Abstract
Esophageal fistula is a critical adverse event in patients treated with chemoradiotherapy (CRT) for locally advanced esophageal cancer. However, risk factors associated with esophageal fistula formation in patients receiving CRT have not yet been elucidated.
We retrospectively analyzed data obtained from 140 patients who were enrolled in a phase II/III trial comparing low-dose cisplatin with standard-dose cisplatin administered in combination with 5-flurouracil and concomitant radiotherapy. Inclusion criteria were performance status (PS) 0 to 2 and histologically proven thoracic esophageal cancer clinically diagnosed as T4 and/or unresectable lymph node metastasis for which definitive CRT was applicable. Risk factors for esophageal fistula were examined with univariate analysis using Fisher exact test and multivariate analysis using logistic regression models.
Esophageal fistula was observed in 31 patients (22%). Of these, 6 patients developed fistula during CRT. Median time interval between the date of CRT initiation and that of fistula diagnosis was 100 days (inter quartile range, 45–171). Esophageal stenosis was the only significant risk factor for esophageal fistula formation both in univariate (P = 0.026) and in multivariate analyses (odds ratio, 2.59; 95% confidence interval, 1.13–5.92, P = 0.025). Other clinicopathological factors, namely treatment arm, age, sex, PS, primary tumor location, T stage, lymph node invasion to adjacent organs, blood cell count, albumin level, and body mass index, were not risk factors fistula formation.
Esophageal stenosis was a significant risk factor for esophageal fistula formation in patients treated with CRT for unresectable locally advanced thoracic esophageal squamous cell carcinoma.
INTRODUCTION
Esophageal cancers that invade adjacent organs either directly (T4) or indirectly via lymph nodes are oncologically considered unresectable. Studies evaluating surgery for esophageal cancers invading adjacent organs showed high morbidity and dismal prognosis with this approach.1–3 Several clinical trials investigating definitive chemoradiotherapy (CRT) for unresectable esophageal cancer demonstrated improved patient outcomes with median survival times between 9 and 13 months, which were comparable with those achieved by surgery and/or CRT.4–8 Thus, definitive CRT is considered as the standard therapy for T4 esophageal cancer and/or unresectable lymph node.
Anatomically, thoracic esophagus is surrounded by the aorta, pericardium, trachea, bronchi, lungs, and vertebrae, which are often invaded by advanced esophageal cancers. Subsequent formation of esophageal fistulae to the adjacent organs is associated with high rates of morbidity and mortality. Furthermore, CRT can also induce fistula formation by damaging the walls of the esophagus and adjacent organs.9,10 The incidence of esophageal fistula formation in patients treated with CRT for T4 esophageal cancer was reported to be 10% to 12%.4–6
Albeit the critical importance of esophageal fistulae in these patients, associated risk factors are yet to be elucidated. Thus, we aimed to identify the risk factors for esophageal fistula formation in patients treated with CRT for locally advanced unresectable esophageal cancer by exploratory analyses using the long-term follow-up data from JCOG0303, a phase II/III randomized clinical trial comparing 2 CRT protocols.
MATERIALS AND METHODS
Summary of JCOG0303
JCOG0303 was a phase II/III clinical trial comparing patient outcomes with 2 different doses of cisplatin administered in combination with 5-flurouracil (CF) and concomitant radiotherapy for locally advanced esophageal cancer. Inclusion criteria were Eastern Cooperative Oncology Group performance status 0 to 2; histologically proven squamous cell, adenosquamous, or basaloid carcinoma; thoracic esophageal cancer clinically diagnosed as T4 and/or unresectable lymph node metastasis due to invasion into an adjacent organ for which definitive CRT was indicated; absence of esophageal fistulae; and absence of metastasis to sites other than the baseline lymph node(s). Patients were randomly assigned to standard dose CF (70 mg/m2 cisplatin given on days 1 and 29 in combination with a continuous infusion of 700 mg/m2 5-FU given on days 1–4 and 29–32) or low-dose CF (a 1-h infusion of 4 mg/m2 cisplatin before radiotherapy, combined with a continuous infusion of 200 mg/m2 5-FU on the first 5 days of each week). Radiotherapy was prescribed to a total dose of 60 Gy in 30 fractions and was started on day 1 concomitantly with chemotherapy in both groups. The primary endpoint was overall survival.
The study protocol was approved by the institutional review board of each institution, and written informed consent was obtained from all participants before randomization. A total of 142 patients were enrolled. There were no significant differences in overall survival (hazard ratio, 1.05; 80% confidence interval [CI], 0.78–1.41) and toxicities between the 2 treatment arms. Detailed results of JCOG0303 were reported elsewhere.7
Definition of Esophageal Fistula
Esophageal fistulae were graded based on the National Cancer Institute Common Toxicity Criteria version 2.0. Data on all esophageal fistulae after CRT initiation were collected regardless of the time interval from CRT initiation. The details of esophageal fistulae were described in the case report forms; however, the type of esophageal fistulae (esophagorespiratory, esophagoarterial, or unknown) was not systematically collected. Therefore, we retrospectively reviewed the clinical course and determined the class in each case. In patients for whom details of the esophageal fistulae were not described in the case report forms, queries to site investigators were submitted via electronic mail.
Statistical Consideration
The background characteristics of patients with esophageal fistulae were compared with those who did not develop esophageal fistulae. From clinical point of view, 9 variables were selected for univariate and/or multivariate analysis. Univariate analysis was performed by Fisher exact test for categorical variables, Wilcoxon rank-sum test for continuous variables, and multivariate analysis by logistic regression model for patient variables: treatment arm; leukocyte count, >10,000/mm2; hemoglobin, >12 g/dL; albumin, >3.5 g/dL; primary tumor location in upper-, mid-, or lower-thoracic esophagus; tumor stage, T4 or non-T4; presence of adjacent organ invasion via lymph node(s); presence of symptoms of esophageal stenosis; and body mass index (BMI) of >20. All tests were 2 sided, and P values of <0.05 were considered statistically significant for all analyses. Statistical analyses were performed using SAS software, release 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
A total of 142 patients were registered to JCOG0303 between April 2004 and September 2009. The median observation time of all patients and survivors was 13.6 and 65.1 months, respectively. Of 142 patients enrolled in JCOG0303, patients were randomized to the standard-dose CF arm (n = 71) and the low-dose CF arm (n = 71). Excluding 1 patient in each arm who did not receive protocol treatment, the data of a total of 140 patients (n = 70 per arm) were analyzed in this study. Protocol treatment was completed in 57 patients (81%) in the standard-dose CF arm and in 61 patients (87%) in the low-dose CF arm. There were 32 cases of esophageal fistula formation after initiation of protocol treatment (15 cases in the standard-dose CF arm and 17 cases in the low-dose CF arm). Of these, 1 case was excluded from further analysis as the cause of fistula was determined as endoscopic intervention.
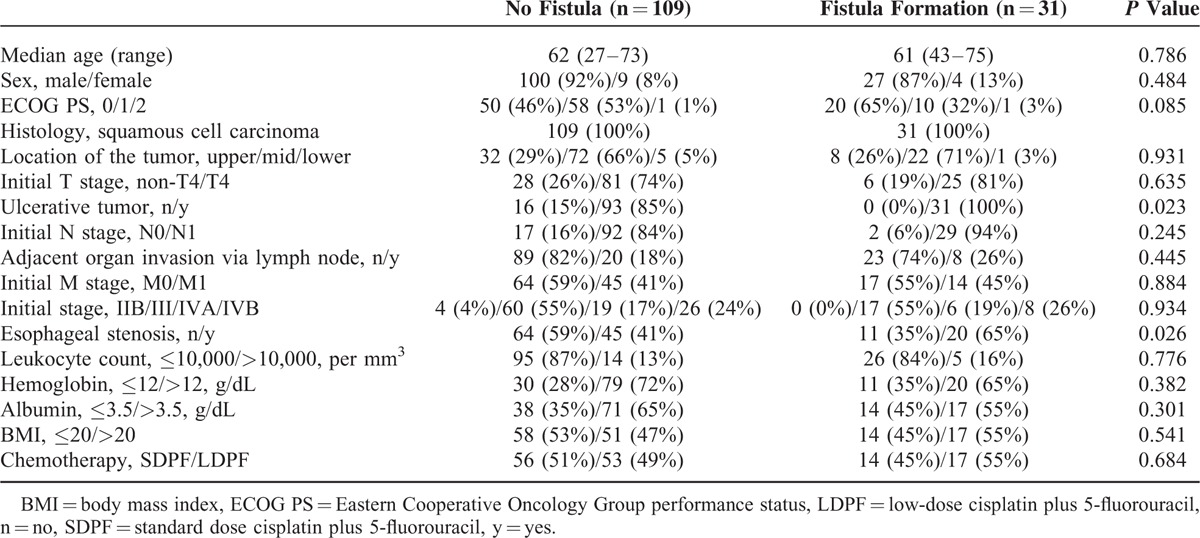
Background characteristics of patients who developed esophageal fistulae and those who did not were as follows: clinical T4, 81% and 74%; adjacent organ invasion via lymph node(s), 26% and 18%; and esophageal stenosis, 65% and 41%, respectively. There was basically no significant difference in patient background characteristics between the 2 groups but the proportion of patients with esophageal stenosis or ulcerative type tumor was significantly greater in the fistula formation group (Table 1). The majority of tumors (89%) included in our analyses was of the ulcerative type.
TABLE 1.
Clinicopathological Features of Patients
Characteristics of Esophageal Fistula
The number of patients who experienced fistula formation during the protocol treatment was 6, and 25 patients developed fistulae after treatment completion. Median time interval between the CRT start date and the date of esophageal fistula diagnosis was 100 days (range, 3–1068; interquartile range, 45–171 days).
The types of esophageal fistulae observed in this trial were esophagorespiratory, esophagoarterial, and not confirmed in 22, 6, and 3 patients, respectively. Of these 31 patients, 21 (68%) died from esophageal fistula-related adverse events. The most common cause for fistula-related death was bleeding. Specifically, esophagoarterial fistulae were present in 7 patients, whereas bleeding subsequent to the formation of esophagorespiratory fistulae was the cause of death in 6 cases. The second most common cause of mortality was respiratory infection due to esophagorespiratory fistula formation, which occurred in 4 cases.
Risk Factors for Esophageal Fistula Formation
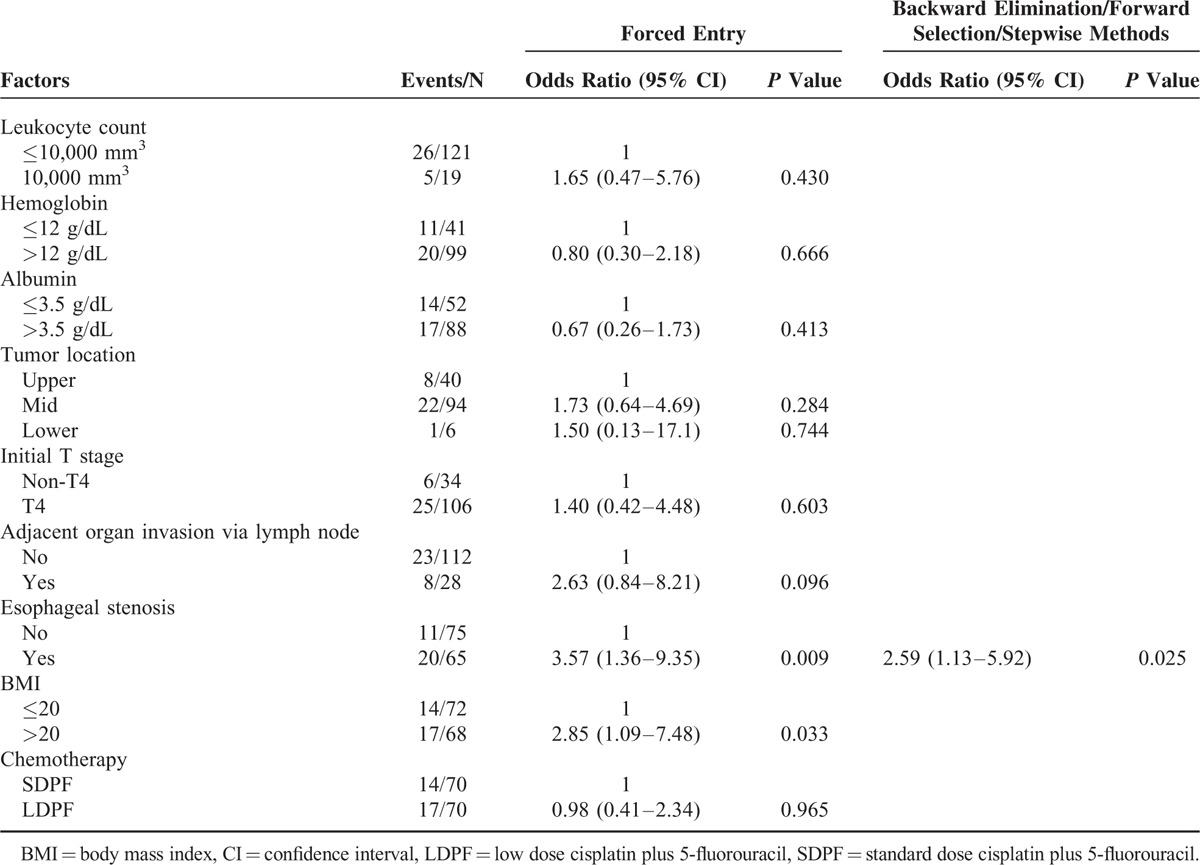
Table 2 shows the results of multivariate analyses of the risk factors for the formation of esophageal fistulae. Among the tested 9 variables (treatment arm, leukocyte count, hemoglobin level, albumin level, primary tumor location, T stage, adjacent organ invasion via metastatic lymph node(s), esophageal stenosis, and BMI level), esophageal stenosis was the only potential risk factor by univariate analysis (P = 0.026). Multivariate analysis revealed esophageal stenosis as the only significant risk factor by the forward selection, backward elimination, or stepwise methods (P = 0.025; odds ratio [OR], 2.59; 95% CI, 1.13–5.92). A BMI of >20 was a significant risk factor only when performed by the forced entry method (P = 0.033; OR, 2.85; 95% CI, 1.09–7.48).
TABLE 2.
Multivariate Analysis of Risk Factors for Esophageal Fistula Formation
DISCUSSION
To date, data on the risk factors for esophageal fistula formation in patients treated with CRT for advanced esophageal cancer are lacking. This is the first report that analyzed prospectively collected data to explore risk factors for the formation of esophageal fistulae in patients with locally advanced T4 esophageal cancer patients and/or unresectable lymph node metastasis who received definitive CRT. Our analysis revealed that esophageal stenosis was the only risk factor for esophageal fistula formation among a total of 9 clinical variates in this setting. Patients with esophageal stenosis before CRT had more than twice the risk of esophageal fistula formation, and esophageal fistula was associated with a high mortality rate of 68% in our study. These findings have significant implications for clinical practice; clinicians need to be aware of the risk for fistulae during CRT treatment of patients with esophageal stenosis.
Among the limited number of studies investigating the risk of treatment-related esophageal fistula formation, a retrospective analysis of 48 patients with T4 esophageal cancer by Taniguchi et al11 indicated that lower total serum cholesterol level was a risk factor for esophagoaortic fistula formation. Unfortunately, as serum total cholesterol was not collected during the JCOG0303 trial, we could not assess this parameter in the present study.
The incidence of esophageal fistula formation was reported as 10% to 12% in previous studies on definitive CRT for patients with T4 esophageal cancer and/or unresectable lymph node(s).4–6 In JCOG0303, the incidence was 22% (31 of 140), which appeared to be higher than those reported in previous studies.7 However, the incidence of esophageal fistula formation within 90 days from the initiation of protocol treatment in JCOG0303 was only 9% (13 of 140). Thus, the higher incidence should be attributed to the longer follow-up period in this study.
Among those who developed esophageal fistulae in this study, 68% died from related complications. Countermeasures against this life-threatening adverse event in CRT for esophageal cancer are of critical importance. Recently, a retrospective study on the data of prospective studies indicated that induction chemotherapy for locally advanced esophageal cancer could improve dysphagia,12 as was observed in 16% of a total of 161 patients analyzed in the study. Although it is not clear whether induction chemotherapy reduces the incidence of CRT treatment-related esophageal fistulae through improvement of esophageal stenosis, elimination of all risk factors for esophageal fistula will be beneficial. Therefore, future studies investigating the association between induction chemotherapy and incidence of fistulae following CRT are warranted. One clinical trial that prospectively investigated induction chemotherapy for T4 esophageal cancer and/or unresectable lymph node(s) showed favorable results.13 The treatment in that trial consisted of induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil administered for up to 3 cycles, followed by CRT. Most of the patients (88%) completed the protocol treatment. In addition, the incidence of esophageal fistula formation was 3%, and the median survival time was 24 months. Despite the small number of patients, the lack of data on esophageal stenosis at baseline, and short follow-up period, the outcomes of that trial suggested that the incidence of esophageal fistula formation was decreased by induction chemotherapy.
There are several limitations in this study. First, the incidence of esophageal fistula formation was not high enough to investigate other factors as covariates for risk. In fact, although ulcerative type tumor was a candidate risk factor, it was excluded from the analysis because there was no patient who had nonulcerative tumor in fistula formation group. Second, the definition of esophageal stenosis was not clearly described in the protocol. Investigators judged stenosis from clinical symptoms and/or patient reports and not from objective measures such as dysphagia score.14 Future studies investigating CRT for patients with T4 esophageal cancer and/or unresectable lymph node(s) will define esophageal stenosis according to clear and established criteria to confirm esophageal stenosis as a risk factor.
In conclusion, our analysis indicated esophageal stenosis as an independent risk factor for esophageal fistula formation in patients with T4 esophageal squamous cell carcinoma and/or unresectable lymph node(s) receiving definitive CRT. The findings from the current as well as previously published studies suggest that induction chemotherapy before CRT might reduce the risk of esophageal fistula formation by relieving esophageal stenosis. This strategy should further be studied in a future prospective trial. Currently, we are conducting a separate prospective trial to demonstrate the superiority of CRT with induction chemotherapy to that without induction chemotherapy.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CF = cisplatin administered with 5-fluorouracil, CRT = chemoradiotherapy, HR = hazard ratio, JCOG = Japan Clinical Oncology Group, PS = performance status.
This study was supported by the following National Cancer Center Research and Development Funds: 23-A-16, 23-A-18, and 26-A-4.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Matsubara T, Ueda M, Kokudo N, et al. Role of esophagectomy in treatment of esophageal carcinoma with clinical evidence of adjacent organ invasion. World J Surg 2001; 25:279–284. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda K, Ishida K, Sato N, et al. Chemoradiotherapy followed by surgery for thoracic esophageal cancer potentially or actually involving adjacent organs. Dis Esophagus 2001; 14:197–201. [DOI] [PubMed] [Google Scholar]
- 3.Fujita H, Sueyoshi S, Tanaka T, et al. Esophagectomy: is it necessary after chemoradiotherapy for a locally advanced T4 esophageal cancer? Prospective nonrandomized trial comparing chemoradiotherapy with surgery versus without surgery. World J Surg 2005; 29:25–30.discussion 1. [DOI] [PubMed] [Google Scholar]
- 4.Ohtsu A, Boku N, Muro K, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol 1999; 17:2915–2921. [DOI] [PubMed] [Google Scholar]
- 5.Ishida K, Ando N, Yamamoto S, et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol 2004; 34:615–619. [DOI] [PubMed] [Google Scholar]
- 6.Ishikura S, Ohtsu A, Shirao K, et al. A phase I/II study of nedaplatin and 5-fluorouracil with concurrent radiotherapy in patients with T4 esophageal cancer: Japan Clinical Oncology Group trial (JCOG9908). Esophagus 2005; 2:133–137. [Google Scholar]
- 7.Shinoda M, Ando N, Kato K, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci 2015; 106:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita H. A history of surgery for locally-advanced (T4) cancer of the thoracic esophagus in Japan and a personal perspective. Ann Thorac Cardiovasc Surg 2013; 19:409–415. [DOI] [PubMed] [Google Scholar]
- 9.Gabrail NY, Harrison BR, Sunwoo YC. Chemo-irradiation induced aortoesophageal fistula. J Surg Oncol 1991; 48:213–215. [DOI] [PubMed] [Google Scholar]
- 10.Sivaraman SK, Drummond R. Radiation-induced aortoesophageal fistula: an unusual case of massive upper gastrointestinal bleeding. J Emerg Med 2002; 23:175–178. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi H, Yamazaki K, Boku N, et al. Risk factors and clinical courses of chemoradiation-related arterio-esophageal fistula in esophageal cancer patients with clinical invasion of the aorta. Int J Clin Oncol 2011; 16:359–365. [DOI] [PubMed] [Google Scholar]
- 12.Won E, Ilson DH, Herrera J, et al. Can induction chemotherapy improve dysphagia in locally advanced esophageal/GEJ cancer? J Clin Oncol. 2014; 32(S):suppl; abstr 148. [Google Scholar]
- 13.Satake M, Tahara M, Mochizuki S, et al. Phase I/II trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil (DCF) followed by concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. J Clin Oncol. 2013; 31:suppl; abstr 4074. [Google Scholar]
- 14.Ogilvie AL, Dronfield MW, Ferguson R, et al. Palliative intubation of oesophagogastric neoplasms at fibreoptic endoscopy. Gut 1982; 23:1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]


