Abstract
Treatment of periodontal diseases has been associated with benefit outcomes for patients with chronic obstructive pulmonary disease (COPD). However, no population-based cohort study has been conducted. We evaluated this relationship by retrospective cohort study using a large population data.
Using the National Health Insurance claims data of Taiwan, we identified 5562 COPD patients with periodontal diseases who had received periodontal treatment as the treatment group. The comparison group was selected at a 1:1 ratio matched by the propensity score estimated with age, sex, date of COPD diagnosis and periodontal treatment, and comorbidities. Both groups were followed up for 5 years to compare risks of acute exacerbation, pneumonia, and acute respiratory failure.
The incidence rates of adverse respiratory events were significantly lower in the treatment group than in the comparison group: 3.79 versus 4.21 per 100 person-years for emergency room visits, 2.75 versus 3.65 per 100 person-years for hospitalizations, and 0.66 versus 0.75 per 100 person-years for intensive care unit admissions. The treatment group also had a 37% reduced risk of deaths (1.81 vs 2.87 per 100 person-years), with an adjusted hazard ratio of 0.57 (95% confidence interval 0.52–0.62).
Periodontal treatment for COPD patients could reduce the risk of adverse respiratory events and mortality. The adequate periodontal health care is important for COPD patients with periodontal diseases.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder with chronic inflammation in the lungs and airways. Persistent airflow limitation is a common condition, usually progressive, for patients with COPD. Smoking and other noxious pollutant exposure can destruct parenchymal tissue and disrupt the repair mechanism, leading to COPD.1 A clinical diagnosis of COPD is generally made for any individual with shortness of breath, chronic cough, or sputum production, especially for individuals with a history of smoking or other noxious particle exposure. Spirometry is usually required to evaluate the presence of persistent airflow limitation.
Periodontal disease is any disorder of the soft tissue surrounding and supporting the teeth, such as gingivitis and periodontitis, mainly caused by a bacterial biofilm accumulating on the teeth.2 Other genetic and environmental factors also contribute to these conditions.3,4 Several studies have demonstrated an association between COPD and periodontal diseases.5–7 A recent study in Taiwan showed that patients with COPD are 1.2-fold more likely than the general population to develop periodontal diseases (32.2 vs 26.4 per 1000 person-years).8
Periodontal diseases are also chronic inflammatory disorders associating with systemic inflammation, with the pathophysiological characteristics similar to COPD.9 Studies have revealed that periodontal treatment can improve the outcomes of COPD.10–12 Their findings suggest that the improved periodontal health can reduce the risk of COPD exacerbation. In a prospective study, Kucukcoskun et al11 found that patients with both COPD and periodontitis receiving periodontal treatment have reduced COPD exacerbations. A recent randomized controlled trial also reported that periodontal therapy for COPD patients with periodontitis can not only improve the pulmonary function but also reduce the exacerbation events.12 However, these studies involved small sample sizes focusing on the beneficial effects of pulmonary function and acute exacerbations.
We, therefore, proposed to further investigate whether periodontal treatment for COPD patients with periodontal diseases can reduce the risk of adverse respiratory events including acute exacerbation, pneumonia, and acute respiratory failure (ARF), using the National Health Insurance (NHI) database of Taiwan, which is a nationwide longitudinal data with medical claims of 23 million people. Several studies used the reliable claims data to investigate issues related to COPD and periodontal diseases.13–16
METHODS
Data Source
This study obtained the NHI claims data from the National Health Research Institutes (NHRI) of Taiwan. Reformed in 1995, the single-payer health insurance program has covered approximately 99% of the total population of Taiwan.17 NHRI has cooperated with the Bureau of National Health Insurance to establish several data sets for research. We used the Longitudinal Health Insurance Database 2000 (LHID2000), which contained medical records of 1,000,000 insured people randomly selected from all people registered in 2000. The database was updated annually, with all personal identifications encrypted to protect patient's privacy. The International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) was used to identify diagnoses of diseases. This study was approved by the Research Ethic Committee, China Medical University and Hospital (CMUH-104-REC2-115).
Study Design
All patients with a history of COPD (ICD-9-CM codes 491, 492, and 496) in 2000 to 2006 were identified as the potential study population (N = 126,251). Among them, we identified 21,066 patients with periodontal diseases (ICD-9-CM code 523) who had received periodontal treatment and 23,878 patients without periodontal diseases (Figure 1). The treatment of periodontal diseases, depending on the severity of the diseases, included basic form of subgingival curettage (scaling [91004C], root planing [91006C, 91007C, 91008C]) in phase I and invasive form of periodontal flap surgery (91009B, 91010B) in phase II. Subgingival curettage procedure removes the plaque through a deep-cleaning method. Scaling is to scrape off the tartar above and below the gum line. Root planing is to get rid of rough spots on the tooth root. Periodontal flap surgery is provided to patients with severe condition if the phase I efforts cannot cure the diseases. Gums are separated from the teeth and folded back temporarily during the surgery, which cleans the tooth roots and repairs the damaged bone. In the phase I treatment, patients may make 4 to 6 visits in 1 week or 2 weeks. The phase II surgical treatment is retained for severe cases.18,19
FIGURE 1.
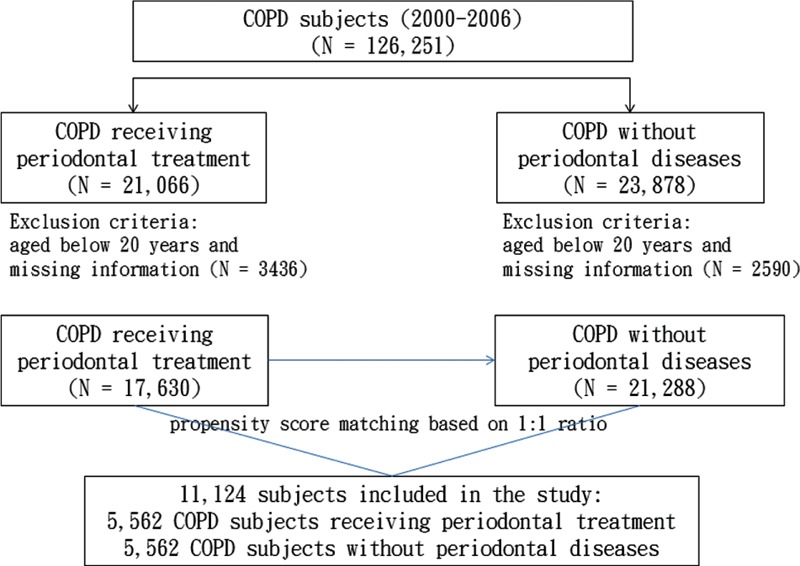
Flow chart showing selection of study subjects.
We calculated propensity score for each patient aged 20 years and above with completed demographic information as the potential study population. The propensity score was calculated using a logistic regression to estimate the probability of the treatment assignment based on the baseline variables, including age, sex, year of COPD diagnosis and periodontal treatment, and comorbidities of hypertension (ICD-9-CM codes 401–405), diabetes (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), chronic kidney disease (CKD) (ICD-9 code 585), smoking-related diseases (smoking-related cancers [ICD-9-CM codes 140–149, 150, 151, 154, 155, 156, 162, 180], stroke [ICD-9-CM codes 430–438], and coronary artery disease [CAD] (ICD-9-CM codes 410–414]), tobacco use disorders (ICD-9-CM code 305.1), and alcohol-related diseases (ICD-9-CM codes 291, 303, 305.0, 571.0–571.3, 790.3, and V11.3).20,21 The C-statistic of the logistic regression model was 0.52. A group of COPD patients with periodontal treatment (n = 5562) and a comparison group of COPD patients without periodontal diseases (n = 5562) were established and matched by the propensity score.
For each subject, follow-up person-years were counted from the inclusion date until an adverse respiratory event diagnosed, or a maximum of 5 years, or until the subject was censored due to death or withdrawal from the insurance system. The primary outcomes of adverse respiratory events in this study were defined as the acute exacerbation of COPD, pneumonia (ICD-9-CM code 486), and ARF (ICD-9-CM code 518.81).22 Emergency room (ER) uses and hospitalization records for these events and deaths were assessed.
Statistical Analysis
Data analysis first compared distributions of baseline demographic status and comorbidities between the treatment and comparison groups, using chi-square test to examine categorical variables and Wilcoxon rank-sum test to compare continuous variables. The demographic variables included age, sex, urbanized level of subject's resident area, occupation, and income. Based on the population density, urbanization levels were stratified into 4 levels; 1 indicated the highest urbanization and 4 the lowest. Occupations were grouped into white collar, blue collar, and other groups; retired, unemployed, or low-income populations were included in the other group. The variables of comorbidity included hypertension, diabetes, hyperlipidemia, CKD, smoking-related diseases (smoking-related cancers, stroke, and CAD), tobacco use disorders, and alcohol-related diseases.
The follow-up person-years were calculated to assess the incidence density rates of adverse respiratory events by the ER use and hospitalization. The Poisson regression model was used to assess the treatment group to the comparison group incidence rate ratios (IRRs) and the 95% confidence interval (CI). Incidence rates of adverse respiratory events and the corresponding IRRs were also calculated by age, sex, and comorbidities. We further estimated all-cause mortality rates for the 2 groups and the associated rate ratios (RRs). Multivariable Poisson regression model was also used to calculate adjusted IRR or RR after controlling for age, sex, residential area, occupation, income, and comorbidities of hypertension, diabetes, hyperlipidemia, CKD, smoking-related diseases (smoking-related cancers, stroke, and CAD), tobacco use disorders, and alcohol-related diseases. Data analyses were performed using SAS Version 9.4 (SAS Institute Inc., Carey, NC), and P < 0.05 was considered statistically significant by the 2-sided t test.
RESULTS
Comparisons of Baseline Characteristics Between Treatment and Comparison Groups
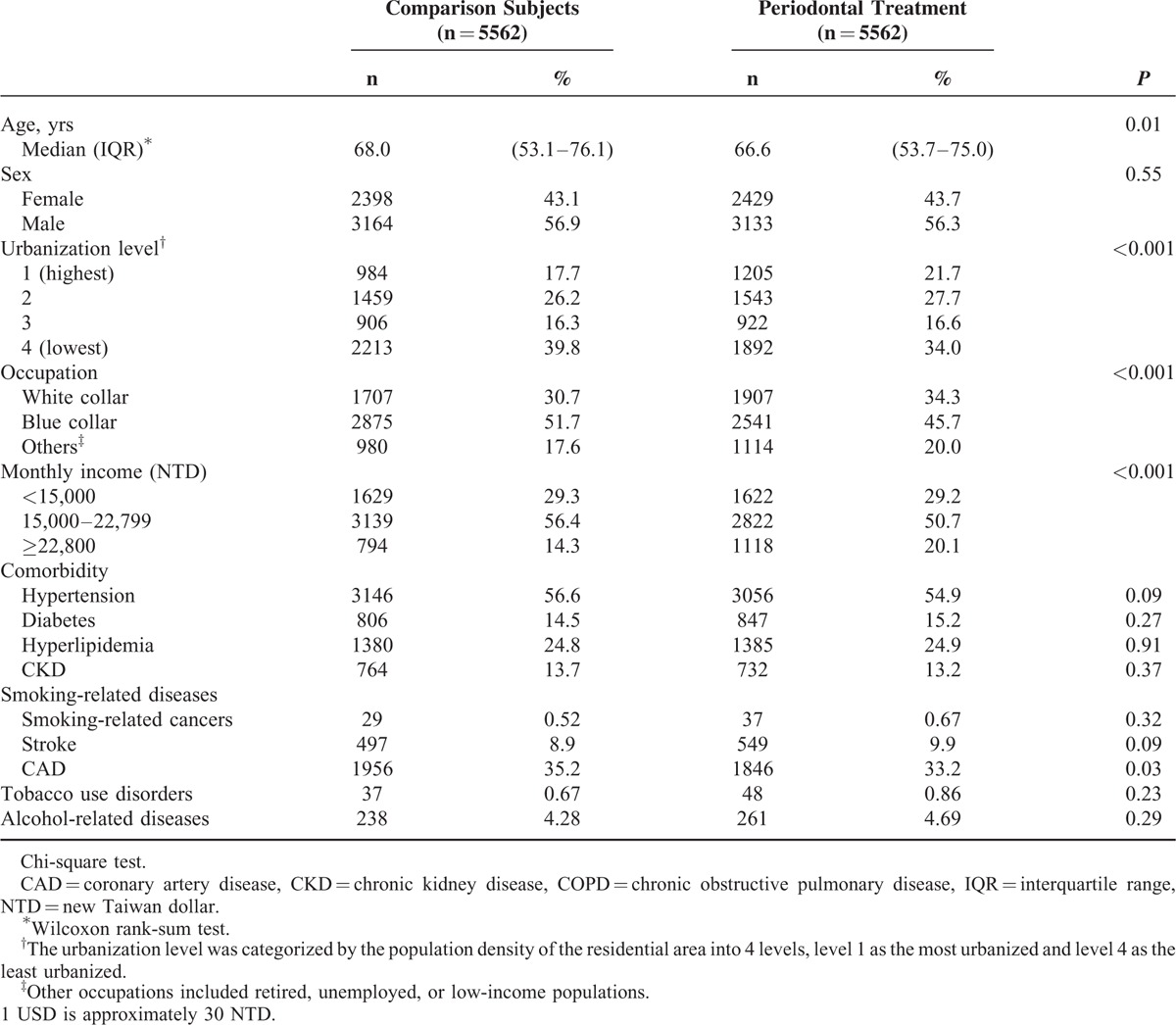
Table 1 shows that over 56% of study populations were men. The periodontal treatment group was slightly younger, but tended to live in more urbanized areas, and to have white collar jobs and better income. Most of the baseline comorbidities were not different significantly between the 2 groups, except CAD.
TABLE 1.
Baseline Demographic Status and Comorbidity in COPD Patients With Periodontal Diseases Underwent Treatment and Comparison Subjects
Comparison of Adverse Respiratory Events Between Treatment and Comparison Groups
During the 5-year follow-up period, all the 3 types of incident adverse respiratory events were lower in the treatment group than in the comparison group for ER uses and hospitalizations. The overall ER use rate for adverse respiratory events were 3.79 versus 4.21 per 100 person-years, respectively, with an adjusted IRR of 0.87 (95% CI 0.80–0.95) (Table 2). The corresponding overall hospitalization rates of adverse respiratory events in the 2 groups were 2.75 versus 3.65 per 100 person-years, with an adjusted IRR of 0.74 (95% CI 0.69–0.80). The incident intensive care unit (ICU) admission was also lower in the treatment group, with an adjusted IRR of 0.84 (95% CI 0.75–0.94).
TABLE 2.
Poisson Regression Analysis Estimated Periodontal Treatment Group to Comparison Group Incidence Rate Ratios of Adverse Respiratory Events Associated With Emergency Use and Hospitalization
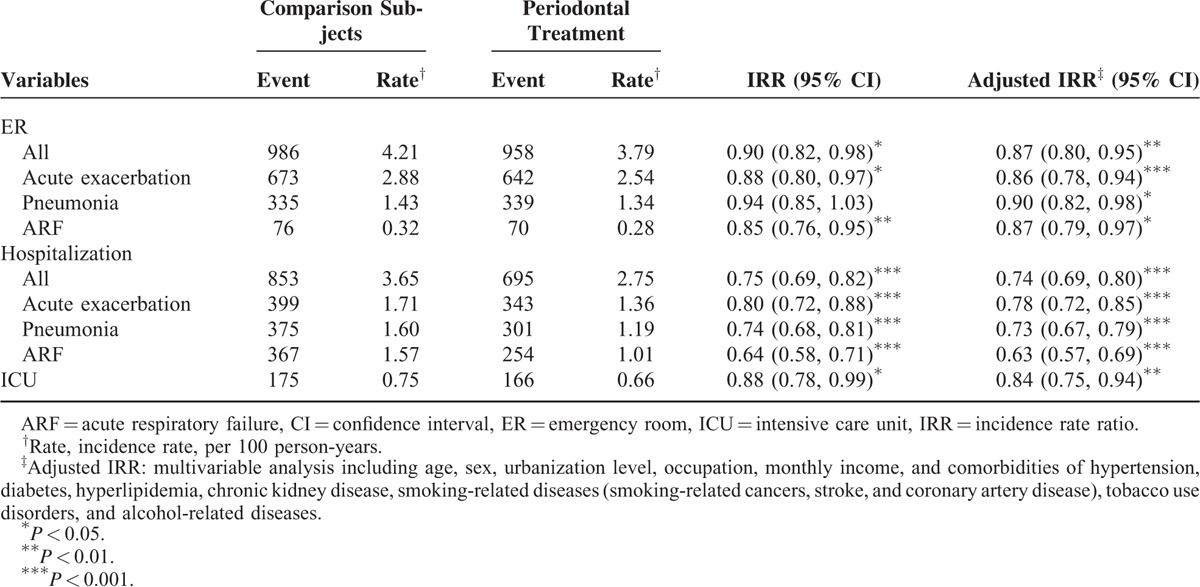
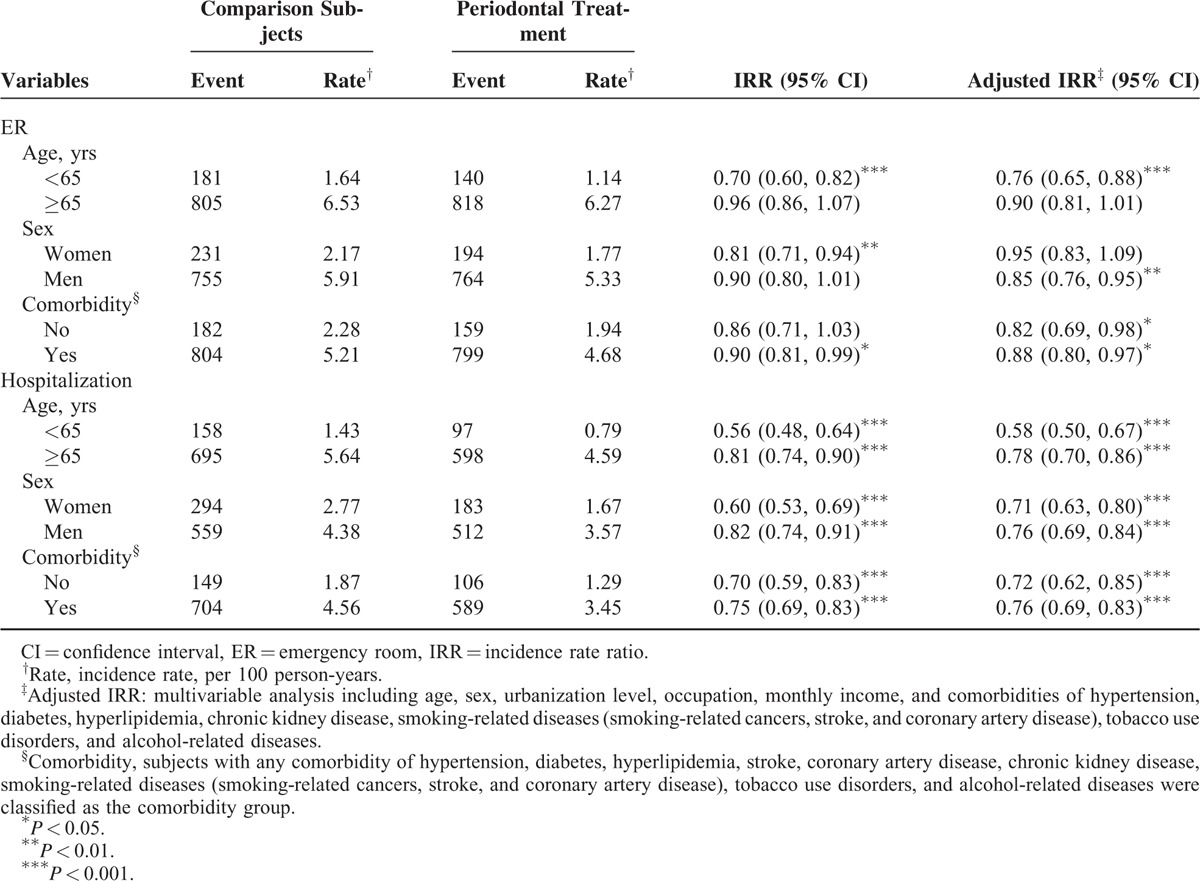
Table 3 shows the overall incident adverse respiratory events and the treatment group to the comparison group IRRs by age, sex, and presence or absence of comorbidity. The risk measures of adverse respiratory events were all lower in the treatment group than in the comparison group. The adjusted IRRs showed that the difference between groups were significant for younger group, men, and those with comorbidity.
TABLE 3.
Poisson Regression Analysis Estimated Periodontal Treatment Group to Comparison Group Incidence Rate Ratios of Adverse Respiratory Events Associated With Emergency Use and Hospitalization by Age, Sex, and Comorbidity
Comparison of Mortality Between Treatment and Comparison Groups
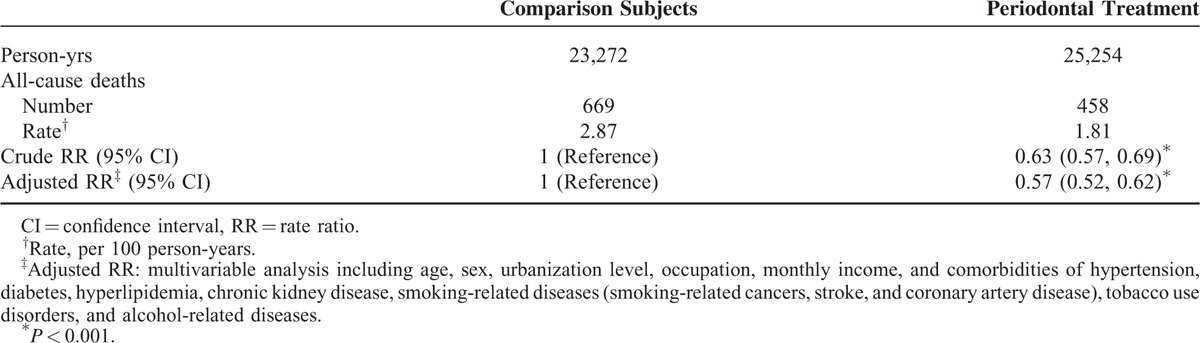
Moreover, the all-cause mortality was 37% lower in the treatment group than in the comparison group (1.81 vs 2.87 per 100 person-years), with an adjusted RR of 0.57 (95% CI 0.52–0.62) (Table 4).
TABLE 4.
Mortality Rate and Poisson Regression Analysis Estimated Periodontal Treatment Group to Comparison Group Rate Ratio
DISCUSSION
To the best of our knowledge, this is the first population-based cohort study to prove periodontal treatment is associated with reduced risks of adverse respiratory events in COPD populations. The incident acute exacerbations, pneumonia, and ARF in COPD patients with periodontal diseases receiving periodontal treatment were all reduced. Furthermore, we found that COPD patients in the treatment group have less medical demands either in ER visits, hospitalizations, and even ICU admissions for these adverse respiratory events. In addition, our study showed that the all-cause mortality rate was significantly lower in periodontal treatment group than in the comparison group.
It is well known that COPD is a disorder of systemic inflammation, which is characterized with systemic oxidative stress, activated circulating inflammatory cells, and increased inflammatory cytokines.23 Periodontal diseases are also disorders of systemic inflammation mainly associated with host immune and inflammatory responses to oral pathogens.24 Mechanisms have been proposed to explain how periodontal diseases could influence the status of COPD. In a recent literature review, Linden et al25 have indicated that aspirated plaque organisms, circulating inflammatory substances, and even plaque organisms in the periodontal lesions play an important role in COPD inflammation.
Periodontal treatment can reduce the dental plaque and the hematogenous dissemination of inflammatory mediators. The mouth is an important reservoir for the potential respiratory pathogens. Aspirating a large amount of virulent pathogen-contaminated saliva into the lung may cause pneumonia or acute exacerbation of COPD.26 A previous study has found in the sputum of patients with a COPD exacerbation an elevated antibody level against Fusobacterium nucleatum and Prevotella intermedia.27 These aspirated anaerobic organisms are likely implicated in periodontal diseases. Periodontal treatment can effectively reduce plaque and oral mucosa colonization. In addition, periodontal treatment can reduce serum level of inflammatory biomarkers. It may improve the systemic inflammatory process.28 Therefore, the treatment may help to prevent airway inflammation and exacerbations.
In a 1-year prospective controlled trial, Kucukcoskun et al11 found the periodontal treatment could reduce exacerbation frequency in COPD patients (n = 20) from 3.00 ± 1.83 to 1.95 ± 1.46. The median of exacerbation values declined from 3 to 2 in the treatment group and increased from 2 to 3 in the control group. In another 2-year follow-up randomized controlled trial, Zhou et al12 also found that the periodontal disease treatment improved lung function slightly in COPD patients after 1-year and 2-year follow-up. In addition, they found the periodontal treatment for COPD patients with periodontal diseases reduced those with frequent exacerbation from 15/40 to 8/40 within 1 year. In the present population study, we observed a similar benefit for COPD patients with periodontal treatment in a much greater sample size.
In an ideal study design, the comparison group in the present study should be COPD patients with periodontal diseases, but without periodontal treatment. However, in an insurance system, as long as a person is diagnosed with disease, the relative treatment is prescribed. Even in a prospective study design, it would be an ethic violation if the disease is not treated in the comparison group. Therefore, we selected COPD patients without periodontal diseases for our comparison group.
Previous studies have reported that 50% to 90% of adults worldwide suffer from periodontal diseases (depending on the definition of the medical term).2 However, the present study reflected a “real world” scenario wherein periodontal diseases were diagnosed by a real medical consultation. Patients with periodontal diseases in the treatment group were believed to have greater disease severity. Therefore, a superior outcome in COPD population with periodontal treatment reflects that it is of a great clinical significance to improve the periodontal health.
The strength of this study is using a longitudinal population-based data to evaluate the benefit of treating the periodontal diseases for COPD patients. A retrospective cohort study using insurance or register data is a suitable and economical alternative. However, there are several limitations to be considered when interpreting the present findings. First, diseases diagnosed by physician were coded with the ICD-9-CM algorithm. To prevent coding errors, we included only diseases with at least 2 physician visits within 1 year. This is particularly accurate for periodontal diseases. Furthermore, reimbursement claims were randomly selected for evaluation by an ad hoc committee to prevent violation. Second, NHI research database does not provide detailed information on the severity/scores of periodontal diseases and COPD, family history, education, body mass index, environmental factors, smoking habits, alcohol consumption, and diet preference. Information on image reports, laboratory tests, and pulmonary function tests was also unavailable for our study.29 Third, this study was conducted based on the data of a single ethnic population. It is not clear whether our findings can be generalized to other population groups. However, the beneficial treatment effectiveness found in this study would be worthwhile to verify with study in other population.
CONCLUSIONS
This study suggests that periodontal treatment is associated with a lower risk of adverse respiratory events in COPD population of Taiwan. The benefits include not only reduced uses of emergency services, hospitalization, and ICU admission but also reduced mortality. The establishment and maintenance of periodontal health for patients with both COPD and periodontal diseases may be an important issue.
Footnotes
Abbreviations: ARF = acute respiratory failure, CAD = coronary artery disease, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, ER = emergency room, ICD-9-CM = International Classification of Disease, 9th Revision, Clinical Modification, ICU = intensive care unit, IQR = interquartile range, IRR = incidence rate ratio, LHID2000 = Longitudinal Health Insurance Database 2000, NHI = National Health Insurance, NHRI = National Health Research Institutes.
F-CS and C-HK contributed equally.
Authors’ contributions: Conception and design—T-CS, P-YC, C-HC, and C-YT; administrative support—T-CH, C-MS, W-HH, F-CS, and C-HK; collection and assembly of data—all authors; data analysis and interpretation—T-CS, C-LL, F-CS, and C-HK; manuscript writing—T-CS, P-YC, C-LL, F-CS, and C-HK; manuscript revision—T-CS, P-YC, C-LL, F-CS, and C-HK; final approval of manuscript—all authors.
Funding: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Conflict of interest: There are no competing interests.
REFERENCES
- 1.Global strategy for diagnosis, management, and prevention of COPD. December 2015. Available at: http://www.goldcopd.org. [DOI] [PubMed] [Google Scholar]
- 2.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366:1809–1820. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 2004; 34:9–21. [DOI] [PubMed] [Google Scholar]
- 4.Jordan RC. Diagnosis of periodontal manifestations of systemic diseases. Periodontol 2000 2004; 34:217–229. [DOI] [PubMed] [Google Scholar]
- 5.Zeng XT, Tu ML, Liu DY, et al. Periodontal disease and risk of chronic obstructive pulmonary disease: a meta-analysis of observational studies. PLoS One 2012; 7:e46508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledić K, Marinković S, Puhar I, et al. Periodontal disease increases risk for chronic obstructive pulmonary disease. Coll Antropol 2013; 37:937–942. [PubMed] [Google Scholar]
- 7.Öztekin G, Baser U, Kucukcoskun M, et al. The association between periodontal disease and chronic obstructive pulmonary disease: a case control study. COPD 2014; 11:424–430. [DOI] [PubMed] [Google Scholar]
- 8.Shen TC, Chang PY, Lin CL, et al. Risk of periodontal diseases in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study. Medicine 2015; 94:e2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usher AK, Stockley RA. The link between chronic periodontitis and COPD: a common role for the neutrophil? BMC Med 2013; 11:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Zhang W, Zhang J, et al. Oral hygiene, periodontal health and chronic obstructive pulmonary disease exacerbations. J Clin Periodontol 2012; 39:45–52. [DOI] [PubMed] [Google Scholar]
- 11.Kucukcoskun M, Baser U, Oztekin G, et al. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J Periodontol 2013; 84:863–870. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Han J, Liu Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol 2014; 41:564–572. [DOI] [PubMed] [Google Scholar]
- 13.Shen TC, Chung WS, Lin CL, et al. Does chronic obstructive pulmonary disease with or without type 2 diabetes mellitus influence the risk of lung cancer? Result from a population-based cohort study. PLoS One 2014; 9:e98290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen TC, Chen WC, Lin CL, et al. The risk of erectile dysfunction in chronic obstructive pulmonary disease: a population-based cohort study in Taiwan. Medicine 2015; 94:e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CF, Lin MC, Lin CL, et al. Non-apnea sleep disorder increases the risk of periodontal disease: a retrospective population-based cohort study. J Periodontol 2014; 85:e65–e71. [DOI] [PubMed] [Google Scholar]
- 16.Wen BW, Tsai CS, Lin CL, et al. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM 2014; 107:283–290. [DOI] [PubMed] [Google Scholar]
- 17.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015; 175:1527–1529. [DOI] [PubMed] [Google Scholar]
- 18.Huang ST, Lin CL, Yu TM, et al. Intensive periodontal treatment reduces risk of infection-related hospitalization in hemodialysis population: a nationwide population-based cohort study. Medicine 2015; 94:e1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aljateeli M, Koticha T, Bashutski J, et al. Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: a randomized clinical trial. J Clin Periodontol 2014; 41:693–700. [DOI] [PubMed] [Google Scholar]
- 20.Chung CW, Wang JD, Yu CF, et al. Lifetime medical expenditure and life expectancy lost attributable to smoking through major smoking related diseases in Taiwan. Tob Control 2007; 16:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai SW, Lin CL, Liao KF. Atorvastatin use associated with acute pancreatitis: a case-control study in Taiwan. Medicine 2016; 95:e2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung WS, Lai CY, Lin CL, et al. Adverse respiratory events associated with hypnotics use in patients of chronic obstructive pulmonary disease: a population-based case-control study. Medicine 2015; 94:e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glass RT, Conrad RS, Bullard JW, et al. Evaluation of microbial flora found in previously worn prostheses from the Northeast and Southwest regions of the United States. J Prosthet Dent 2010; 103:384–389. [DOI] [PubMed] [Google Scholar]
- 24.Barros SP, Suruki R, Loewy ZG, et al. A cohort study of the impact of tooth loss and periodontal disease on respiratory events among COPD subjects: modulatory role of systemic biomarkers of inflammation. PLoS One 2013; 8:e68592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Clin Periodontol 2013; 40 suppl 14:S8–S19. [DOI] [PubMed] [Google Scholar]
- 26.Devlin J. Patients with chronic obstructive pulmonary disease: management considerations for the dental team. Br Dent J 2014; 217:235–237. [DOI] [PubMed] [Google Scholar]
- 27.Brook I, Frazier EH. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest 2003; 124:832–833. [DOI] [PubMed] [Google Scholar]
- 28.Koromantzos PA, Makrilakis K, Dereka X, et al. Effect of non-surgical periodontal therapy on C-reactive protein, oxidative stress, and matrix metalloproteinase (MMP)-9 and MMP-2 levels in patients with type 2 diabetes: a randomized controlled study. J Periodontol 2012; 83:3–10. [DOI] [PubMed] [Google Scholar]
- 29.Shen TC, Lin CL, Wei CC, et al. Risk of obstructive sleep apnea in adult patients with asthma: a population-based cohort study in Taiwan. PLoS One 2015; 10:e0128461. [DOI] [PMC free article] [PubMed] [Google Scholar]


