Abstract
Atrial fibrillation (AF) is one of the most frequent arrhythmias in clinical practice. Previous studies have reported the influence of AF on patients with heart failure (HF). The effect of AF on the non-HF critically ill patients in a medical intensive care unit (ICU) remains largely unclear. The study aimed to investigate the impact of AF presenting on ICU admission on the weaning outcome of non-HF mechanically ventilated patients in a medical ICU.
A retrospective observational case–control study was conducted over a 1-year period in a medical ICU at Taipei Veterans General Hospital, a tertiary medical center in north Taiwan. Non-HF mechanically ventilated patients who were successful in their spontaneous breathing trial and underwent ventilator discontinuation were enrolled. The primary outcome measure was the ventilator status after the first episode of ventilator discontinuation.
A total of 285 non-HF patients enrolled were divided into AF (n = 62) and non-AF (n = 223) groups. Compared with the non-AF patients, the AF patients were significantly associated with old age (P = 0.002), a higher rate of acute respiratory distress syndrome causing respiratory failure (P = 0.015), a higher percentage of sepsis before liberation from mechanical ventilation (MV) (P = 0.004), and a higher serum level of blood urea nitrogen on the day of liberation from MV (P = 0.003). Multivariate logistic regression analysis demonstrated that AF independently increased the risk of weaning failure [adjusted odds ratio (AOR), 3.268; 95% confidence interval (CI), 1.254–8.517; P = 0.015]. Furthermore, the AF patients were found to be independently associated with a high rate of ventilator dependence (log rank test, P = 0.026), prolonged total ventilator use (AOR, 1.979; 95% CI, 1.032–3.794; P = 0.040), increased length of ICU stay (AOR, 2.256; 95% CI, 1.049–4.849; P = 0.037), increased length of hospital stay (AOR, 2.921; 95% CI, 1.363–6.260; P = 0.006), and increased ICU mortality (AOR, 4.143; 95% CI, 1.381–12.424; P = 0.011).
AF on ICU admission is an independent risk factor for weaning failure and significantly associated with poor hospital outcome in non-HF mechanically ventilated patients in a medical ICU.
INTRODUCTION
Atrial fibrillation (AF) is recognized as one of the most frequent arrhythmias in clinical practice.1–4 The prevalence of AF ranges from 6% to 26% in noncardiac adult medical ICUs.5–8 In critically ill patients, AF is associated with increased mortality and a prolonged ICU stay, and increased hospital stay.6,9–11 Numerous studies reporting the influence of AF on short- and long-term patient outcomes have been conducted in surgical and mixed ICUs where postoperative and/or heart failure (HF) patients are enrolled.12–18 Few studies focus on the non-HF critically ill patient of medical ICUs and investigate the impact of AF on the clinical outcome in this patient population. Therefore, the influence of AF on non-HF patients in a medical ICU remains incompletely understood.
The weaning outcome in critically ill patients receiving mechanical ventilation (MV) is frequently studied and potentially influenced by AF. The irregular rhythm and chaotic atrial activity associated with AF can cause various potentially deleterious hemodynamic consequences that may exert a negative influence on the weaning process of mechanically ventilated patients.3,14 In patients with exacerbated chronic respiratory failure, Marcelino et al19 recently reported that patients presenting with AF on ICU admission require a significantly long length of MV. However, the influence of AF on ventilator outcomes, including successful weaning of ventilator, ventilator days, and ventilator dependence, in a medical ICU has not yet been investigated and remains unknown.
In the present study, we conducted an observational retrospective case-control study to investigate the influence of AF on the weaning outcome in non-HF mechanically ventilated patients in a medical ICU. We hypothesized that AF on ICU admission may have an adverse effect on the weaning outcome and increase the burden of respiratory critical care in this patient group.
METHODS
Design of the Study
A retrospective observational case–control study was conducted in a 35-bed medical ICU of Taipei Veterans General Hospital, a tertiary medical center in Taiwan. The study was approved by the Institutional Ethical Review Board of Taipei Veterans General Hospital (approval number: VGHTPE-IRB No. 2015-02-006AC), and informed consent was waived for this retrospective study. Patients who were admitted to the ICU for intensive critical care and ventilator weaning during the study period between January 2011 and December 2011 were reviewed. Non-HF mechanically ventilated patients who were successful in their spontaneous breathing trial (SBT) and underwent ventilator discontinuation were enrolled. Patients were excluded from the study if they were receiving noninvasive positive pressure ventilation (NIPPV) on ICU admission, they had a diagnosis of HF on ICU admission, they had a history of cardiac surgery, they had a history of arrhythmia except AF, there was newly diagnosed HF during the hospitalization, AF was terminated within 7 days of onset or there was a new onset of AF during the ICU stay, the occurrence of AF was due to medication and/or electrolyte imbalance, and this was a repeat ICU admission. To evaluate the effect of AF on the weaning outcome, patients with factors known to influence the weaning process and SBT were also excluded. The enrolled patients were divided into the AF and non-AF groups (Figure 1).
FIGURE 1.
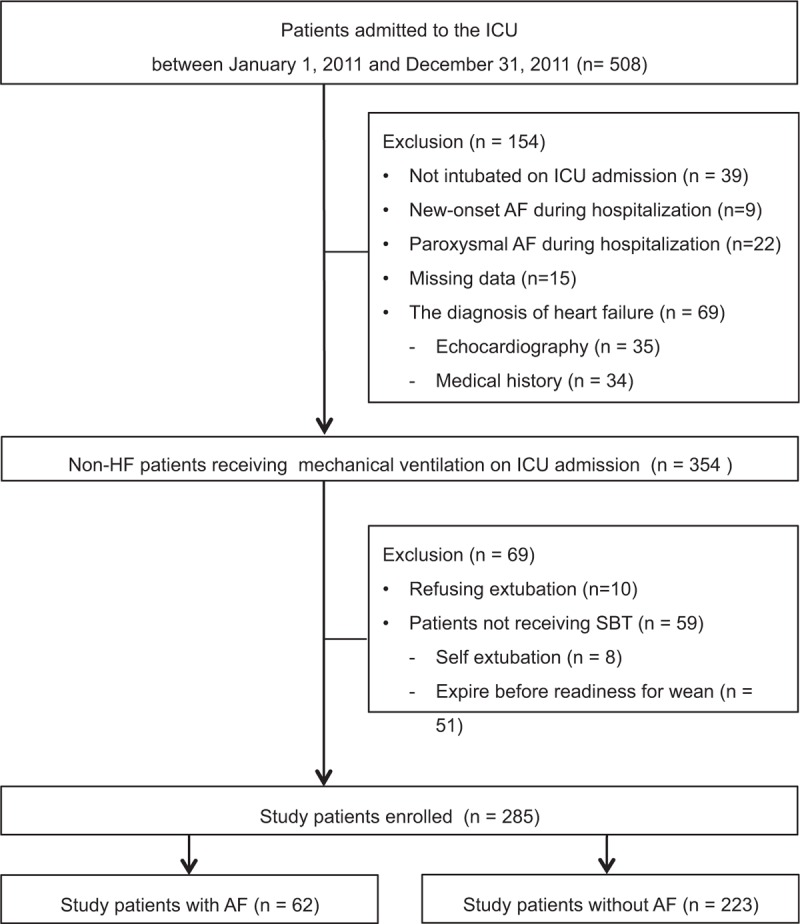
Flow chart of the study. ICU = intensive care unit, HF = heart failure, SBT = spontaneous breathing trial, RFW = readiness for weaning, AF = atrial fibrillation.
Subjects and Measurements
The departmental log of the ICU was used to identify the patients. The medical charts of all consecutive appropriate patients admitted to the ICU during the study period were carefully reviewed and recorded. The following information was collected, namely age, sex, Acute Physiology and Chronic Health Evaluation (APACHE) II score on ICU admission,20 body mass index, the cause(s) of respiratory failure, comorbidities, the patient's laboratory data on ICU admission, the patient's laboratory data on the day of liberation from MV, any ICU events, the weaning parameters on the day of readiness for weaning (RFW), the length of ICU stay and the length of hospital stay. The weaning outcome, ICU mortality, and hospital mortality were also recorded. If the patient enrolled had several episodes of attempted weaning from MV, only the first episode was analyzed. The primary outcome measure was the status of ventilator status after the first episode of ventilator discontinuation.
Weaning Protocol
When the patients recovered from the disease(s) causing respiratory failure, they were assessed as to whether they met the following criteria: adequate cough ability and/or an acceptable conscious state, adequate oxygenation: PaO2 ≥ 60 mm Hg and FIO2 ≤ 40% with PEEP ≤ 8 cmH2O or PaO2/FiO2 > 150 mm Hg, afebrile (BT < 38°C), a stable cardiovascular status: HR ≤ 140/min, SBP 90–160 mm Hg with no or minimal vasopressors, a stable metabolic status with acceptable electrolyte levels, no significant respiratory acidosis, and an adequate mental state with spontaneous breathing ability. Subsequently, the patient's rapid shallow breathing index (RSBI, respiratory rate/tidal volume ratio, breaths per liter per minute) was checked. At least 2 physicians were used to confirm the criteria for weaning every day. After the RSBI of patients was less than 105 breaths per liter per minute, the patients were considered suitable for RFW. These patients then received a T-piece or pressure support ventilation of 5 cmH2O with PEEP of 5 cmH2O for 60 minutes as SBT. Data regarding their arterial blood gas before SBT within 24 hours and immediately after SBT were recorded. The heart rate of the study patients was also recorded before and immediately after SBT. For those patients who could tolerate SBT without any symptoms or signs of respiratory distress, we performed extubation or discontinuation of MV. We did not routinely use NIPPV after weaning from MV.
Definitions
AF was diagnosed based on electrocardiogram characteristics including: absence of distinct repeating P waves, irregular RR intervals (when atrioventricular conduction is present), and irregular atrial activity.3 In this study, we enrolled patients with AF for more than 7 days (chronic AF) instead of paroxysmal AF and new-onset AF. HF was confirmed by a cardiologist and the diagnosis was based on the patient's clinical symptoms/signs, echocardiogram, or cardiac nuclear medicine examination.21 Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition.22 Acute kidney injury was defined according to the glomerular filtration rate criteria of the “Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease” (RIFLE) classification.23 The criteria for a diagnosis of respiratory distress during SBT were as follows: a respiratory rate of more than 35 breaths per minute, accessory muscle use or paradoxical movement, a persistent saturation of less than 88% on a pulse oximeter, a systolic or diastolic blood pressure that increased by 10%, and a heart rate of more than 130 beats per minute. Weaning failure was defined as the need for reintubation or reconnecting tracheotomized patients to the ventilator within 72 hours after weaning from MV, or receiving NIPPV within 72 hours after weaning from MV.
Statistical Analysis
All data are expressed as the mean ± standard deviation (SD) or as a percentage (%). For numerical variables, we used the Mann–Whitney U test for nonnormally distributed variables and the independent t test for normally distributed variables. Categorical variables were compared using the Chi-square test or Fisher exact test. Additionally, multivariate logistic regression analyses were used to calculate the crude odds ratios (OR) and adjusted odds ratio (AOR) of each dependent variable for the AF patients, with non-AF patients as the reference group. A multivariate logistic regression model was used via the enter technique, and the variables used during the univariate analysis were total ventilator days, length of ICU stay, length of hospital stay, weaning failure, ICU mortality, and hospital mortality. We also analyzed the ventilator dependence of the study patients by the Kaplan–Meier method and the log-rank test. These statistical analyses were carried out using SPSS, version 19.0 (SPSS, Inc., Chicago, IL). The results were considered significant at P < 0.05 and all P values were 2-sided.
RESULTS
Demographic Characteristics of the Study Patients
During this 1-year study period, 508 consecutive patients were admitted to the ICU and reviewed. A total of 223 patients were excluded according to the exclusion criteria, and 285 non-HF patients who received MV support on ICU admission and underwent ventilator discontinuation were enrolled in this study (Figure 1). The overall rate of weaning failure in these study patients was 25.3%, and the ICU and hospital mortalities were 19.3% and 29.8%, respectively. The mean age and APACHE II on ICU admission of the study patients were 77.4 (±14.5) years and 18.1 (±4.9), respectively. The most common comorbidities were hypertension (54%), stroke (40%), and diabetes mellitus (DM) (30%). The mean length of total ventilator use, the length of ICU stay, and length of hospital stay were 22.7 (±20.2), 26.2 (±17.3), and 54.8 (±46.2) days, respectively.
Clinical Characteristics of the Study Patients With and Without AF on ICU Admission
The study patients were divided into AF (n = 62) and non-AF (n = 223) groups according to the occurrence of AF on ICU admission. Compared with the non-AF patients, the AF patients were significantly associated with an older age (82.3 ± 8.3 years vs 76.0 ± 15.5 years, P = 0.002) and a higher rate of ARDS as the cause of respiratory failure (14.5% vs 5.4%, P = 0.015) (Table 1). We found that the AF patients were significantly associated with a higher percentage of sepsis before liberation from MV (54.8% vs 34.5%, P = 0.004) and a higher serum level of blood urea nitrogen on the day of liberation from MV (69.8 ± 49.0 mg/dl vs 52.7 ± 41.9 mg/dl, P = 0.003) (Table 2) than the non-AF patients. Moreover, the AF patients were significantly associated with a prolonged number of MV days before RFW (19.2 ± 13.2 days vs 18.4 ± 32.2 days, P = 0.040), a longer length of ICU stay (24.7 ± 16.0 days vs 20.7 ± 15.4 days, P = 0.020), and a longer length of hospital stay (65.6 ± 47.4 days vs 51.8 ± 45.7 days, P = 0.004), as well higher rates of ICU mortality (30.6% vs 16.1%, P = 0.010), hospital mortality (41.9% vs 26.5%, P = 0.020), and weaning failure (37.1% vs 22.0%, P = 0.020), as compared with the non-AF patients (Table 2 and Figure 2). Notably, AF on ICU admission contributed to an approximate 15% excess rate of ICU mortality, hospital mortality, and weaning failure among this patient population.
TABLE 1.
Baseline Characteristics of the Study Patients With and Without AF on ICU Admission
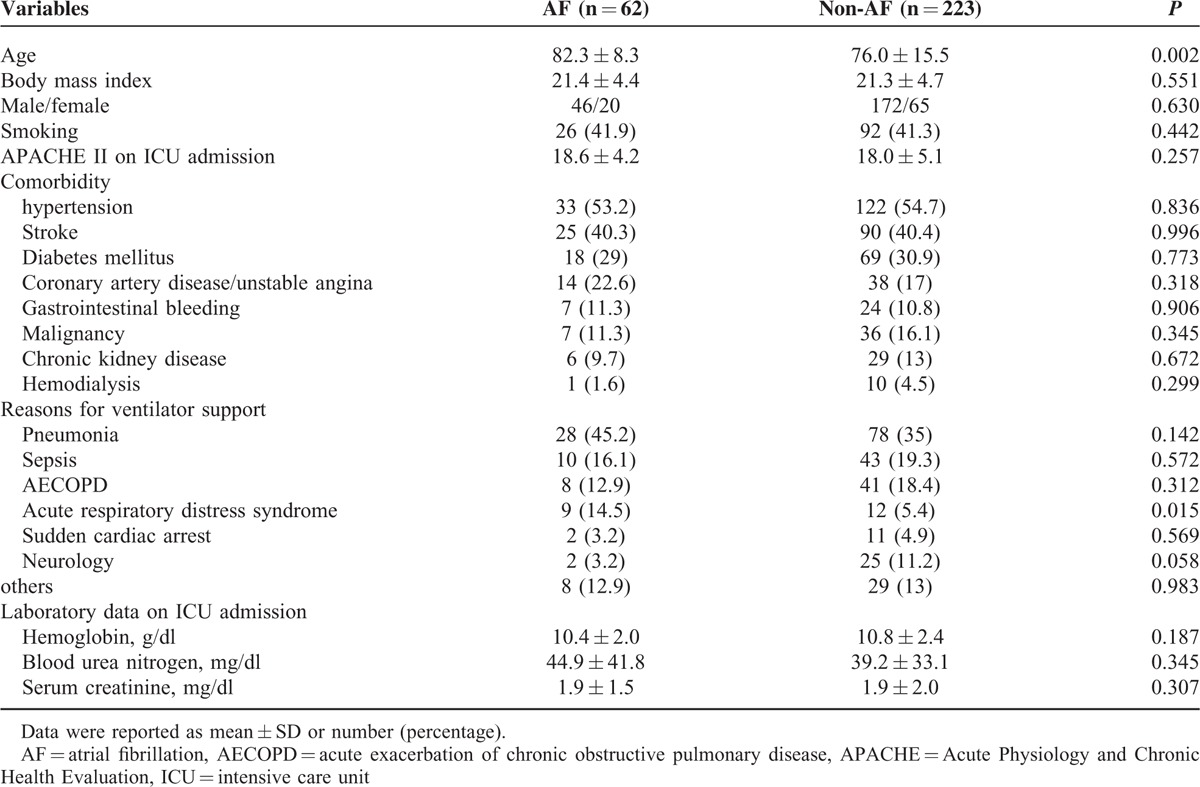
TABLE 2.
Clinical Characteristics and Patient Outcomes in the Study Patients With and Without AF on ICU Admission
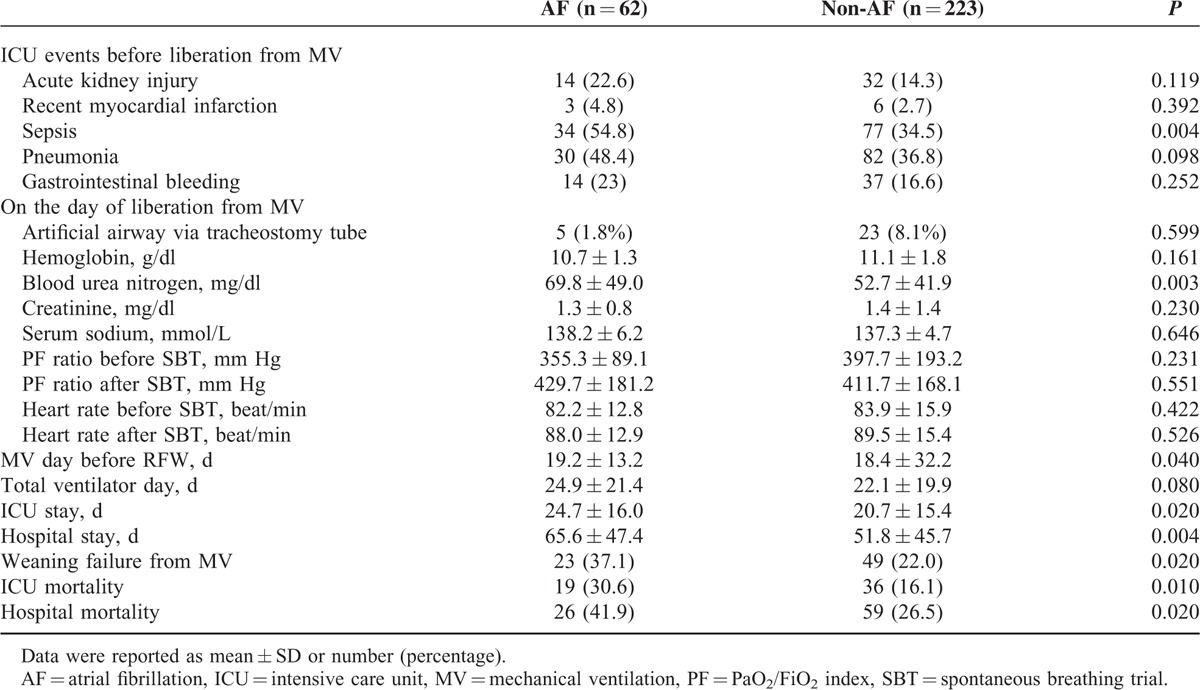
FIGURE 2.
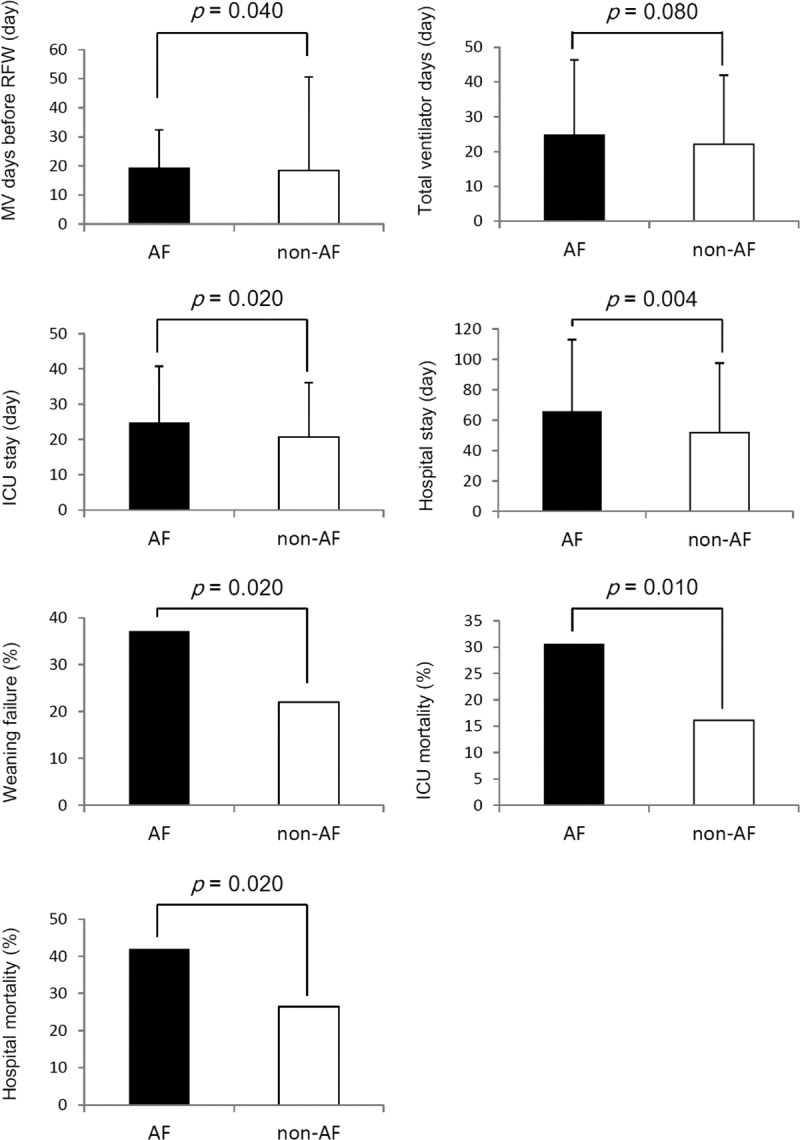
Clinical outcomes based on the occurrence of AF on ICU admission among non-HF mechanically ventilated patients. MV = mechanical ventilation, RFW = readiness for weaning, ICU = intensive care unit, AF = atrial fibrillation.
AF on ICU Admission Is Independently Associated With a Poor Hospital Outcome
The effect of AF on ICU admission among non-HF patients with MV was further analyzed by multivariate logistic regression and the ORs and AORs for patient outcomes are shown in Table 3. Compared with the non-AF patients, AF on ICU admission was independently associated with the risk of poor patient outcomes, including prolonged total ventilator days (AOR, 1.979; 95% confidence interval (CI), 1.032–3.794; P = 0.040), increased ICU stay (AOR, 2.256; 95% CI, 1.049–4.849; P = 0.037), increased hospital stay (AOR, 2.921; 95% CI, 1.363–6.260; P = 0.006), increased ICU mortality (AOR, 4.143; 95% CI, 1.381–12.424; P = 0.011), and increased weaning failure (AOR, 3.268; 95% CI, 1.254–8.517; P = 0.015) (Figure 3).
TABLE 3.
Multivariate Logistic Regression Analysis for Patient Outcomes in the Study Patients With and Without AF on ICU Admission
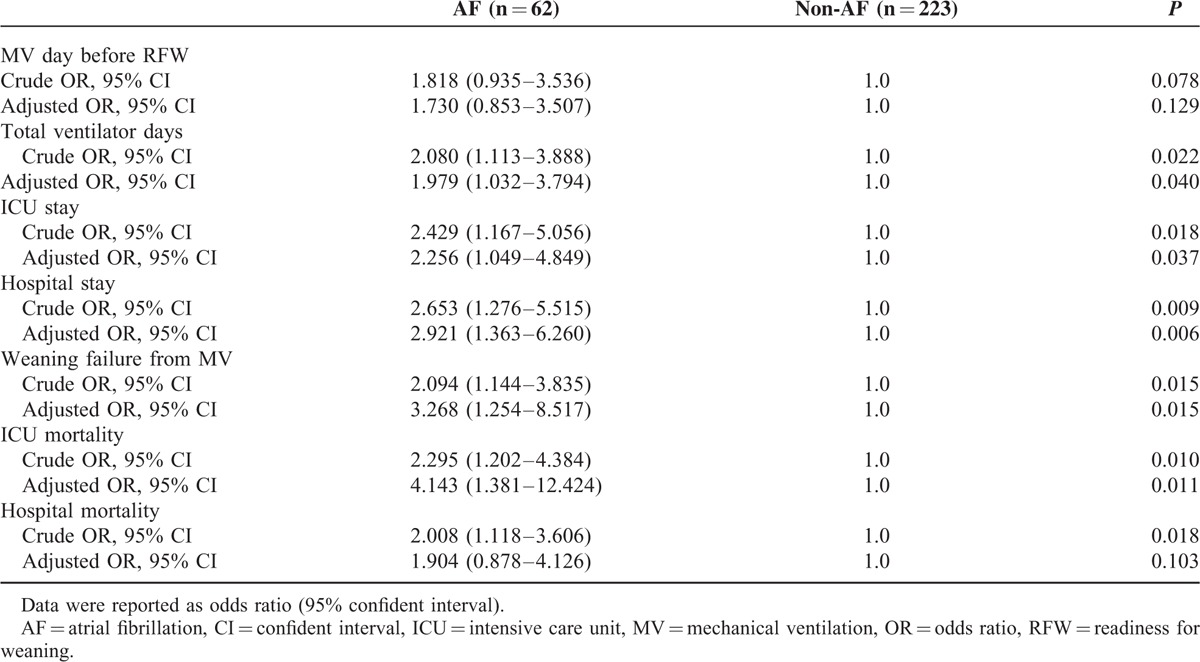
FIGURE 3.
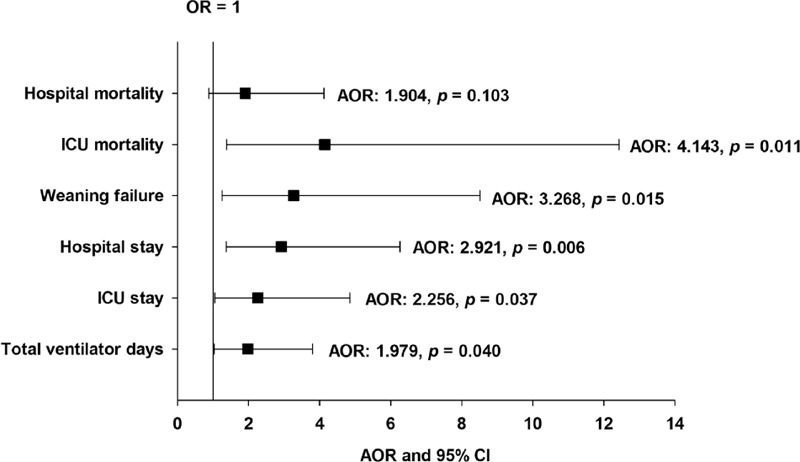
Results of multivariate logistic regression analysis of the clinical outcomes among patients with AF on ICU admission. Patients without AF were used as the reference group. AOR = adjusted odds ratio, CI = confidence interval, ICU = intensive care unit, OR = odds ratio.
AF on ICU Admission Adversely Influences Ventilator Dependence Rate
Results of Kaplan–Meier analysis showed that the probability of ventilator dependence within 30 days of ventilator use among AF patients was higher than that among non-AF patients (log-rank test, P = 0.026) (Figure 4). This result implied that AF on ICU admission may hamper the weaning process and contribute to an increased probability of ventilator dependence.
FIGURE 4.
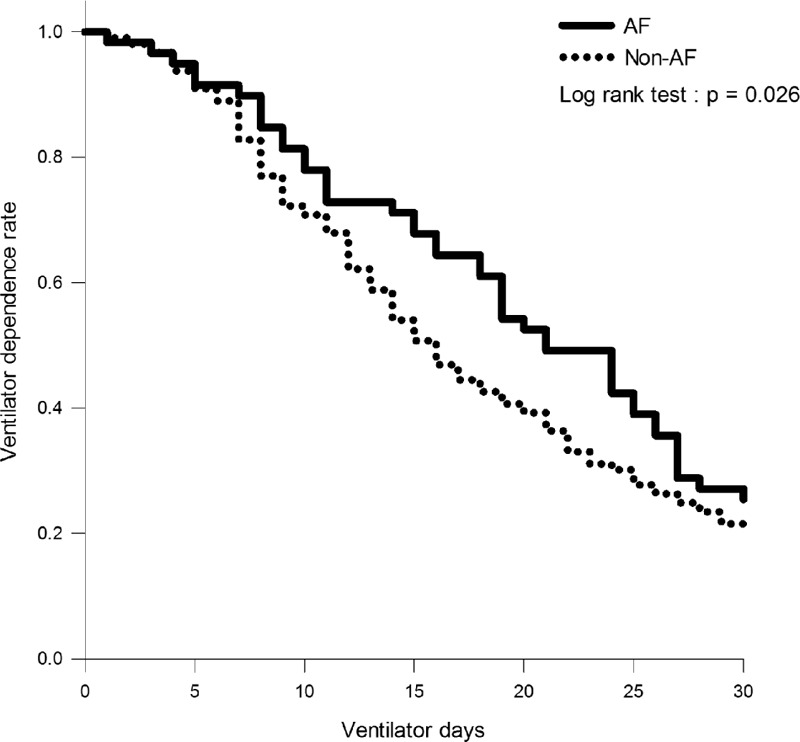
Kaplan–Meier curves illustrating the effect of AF on ICU admission on the risk of ventilator dependence among non-HF mechanically ventilated patients. HF = heart failure, AF = atrial fibrillation.
DISCUSSION
AF, one of the most frequent arrhythmias in clinical practice, is known to adversely affect various hospital outcomes in critically ill patients.5–11,24–27 This study focused on the non-HF mechanically ventilated patients of medical ICU and found that AF on ICU admission was independently associated with prolonged total ventilator use, increased length of ICU stay, increased length of hospital stay, and increased ICU mortality, as compared with non-AF patients. Importantly, AF on ICU admission independently increased the risk of weaning failure and significantly contributed to a higher rate of ventilator dependence as compared with those patients without AF on ICU admission.
Clinically, the factors commonly associated with the development of AF include both cardiac and noncardiac conditions. The cardiac factors associated with AF development are HF, structural heart disease, ischemic heart disease, and hypertension.3,4 Recently, D’Ascia et al28 enrolled 58 non-AF patients who received cardiac resynchronization therapy for nonischemic idiopathic dilated cardiomyopathy and severe HF, and they reported that device implantation nonresponders had an increased risk of new-onset AF development. According to this result, the authors concluded that structural atrial remodeling, left ventricular systolic function, and the degree of mitral regurgitation can simultaneously contribute to the development of atrial arrthymia.28,29 Therefore, electrical remodeling and structural remodeling are among the most important pathophysiological mechanisms for AF development.28–31
The noncardiac factors associated with the development of AF include old age, DM, chronic kidney disease (CKD), sepsis and chronic obstructive pulmonary disease.3,11,32–34 An advanced age is a well-known risk factor for AF development among critically ill patients with HF, trauma, long-term hemodialysis, and sepsis.9–11,14,17,18 Cardiac aging and the molecular mechanism of mitochondrial oxidative stress in promoting AF have been well investigated by Xie et al.35 The prevalence of AF increases with age, and this age-dependent increase in oxidative stress can promote the development of AF.35 In our non-HF mechanically ventilated patients, we confirmed that old age was still associated with AF development. In addition, we found that AF patients had a higher percentage of respiratory failure due to ARDS than non-AF patients. To the best of our knowledge, no previous study has reported ARDS as a risk factor for the AF development. We assumed that ARDS-related systemic inflammation participates in the pathogenesis of AF and contributes to the development of dysrhythmia. Our findings were consistent with a previous observation that suggested severe sepsis increases the risk of developing newly diagnosed AF.11 These findings indicated that AF may be a consequence of a severe systemic inflammation response in patients with ARDS and severe sepsis. However, other potentially predisposing factors for the development of AF, which include DM, CKD, coronary artery disease, and ischemic heart disease, did not show a statistical difference between the AF and non-AF groups in our study. This finding may be explained by the fact that we excluded HF patients and reduced the effect of these factors on AF development.
Cardiac ischemia and left ventricular dysfunction have been reported as predictors of weaning failure,36–42 but whether cardiac dysrhythmia influences weaning outcome remains unclear. Only one study reported that AF is associated with a prolonged duration of MV in patients with exacerbated chronic respiratory failure.19 The effect of AF alone on the weaning outcome in non-HF critically ill patients remains uncertain. Our study is the first to report that AF on ICU admission independently increased the risk of weaning failure by threefold in non-HF mechanically ventilated patients in a medical ICU. In addition, no significant difference in baseline heart rate before SBT was observed between the AF and non-AF groups. Moreover, there was no significant difference in the occurrence of tachycardia after SBT across both groups. A previous study determined the cardiac origin of weaning failure and identified diastolic dysfunction of the left ventricle with relaxation impairment as a predictor of weaning failure.41 Our findings indicated that AF-related irregular rhythm and chaotic atrial activity, but not relaxation impairment caused by a rapid ventricular response during weaning processes, played a dominant role that led to deleterious hemodynamic consequences when mechanically ventilated AF patients underwent weaning.3,14,41,43 Accordingly, AF-related dysrhythmia may affect weaning processes and contribute to weaning failure.
Previous studies have reported that AF worsens hospital outcomes in critically ill patients, including a higher rate of MV support, a longer length of ICU stay, a longer length of hospital stay, increased cardiovascular mortality, and increased noncardiovascular mortality.5–11,24–27 However, the influence of AF on hospital outcomes in mechanically ventilated ICU patients without HF remains largely unclear. In this patient population, we found that AF on ICU admission was significantly associated with increased morbidity because of its relation to more septicemia cases, as well as a high rate of renal dysfunction. Furthermore, AF on ICU admission was significantly associated with a range of hospital outcomes, including prolonged ventilation use, increased ICU stay, increased hospital stay, and increased ICU mortality. Although the mechanism by which AF is associated with deterioration in hospital outcomes in critically ill patients is complex, there is growing evidence indicating that AF is an inflammatory disorder linked to an increased risk of AF-related complications.44,45 Therefore, we suggested that AF-related inflammation may have a critical influence on patient outcomes and may be potentially implicated in the development of sepsis and in renal function impairment. Intensivists should pay attention to mechanically ventilated patients with AF on ICU admission and be alerted to the AF-related respiratory care burden.
This study had several limitations. First, our investigation was a retrospective observational study, and the etiologies of AF could not be evaluated thoroughly. The observational design precludes causal statements about the relationship between AF and hospital outcomes. Furthermore, the groups differed significantly in terms of age, incidence of sepsis, and ARDS; we could not determine whether AF “causes” long hospital stays, ICU stays, and an increase in mortality. Second, the diagnosis of HF was challenging and dependent on the patient's history, their clinical symptoms and signs, the physical examination, and the findings of echocardiography. A previous study suggested that no standard value exists for the diagnosis of HF in the elderly and such subjects may have an atypical presentation.46 Third, only some of the patients received echocardiography. We could not confirm if the prevalence of diastolic dysfunction was higher among patients with AF and contributed to AF development. Fourth, we used electrocardiography as the standard for AF diagnosis. Holter examination and continuous monitoring offered by implanted devices were not used for AF diagnosis, and overall AF burden might not be assessed adequately. Finally, our research was carried out at Veterans General Hospital. Therefore, the average age of the study population is relatively old. The clinical features and effect of AF on younger patients still require further study.
In conclusion, we found that AF on ICU admission was an independent risk factor for weaning failure and also significantly associated with poor hospital outcomes of non-HF patients receiving MV in a medical ICU. Intensivists should be alerted regarding this important issue in the respiratory care of critically ill patients.
Acknowledgments
The authors thank all the health care workers of the RCUB at the Taipei Veterans General Hospital for their valuable contribution to the patient care. The authors are grateful to Dr. Ralph Kirby, Department of Life Sciences, National Yang-Ming University, and Dr. Hollie, Department of Enago, Crimson Interactive, Inc. (USA) for his help with language editing.
Footnotes
Abbreviations: AF = atrial fibrillation, AOR = adjusted odds ratio, APACHE II = Acute Physiology and Chronic Health Evaluation II, ARDS = acute respiratory distress syndrome, CI = confidence interval, CKD = chronic kidney disease, HF = heart failure, ICU = intensive care unit, MV = mechanical ventilation, NIPPV = noninvasive positive pressure ventilation, OR = odds ratio, RFW = readiness for weaning, RIFLE = Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease, RSBI = rapid shallow breathing index, SBT = spontaneous breathing trial, SD = standard deviation.
Author's contributions: YHT contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, wrote and revised the manuscript. HKK contributed to the conception and design of the study, analysis and interpretation of data, revised the manuscript. YCT contributed to the conception and design of the study, analysis and interpretation of data, revised the manuscript. YHL contributed to the conception and design of the study, revised the manuscript. YRK contributed to the conception and design of the study, analysis and interpretation of data, wrote and revised the manuscript. All authors read and approved the final manuscript.
This study was supported by grants MOST 104-2320-B-010-014-MY3 from Ministry of Science and Technology, Taiwan, a grant from Ministry of Education, Aim for the Top University Plan, Taiwan, and a grant 98DHA0100384 from Taipei Veterans General Hospital.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 2012; 9:632.e621–696.e621.22386883 [Google Scholar]
- 2.Camm AJ, et al. European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 3.Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2016; 67:1575–1623. [DOI] [PubMed] [Google Scholar]
- 4.Ng RR, Tan GH, Liu W, et al. The association of acute kidney injury and atrial fibrillation after cardiac surgery in an Asian prospective cohort study. Medicine 2016; 95:e3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaver CM, Chen W, Janz DR, et al. Atrial fibrillation is an independent predictor of mortality in critically ill patients. Crit Care Med 2015; 43:2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian SA, Schorr C, Ferchau L, et al. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care 2008; 23:532–536. [DOI] [PubMed] [Google Scholar]
- 7.Kanji S, Williamson DR, Yaghchi BM, et al. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care 2012; 27:326.e1–326.e8. [DOI] [PubMed] [Google Scholar]
- 8.Salman S, Bajwa A, Gajic O, et al. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med 2008; 23:178–183. [DOI] [PubMed] [Google Scholar]
- 9.Seguin P, Laviolle B, Maurice A, et al. Atrial fibrillation in trauma patients requiring intensive care. Intensive Care Med 2006; 32:398–404. [DOI] [PubMed] [Google Scholar]
- 10.Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis 2008; 51:255–262. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systemic review. Crit Care 2014; 18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997; 337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson WG, Stevenson LW. Atrial fibrillation and heart failure—five more years. N Engl J Med 2004; 351:2437–2440. [DOI] [PubMed] [Google Scholar]
- 14.Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 1998; 32:695–703. [DOI] [PubMed] [Google Scholar]
- 15.Tveit A, Flonaes B, Aaser E, et al. No impact of atrial fibrillation on mortality risk in optimally treated heart failure patients. Clin Cardiol 2011; 34:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, et al. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ Heart Fail 2012; 5:191–201. [DOI] [PubMed] [Google Scholar]
- 17.Rivero-Ayerza M, Scholte Op Reimer W, Lenzen M, et al. New-onset atrial fibrillation is an independent predictor of in-hospital mortality in hospitalized heart failure patients: results of the EuroHeart Failure Survey. Eur Heart J 2008; 29:1618–1624. [DOI] [PubMed] [Google Scholar]
- 18.Swedberg K, Olsson LG, Charlesworth A, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J 2005; 26:1303–1308. [DOI] [PubMed] [Google Scholar]
- 19.Marcelino P, Germano N, Nunes AP, et al. [Cardiac influence on mechanical ventilation time and mortality in exacerbated chronic respiratory failure patients. The role of echocardiographic parameters]. Rev Port Pneumol 2006; 12:131–146. [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829. [PubMed] [Google Scholar]
- 21.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:e391–e479. [DOI] [PubMed] [Google Scholar]
- 22.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 23.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinelt P, Karth GD, Geppert A, et al. Incidence and type of cardiac arrhythmias in critically ill patients: a single center experience in a medical-cardiological ICU. Intensive Care Med 2001; 27:1466–1473. [DOI] [PubMed] [Google Scholar]
- 25.Echahidi N, Pibarot P, O’Hara G, et al. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008; 51:793–801. [DOI] [PubMed] [Google Scholar]
- 26.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med 2001; 135:1061–1073. [DOI] [PubMed] [Google Scholar]
- 27.Sahin I, Ozkaynak B, Karabulut A, et al. Impact of coronary collateral circulation and severity of coronary artery disease in the development of postoperative atrial fibrillation. Interact Cardiovasc Thorac Surg 2014; 19:394–397. [DOI] [PubMed] [Google Scholar]
- 28.D’Ascia SL, D’Ascia C, Marino V, et al. Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. Int J Clin Pract 2011; 65:1149–1155. [DOI] [PubMed] [Google Scholar]
- 29.Santulli G, D’Ascia SL, D’Ascia C. Development of atrial fibrillation in recipients of cardiac resynchronization therapy: the role of atrial reverse remodelling. Can J Cardiol 2012; 28:245.e17.author reply 245.e17–245.e18. [DOI] [PubMed] [Google Scholar]
- 30.Santulli G, Iaccarino G, De Luca N, et al. Atrial fibrillation and microRNAs. Front Physiol 2014; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Sharma D, Li G, et al. Atrial remodeling: new pathophysiological mechanism of atrial fibrillation. Med Hypotheses 2013; 80:53–56. [DOI] [PubMed] [Google Scholar]
- 32.Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123:2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conway DS, Buggins P, Hughes E, et al. Predictive value of indexes of inflammation and hypercoagulability on success of cardioversion of persistent atrial fibrillation. Am J Cardiol 2004; 94:508–510. [DOI] [PubMed] [Google Scholar]
- 34.Marott SC, Nordestgaard BG, Zacho J, et al. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol 2010; 56:789–795. [DOI] [PubMed] [Google Scholar]
- 35.Xie W, Santulli G, Reiken SR, et al. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep 2015; 5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurford WE, Favorito F. Association of myocardial ischemia with failure to wean from mechanical ventilation. Crit Care Med 1995; 23:1475–1480. [DOI] [PubMed] [Google Scholar]
- 37.Chatila W, Ani S, Guaglianone D, et al. Cardiac ischemia during weaning from mechanical ventilation. Chest 1996; 109:1577–1583. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava S, Chatila W, Amoateng-Adjepong Y, et al. Myocardial ischemia and weaning failure in patients with coronary artery disease: an update. Crit Care Med 1999; 27:2109–2112. [DOI] [PubMed] [Google Scholar]
- 39.Richard C, Teboul JL, Archambaud F, et al. Left ventricular function during weaning of patients with chronic obstructive pulmonary disease. Intensive Care Med 1994; 20:181–186. [DOI] [PubMed] [Google Scholar]
- 40.Caille V, Amiel JB, Charron C, et al. Echocardiography: a help in the weaning process. Critical Care 2010; 14:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moschietto S, Doyen D, Grech L, et al. Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care 2012; 16:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007; 29:1033–1056. [DOI] [PubMed] [Google Scholar]
- 43.Lim HS, Hamaad A, Lip GY. Clinical review: clinical management of atrial fibrillation—rate control versus rhythm control. Crit Care 2004; 8:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J 2006; 27:136–149. [DOI] [PubMed] [Google Scholar]
- 45.Korantzopoulos P, Kolettis TM, Kountouris E, et al. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol 2005; 102:321–326. [DOI] [PubMed] [Google Scholar]
- 46.Shamsham F, Mitchell J. Essentials of the diagnosis of heart failure. Am Fam Physician 2000; 61:1319–1328. [PubMed] [Google Scholar]


