Abstract
The purpose of this study is to compare the clinical features and treatment outcomes among patients with bacteremic urinary tract infection (UTI) caused by multidrug-resistant (MDR) and non-MDR Enterobacteriaceae and to identify whether MDR pathogens were independently associated with severe sepsis or septic shock at presentation.
The clinical data of adult patients visiting and being treated at Chia-Yi Christian Hospital due to bacteremic UTI caused by Enterobacteriaceae from January 2006 to August 2015 were retrospectively analyzed.
A total of 585 patients were enrolled. Among them, 220 (37.6%) were caused by the MDR Enterobacteriaceae. A total of 206 patients (35.2%) developed severe sepsis or septic shock at presentation. Patients in the MDR group tend to be male and have a past history of gout, recurrent UTI, prior hospitalization, hydronephrosis, renal stone, ureteral stone, indwelling urinary catheter, newly development of renal dysfunction, severe sepsis or septic shock, intensive care unit (ICU) admission, receipt of ineffective empirical therapy, longer hospital stay, and higher in-hospital mortality (2.7% vs 1.9%, P = 0.569). Using multivariate logistic regression analysis, it is revealed that independent predictors associated with severe sepsis or septic shock at presentation were liver cirrhosis (OR 2.868; 95% CI 1.439–5.716; P = 0.003), indwelling urinary catheter (OR 1.936; 95% CI 1.238–3.027; P = 0.004), and MDR Enterobacteriaceae (OR 1.447; 95% CI 1.002–2.090; P = 0.049).
Multidrug resistance was associated with the development of severe sepsis or septic shock upon presentation among patients with bacteremic UTI caused by Enterobacteriaceae. Therefore, empirical antibiotics therapy for patients with UTI presented with severe sepsis and/or septic shock should be more broad-spectrum to effectively cover MDR Enterobacteriaceae.
INTRODUCTION
Urinary tract infection (UTI) is one of the most frequently encountered bacterial infections presenting to hospitals and up to 60% of women will experience at least 1 episode of symptomatic UTI during their lifespan in the United States.1 Positive blood cultures at the time of presentation were reported among 15% to 25% of UTI episodes,2–4 but more than 40% of patients with complicated acute pyelonephritis developed bacteremia in Taiwan.5 In general, bacteremia is associated with severe sepsis or septic shock (hereafter using “critical sepsis” to stand for both severe sepsis and septic shock), and around 26% to 33% of patients with bacteremic UTI presented with critical sepsis at presentation.6,7 Of special concerns, the case fatality rate (CFR) of patients who developed UTI concurrently with critical sepsis may be up to 30% to 50%.8 Facing patients developing severe acute bacterial infections, it is recommended that empirical therapy should effectively cover over 80% to 90% possible pathogens to ensure a better outcome.9 It has been argued that drug-resistant bacteria might play an important role in the severity of initial presentations or clinical outcomes of UTI. Some studies demonstrated that drug resistance did not influence the severity of infection at presentation.7,10 However, other studies did disclose a positive correlation.11,12 If drug resistance does matter, it would lead to an additional concern on the selection of empirical therapy for patients with severe UTI. The aim of this study is to compare the clinical characteristics of patients with bacteremic UTI caused by multidrug-resistant (MDR) and non-MDR Enterobacteriaceae, and to identify whether MDR uropathogens were one of the associated factors with critical sepsis at presentation in these patients.
MATERIALS AND METHODS
Study Design and Settings
This retrospective study was conducted in Chia-Yi Christian Hospital (CYCH), a 1000-bed regional teaching hospital in southern Taiwan. The list of adult patients (aged 18 years or more) receiving treatment at CYCH between January 2006 and August 2015 due to bacteremic UTI was retrieved first from the hospital's databank. The inclusion criteria in this study were: at least one of the following urinary tract symptoms, including dysuria, suprapubic tenderness, flank pain, or fever; bacterial growth ≧105 colony-forming units (cfu)/mL in midstream urine or ≧103 cfu/mL in a single catheter urine specimen; and isolation of the same Enterobacteriaceae from both urine and blood culture.13 UTI concurrently combined with other systemic infections, including pulmonary infection, pressure sores, cellulitis, enterocolitis, liver abscess, peritonitis, osteomyelitis, biliary tract infection, pelvic inflammatory disease, pulmonary tuberculosis, catheter-related infection, patients on dialysis therapy, and other diseases, were excluded. Finally, only those patients with monoclonal UTI and bacteremia caused by Enterobacteriaceae without other infectious sources identified by clinicians were enrolled. A part of patients (from 2006 to August 2012) enrolled by the present study has also been included in a previous study to investigate the risk factors for septic shock.14 In that study, the information and effect of MDR pathogens were not collected and analyzed, and also the outcome of interest was different. A standardized case report form was used to collect the following clinical information: age, gender, underlying diseases, urological history, clinical manifestations and initial laboratory data at presentation, causative microorganisms, antimicrobial resistance patterns, antibiotic treatment, length of hospital stay, and in-hospital mortality. Comorbidities were recorded according to the score proposed by Charlson et al.15 The severity of bacteremic UTI was assessed when the positive blood culture was also evaluated by using the Pitt bacteremia score.16
Definitions
Presence of 2 or more of the followings, including fever or hypothermia, tachycardia, tachypnea, and leukocytosis or leukopenia, was considered as sepsis.17 Severe sepsis was defined as sepsis associated with at least 1 organ dysfunctions or tissue hypoperfusion, such as renal dysfunction (serum creatinine >2 mg/dL in patients without prior renal insufficiency or an increase of 0.5 mg/dL of basal creatinine levels in patients with chronic renal insufficiency), hematologic dysfunction (platelet count <105/μL), and central nervous system dysfunction (deterioration in consciousness level).18 Septic shock was defined as either systolic blood pressure ≤90 mm Hg or mean arterial pressure ≤70 mm Hg. Leukocytosis was defined as a white blood cell count ≥12 K/μL, thrombocytopenia as a platelet count ≤105/μL, and a markedly elevated C-reactive protein (CRP) level as >10 mg/dL. Infections of the ureter or kidney, including renal abscess demonstrated by renal image or acute pyelonephritis, were classified as upper UTI and others were categorized into lower UTI.19 History of recurrent UTI was described as one or more episode in the past prior to the current hospitalization. Empirical therapy was defined as antibiotics given before the blood culture and its susceptibility testing results were available whereas definitive therapy was defined as effective therapy prescribed according to the results of final blood cultures and susceptibility testing. Empirical therapy was further classified into 2 groups: effective empirical therapy and ineffective empirical therapy. The former was defined as empirical antibiotics prescribed being actively against the causative pathogen according to in vitro susceptibility testing; the latter was defined by empirical antibiotics being ineffectively against it. Imaging study included renal echography, intravenous pyelography, and computerized tomography scans. The study was approved by the ethics review boards of the hospital.
Microbiologic Studies
All bacterial isolates from urine and blood samples were identified using automated Vitek version 2.0 (Biomerieux, France). The antimicrobial susceptibilities, including ampicillin/sulbactam, piperacillin/tazobactam (TZP), cefazolin, cefuroxime, cefotaxime, cefepime, ertapenem, meropenem, levofloxacin, gentamicin, amikacin, and trimethoprim/sulfamethoxazole, were determined using disk diffusion method according to the recommendation of the Clinical and Laboratory Standards Institute20 and the results were interpreted using the criteria suggested by Clinical and Laboratory Standards Institute.21 MDR strains were defined as isolates resistant to 3 or more antibiotic classes among tested antimicrobial agents according to international expert recommendations.22
Statistical Analysis
Continuous variables were described as means and standard deviations, and analyzed using a Mann–Whitney U test. Categorical variables were expressed as frequency and proportions and compared with Chi-square test or Fisher exact test if the expected number was less than or equal to 10. All tests were 2-tailed and a P-value <0.05 was considered statistically significant. Multivariate logistic regression analysis was performed using the backward stepwise method to identify the independent associated factors for critical sepsis. Variables with P < 0.2 in univariate analysis were included in the multivariate model to control for confounding. Statistical analyses were performed using the SPSS 17.0 software (International Business Machines Corp., Armonk, NY).
RESULTS
Study Population and Microbiological Results
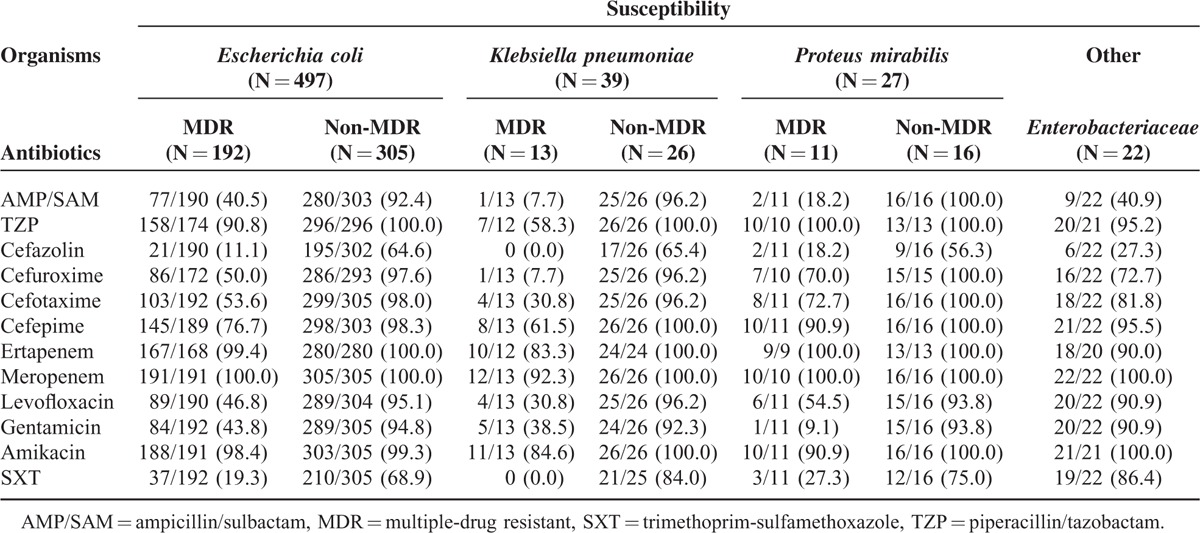
Initially, a total of 2553 patients with the diagnosis of UTI were recruited, but 377 patients among them were excluded because they had other systemic infections concurrently. Of 2176 cases with only UTI, 1561 patients without bacteremic UTI were excluded subsequently. Later 615 adult patients with bacteremic UTI were screened during the study period, and 30 of them were excluded because the isolates did not belong to Enterobacteriaceae family. Finally, 585 patients were enrolled in this study, and 220 (37.6%) were infected with MDR Enterobacteriaceae (Figure 1). Among these patients, around three-fourths were female and the mean ± SD of age was 69 ± 15 years (Table 1). The 3 leading comorbidities were hypertension, diabetes mellitus, and cerebrovascular accident. Among the 408 patients undergoing image studies, 112 patients were found to have hydronephrosis. Approximately 20% of these patients developed renal dysfunction due to the UTI episodes. Also, about 20% of these patients had indwelled urinary catheter on presentation. Critical sepsis at presentation was identified in 206 (35.2%) patients: 166 with severe sepsis and 40 with septic shock. Ninety-nine (16.9%) patients were admitted to intensive care unit (ICU) because of the UTI episodes. Among all of these Enterobacteriaceae isolates, Escherichia coli were the most common microorganism (497/585, 85.0%). Other uropathogens included Klebsiella pneumoniae (39 patients), Proteus mirabilis (27), Citrobacter sp (8), Enterobacter sp (8), Serratia marcescens (5), and Providencia rettgeri (1). To sum up, the in vitro susceptibilities to ampicillin/sulbactam, TZP, cefazolin, cefuroxime, cefotaxime, cefepime, ertapenem, meropenem, levofloxacin, gentamicin, amikacin, and trimethoprim/sulfamethoxazole were 72.3%, 96.0%, 45.6%, 79.5%, 79.7%, 90.5%, 99.0%, 99.8%, 77.0%, 74.9%, 98.6%, and 51.7%, respectively. The detailed resistance patterns stratified by different microorganisms are shown in Table 2. A total of 432 patients (75.1%; data available only in 575 patients) received effective empirical therapy, and the mean ± SD of hospital stay was 10 ± 5.79 days. Overall, the crude in-hospital mortality rate was 2.2%.
FIGURE 1.
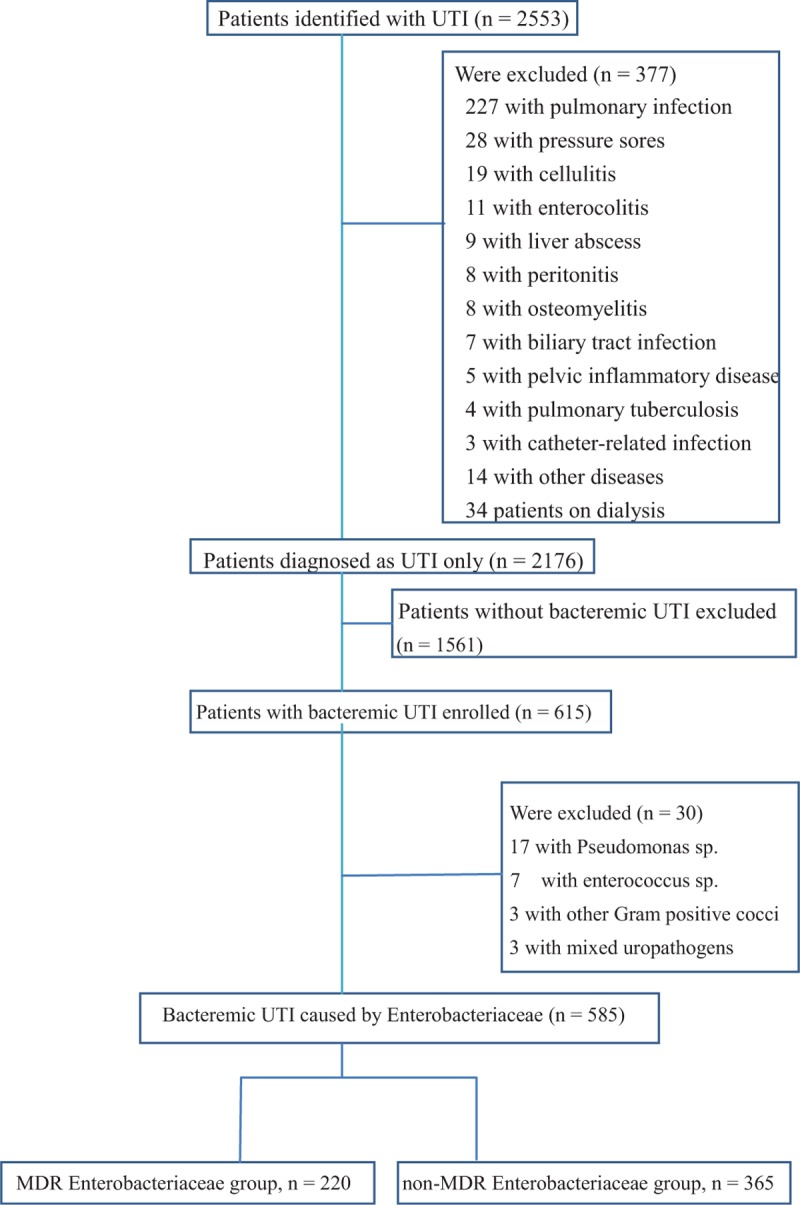
Flow chart of bacteremic urinary tract infection included in the analysis.
TABLE 1.
Clinical Characteristics of MDR Enterobacteriaceae and Non-MDR Enterobacteriaceae in Patients With Bacteremic Urinary Tract Infection
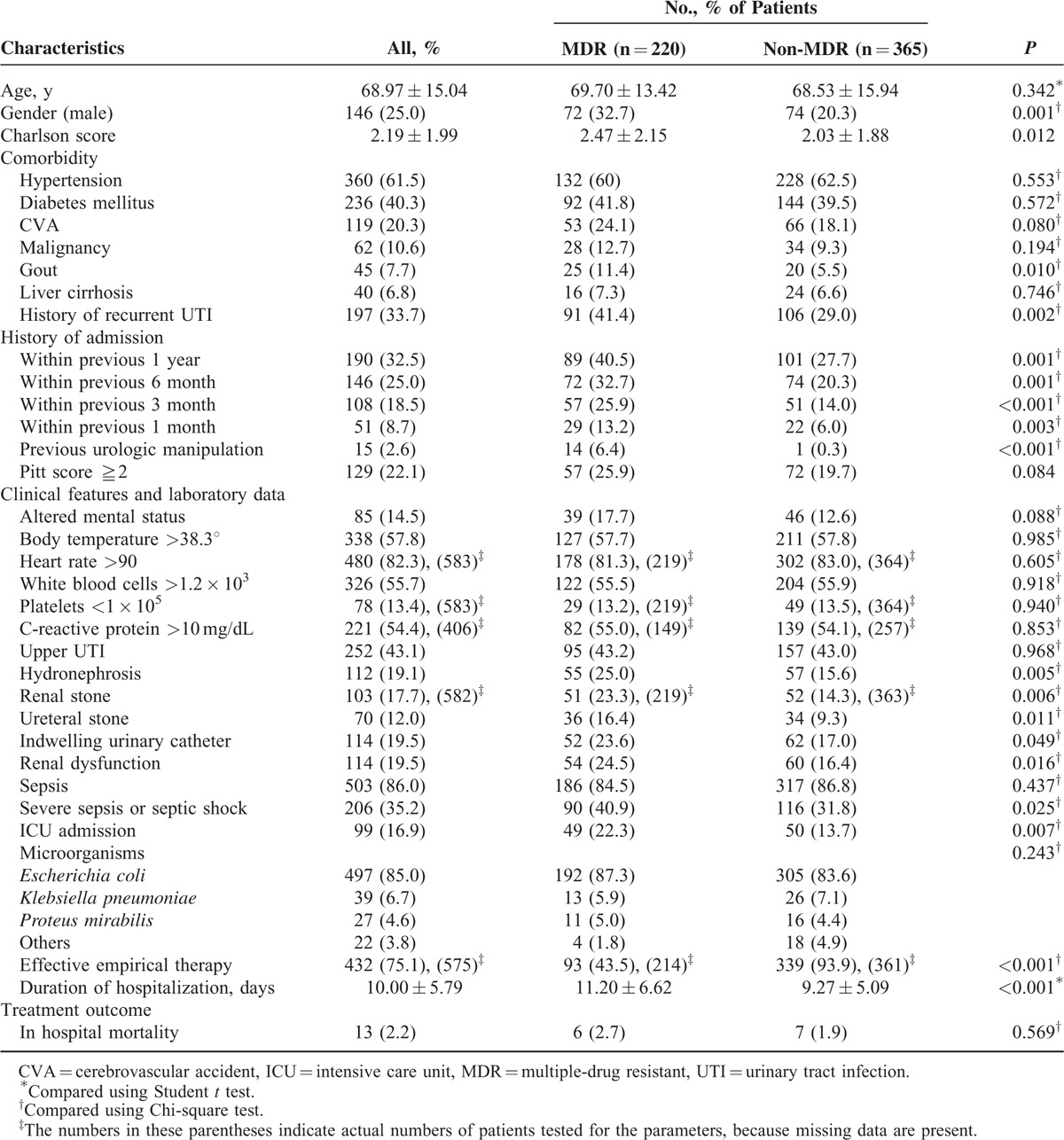
TABLE 2.
Distribution of Uropathogens and Antimicrobial Susceptibility in Patients With Bacteremic Urinary Tract Infection Caused by Enterobacteriaceae
Comparison of Clinical Characteristics of MDR and Non-MDR Bacteremic UTI Patients
The comparison of clinical, laboratory, and microbiological variables between these 2 groups are also demonstrated in Table 1. The mean age is not different between the 2 groups but the patients in the MDR group had a tendency toward being male gender. Higher Charlson comorbidities index score was observed in MDR group. Patients in the MDR group were also more likely to have a past history of gout, recurrent UTI, prior hospitalization, and previous invasive urologic procedure within 30 days.
As for clinical manifestations and laboratory data, more patients in the MDR group presented with consciousness change but without statistical significance (P = 0.088). Laboratory parameters indicating severe diseases such as leukocytosis, thrombocytopenia, and markedly elevated CRP level did not differ between these 2 groups. However, hydronephrosis, urolithiasis including renal and ureteral stone, and presence of indwelling urinary catheter were much more frequently observed in MDR patients. Furthermore, patients with MDR Enterobacteriaceae were more likely to develop renal dysfunction, critical sepsis (40.9% vs 31.8%, P = 0.025), and to be admitted to ICU. E coli, K pneumoniae, and P. mirabilis were the most frequently isolated causative microorganisms in both groups without significant differences between them.
Concerning treatment, significantly much smaller portion of patients in the MDR group were prescribed with effective empirical therapy (43.5% vs 93.9%, P < 0.001), and the hospital stay was longer for patients of the MDR group compared with those of the non-MDR group (mean 11.20 vs 9.27 days, P < 0.001). The in-hospital mortality was slightly higher in the MDR patients than the non-MDR patients without significant difference (2.7% vs 1.9%, P = 0.569). Among these mortality cases, 30.8% (4/13) did not receive effective empirical therapy, and all the 4 patients belonged to the MDR group (4/6 vs 0/7, P = 0.021).
Factors Related to Critical Sepsis in Patients With Bacteremic UTI
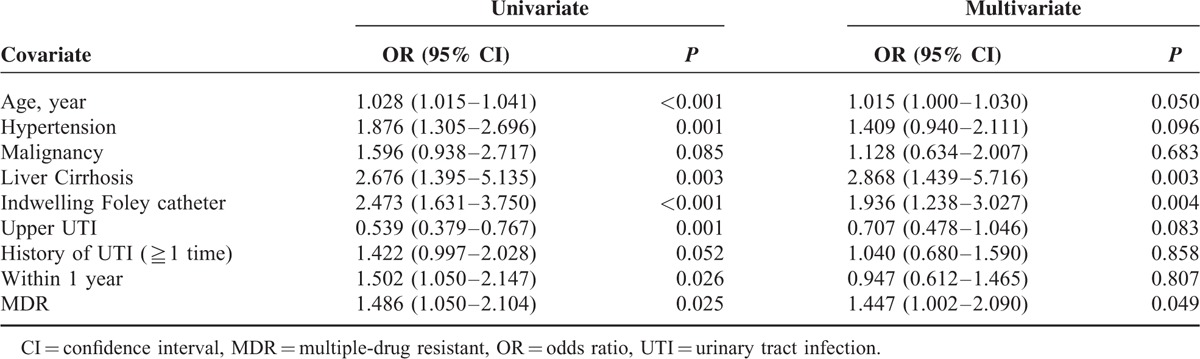
In order to identify whether MDR Enterobacteriaceae was one of the associated factors for critical sepsis in patients with bacteremic UTI, logistic regression analysis was performed using potential factors predicted by urivariate analysis (Table 3). Age, hypertension, liver cirrhosis, indwelling urinary catheter, history of prior admission within 1 year, upper UTI, and MDR Enterobacteriaceae were all associated with critical sepsis in univariate analysis (Table 3). Multivariate analysis revealed that liver cirrhosis (OR 2.868; 95% CI 1.439–5.716, P = 0.003), indwelling urinary catheter (OR 1.936; 95% CI 1.238–3.027, P = 0.004), and MDR Enterobacteriaceae (OR 1.447; 95% CI 1.002–2.090, P = 0.049) were significant predictors for critical sepsis.
TABLE 3.
Results of the Logistic Regression Model for Factors Related to Critical Sepsis
DISCUSSION
This study was undertaken to compare the clinical characteristics between patients with bacteremic UTI caused by MDR or non-MDR Enterobacteriaceae, and investigate the predictors for critical sepsis on presentation among these patients. We found that patients in the MDR group have distinct clinical manifestation in gender, comorbidities, past histories, urinary tract abnormalities and were associated with worse clinical course, including development of critical sepsis, ICU admission, longer hospital stay, and higher in-hospital mortality. Liver cirrhosis, presence of indwelling urinary catheter, and MDR Enterobacteriaceae were independently associated with critical sepsis at admission.
Most prior studies23,24 demonstrated that the male gender was more frequently observed in patients with infection caused by drug-resistant pathogens, which supported our findings. Complicated UTIs, including presence of hydronephrosis, urinary tract abnormalities, and need for indwelling urinary catheter due to obstructive uropathy, have been shown to be associated with high drug resistance rates,25,26 which were also demonstrated in our study. Hence, detailed obtainment of urologic history, especially for male patients, would alert clinicians about the possibilities of drug-resistant infections. In terms of clinical features, 1 prior study showed shock on presentation among patients with bloodstream infections was significantly more common in those caused by drug-resistant bacteria.27 Our report also found that patients in the MDR group had a much higher Charlson comorbidities index score and a more severe diseases on initial presentation. Because of more complicated underlying and severe clinical conditions, tendency to develop renal dysfunction and critical sepsis in patients of MDR group was anticipated. Therefore, it seems to be reasonable that ICU admission was also more common in patients with drug-resistant uropathogens, just as reported by Oliveira et al.23 Therefore, for those patients with complex underlying diseases and serious clinical manifestations, clinicians should cautiously consider to use an empirical therapy with coverage of MDR pathogens.
In our study, the major 3 empirical antimicrobial agents prescribed were 1st- or 2nd-generation cephalosporins (39.5%), fluoroquinolones (27.9%), and 3rd-generation cephalosporins (23.6%). Because the susceptible rates of the MDR Enterobacteriaceae in this study to antibiotics belonging to the 3 above-mentioned classes were much less than non-MDR Enterobacteriaceae, the proportion of patients in MDR group receiving effective empirical therapy was therefore much smaller compared with that in non-MDR group. Such a finding has been demonstrated by several other reports.23,28 Good understanding of local epidemiology might be helpful for physicians to administer adequate antibiotics initially, even in the MDR group. The significant difference in hospital stay between the MDR group and the non-MDR group has also been disclosed by previous comparable observations,23,24,29 which might raise the medical costs. The crude in-hospital mortality measured in our study was lower than several prior reports.6,7 This could be due to the fact that less patients presenting with septic shock were enrolled in the present study (7% vs 15%–26%). More importantly, higher in-hospital mortality rate was noted in patients with bacteremic UTI caused by MDR pathogens. These might be partly attributed to that more patients in MDR group received inadequate empirical antibiotic therapy, as suggested by several prior studies.23,24,28,29 Thus, identifying the associated factors of getting MDR pathogens infections and prescribing appropriate empirical antimicrobial agents is critical in reducing hospitalization days and mortality rates.
Several important predictors for critical sepsis at presentation among patients with bacteremic UTI had been identified in our study. These included liver cirrhosis, indwelling urinary catheter, and MDR Enterobacteriaceae. Lee et al6 demonstrated that liver cirrhosis was an independent risk factor for the occurrence of septic shock among patients with bacteremic acute pyelonephritis, which is in agreement with our findings. It is well-understood that impairment of humoral and cell-mediated immunity often occurred in patients with liver cirrhosis.30 Thus, much more severe clinical manifestations were anticipated in cirrhotic patients with bacteremic UTI. Moreover, the presence of indwelling urinary catheter might imply a severe underlying disease (Charlson comorbidity index score, 3.13 ± 2.28 vs 1.97 ± 1.85, P < 0.001) or poor general condition.7,31 Therefore, it was intuitive that presence of indwelling urinary catheter could be an independent factor associated with critical sepsis in bacteremic UTI. In other words, when those cirrhotic patients with indwelling urinary catheters were admitted due to UTI, it is important to consider following the guidelines of surviving sepsis campaign, as they are at risk of developing critical sepsis.
The debate whether MDR pathogens had effect on clinical manifestations upon presentation had been noted for a time. Ortega et al27 have demonstrated that shock on presentation was significantly more frequently noted in cefotaxime-resistant Klebsiella bloodstream infection than cefotaxime-susceptible isolates. Notwithstanding, 1 prospective, large hospitalized cohort study conducted in Spain showed a different result.7 The reasons why such a difference occurred between the 2 studies might be due to different study population and different criteria for enrollment. Ortega et al also proposed that drug-resistant pathogens might be more virulent.27 However, some authors demonstrated that the expression of drug resistance in some bacterial pathogens may be associated with extra biological costs, leading to the attenuation of their virulence.32 In addition, the P value of the association between MDR pathogens and critical sepsis revealed by our present study was only of borderline significance (0.049) even in the multivariate model. Also, an association between poor host factors and MDR pathogens was found in the current study, and relatively critical status upon presentation may be affected by these host or environmental factors. Therefore, further studies are needed to conclude that MDR pathogens infections are one of the significant predictors for critical sepsis. However, as critical sepsis at presentation might be a surrogate marker for contracting MDR pathogens by current available evidence, clinicians should consider prescribing more broad-spectrum antimicrobial agents, such as TZP, carbapenems, and 4th-generation cephalosporins, as initial therapy for UTI patients with severe sepsis.
There were some limitations in our study. First, clinical information was collected retrospectively and several biases as well as missing data would be unavoidable. Second, this study was performed only in 1 single institution and hence generalization should be handled carefully. Third, we did not analyze virulence genes or phylogenetic groups of these Enterobacteriaceae; therefore, the correlation of virulence factors of MDR uropathogens and host clinical presentations should be further investigated. Fourth, because of the low mortality rate, multivariate analysis to identify factors associated with mortality could not be conducted. Finally, previous antibiotic exposure, which would have a great impact on subsequent acquirement of MDR pathogens infection or colonization, was not evaluated in the present study. Nonetheless, it was usually difficult to collect information about prior antibiotics exposure from patients going to emergency department for acute infectious diseases. It will be helpful for clinical physicians in decision-making about choices of antibiotics if there was any surrogate marker that could be easily collected or identified to indicate the risk of presence of MDR pathogens. In this way, disease severity, as disclosed by the present study, would be a useful parameter to predict whether MDR pathogens were the etiology or not.
In conclusion, different clinical characteristics could be found between the MDR group and the non-MDR group, and the former had more severe disease at presentation, including a much higher frequency of critical sepsis, ICU admission, longer hospitalization, and in-hospital mortality. Patients in MDR group were independently associated with critical sepsis at presentation. When we treated patients with bacteremic UTI conforming with the criteria of critical sepsis at initial presentation, empirical prescription with more broad-spectrum antibiotics covering potential drug resistance pattern, such as TZP, carbapenems, or 4th-generation cephalosporins, should be considered.
Footnotes
Abbreviations: ICU = intensive care unit, MDR = multidrug-resistant, TZP = piperacillin/tazobactam, UTI = urinary tract infection.
Y-CL and C-YH contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002; 113:5S–13S. [DOI] [PubMed] [Google Scholar]
- 2.Velasco M, Martínez JA, Moreno-Martínez A, et al. Blood cultures for women with uncomplicated acute pyelonephritis: are they necessary? Clin Infect Dis 2003; 37:1127–1130. [DOI] [PubMed] [Google Scholar]
- 3.Bahagon Y, Raveh D, Schlesinger Y, et al. Prevalence and predictive features of bacteremic urinary tract infection in emergency department patients. Eur J Clin Microbiol Infect Dis 2007; 26:349–352. [DOI] [PubMed] [Google Scholar]
- 4.Van Nieuwkoop C, Bonten TN, Wout JW, et al. Risk factors for bacteremia with uropathogen not cultured from urine in adults with febrile urinary tract infection. Clin Infect Dis 2010; 50:e69–e72. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, Fang HC, Chou KJ, et al. The clinical impact of bacteremia in complicated acute pyelonephritis. Am J Med Sci 2006; 332:175–180. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Lee YM, Cho JH. Risk factors of septic shock in bacteremic acute pyelonephritis patients admitted to an ER. J Infect Chemother 2012; 18:130–133. [DOI] [PubMed] [Google Scholar]
- 7.Shaw E, Benito N, Rodríguez-Baño J, et al. Risk factors for severe sepsis in community-onset bacteraemic urinary tract infection: impact of antimicrobial resistance in a large hospitalised cohort. J Infect 2015; 70:247–254. [DOI] [PubMed] [Google Scholar]
- 8.Munford RS, Suffredini AF. Mandell GL, Bennett JE, Dolin R. Sepsis, Severe Sepsis, and Septic Shock.Chapter 75. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases The Churchill Livingstone Elsevier, 8th edPhiladelphia, PA:2015. [Google Scholar]
- 9.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 10.Piatti G, Mannini A, Balistreri M, et al. Virulence factors in urinary Escherichia coli strains: phylogenetic background and quinolone and fluoroquinolone resistance. J Clin Microbiol 2008; 46:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman JT, McBride SJ, Nisbet MS, et al. Bloodstream infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary care hospital in New Zealand: risk factors and outcomes. Int J Infect Dis 2012; 16:e371–e374. [DOI] [PubMed] [Google Scholar]
- 12.Park YS, Bae IK, Kim J, et al. Risk factors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli bacteremia. Yonsei Med J 2014; 55:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honkinen O, Jahnukainen T, Mertsola J, et al. Bacteremic urinary tract infection in children. Pediatr Infect Dis J 2000; 19:630–634. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao CY, Yang HY, Chang CH, et al. Risk factors for development of septic shock in patients with urinary tract infection. Biomed Res Int 2015; 2015:717094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 16.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 1999; 11:7–12. [DOI] [PubMed] [Google Scholar]
- 17.American College of Chest Physician/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992; 20:864–874 [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao CY, Yang HY, Hsiao MC, et al. Risk factors for development of acute kidney injury in patients with urinary tract infection. PLoS One 2015; 10:e0133835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement M100-S17. Wayne, PA: CLSI; 2007. [Google Scholar]
- 21.Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement M100-S23. Wayne, PA: CLSI; 2013. [Google Scholar]
- 22.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira MC, Oliveira CR, Gonçalves KV, et al. Enterobacteriaceae resistant to third generation cephalosporins upon hospital admission: risk factors and clinical outcomes. Braz J Infect Dis 2015; 19:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu YH, Chen PL, Hung YP, et al. Risk factors and clinical impact of levofloxacin or cefazolin nonsusceptibility or ESBL production among uropathogens in adults with community-onset urinary tract infections. J Microbiol Immunol Infect 2014; 47:197–203. [DOI] [PubMed] [Google Scholar]
- 25.Arslan H, Azap OK, Ergönül O, et al. Urinary Tract Infection Study Group. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J Antimicrob Chemother 2005; 56:914–918. [DOI] [PubMed] [Google Scholar]
- 26.Alós JI, Serrano MG, Gómez-Garcés JL, et al. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect 2005; 11:199–203. [DOI] [PubMed] [Google Scholar]
- 27.Ortega M, Marco F, Soriano A, et al. Cefotaxime resistance and outcome of Klebsiella spp bloodstream infection. Eur J Clin Microbiol Infect Dis 2011; 30:1599–1605. [DOI] [PubMed] [Google Scholar]
- 28.Peralta G, Sánchez MB, Garrido JC, et al. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J Antimicrob Chemother 2007; 60:855–863. [DOI] [PubMed] [Google Scholar]
- 29.Mauldin PD, Salgado CD, Hansen IS, et al. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother 2010; 54:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thulstrup AM, Sorensen HT, Schonheyder HC, et al. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis 2000; 31:1357–1361. [DOI] [PubMed] [Google Scholar]
- 31.Shigemura K, Tanaka K, Osawa K, et al. Clinical factors associated with shock in bacteremic UTI. Int Urol Nephrol 2013; 45:653–657. [DOI] [PubMed] [Google Scholar]
- 32.Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in bacterial world? Clin Microbiol Rev 2013; 26:185–230. [DOI] [PMC free article] [PubMed] [Google Scholar]


