Abstract
Renal dysfunction predicts all-cause mortality in general population. However, the prevalence of renal insufficiency and its relationship with mortality in cancer patients are unclear.
We retrospectively studied 9465 patients with newly diagnosed cancer from January 2010 to December 2010. Renal insufficiency was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation. The hazard ratio (HR) of all-cause mortality associated with baseline eGFR was assessed by Cox regression.
Three thousand sixty-nine patients (32.4%) exhibited eGFR <90 mL/min/1.73 m2 and 3% had abnormal serum creatinine levels at the time of diagnosis. Over a median follow-up of 40.5 months, 2705 patients (28.6%) died. Compared with the reference group (eGFR ≥ 60 mL/min/1.73 m2), an elevated all-cause mortality was observed among patients with eGFR < 60 mL/min/1.73 m2 stratified by cancer stage in the entire cohort, the corresponding hazard ratios were 1.87 (95% CI, 1.41–2.47) and 1.28 (95% CI, 1.01–1.62) for stage I to III and stage IV, respectively. However, this relationship was not observed after multivariate adjustment. Subgroup analysis found that eGFR < 60 mL/min/1.73 m2 independently predicted death among patients with hematologic (adjusted HR 2.93, 95% CI [1.36–6.31]) and gynecological cancer (adjusted HR 2.82, 95% CI [1.19–6.70]), but not in those with other cancer. Five hundred fifty-seven patients (6%) had proteinuria. When controlled for potential confounding factors, proteinuria was a risk factor for all-cause mortality among patients in the entire cohort, regardless of cancer stage and eGFR values. When patients were categorized by specific cancer type, the risk of all-cause death was only significant in patients with digestive system cancer (adjusted HR, 1.85 [1.48–2.32]).
The prevalence of renal dysfunction was common in patients with newly diagnosed cancer. Patients with eGFR < 60 mL/min/1.73 m2 or proteinuria were associated with increased risk for all-cause mortality, this relation depended on cancer site.
INTRODUCTION
Worldwide, it is estimated that there will be 26.4 million new cancer cases and 17.0 million cancer-related deaths by 2030.1,2 Although advances have been made in therapy, 5-year survival rates have improved among patients without comorbidity, but not those with comorbidity.3,4 Thus, coexistence of chronic disease or comorbid conditions become the major determinants of outcome in cancer patients.5–8
Several previous studies have demonstrated that renal dysfunction occur frequently in patients with cancer.9–12 The renal insufficiency and anticancer medications (IRMA) study showed that 52.9% exhibited an estimated glomerular filtration rate (eGFR) <90 mL/min/1.73 m2, calculated according to the Modification of Diet in Renal Disease (MDRD) formula. Recently, a prospective population-based cohort study reported that 73.3% subjects had eGFR <75 mL/min/1.73 m2, on the basis of the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI). However, limited data are available regarding the prevalence of renal impairment among cancer patients in China.
Few studies to date have investigated the impact of reduced renal function on survival among cancer patients.10,13–18 Nevertheless, the results are inconsistent. Some studies provided supportive information of inverse association with different cutoff value of the eGFR,10,13–15 whereas others suggested there was no relationship.16,17 Notably, cancer per se may have a strong influence on mortality rather than a reduced kidney function, previous studies suggested that the association of eGFR and mortality varied by type of cancer.10,19 Thus, the heterogeneous findings may be due to differences in patient's age, tumor type and stage, control for renal function status, anticancer treatment modality, and relatively small sample size.11,20–24
In this study, we examined the renal function status at the time of diagnosis without any anticancer treatment and explored the association of renal dysfunction with all-cause mortality among cancer patients.
METHODS
Patients
This study was approved by the institutional review boards of the First Affiliated Hospital and Cancer Center of Sun Yat-sen University. All participants provided their written informed consent before inclusion.
A total of 10,465 cancer patients in Cancer Center of Sun Yat-sen University between January 1, 2010 and December 31, 2010 were retrieved from computerized hospital database. Patients older than 18 years with newly diagnosed, untreated cancer were enrolled. Patients were excluded if they had no kidney function data (n = 1000).
Patients were categorized according to either baseline eGFR, the presence of proteinuria, or cancer type to examine the association between eGFR and mortality.
Demographic and Clinical Data
All data were obtained at the time of diagnosis, including age, gender, body mass index, history of hypertension, diabetes mellitus (DM), cardiovascular disease (CVD), smoking (we only considered ever smoking, regardless of smoking amount, frequency, and duration), blood pressure, type of tumor, and laboratory parameters. All biochemical and hematological tests were measured in the biochemical laboratory of Cancer center of Sun Yat-sen University.
Renal function was evaluated by eGFR with the CKD-EPI equation, which has been indicated to be more accurate than the MDRD formula in various populations including Asian, as previously described.25–27 In our study, RI was defined as eGFR < 60 mL/min/1.73 m2. The presence of proteinuria was defined as albumin 1+ or greater. Cancer stage was based on the Union for International Cancer Control/American Joint Committee on Cancer TNM (tumor-node-metastasis) classification system.
Outcomes
Our primary outcomes were all-cause mortality. Survival was defined as the time from enrollment to all-cause death or administrative censoring (i.e., loss to follow-up or end of the study period) at March 31, 2015.
Statistical Analyses
Continuous data were expressed as mean ± standard deviation or median (interquartile range, IQR), and categorical variables were expressed as percentages. Baseline characteristics among 3 eGFR groups were compared using ANOVA, Kruskal-Wallis rank sum test, or Chi-square test as appropriate. Multiple comparisons were performed using t test or Wilcoxon rank sum test with P value adjusted by Bonferroni method. Univariable and multivariable logistic regression models were used to assess the association of independent variables with eGRF < 60 mL/min/1.73 m2 and odds ratios (ORs) and 95% confidence intervals (CIs) were derived. Those covariates with a P < 0.2 in univariable analysis were included in the multivariable logistic regression model using enter method. To evaluate the relationship between eGFR levels and mortality, a log-rank test and the Kaplan-Meier curve method were used. Univariable and Multivariable Cox proportional hazards regression analyses were performed to investigate the association of baseline eGFR and proteinuria with all-cause mortality. Patients with an eGFR level of 60 mL/min/1.73 m2 or more were regarded as the reference group. The relationship between eGFR level or proteinuria and risk of all-cause mortality was calculated in a multivariable Cox regression model adjusted for potential confounding factors.
Given that the effect of renal function on prognosis may be varied due to different tumor types, we performed subgroup analyses to evaluate the relationship of eGFR levels or proteinuria and all-cause mortality in the common types of cancer in our study. Hazard ratios (HRs) and 95% CIs were derived from COX models. A P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software, version 13.0 (SPSS Inc, Chicago, IL).
RESULTS
Baseline Characteristics of Study Cohort
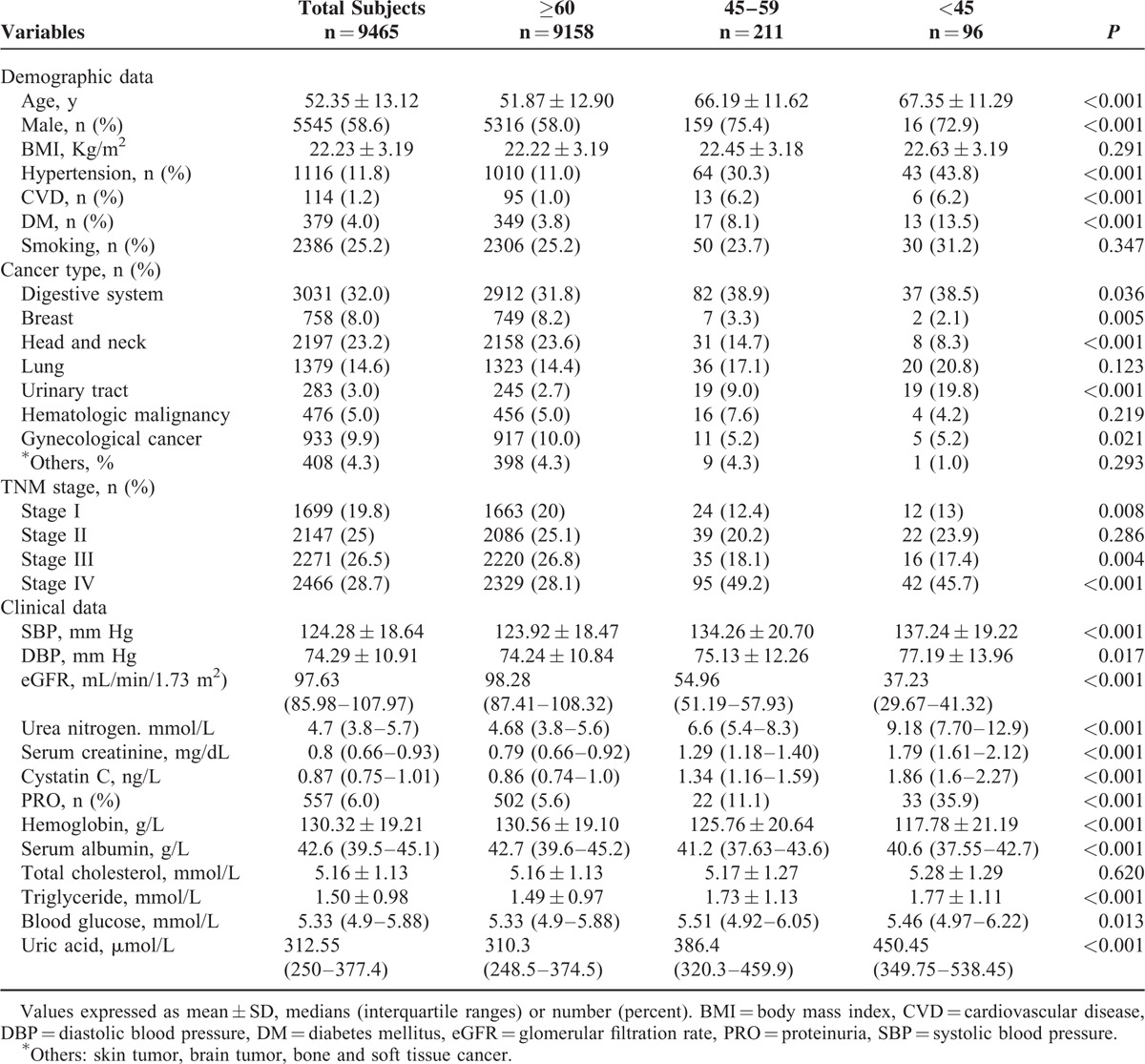
Table 1 showed the baseline demographic and clinical characteristics of the entire cohort and stratified by eGFR levels. Of the 9465 patients, the mean age was 52.35 ± 13.12 years, 58.6% male, with a predominance of digestive system cancer, head and neck cancer, and lung cancer across all eGFR levels. Five hundred fifty-seven patients (6%) had proteinuria. The mean baseline eGFR was 96.18 mL/min/1.73 m2. Participants who had eGFR of 90 or above, 60 to 89, and 45 to 59 mL/min/1.73 m2 were 67.6%, 29.2%, and 2.2% of all participants, respectively. In contrast, 3% had abnormal serum creatinine levels. Compared with patients with eGFR of 60 mL/min/1.73 m2 or more, those with lower eGFR were much older, had more coexisted diseases such as hypertension, CVD, and DM, presence of proteinuria, higher systolic pressure, lower levels of hemoglobin and serum albumin, and higher levels of serum triglyceride, blood glucose, and uric acid.
TABLE 1.
Baseline Data of Patients Stratified by eGFR (mL/min/1.73 m2)
Variables Related With eGFR < 60 mL/min/1.73 m2
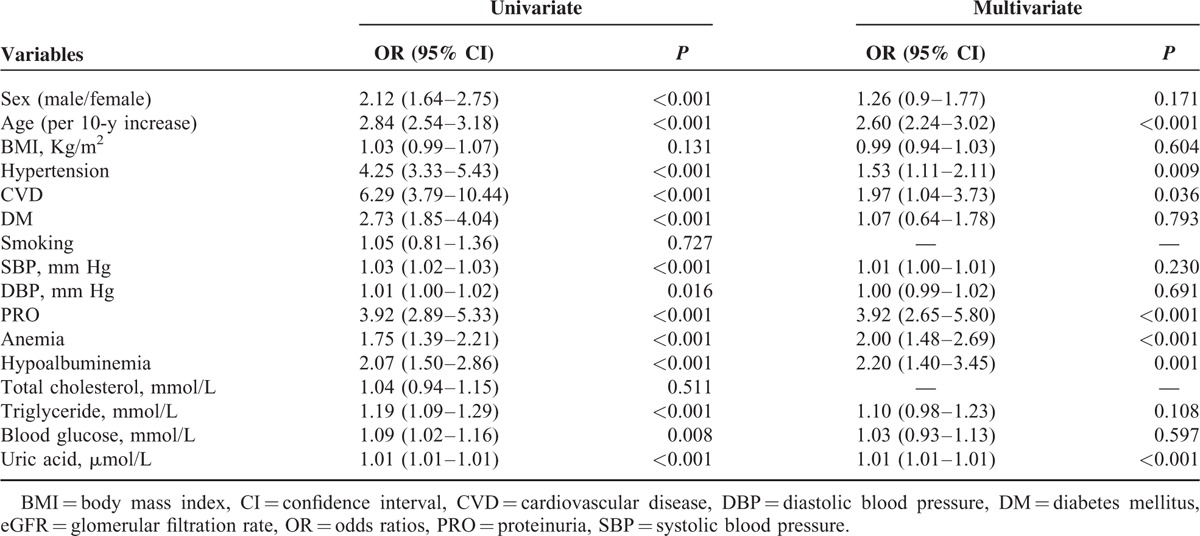
As shown in Table 2, univariate logistic analysis showed that older age, male gender, presence of CVD, hypertension, or DM, proteinuria, hypoalbuminemia, anemia, blood glucose, serum triglyceride, and uric acid were associated with eGFR < 60 mL/min/1.73 m2. Multivariable logistic model revealed that age (OR, 2.60; 95% CI, 2.24–3.02, per 10-year increase), history of hypertension (OR, 1.53; 95% CI, 1.11–2.11) and CVD (OR, 1.97; 95% CI, 1.04–3.73), proteinuria (OR, 3.92; 95% CI, 2.65–5.80), hypoalbuminemia (OR, 2.20; 95% CI, 1.40–3.45), anemia (OR, 2.00; 95% CI, 1.48–2.69), and uric acid (OR, 1.01; 95% CI, 1.01–1.01, per 1 μmol/L higher) were independently related to eGFR < 60 mL/min/1.73 m2.
TABLE 2.
Odds Ratios for Prevalence of eGFR < 60 mL/min/1.73 m2
Renal Function and All-Cause Mortality
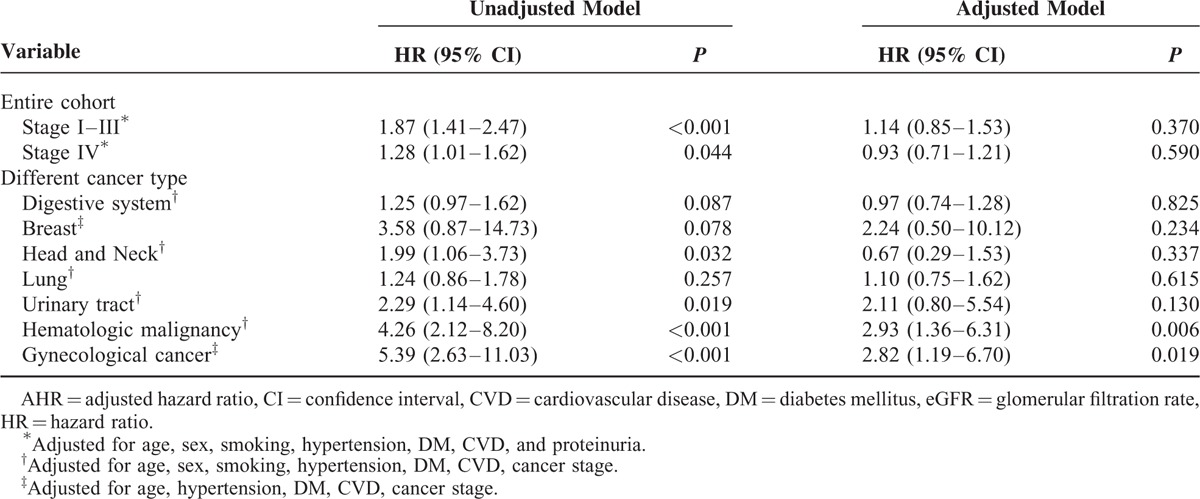
During a median follow-up of 40.5 months (IQR: 14.3, 51.4), 2705 patients (28.6%) died. As shown in Table 3, the overall crude mortality rate was 101.44 deaths per 1000 person-years (PYs). There was a significant increase in all-cause mortality with decreasing values of eGFR (P for trend < 0.001). Patients with an eGFR < 60 mL/min/1.73 m2 showed the highest mortality (188.4 deaths per 1000 PYs), whereas those with eGFR levels of at least 60 mL/min/1.73 m2 revealed the lowest mortality (99.64 deaths per 1000 PYs). Similar trends were observed in certain site-specific cancers, with the exception of digestive system, breast and lung cancers. To evaluate patient survival and mortality risk, eGFR was divided into 2 groups based upon the cutoff value of at least 60 mL/min/1.73 m2. Because of relatively few cancer patients with stage I, II, and III, patients were categorized into 1 group for analysis. Figure 1 displayed the Kaplan-Meier survival curves for all-cause mortality stratified by eGFR and cancer stage categories during 5 years of follow-up. Patients with eGFR < 60 mL/min/1.73 m2 were likely to have worse overall rates of survival than those with eGFR at least 60 mL/min/1.73 m2, regardless of cancer stage I to III (Figure 1A) and IV (Figure 1B) in the entire cohort. The HRs for all-cause mortality associated with eGFR were presented in Table 4, with eGFR of at least 60 mL/min/1.73 m2 as the reference. In unadjusted model, eGFR levels <60 mL/min/1.73 m2 was associated with increased risk of all-cause mortality stratified by cancer stage in the entire cohort, the corresponding hazard ratios were 1.87 (95% CI, 1.41–2.47) and 1.28 (95% CI, 1.01–1.62) for stage I to III and stage IV, respectively. However, this relationship was not observed after controlling for age, sex, history of hypertension, CVD, DM, or smoking, and proteinuria.
TABLE 3.
The Cumulative Incidence Death Rates in the Subgroups Classified by Cancer Type
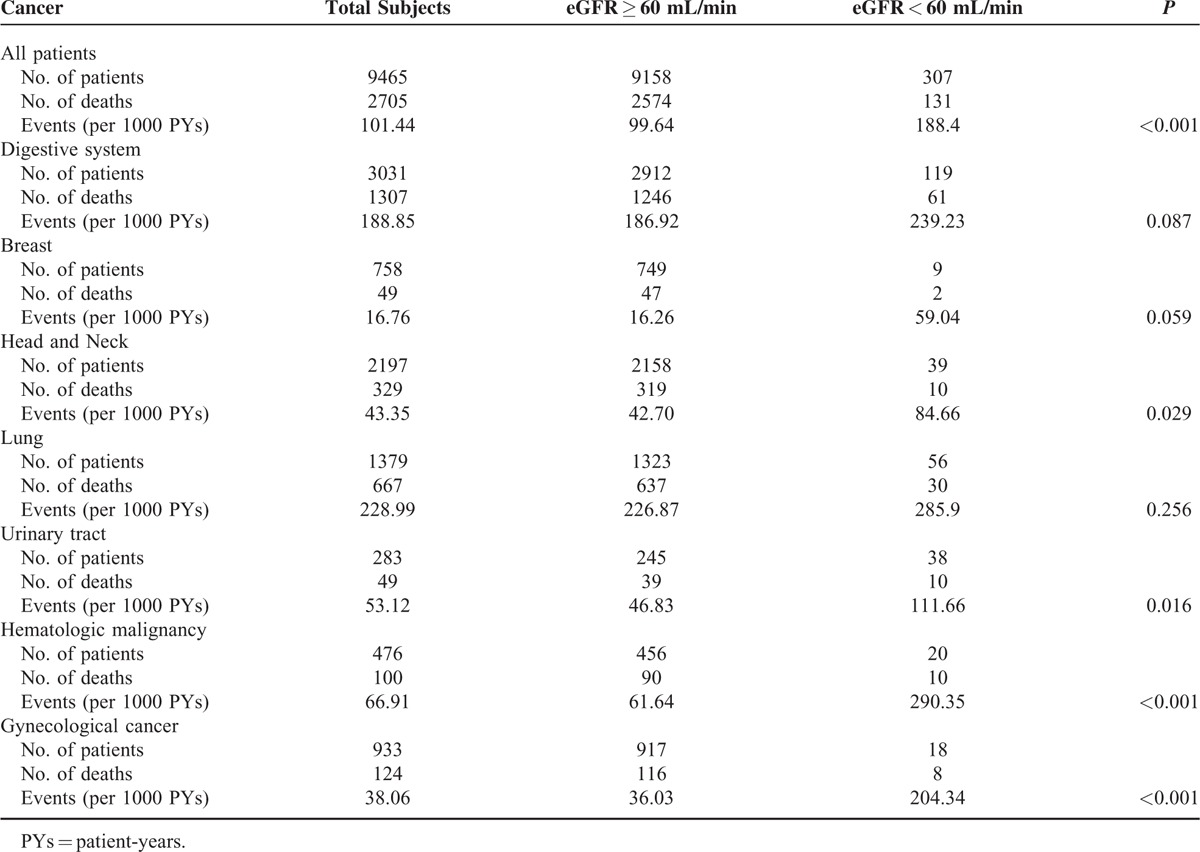
FIGURE 1.
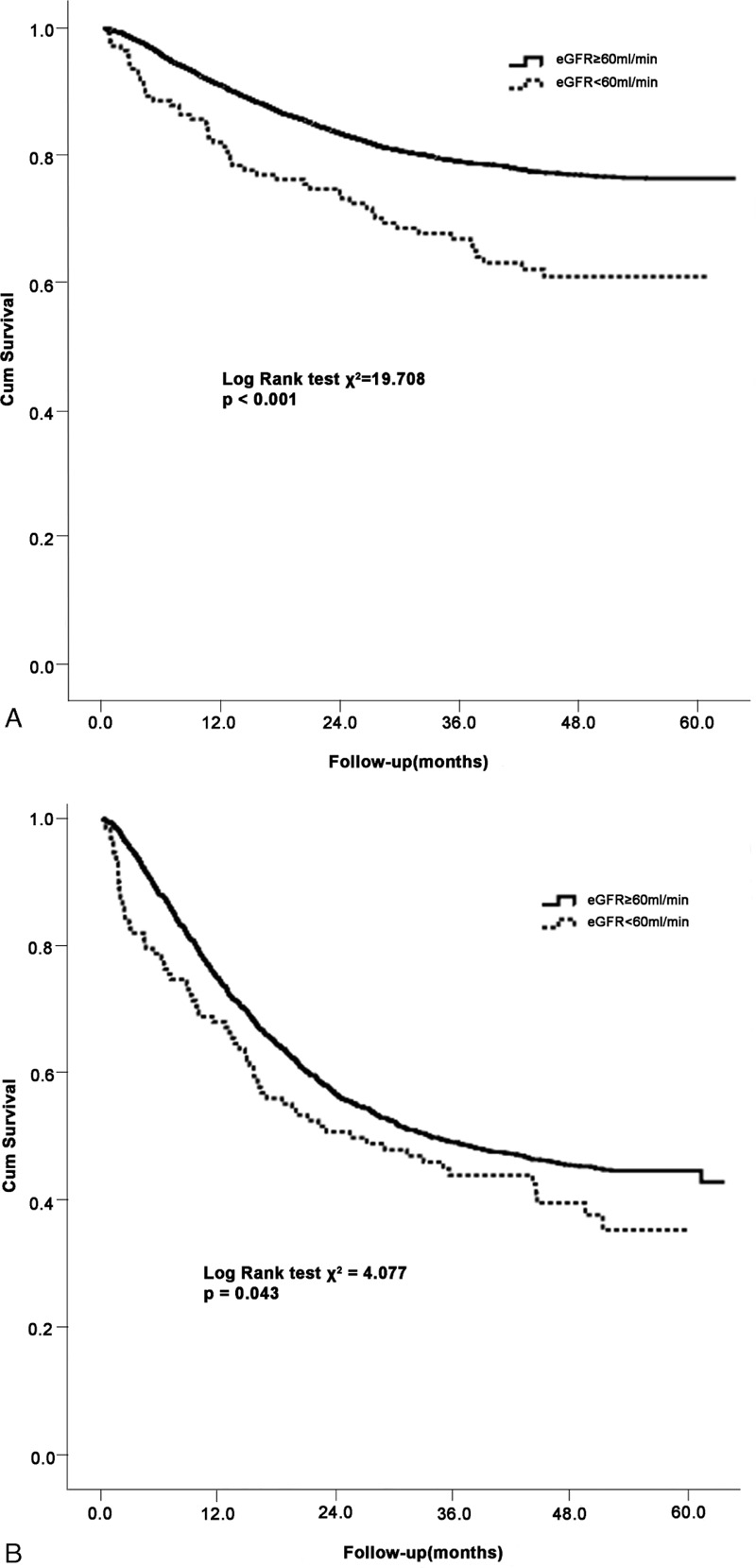
Kaplan-Meier survival curves for all-cause mortality stratified by eGFR at least 60 or <60 mL/min/1.73 m2 among patients with cancer stage I to III (A) and stage IV (B). eGFR = estimated glomerular filtration rate.
TABLE 4.
Adjusted HRs of All-Cause Mortality for Participants With eGFR < 60 mL/min compared with eGFR ≥ 60 mL/min
Association of Renal Function With Mortality by Primary Cancer
We further investigate whether the risk of reduced renal function-related mortality varies by primary site of cancer. Given that relative small sample size and low event rate among patients with eGFR < 45 mL/min/1.73 m2 (Table 1), eGFR was categorized into 2 groups: at least 60 and <60 mL/min/1.73 m2. As shown in Table 4, compared with eGFR at least 60 mL/min/1.73 m2, eGFR < 60 mL/min/1.73 m2 was associated with an increased risk of all-cause mortality among patients who diagnosed with hematologic malignancy and gynecological cancer (adjusted HR [AHR], 2.93 [1.36–6.31] and 2.82 [1.19–6.70], respectively) after additional adjustments for cancer stage, but no significant link between eGFR and other cancer types.
Association of Proteinuria With Mortality
Proteinuria is a marker for progression of chronic kidney disease. We thus evaluated the relationship between proteinuria and all-cause mortality in cancer patients. The HRs for all-cause mortality associated with proteinuria were presented in Table 5. Proteinuria was not associated with an increased risk of all-cause mortality in stage I to III cancer patients (AHR 1.26, 95% CI [0.99–1.59]), as shown in Kaplan-Meier survival curves, the overall survival rate was similar between patients with and without proteinuria (Figure 2A). However, in stage IV patients, proteinuria was a risk factor for mortality, with adjusted hazard ratios of 1.50 (1.17–1.92), in multivariate adjusted Cox proportional hazards models (Figure 2B). When patients were categorized by specific cancer type, the risk of all-cause death was only significant in subjects with digestive system cancer (AHR, 1.85 (1.48–2.32)] after additional adjustments for cancer stage. In contrast, this relationship was not significant for patients with other cancer types. The mortality risk for patients with proteinuria was significantly higher than the risk for those without proteinuria, with adjusted hazard ratios of 1.32 (1.10–1.59) and 1.67 (1.05–2.66) for eGFR at least 60 mL/min/1.73 m2 and <60 mL/min/1.73 m2, respectively, regardless of eGFR values.
TABLE 5.
AHRs of All-Cause Mortality for Participants With Proteinuria Compared With Non-Proteinuria
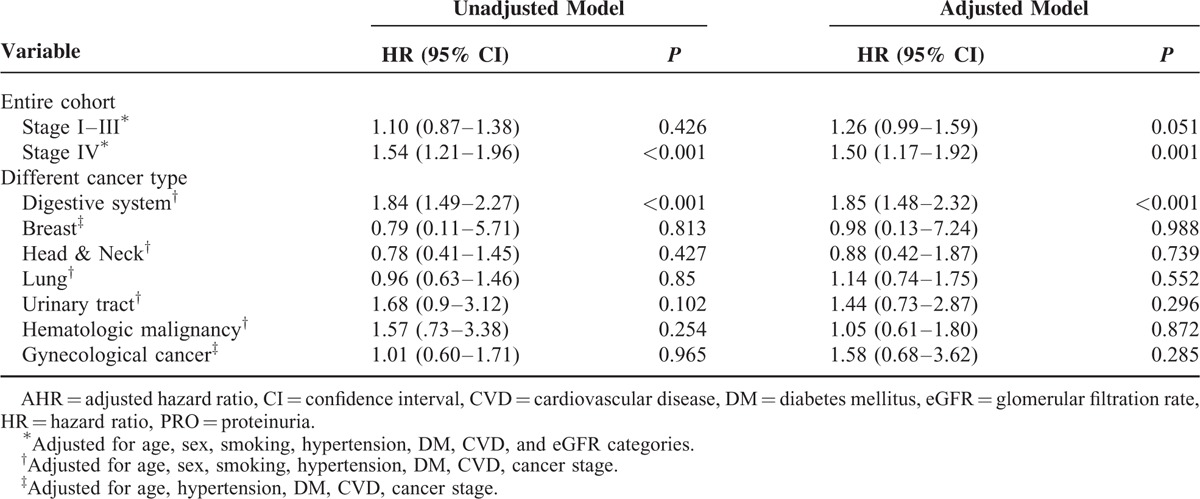
FIGURE 2.
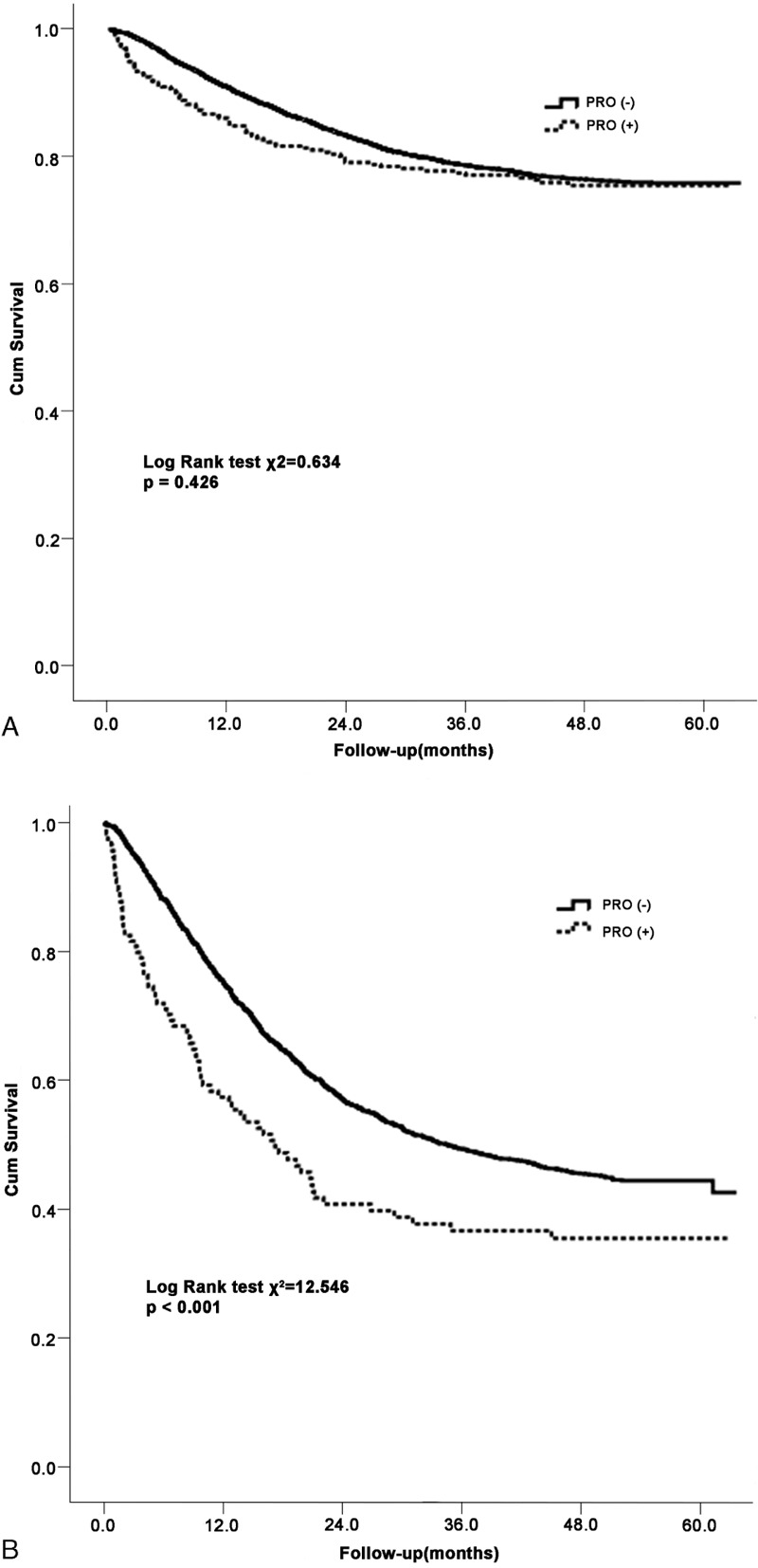
Kaplan-Meier survival curves for all-cause mortality according to proteinuria in patients with cancer stage I to III (A) and stage IV (B).
DISCUSSION
In the present study, we demonstrated that 32.4% of patients with newly diagnosed cancer exhibited renal insufficiency. Renal function was inversely related to all-cause mortality. Compared with eGFR at least 60 mL/min/1.73 m2, eGFR < 60 mL/min/1.73 m2 was an independent predictor of elevated death. This relationship between renal dysfunction and all-cause mortality risk depended on cancer site. After adjustment for confounders, eGFR < 60 mL/min/1.73 m2 was associated with increased mortality risk among patients with hematologic malignancy and gynecological cancer. In addition, proteinuria was a risk factor for mortality among patients with digestive system cancer.
Previous studies showed that renal insufficiency is a frequent comorbidity in cancer patients, despite normal serum creatinine levels in most patients.9,10,28–31 IRMA study, a French national observation of 4684 cancer patients, reported that 7.2% of patients had elevated serum creatinine, whereas 57.4% and 52.9% exhibited an eGFR <90 mL/min/1.73 m2 based on the Cockcroft-Gault formula and the MDRD formula, respectively. The Belgian Renal Insufficiency and Anticancer Medications (BIRMA) study from 7 participating centers showed that 64% had a reduced GFR when estimating the GFR with the MDRD formula. Further, according to CKD-EPI equation, a study from Australian found that 73.3% subjects had eGFR <75 mL/min/1.73 m2. In the present study, we observed that 3% patients had abnormal serum creatinine levels. The prevalence of eGFR < 90 mL/min/1.73 m2 among cancer patients at the time of diagnosis was 32.4% on basis of CKD-EPI equation, which was lower than that in the IRMA and BIRMA studies.9,28 This apparent discrepancy might be explained by the different populations studied, including age, cancer type, other comorbid status, and influence of treatment.11,20–24 Because we enrolled incident rather than prevalent patients and eGFR was obtained at the time of diagnosis, the impact of anticancer treatment on renal function could rule out. In addition, we assessed eGFR using CKD-EPI equation, which has been shown to be more accurately categorized the risk for mortality than did the MDRD formula.25-27
Despite a high prevalence of renal dysfunction in cancer patients, its effect on mortality risk is less well investigated. A larger retrospective analysis of 8223 patients with different malignancies showed that lower eGFR was an independent risk factor for death. Compared with subjects with eGFR at least 60 mL/min/1.73 m2, those with eGFR 30 to 59 and <30 mL/min/1.73 m2 conferred 1.12- and 1.75-fold greater mortality risk, respectively.13 However, they did not demonstrate the distribution of patients by eGFR intervals and examine the impact of cancer stage on mortality. A previous study of 3379 patients with colorectal cancer by Kim et al32 found that renal insufficiency defined as an eGFR < 60 mL/min/1.73 m2 was not significantly associated with mortality both in early stage and advanced stage colorectal cancer. Another study17 investigating various forms of cancer, no significant difference was observed in mortality risk across eGFR levels, compared with eGFR of >90 mL/min/1.73 m2. Our results were somewhat consistent with those previous study that eGFR < 60 mL/min/1.73 m2 was not a risk factor for all-cause mortality among the patients in the entire cohort stratified by cancer stage (I/II/III and IV). These inconsistent results may be due to the sample size in the various eGFR levels, threshold value of eGFR, cancer stage, and follow-up periods.
Previous studies suggested that the influence of eGFR on mortality may vary by the type of cancer.10,16,30 A prospective population-based cohort study by Samuel et al showed eGFR < 60 mL/min/1.73 m2 was a significant risk factor for death from cancer. This eGFR-related mortality was affected by primary site of cancer, with the greatest risk for breast and urinary tract cancer deaths. By contrast, a case-matched, retrospective study of patients with breast cancer suggested that eGFR < 60 mL/min/1.73 m2 appeared to have no significant impact on patient survival.16 Meanwhile, a retrospective cohort study of patients with colorectal cancer after surgical resection suggested that those with eGFR <15 mL/min/1.73 m2 had a poorer overall survival.15 Similar result was also observed in patients with gastric cancer after gastrectomy.14 In agreement with previous studies, our data revealed that eGFR < 60 mL/min/1.73 m2 was associated with higher risk for all-cause mortality among patients with hematologic malignancy and gynecological cancer than that of eGFR > 60 mL/min/1.73 m2, whereas no obvious relationship between renal function and death was observed for other cancers. Despite association of reduced renal function, specific cancer site, and mortality, patients were not stratified by the severity of primary cancer to determine the shape of the renal function-mortality relationship. Moreover, cancer per se may have a strong influence on mortality rather than a reduced kidney function.15,33–35 Therefore, the mechanisms underlying the impact of renal function on mortality among patients with different primary cancer are complex and remain to be elucidated.
Proteinuria is a critical marker of renal disease, but its effect on mortality in cancer patients is unclear. A previous study found that cumulative incidence rates for death from cancer increased among patients with proteinuria.13 Another retrospective analysis with colorectal cancer showed that proteinuria was an important risk factor for mortality in early-stage cancer patients, but not in advanced-stage patients32. Similar with previous study, we found that proteinuria was a risk factor for all-cause mortality in stage IV cancer patients in the entire cohort after controlled confounder, but not in patients with stage I to III. Further, proteinuria showed an increased risk of all-cause mortality among patients with digestive system cancer after adjustments stage, compared with those without proteinuria. These results suggest that assessing and monitoring proteinuria in cancer patients are crucial because of its potential effect on survival.
Our study had several limitations. First, we only used single measurement of renal function at the time of diagnoses, we were unable to determine the association of eGFR change over time with mortality. Second, the number of populations with eGFR <45 mL/min/1.73 m2 was relatively small, which may limit the power of the analysis. Third, the treatment modalities were not considered in our study; they may also affect clinical outcomes. Finally, a retrospective observational study cannot completely exclude the possibility of unmeasured confounders.
In conclusion, renal insufficiency is frequent in patients with cancer at the time of diagnosis. Both eGFR < 60 mL/min/1.73m2 and proteinuria are associated with increased risk for all-cause mortality, which depend on cancer site. Thus, prevention, diagnosis, and management of renal insufficiency may prepare patients for further oncologic therapy and reduce mortality among cancer patients. Nevertheless, these findings need to be confirmed in large and prospective studies.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, BIRMA = the Belgian Renal Insufficiency and Anticancer Medications, BMI = body mass index, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, CVD = cardiovascular disease, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, IRMA = the renal insufficiency and anticancer medications, MDRD = the modification of diet in renal disease, PRO = proteinuria, RI = renal insufficiency, TNM = tumor-node-metastasis, UICC = Union for International Cancer Control.
YY and H-YL contributed equally to this work.
This work was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant no. 2011BAI10B05), the National Basic Research Program of China (grant no. 2012CB517700-2012CB517706), the Guangzhou Committee of Science and Technology, China (grant no. 2010U1-E00831), and 5010 Clinical Program of Sun Yat-sen University (grant no. 2007007). The funders had no role in study design, data collect, and analysis. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.DVM AJ, Bray F, Melissa M, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Are C, Rajaram S, Are M, et al. A review of global cancer burden: trends, challenges, strategies, and a role for surgeons. J Surg Oncol 2013; 107:221–226. [DOI] [PubMed] [Google Scholar]
- 3.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol 2010; 28:4086–4093. [DOI] [PubMed] [Google Scholar]
- 4.Khan R, Apewokin S, Grazziutti M, et al. Renal insufficiency retains adverse prognostic implications despite renal function improvement following Total Therapy for newly diagnosed multiple myeloma. Leukemia 2015; 29:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004; 291:2441–2447. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Kim HS, Kim W, et al. Comorbidity is independently associated with poor outcome in extremity soft tissue sarcoma. Clin Orthop Surg 2015; 7:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non-small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group Trials. J Clin Oncol 2008; 26:54–59. [DOI] [PubMed] [Google Scholar]
- 8.Wu CC, Hsu TW, Chang CM, et al. Age-adjusted Charlson Comorbidity Index Scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine 2015; 94:e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management. Cancer 2007; 110:1376–1384. [DOI] [PubMed] [Google Scholar]
- 10.Iff S, Craig JC, Turner R, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis 2014; 63:23–30. [DOI] [PubMed] [Google Scholar]
- 11.Launay-Vacher V, Etessami R, Janus N, et al. Lung cancer and renal insufficiency: prevalence and anticancer drug issues. Lung 2008; 187:69–74. [DOI] [PubMed] [Google Scholar]
- 12.Pontes Lde B, Antunes YP, Bugano DD, et al. Prevalence of renal insufficiency in elderly cancer patients in a tertiary cancer center. Einstein (São Paulo) 2014; 12:300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol 2011; 33:121–130. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Takayama T, Wakatsuki K, et al. Short-term and long-term outcomes after gastrectomy for gastric cancer in patients with chronic kidney disease. World J Surg 2013; 38:1453–1460. [DOI] [PubMed] [Google Scholar]
- 15.Nozawa H, Kitayama J, Sunami E, et al. Impact of chronic kidney disease on outcomes of surgical resection for primary colorectal cancer: a retrospective cohort review. Dis Colon Rectum 2012; 55:948–956. [DOI] [PubMed] [Google Scholar]
- 16.Dubose AC, Chu QD, Li BD, et al. Is chronic kidney disease an independent risk factor for mortality in breast cancer? J Surg Res 2013; 184:260–264. [DOI] [PubMed] [Google Scholar]
- 17.Königsbrügge O, Lötsch F, Zielinski C, et al. Chronic kidney disease in patients with cancer and its association with occurrence of venous thromboembolism and mortality. Thromb Res 2014; 134:44–49. [DOI] [PubMed] [Google Scholar]
- 18.Currie A, Malietzis G, Askari A, et al. Impact of chronic kidney disease on postoperative outcome following colorectal cancer surgery. Colorectal Dis 2014; 16:879–885. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao LT, Yang CF, Yang SH, et al. Chronic kidney disease stage 5 as the prognostic complement of International Staging System for multiple myeloma. Eur J Haemato 2012; 88:159–166. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Ueno M, Ohkawa S, et al. Renal toxicity associated with weekly cisplatin and gemcitabine combination therapy for treatment of advanced biliary tract cancer. Oncology 2014; 87:30–39. [DOI] [PubMed] [Google Scholar]
- 21.Launay-Vacher V, Ayllon J, Janus N, et al. Drug management of prostate cancer: prevalence and consequences of renal insufficiency. Clin Genitourin Cancer 2009; 7:83–89. [DOI] [PubMed] [Google Scholar]
- 22.Lauritsen J, Mortensen MS, Kier MG, et al. Renal impairment and late toxicity in germ-cell cancer survivors. Ann Oncol 2015; 26:173–178. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Bonventre JV, Parrish AR. The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci 2014; 15:15358–15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung JS, Son NH, Lee SE, et al. Overall survival and renal function after partial and radical nephrectomy among older patients with localised renal cell carcinoma: a propensity-matched multicentre study. Eur J Cancer 2015; 51:489–497. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Me 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal SK, Ruel N, Villegas S, et al. CKD-EPI and Cockcroft-Gault equations identify similar candidates for neoadjuvant chemotherapy in muscle-invasive bladder cancer. PLoS One 2014; 9:e94471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer 2010; 103:1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aapro M, Launay-Vacher V. Importance of monitoring renal function in patients with cancer. Cancer Treatment Rev 2012; 38:235–240. [DOI] [PubMed] [Google Scholar]
- 30.Christensen A, M⊘ller JB, Hasselbalch HC. Chronic kidney disease in patients with the Philadelphia-negative chronic myeloproliferative neoplasms. Leuk Res 2014; 38:490–495. [DOI] [PubMed] [Google Scholar]
- 31.Choi M, Sun CL, Kurian S, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer 2008; 113:1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MJ, Kang YU, Kim CS, et al. Proteinuria as a risk factor for mortality in patients with colorectal cancer. Yonsei Med J 2013; 54:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel P, Henry LL, Ganti AK, et al. Clinical course of lung cancer in patients with chronic kidney disease. Lung Cancer 2004; 43:297–300. [DOI] [PubMed] [Google Scholar]
- 34.de Souza JA, Saliba RM, Patah P, et al. Moderate renal function impairment does not affect outcomes of reduced-intensity conditioning with fludarabine and melphalan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15:1094–1099. [DOI] [PubMed] [Google Scholar]
- 35.Oshima K, Kanda Y, Nanya Y, et al. Allogeneic hematopoietic stem cell transplantation for patients with mildly reduced renal function as defined based on creatinine clearance before transplantation. Ann Hemato 2012; 92:255–260. [DOI] [PubMed] [Google Scholar]


