrous-myofibroblastic tumor mimicking myositis ossificans in a 22-month-old girl. J Pediatr Surg 2011; 46:E5–E8. [DOI] [PubMed] [Google Scholar]
- 30.Hainaut P, Lesage V, Weynand B, et al. Calcifying fibrous pseudotumor (CFPT): a patient presenting with multiple pleural lesions. Acta Clin Belg 1999; 54:162–164. [DOI] [PubMed] [Google Scholar]
- 31.Cao KX1, Rosenberg AE, Hakim J, et al. Axillary calcifying fibrous tumor (CFT) in an 8 year old girl. J Pediatr Surg 2012; 47:2341–2344. [DOI] [PubMed] [Google Scholar]
- 32.Gamsizkan M1, Yildirim C, Daş K, et al. Calcifying fibrous tumor: a case report. Turk Patoloji Derg 2015; 31:141–144. [DOI] [PubMed] [Google Scholar]
- 33.Maeda A, Kawabata K, Kusuzaki K. Rapid recurrence of calcifying fibrous pseudotumor (a case report). Anticancer Res 2002; 22:1795–1797. [PubMed] [Google Scholar]
- 34.Bell DM, Dekmezian RH, Husain SA, et al. Oral calcifying fibrous pseudotumor: case analysis and review. Head Neck Pathol 2008; 2:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Rosa MR, de Oliveira JM, Dias-Ribeiro E, et al. Large calcifying epithelial odontogenic tumor with extension into the maxillary sinus: a case report. Gen Dent 2011; 59:e38–e40. [PubMed] [Google Scholar]
- 36.Li C, Men Y, Gao N, et al. Calcifying fibrous pseudotumour of the tongue: report of a rare case. Br J Oral Maxillofac Surg 2014; 52:974–976. [DOI] [PubMed] [Google Scholar]
- 37.Lee SW, Yeh HZ, Chang CS. Calcifying fibrous pseudotumor of the esophagus. J Chin Med Assoc 2010; 73:599–601. [DOI] [PubMed] [Google Scholar]
- 38.Nathoo N, Viloria A, Iwenofu OH, et al. Calcifying fibrous tumor of the spine. World Neurosurg 2012; 77:592. [DOI] [PubMed] [Google Scholar]
- 39.Ammar A, El Hammami S, Horchani H, et al. Calcifying fibrous pseudotumor of the pleura: a rare location. Ann Thorac Surg 2003; 76:2081–2082. [DOI] [PubMed] [Google Scholar]
- 40.Rudez I1, Legac A, Baric D, et al. Asymptomatic calcifying fibrous pseudotumor compressing heart cavities. Ann Thorac Surg 2011; 91:291–293. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Zhang W, Wang X. Calcifying fibrous tumour originating from the right cardiac ventricle in a child. Cardiol Young 2014; 24:161–163. [DOI] [PubMed] [Google Scholar]
- 42.Soyer T, Ciftci AO, Güçer S, et al. Calcifying fibrous pseudotumor of lung: a previously unreported entity. J Pediatr Surg 2004; 39:1729–1730. [DOI] [PubMed] [Google Scholar]
- 43.Mangat A, Schiller C, Mengoni P, et al. Calcifying fibrous pseudotumor of the breast. Breast J 2009; 15:299–301. [DOI] [PubMed] [Google Scholar]
- 44.Khoury J, Jerushalmi J, Naser E, et al. Calcifying fibrous pseudotumor. A rare benign tumor detected on bone scintography. Clin Nucl Med 2002; 27:799–800. [DOI] [PubMed] [Google Scholar]
- 45.Beg MH, Khan SA, Ahmad R. Calcifying fibrous pseudo tumor arising from adrenal gland- a rare entity. Indian J Thorac Cardiovasc Surg 2010; 26:273–274. [Google Scholar]
- 46.Bobbio A, MAzzeo A, Carbognani P, et al. An unusual case of calcifying fibrous pseudotumor of the cervicothoracic junction. Eur J Cardiothorac Surg 2008; 34:1123–1125. [DOI] [PubMed] [Google Scholar]
- 47.Mito K, Kashima K, Daa T, et al. Multiple calcifying fibrous tumors of the pleura. Virchows Arch 2005; 446:78–81. [DOI] [PubMed] [Google Scholar]
- 48.Sleigh KA, Lai W, Keen CE, et al. Calcifying fibrous pseudotumours: an unusual case with multiple pleural and mediastinal lesions. Interact Cardiovasc Thorac Surg 2010; 10:652–653. [DOI] [PubMed] [Google Scholar]
- 49.Chauhan KR, Shah HU, Trivedi PP, et al. Calcifying fibrous pseudotumor of the mediastinum: a rare case report. Indian J Pathol Microbiol 2014; 57:155–156. [DOI] [PubMed] [Google Scholar]
- 50.Vasilakaki T, Skafida E, Tsavaria A, et al. Gastric calcifying fibrous tumor: a very rare case report. Case Rep Oncol 2012; 5:455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wesecki M, Radziuk D, Niemiec S, et al. Calcifying fibrous tumor of the small bowel mesentery in a 27-year old male patient - case report. Pol Przegl Chir 2014; 86:436–439. [DOI] [PubMed] [Google Scholar]
- 52.Lee JC, Lien HC, Hsiao CH. Coexisting sclerosing angiomatoid nodular transformation of the spleen with multiple calcifying fibrous pseudotumors in a patient. J Formos Med Assoc 2007; 106:234–239. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Yan L, Yang J, et al. Obstructive jaundice secondary to haemobilia from calcifying fibrous pseudotumor. Dig Liver Dis 2011; 43:e17. [DOI] [PubMed] [Google Scholar]
- 54.Emanuel P, Qin L, Harpaz N. Calcifying fibrous tumor of small intestine. Ann Diagn Pathol 2008; 12:138–141. [DOI] [PubMed] [Google Scholar]
- 55.Lau SK, Weiss LM. Calcifying fibrous tumor of the adrenal gland. Hum Pathol 2007; 38:656–659. [DOI] [PubMed] [Google Scholar]
- 56.Sudhakar S, Mistry Y, Dastidar A, et al. Calcifying fibrous tumour: an unusual omental lesion. Pediatr Radiol 2008; 38:1246–1248. [DOI] [PubMed] [Google Scholar]
- 57.Attila T, Chen D, Gardiner GW, et al. Gastric calcifying fibrous tumor. Can J Gastroenterol 2006; 20:487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim DH, Min KW, Kim DH, et al. Traumatic bowel perforation and inguinal hernia masking a mesenteric calcifying fibrous tumor. J Pathol Transl Med 2015; 49:267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puccio F, Solazzo M, Marciano P, et al. Laparoscopic resection of calcifying fibrous pseudotumor of the gastric wall. A unique case report. Surg Endosc 2001; 15:1227. [DOI] [PubMed] [Google Scholar]
- 60.Konstantakos AK, Shuck JM. Calcifying fibrous pseudotumor of the anterior parietal peritoneum: treatment by laparoscopic resection. Surgery 2005; 137:257–259. [DOI] [PubMed] [Google Scholar]
- 61.Suh JH, Shin OR, Kim YH. Multiple calcifying fibrous pseudotumor of the pleura. J Thorac Oncol 2008; 3:1356–1358. [DOI] [PubMed] [Google Scholar]
- 62.Ellouze S, Chaari C, Gouiaa N, et al. Tumeur Fibreuse Calcifiante de l’ intestin grêle. Ann Pathol 2010; 30:409–410. [DOI] [PubMed] [Google Scholar]
- 63.Larousserie F. Tumeurs et pseudotumeurs digestives dont on parle rarement. Cas N° 2 : Tumeur fibreuse calcifiante. Ann Pathol 2013; 33:255–258. [DOI] [PubMed] [Google Scholar]
- 64.Bajpai J, Rekhi B, Iyer K, et al. Multifocal calcifying fibrous tumor of the mesentery: an unusual case report with literature review and therapeutic implications. J Cancer Res Ther 2011; 7:500–502. [DOI] [PubMed] [Google Scholar]
- 65.Fan SF, Yang H, Li Z, et al. Gastric calcifying fibrous pseudotumour associated with an ulcer: report of one case with a literature review. Br J Radiol 2010; 3:e188–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larson BK, Balzer B, Goldwasser J, et al. Calcifying fibrous tumor: an unrecognized IgG4–related disease? APMIS 2015; 123:72–76. [DOI] [PubMed] [Google Scholar]
- 67.Nair N, Chen F, Klimstra D, et al. Multiple calcifying fibrous tumors: an incidental finding. Surgery 2011; 150:568–569. [DOI] [PubMed] [Google Scholar]
- 68.Delbecque K, Legrand M, Boniver J, et al. Calcifying fibrous tumour of the gastric wall. Histopathology 2004; 44:399–400. [DOI] [PubMed] [Google Scholar]
- 69.Farah RB, Dimet S, Bidault AT, et al. Multiple peritoneal calcifying fibrous tumors revealed by ischemic colitis. Ann Diagn Pathol 2007; 6:460–463. [DOI] [PubMed] [Google Scholar]
- 70.Mourra N, Bell S, Parc R, et al. Calcifying fibrous pseudotumour: first case report in the gallbladder. Histopathology 2004; 44:84–86. [DOI] [PubMed] [Google Scholar]
- 71.Shi Q, Xu M, Zhong Y, et al. The laparoscopic-endoscopic cooperative surgery for the colonic calcifying fibrous tumor: one case report. J Laparoendosc Adv Surg Tech A 2012; 22:996–999. [DOI] [PubMed] [Google Scholar]
- 72.Gatt N, Falzon S, Ratynska M. Multifocal peritoneal calcifying fibrous tumour: incidental finding at cholecystectomy. BMJ Case Rep 2011. 2011.doi: 10.1136/bcr.05.2011.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang HH, Chai CY, Lin YH, et al. Jejunal and multiple mesenteric calcifying fibrous pseudotumor induced jejunojejunal intussusception. J Formos Med Assoc 2007; 106:485–489. [DOI] [PubMed] [Google Scholar]
- 74.Lynnhtun K, Achan A, Lam V. IgG4 related pseudotumor (calcifying fibrous tumor) of adrenal gland. Pathology 2013; 45:519–521. [DOI] [PubMed] [Google Scholar]
- 75.Senol S, Ayşe1 BC, Zemheri E, et al. Calcifying fibrous tumor of the large intestine. Indian J Pathol Microbiol 2015; 58:122–123. [DOI] [PubMed] [Google Scholar]
- 76.Peng J, Lin J, Ye L, et al. Gastric calcifying fibrous pseudotumor: report of two cases and review of literature. Chinese J Gastroenterol 2014; 19:703–704. [Google Scholar]
- 77.Murdock T, Lee A, Wilcox R. An unusual cause of upper gastrointestinal bleeding. Clin Gastroenterol Hepatol 2014; 12:e95–e96. [DOI] [PubMed] [Google Scholar]
- 78.Abbadessa B, Narang R, Mehta R, et al. Laparoscopic resection of a gastric calcifying fibrous pseudotumor presenting with ulceration and hematemesis in a teenage patient: A case report and review of the literature. J Surg Radiol 2012; 3:44–47. [Google Scholar]
- 79.Sigel JE, Smith TA, Reith JD, et al. Immunohistochemical analysis of anaplastic lymphoma kinase expression in deep soft tissue calcifying fibrous pseudotumor: evidence of a late sclerosing stage of inflammatory myofibroblastic tumor? Ann Diagn Pathol 2001; 5:10–14. [DOI] [PubMed] [Google Scholar]
- 80.Ağaçkıran Y, Fındık G, Aydoğdu K, et al. An extremely rare case of multiple calcifying tumor of the pleura. Tuberk Toraks 2012; 60:385–388. [PubMed] [Google Scholar]
- 81.Jang KS, Oh YH, Han HX, et al. Calcifying fibrous pseudotumor of the pleura. Ann Thorac Surg 2004; 78:e87–88. [DOI] [PubMed] [Google Scholar]
- 82.Cavazza A, Gelli MC, Agostini L, et al. Calcifying fibrous pseudotumor of pleura: a case report. Pathologica 2002; 94:201–205. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein EB, Savel RH, Sen F, et al. Calcifying fibrous pseudotumor of the neck: diagnostic challenges of a rare benign lesion. Am Surg 2005; 71:1051–1054. [PubMed] [Google Scholar]
- 84.Özkan S, Demirağ F, Yekeler E, et al. Calcifying fibrous tumor of the lungs. Turk J Med Sci 2014; 44:901–903. [DOI] [PubMed] [Google Scholar]
- 85.Peachell M, Mayo J, Kalloger S, et al. Calcifying fibrous pseudotumour of the lung. Thorax 2003; 58:1018–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawahara K, Yasukawa M, Nakagawa K, et al. Multiple calcifying fibrous tumor of the pleura. Virchows Arch 2005; 447:1007–1008. [DOI] [PubMed] [Google Scholar]
- 87.George SA, Abdeen S. Gastric calcifying tumor resembling gastrointestinal stromal tumor: A case report. Iranian J Pathol 2015; 10:306–309. [PMC free article] [PubMed] [Google Scholar]
- 88.Sato S, Ooike N, Yamamoto T, et al. Rare gastric calcifying fibrous pseudotumor removed by endoscopic submucosal dissection. Digest Endosc 2008; 20:84–86. [Google Scholar]
- 89.Voltaggio L, Montgomery E. Gastric mesenchymal lesions other than gastrointestinal stromal tumor. Diagnost Histopathol 2014; 20:228–238. [Google Scholar]
- 90.Giardino AA, Ramaiya NH, Shinagare AB, et al. Case report: Calcifying fibrous tumor presenting as an asymptomatic pelvic mass. Indian J Radiol Imaging 2011; 21:306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eftekhari F, Ater J, Ayala A, et al. Calcifying fibrous pseudotumour of the adrenal gland. Br J Radiol 2001; 74:452–454. [DOI] [PubMed] [Google Scholar]
- 92.Xiao P, Peng JJ, Li Y, et al. Unusual calcifying fibrous tumor in the fallopian tube: review and discussion of the differential diagnosis. Int J Gynecol Pathol 2014; 33:40–44. [DOI] [PubMed] [Google Scholar]
- 93.Bellver Oliver M, Arredondo Chaves J, Queipo Gutierrez P, et al. Calcifying cystic fibrous tumour. A rare form of benign peritoneal carcinomatosis. Cir Esp 2013; 91:338–339. [DOI] [PubMed] [Google Scholar]
- 94.Jain A, Maheshwari V, Alam K, et al. Calcifying fibrous pseudotumor of peritoneum. J Postgrad Med 2007; 53:189–190. [DOI] [PubMed] [Google Scholar]
- 95.Shigematsu H, Sano Y, Kasahara S, et al. Calcifying fibrous tumor arising from the heart. J Thorac Cardiovasc Surg 2006; 132:e21–e22. [DOI] [PubMed] [Google Scholar]
- 96.Jiang K, Nie J, Wang J, et al. Multiple calcifying fibrous pseudotumor of the bilateral pleura. Jpn J Clin Oncol 2011; 41:130–133. [DOI] [PubMed] [Google Scholar]
- 97.Shimizu S, Funakoshi Y, Yoon HE, et al. Small calcifying fibrous pseudotumor of the heart confined to the epicardium. Cardiovasc Pathol 2015; 24:191–193. [DOI] [PubMed] [Google Scholar]
- 98.Jaiswal SS, Agrawal A, Sahai K, et al. Large retroperitoneal calcifying fibrous tumor. Med J Armed Forces India 2013; 69:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill K, Gonzalez-Crussi F, Chou P. Calcifying fibrous pseudotumor versus inflammatory myofibroblastic tumor: a histological and immunohistochemical comparison. Mod Pathol 2001; 14:784–790. [DOI] [PubMed] [Google Scholar]
- 100.Dehner LP, Coffin CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol 1998; 15:161–173. [PubMed] [Google Scholar]
- 101.Yan J, Hao F, Ye Q-Y, et al. Calcifying fibrous tumor: a case report. J Clin Dermatol 2009; 38:451–452. [Google Scholar]
- 102.Shinohara N, Nagano S, Yokouchi M, et al. Bilobular calcifying fibrous pseudotumor in soleus muscle: a case report. J Med Case Rep 2011; 5:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Z, Guo J, Ren W, et al. A gastric calcifying fibrous pseudotumor detected by transabdominal ultrasound after oral administration of an echoic cellulose-based gastrointestinal ultrasound contrast agent. Ltraschall in Med 2014; 35:181–183. [DOI] [PubMed] [Google Scholar]
- 104.Nobili C, Rosso E, Oussoultzoglou E, et al. Image of the month. Hepatic calcifying fibrous pseudotumor. Arch Surg 2011; 146:237–238. [DOI] [PubMed] [Google Scholar]
- 105.Chatelain D1, Lauzanne P, Yzet T, et al. La pseudotumeur fibreuse calcifiante gastrique, une tumeue rare de l’ estomac. Gastroenterol Clin Biol 2008; 32 (5 pt 1):441–444. [DOI] [PubMed] [Google Scholar]
- 106.Weynand B, Draguet A-P, Bernard P, et al. Calcifying fibrous pseudotumour: first case report in the peritoneum with immunostaining for CD34. HIstopathology 1999; 34:86–87. [DOI] [PubMed] [Google Scholar]
- 107.Jang KY, Park HS, Moon WS, et al. Calcifying fibrous tumor of the stomach: a case report. J Korean Surg Soc 2012; 83:56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi Q, Xu MD, Chen T, et al. Endoscopic diagnosis and treatment of calcifying fibrous tumors. Turk J Gastroenterol 2014; 25 suppl 1:153–156. [DOI] [PubMed] [Google Scholar]
- 109.Abbadessa B, Narang R, Mehta R, et al. Laparoscopic resection of a gastric calcifying fibrous pseudotumor presenting with ulceration and hematemesis in a teenage patient. J Surg Radiol 2013; 4:48–51. [Google Scholar]


