Abstract
Pregnancy-induced hypertension (PIH) may be a major predictor of pregnancy-associated intracranial hemorrhage (ICH). However, the relationship between PIH and long-term ICH risk is unknown.
The objective of the study was to determine the association between PIH and ICH and to identify the predictive risk factors.
Patients with newly diagnosed PIH were recruited from the Taiwan National Health Insurance Research Database. PIH patients were divided into gestational hypertension (GH) and preeclampsia groups. The 2 groups were separately compared with matched cohorts of patients without PIH based on age and date of delivery. The occurrence of ICH was evaluated in both cohorts. The overall observational period was from January 1, 2000 to December 31, 2013.
Among the 23.3 million individuals registered in the National Health Insurance Research Database, 28,346 PIH patients, including 7390 with GH and 20,956 with preeclampsia, were identified. The incidences of ICH were increased in both groups (incidence rate ratio [IRR] = 3.72 in the GH group, 95% confidence interval [CI] 3.63–3.81, P < 0.0001 and IRR = 8.21 in the preeclampsia group, 95% CI 8.12–8.31, P < 0.0001, respectively). In addition, according to the results of stratification of follow-up years, both groups were associated with a highest risk of ICH at 1 to 5 years of follow-up (IRR = 11.99, 95% CI 11.16–12.88, P < 0.0001 and IRR = 21.83, 95% CI 21.24–22.44, P < 0.0001, respectively). After adjusting for age, parity, severity of PIH, number of PIH occurrences, gestational age, and comorbidities in the multivariate survival analysis using Cox regression model, age ≥30 years (hazard ratio [HR] 1.99, 95% CI 1.27–3.10, P = 0.0026), patients with preeclampsia (HR 2.18, 95% CI 1.22–3.90, P = 0.0089), multiple PIH occurrences (HR 4.08, 95% CI 1.85–9.01, P = 0.0005), hypertension (HR 4.51, 95% CI 1.89–10.74, P = 0.0007), and obesity (HR 7.21, 95% CI 1.58–32.84, P = 0.0107) were independent risk factors for the development of ICH among patients with PIH.
Patients with PIH, especially those with older age, preeclampsia, and multiple PIH occurrences, may have an increased risk of developing ICH later in life.
INTRODUCTION
Pregnancy-induced hypertension (PIH), which includes gestational hypertension (GH) and preeclampsia, is a leading cause of maternal morbidity and mortality.1,2 Preeclampsia complicates approximately 3% to 5% of pregnancies,3,4 and is generally defined as the de novo development of hypertension and proteinuria arising after 20 weeks of gestation in previously normotensive women.5,6 Although the pathogenesis of preeclampsia remains unclear, the central hypothesis strongly suggests that impaired trophoblast invasion of the spiral arteries occurs during early pregnancy, which contributes to a failure in spiral artery remodeling and subsequent progressive insufficient utero-placental blood flow. As a consequence of placental ischemia, several antiangiogenic factors, reactive oxygen species, and inflammatory cytokines are released, leading to widespread endothelial dysfunction, microangiopathy, and vasospasm, which precede the onset of symptomatic clinical disease.7–11
Pregnancy-associated intracranial hemorrhage (ICH) is an uncommon but potentially life-threatening event that markedly contributes to maternal mortality. Preeclampsia is one of the risk factors for pregnancy-associated ICH.12–17 Patients with pregnancy-associated ICH have poorer prognoses for both the mother and fetus compared with other etiologies.12,18 Possible effects of preeclampsia on systemic vessels include endothelial dysfunction, microangiopathy, and vasospasm. Additionally, high blood pressure persistently damages vessel walls and leads to rupture and bleeding. Concurrent ICH may be the end-stage manifestation of preeclampsia.12 In a study by Bateman et al,15 GH and preeclampsia/eclampsia were significant independent risk factors for ICH during pregnancy, and accounted for 30.5% of such cases. In a study by Oudghiri et al,19 a patient with preeclampsia at 31 weeks of gestation developed a spontaneous subdural hematoma. However, few studies have investigated the relationship between PIH and long-term ICH risk. In addition, the association between GH and subsequent ICH risk was seldom investigated. We hypothesized that a history of PIH, including GH and preeclampsia, increases the risk of subsequent ICH. To test our hypothesis, we designed a nationwide population-based matched cohort study to assess the incidence of ICH among women with a history of PIH.
METHODS
Data Sources
The National Health Insurance program in Taiwan was initiated in 1995, and approximately 98% of the population has utilized this coverage. The National Health Research Institute (NHRI) established a National Health Insurance research database (NHIRD), from which we obtained data for this study. This database contains insurance claims for 23.3 million beneficiaries from 2000 through 2013. The NHRID safeguards the privacy of individuals and provides data to researchers who have ethical approval. We obtained anonymous data from NHRID; therefore, the identities of the patients could not be determined. The Kaohsiung Veterans General Hospital (VGHKS15-EM4–01) Institutional Review Board approved this study.
Study Design and Participants
Patients with PIH who were >20 but <50 years of age were assessed according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 642.3 to 642.6, including patients with GH (ICD-9-CM codes 642.30, 642.31, 642.32, 642.33, and 642.34) and preeclampsia (ICD-9-CM codes 642.40, 642.41, 642.42, 642.43, 642.44, 642.50, 642.51, 642.52, 642.53, and 642.54). To ensure diagnostic validity and to avoid any potential misclassifications, only patients with a diagnosis of PIH and inpatient hospitalization were selected. Patients were not eligible for enrollment in this cohort study if they had a history of gestational diabetes mellitus or ICH. A total of 28,346 PIH patients were assessed for this study. PIH patients were divided into GH and preeclampsia groups. For each patient with GH or preeclampsia included in the PIH cohort, 4 age and the date of delivery-matched patients without PIH were randomly selected from the NHIRD and were included in the comparison cohort. GH group and preeclampsia group were matched with control groups separately. The reason we took the date of delivery for matching because the diagnostic criteria for PIH have changed over time. Furthermore, prevention and management strategies of PIH and diagnostic accuracy of ICH improved over time. Both the GH patients, or preeclampsia patients and comparison patients were followed until the development of ICH, death, or the end of 2013. In our study, sample size was determined to achieve 90% statistical power at 2-sided type I error rate of 5%. Under the assumption, 1:1 matching was not considered because the matching process would provide the maximum possible statistical power at around 65% while analyzing the risk of subsequent ICH development among the patients with GH. Therefore, 1:4 matching was used instead.
Definitions of the Clinical Endpoints and Follow-up
The index date for the patients in the PIH cohort was the date of initial PIH diagnosis. The study endpoint was defined as the date of ICH diagnosis (ICD-9-CM: 430–432) or death during the 13-year follow-up period (2000–2013). The pregnancy characteristics of the patients were recorded, including age, parity, gestational age, gestational number, and comorbidities. The comorbidities in our study included diabetes mellitus (ICD-9-CM: 250), hypertension (ICD-9-CM: 401–405), obesity (ICD-9-CM: 278.0), dyslipidemia (ICD-9-CM: 272), chronic kidney disease (ICD-9-CM: 585 and 403), and chronic obstructive pulmonary disease (COPD) (ICD-9-CM: 491.2, 493.2, and 496).
Statistical Analysis
The incidence of newly diagnosed ICH in the PIH patients and matched controls was assessed. We calculated ICH incidence rates (per 10,000 person-years) and incidence rate ratios (IRRs). The study groups were compared using the chi-square test for categorical variables. A Kaplan–Meier analysis was used to calculate the cumulative incidence rates for ICH between the study cohorts and matched cohorts, and the log-rank test was used to analyze the differences between survival curves. A Cox proportional-hazards model was used to identify the risk factors for ICH in patients with PIH. The qualifying criterion for inclusion in the multivariate analysis was a result in the univariate analysis, with a P value less than 0.1. SAS version 9.4 (SAS System for Windows) was used for data analysis. Comparisons with a P value <0.05 were considered significant.
RESULTS
Participant Characteristics
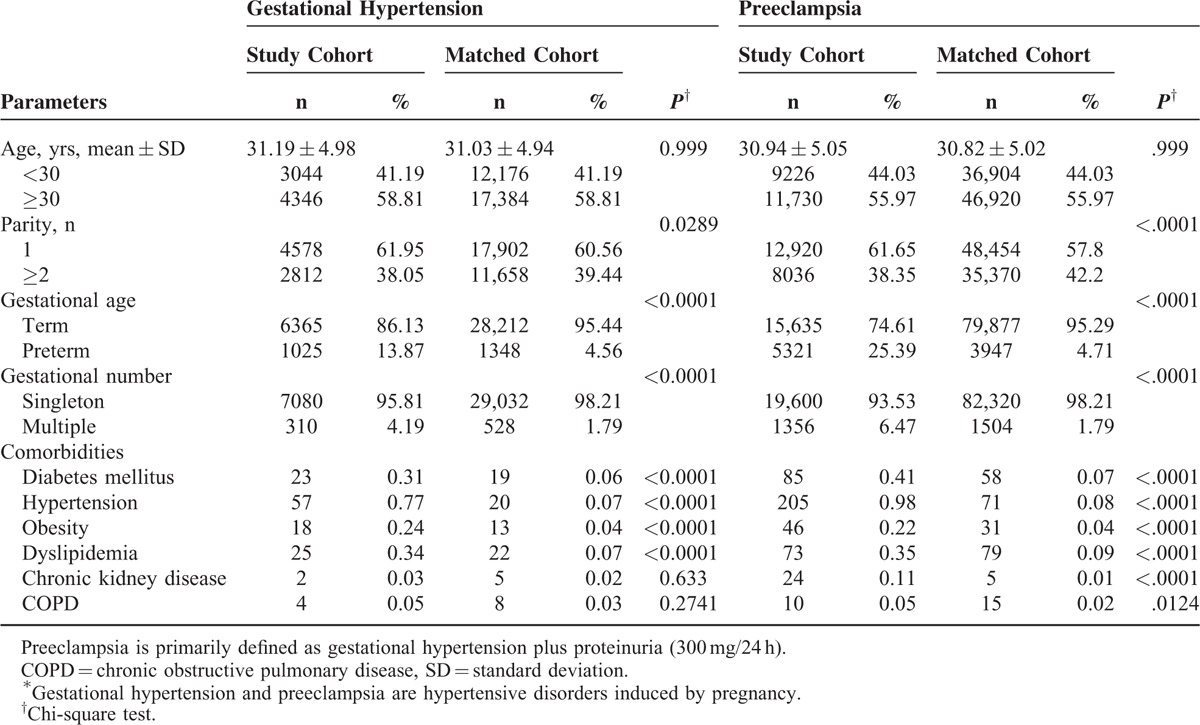
A total of 28,346 patients with PIH (including 7390 with GH and 20,956 with preeclampsia) and matched cohort of 113,384 subjects (including 29,560 matched with GH patients and 83,824 matched with preeclampsia patients) were identified for this study. Table 1 presents the demographics and comorbidities of the PIH patients and matched subjects. The mean patient ages were 31.19 ± 4.98 and 30.94 ± 5.05 years for GH and preeclampsia groups, respectively. The majority of patients were older than 30 years in both the GH (58.81%) and preeclampsia (55.97%) groups. When compared with matched cohorts, patients in both the GH and preeclampsia groups had lower parity, higher preterm birth, and higher multiple pregnancy rates. Furthermore, patients with GH had a higher prevalence of diabetes mellitus (DM), hypertension (HTN), obesity, and dyslipidemia; patients with preeclampsia had a higher prevalence of DM, HTN, obesity, dyslipidemia, chronic kidney disease, and COPD.
TABLE 1.
Baseline Characteristics of Patients With Pregnancy-induced Hypertension∗ and matched cohort
Incidence of ICH
Table 2 shows the risk for ICH in PIH patients stratified by age and follow-up years.
TABLE 2.
Incidence Risk Ratios of Intracranial Hemorrhage in Patients With Pregnancy-induced Hypertension and Matched Cohort
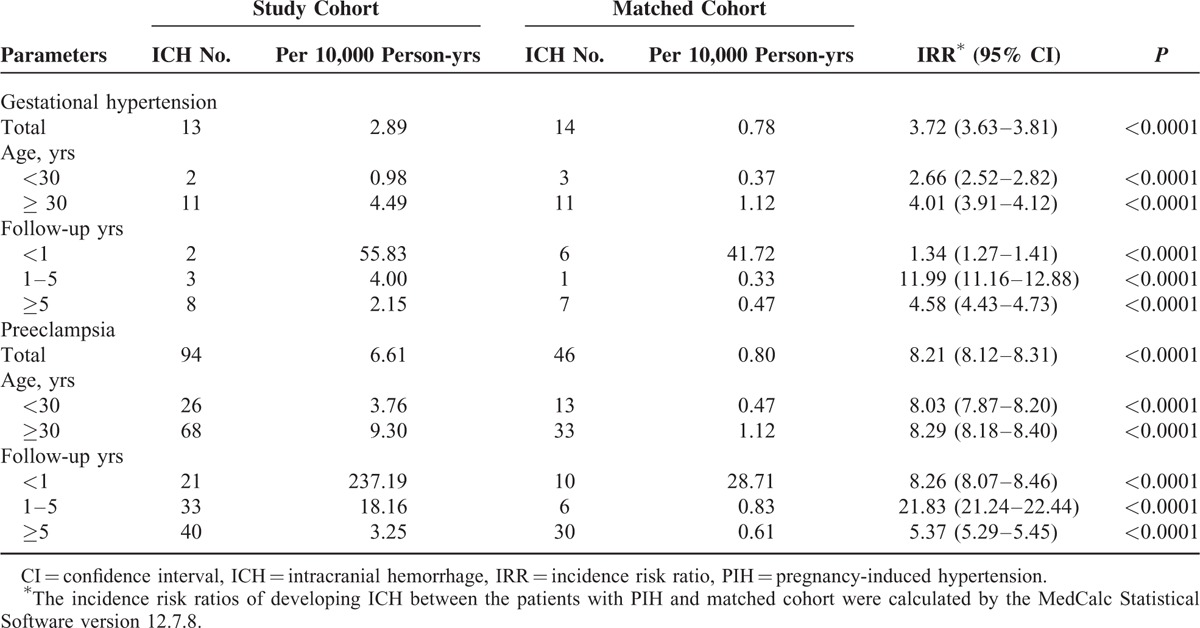
In GH group, during the 13-year follow-up period, the incidence rates for ICH in patients with GH and the matched cohorts were 2.89 and 0.78 per 10,000 person-years, respectively. Patients with GH had a significantly higher risk of ICH than patients without GH (IRR = 3.72, 95% confidence interval [CI] 3.63–3.81, P < 0.0001). After stratifying patients according to age, both patients aged <30 years (IRR = 2.66, 95% CI 2.52–2.82, P < 0.0001) and aged ≥30 (IRR = 4.01, 95% CI 3.91–4.12, P < 0.0001) had a higher risk of ICH than matched cohorts. After stratifying patients according to follow-up duration, the ICH risk was the most pronounced in the 1 to 5-year follow-up (IRR = 11.99, 95% CI 11.16–12.88, P < 0.0001). According to a Kaplan–Meier analysis, GH patients were associated with higher cumulative incidence rates for ICH than patients in the comparison cohort (log-rank P = 0.0002) in Figure 1.
FIGURE 1.
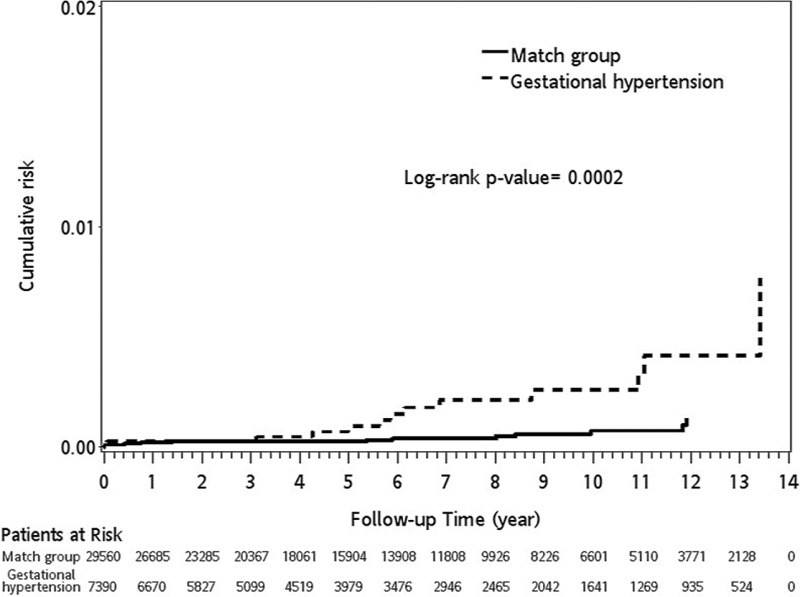
The cumulative incidence (%) of intracranial hemorrhage (ICH) in patients with gestational hypertension (GH) and matched controls. Kaplan–Meier curve comparing the cumulative incidence of ICH over time in GH patients with matched controls. The survival functions are significantly different by the log-rank test, with ICH occurring more frequently in the patients with GH than the matched group (P = 0.0002, log-rank test).
In preeclampsia group, during the 13-year follow-up period, the incidence rates for ICH in patients with preeclampsia and matched subjects were 6.61 and 0.80 per 10,000 person-years, respectively. Patients with preeclampsia had a significantly higher risk of ICH than those without preeclampsia (IRR = 8.21, 95% CI 8.12–8.31, P < 0.0001). After stratifying patients according to age, both patients aged <30 years (IRR = 8.03, 95% CI 7.87–8.20, P < 0.0001) and aged ≥30 (IRR = 8.29, 95% CI 8.18–8.40, P < 0.0001) had a higher risk of ICH than matched cohorts. We also stratified the patients according to follow-up duration and observed that the IRRs were most pronounced in the 1 to 5-year follow-up (IRR = 21.83, 95% CI 21.24–22.44). Based on a Kaplan–Meier analysis, the log-rank test indicated that preeclampsia patients had significantly higher cumulative incidence rates of ICH than patients in the matched cohort (log-rank P < 0.0001) in Figure 2.
FIGURE 2.
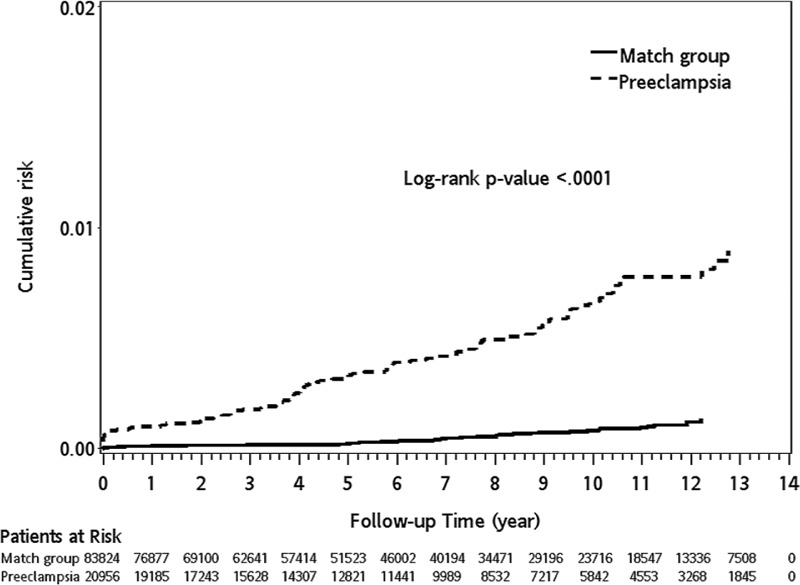
The cumulative incidence (%) of intracranial hemorrhage (ICH) in patients with preeclampsia and matched controls. Kaplan–Meier analysis of the cumulative incidence of ICH over time among patients with preeclampsia and the matched group. The survival curves were compared using the log-rank test. Although the results are similar to those shown in Figure 1, the difference in Figure 2 is more significant by the log-rank test (P < 0.0001) than the results of Figure 1. However, the Kaplan–Meier curve revealed that subsequent ICH occurs much earlier and the overall ICH risk is much higher in the patients with preeclampsia (eg, a more severe form of pregnancy-induced hypertension than gestational hypertension) than the matched group.
Risk Factors for ICH in Patients With PIH
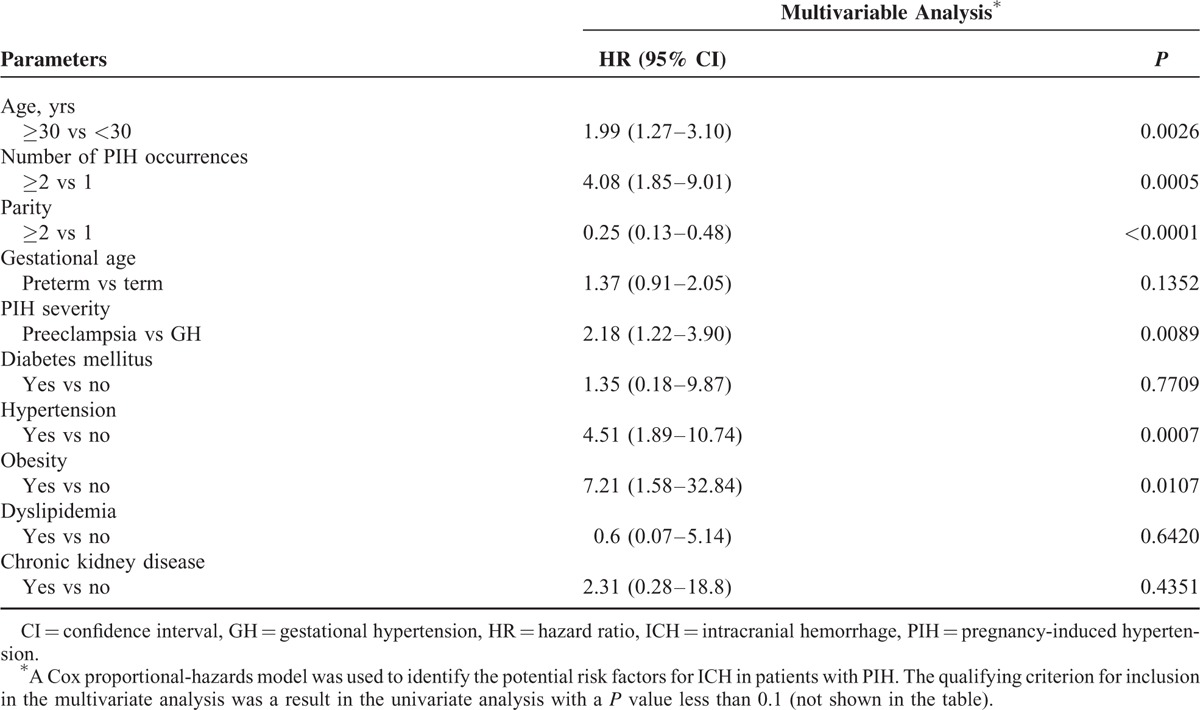
As demonstrated in the multivariate analyses, the independent risk factors for the development of ICH among the PIH patients included age ≥30 years (hazard ratio [HR] = 1.99, 95% CI 1.27–3.10, P = 0.0026), multiple PIH occurrences (HR = 4.08, 95% CI 1.85–9.01, P = 0.0005), PIH severity (HR = 2.18, 95% CI 1.22–3.90, P = 0.0089), hypertension (HR = 4.51, 95% CI 1.89–10.74, P = 0.0007), and obesity (HR = 7.21, 95% CI 1.58–32.84, P = 0.0107). However, multiparity was a protective factor against ICH among patients with PIH (HR = 0.25, 95% CI 0.13–0.48, P < 0.0001).
DISCUSSION
The estimated pregnancy-associated ICH rates range from 3.8 to 18.1 per 100,000 deliveries.15,17,20–22 Mortality rates resulting from pregnancy-associated ICH range from 9% to 38%,13–16,18 and permanent neurologic deficits occur in 40% of patients.15 Preeclampsia plays a pivotal role in the development of pregnancy-associated ICH. The possible contributions7,8,12,18,23–25 included endothelial dysfunction, microangiopathy, and vasospasm of brain vessels; increased cerebral perfusion pressure and brain capillary permeability; disturbances of cerebral blood flow auto-regulation and subsequent cerebral hyperperfusion leading to vasodilation and brain edema; and thrombocytopenia or coagulation factors (as predisposing factors). Notably, preeclampsia is an important etiology of pregnancy-associated ICH. Eclampsia or preeclampsia rates reported in pregnant women with ICH range from 14% to 57.5%.14,16,21 Bateman et al demonstrated that GH (odds ratio [OR] 2.41, 95% CI 1.62–3.59) and preeclampsia/eclampsia (OR 10.39, 95% CI 8.32–12.98) were independent predictors of pregnancy-related intracerebral hemorrhage.15 Although the ICH associated with preeclampsia is typically intraparenchymal, subdural hematomas or subarachnoid hemorrhages have also been observed,19,21,26 including our study. However, most studies followed patients for only 6 weeks after delivery. Thus, the long-term ICH risks after PIH are unknown. After a follow-up of 13 years, the present study indicated an ICH incidence that was 3.72-fold higher in the GH cohort (95% CI 3.63–3.81, P < 0.0001) and 8.21-fold higher in the preeclampsia cohort (95% CI 8.12–8.31, P < 0.0001) than in their respective comparison cohorts (Table 2). This finding supports our hypothesis that PIH is a substantial risk factor for long-term ICH.
The incidence of pregnancy-associated ICH is highest during the postpartum period.15,27 Rapid changes of hormonal and hemodynamic changes during the postpartum period may promote the development ICH due to effects on coagulation and blood vessel walls.15 Nevertheless, some studies have shown that ICH primarily occurred during the antepartum period, especially the third trimester.18 A study by Tang et al28 revealed that preeclampsia or eclampsia significantly increased stroke risk during pregnancy and the first postpartum year. The present study's long-term follow-up revealed that the ICH risk was the most prominent for years 1 to 5 in both the GH (IRR = 11.99, 95% CI 11.16–11.88, P < 0.0001) and the preeclampsia cohorts (IRR = 21.83, 95% CI 21.24–22.44, P < 0.0001) when compared with their respective matched cohorts. These risks remained significant at ≥5 years postpartum (Table 2). We hypothesize that the effects of PIH on brain vessels, including endothelial dysfunction or microangiopathy, are irreversible and progressive, leading to brain vessel rupture and bleeding later in life. Thus, years 1 to 5 after delivery have the most ICH risk in patients with PIH. However, more studies are required to confirm these results.
Intracranial hemorrhage has a worldwide incidence estimated at 10 to 20 per 100,000 deliveries. ICH is associated with the poorest mortality rate of any stroke subtype and results in fatalities in 40% to 50% of patients within 30 days. Functional dependency after ICH is also high (reaching 75%).29–31 The most important risk factor for ICH is hypertension.32–35 A systemic review and meta-analysis revealed hypertension was a central contributor of subarachnoid hemorrhages.36 Obesity seems to have an impact on the risk of ICH. A multicenter, observational study conducted by Pezzini et al37 indicated that obesity was associated with increased risk of deep intracerebral hemorrhage, but had no effect on the risk of lobar intracerebral hemorrhage. A single-center prospective study showed that extremes of body mass index (BMI), which included low BMI (<18.5 kg/m2) and very high BMI (>30.0 kg/m2), increased risk of deep intracerebral hemorrhage, but not lobar intracerebral hemorrhage.38 Matsukawa et al39 demonstrated that patients with pontine hemorrhage had a higher proportion of obese individuals. As demonstrated in our multivariate analyses, hypertension (HR 4.51, 95% CI 1.89–10.74, P = 0.0007) and obesity (HR 7.21, 95% CI 1.58–32.84, P = 0.0107) were independent risk factors for ICH among PIH patients. PIH is also a pivotal risk factor for ICH. As shown in Table 3, our analysis revealed increased PIH severity and occurrences were associated with higher ICH risk among patients with PIH. Additionally, age ≥30 years was an independent risk factor for ICH in PIH patients (HR 1.99, 95% CI 1.27–3.10, P = 0.0026) (Table 3). Advanced maternal age is also an independent predictor of pregnancy-related ICH.15 A systematic review and meta-analysis conducted by van Asch et al31 demonstrated that the incidence of ICH increases with age. However, in our study, multiparity was protective against ICH (HR 0.25, 95% CI 0.13–0.48, P < 0.0001) (Table 3). Our result contrasts the findings of Jung et al,40 who indicated that an increased number of childbirth was related to an increased risk of ICH. More studies are needed to clarify this discrepancy.
TABLE 3.
Analyses of Risk Factors for Subsequent Intracranial Hemorrhage Among the Patients With Pregnancy-induced Hypertension
This study was a longitudinal, large population-based design. Nonetheless, several limitations inherent to the use of insurance claims databases must be considered. First, the diagnosis of PIH in the NHIRD was based on the ICD-9-CM code. Information on blood pressure, proteinuria, and symptoms could not be obtained from the database. Second, many demographic variables were not present in the database, such as socioeconomic status, BMI, lifestyle, smoking status, and family medical history. These factors could have been useful for assessing other factors that may be associated with PIH or ICH. Third, information on the cause of ICH was not available; consequently, we cannot conclude that the etiology of ICH is related to PIH or other factors, such as arteriovenous malformations or cerebral aneurysms. Finally, the diagnostic criteria for PIH have changed over the years, which could lead to heterogeneous populations across studies and may limit comparisons. Despite these limitations, our study was based on a nationwide, population-based database that included nearly all of Taiwan's residents. The large sample size in our study contributed to its substantial statistical power and revealed an obvious association between PIH and ICH with minimal selection biases.
CONCLUSIONS
In conclusion, PIH was associated with a significant increase in ICH risk later in life. Preeclampsia may have higher ICH risk than GH. In addition, older age, multiple PIH occurrences, hypertension, and obesity were independent risk factors for ICH.
Acknowledgment
We are grateful for the use of the National Health Insurance Research Database provided by the Statistic Center of Department of Health and Welfare.
Footnotes
Abbreviations: CI = confidence interval, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, GH = gestational hypertension, HTN = hypertension, ICD-9-CM = International Classification of Diseases, Ninth Revision, clinical modification, ICH = intracranial hemorrhage, IRR = incidence rate ratio, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, PIH = pregnancy-induced hypertension, SD = standard deviation.
This study was supported by grants (VGHKS15-EM4–01) from Kaohsiung Veterans General Hospital and the Taiwan Health Promotion Administration.
Disclosures: The interpretations and conclusions contained herein do not represent those of the Bureau of Health Promotion, Taiwan, R.O.C.
The authors report no conflicts of interest.
REFERENCES
- 1.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014; 2:e323–e333. [DOI] [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet (London, England) 2006; 367:1066–1074. [DOI] [PubMed] [Google Scholar]
- 3.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25:391–403. [DOI] [PubMed] [Google Scholar]
- 4.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013; 209:e512.544 e541-544. [DOI] [PubMed] [Google Scholar]
- 5.Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet (London, England) 2010; 376:631–644. [DOI] [PubMed] [Google Scholar]
- 6.Magee LA, Pels A, Helewa M, et al. Canadian Hypertensive Disorders of Pregnancy Working G. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 2014; 4:105–145. [DOI] [PubMed] [Google Scholar]
- 7.Chaiworapongsa T, Chaemsaithong P, Yeo L, et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nature Rev Nephrol 2014; 10:466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warrington JP, George EM, Palei AC, et al. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension 2013; 62:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy 2002; 21:205–223. [DOI] [PubMed] [Google Scholar]
- 10.Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern-Fetal Neonat Med 2012; 25:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrish MR, Murphy SR, Rutland S, et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 2010; 23:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang ZW, Lin L, Gao WL, et al. A clinical characteristic analysis of pregnancy-associated intracranial haemorrhage in China. Sci Rep 2015; 5:9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott CA, Bewley S, Rudd A, et al. Incidence, risk factors, management, and outcomes of stroke in pregnancy. Obstetrics and gynecology 2012; 120 (2 Pt 1):318–324. [DOI] [PubMed] [Google Scholar]
- 14.Liang CC, Chang SD, Lai SL, et al. Stroke complicating pregnancy and the puerperium. Eur J Neurol 2006; 13:1256–1260. [DOI] [PubMed] [Google Scholar]
- 15.Bateman BT, Schumacher HC, Bushnell CD, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology 2006; 67:424–429. [DOI] [PubMed] [Google Scholar]
- 16.Cantu-Brito C, Arauz A, Aburto Y, et al. Cerebrovascular complications during pregnancy and postpartum: clinical and prognosis observations in 240 Hispanic women. Eur J Neurol 2011; 18:819–825. [DOI] [PubMed] [Google Scholar]
- 17.James AH, Bushnell CD, Jamison MG, et al. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 2005; 106:509–516. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimatsu J, Ikeda T, Katsuragi S, et al. Factors contributing to mortality and morbidity in pregnancy-associated intracerebral hemorrhage in Japan. J Obstet Gynaecol Res 2014; 40:1267–1273. [DOI] [PubMed] [Google Scholar]
- 19.Oudghiri N, Behat M, Elchhab N, et al. Spontaneous subdural hematoma associated with preeclampsia: a case report and litterature review. Pan Afr Med J 2014; 19:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davie CA, O’Brien P. Stroke and pregnancy. J Neurol Neurosurg Psychiatry 2008; 79:240–245. [DOI] [PubMed] [Google Scholar]
- 21.Jeng JS, Tang SC, Yip PK. Incidence and etiologies of stroke during pregnancy and puerperium as evidenced in Taiwanese women. Cerebrovasc Dis (Basel, Switzerland) 2004; 18:290–295. [DOI] [PubMed] [Google Scholar]
- 22.Jaigobin C, Silver FL. Stroke pregnancy. Stroke 2000; 31:2948–2951. [DOI] [PubMed] [Google Scholar]
- 23.Belfort MA, Saade GR, Grunewald C, et al. Association of cerebral perfusion pressure with headache in women with pre-eclampsia. Br J Obstet Gynaecol 1999; 106:814–821. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham FG, Twickler D. Cerebral edema complicating eclampsia. Am J Obstet Gynecol 2000; 182 (1 Pt 1):94–100. [DOI] [PubMed] [Google Scholar]
- 25.Zeeman GG, Hatab M, Twickler DM. Maternal cerebral blood flow changes in pregnancy. Am J Obstet Gynecol 2003; 189:968–972. [DOI] [PubMed] [Google Scholar]
- 26.Djoubairou BO, Onen J, Doleagbenou AK, et al. Chronic subdural haematoma associated with pre-eclampsia: case report and review of the literature. Neurochirurgie 2014; 60:48–50. [DOI] [PubMed] [Google Scholar]
- 27.Salonen Ros H, Lichtenstein P, Bellocco R, et al. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology (Cambridge, Mass) 2001; 12:456–460. [DOI] [PubMed] [Google Scholar]
- 28.Tang CH, Wu CS, Lee TH, et al. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke 2009; 40:1162–1168. [DOI] [PubMed] [Google Scholar]
- 29.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation 2007; 116:e391–e413. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 31.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9:167–176. [DOI] [PubMed] [Google Scholar]
- 32.Thrift AG, McNeil JJ, Forbes A, et al. Three important subgroups of hypertensive persons at greater risk of intracerebral hemorrhage. Melbourne Risk Factor Study Group. Hypertension 1998; 31:1223–1229. [DOI] [PubMed] [Google Scholar]
- 33.Ariesen MJ, Claus SP, Rinkel GJ, et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 2003; 34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 34.Feldmann E, Broderick JP, Kernan WN, et al. Major risk factors for intracerebral hemorrhage in the young are modifiable. Stroke 2005; 36:1881–1885. [DOI] [PubMed] [Google Scholar]
- 35.Inagawa T. Risk factors for primary intracerebral hemorrhage in patients in Izumo City, Japan. Neurosurg Rev 2007; 30:225–234.[discussion 234]. [DOI] [PubMed] [Google Scholar]
- 36.Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36:2773–2780. [DOI] [PubMed] [Google Scholar]
- 37.Pezzini A, Grassi M, Paciaroni M, et al. Obesity and the risk of intracerebral hemorrhage: the multicenter study on cerebral hemorrhage in Italy. Stroke 2013; 44:1584–1589. [DOI] [PubMed] [Google Scholar]
- 38.Biffi A, Cortellini L, Nearnberg CM, et al. Body mass index and etiology of intracerebral hemorrhage. Stroke 2011; 42:2526–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsukawa H, Shinoda M, Fujii M, et al. Impact of body mass index on the location of spontaneous intracerebral hemorrhage. World Neurosurg 2013; 79:478–483. [DOI] [PubMed] [Google Scholar]
- 40.Jung SY, Bae HJ, Park BJ, et al. Parity and risk of hemorrhagic strokes. Neurology 2010; 74:1424–1429. [DOI] [PubMed] [Google Scholar]


