Supplemental Digital Content is available in the text
Abstract
The etiological basis of functional dyspepsia (FD) is incompletely understood. The aim of this study was to evaluate the involvement of nociceptor-related genes and Helicobacter pylori (HP) in the pathogenesis of FD. The expression of nociceptor-related genes was measured in gastric cell lines that were co-cultured with HP. FD patients (n = 117) and controls (n = 55) were enrolled from a tertiary hospital gastroenterology clinic. Expression of the genes nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), and transient receptor potential cation channel subfamily V member 1 (TRPV1) in the gastric mucosa were detected by reverse transcription polymerase chain reaction (RT-PCR), and immunohistochemical staining of TRPV1 was analyzed. These measurements were repeated after 1 year. TRPV1, GDNF, and NGF expression was elevated in gastric cell lines co-cultured with HP. TRPV1 immunostaining was stronger in HP-positive than HP-negative subjects. The FD group showed higher expression levels of TRPV1, GDNF, and NGF and increased TRPV1 immunostaining compared with those of the control group (all P < 0.05). Among 61 subjects who were followed up at 1 year, controls with successful HP eradication and patients whose symptoms had improved both showed significant reductions in the expression of TRPV1 and NGF (all P < 0.05) compared with controls without HP eradication and patients whose symptoms had not improved, respectively. The expression of NGF, GDNF, and TRPV1 may be associated with the pathogenesis of FD. Since HP infection may induce the increased expression of these genes, anti-HP therapy could be beneficial for HP-positive patients with FD.
INTRODUCTION
Functional dyspepsia (FD) is one of the most common gastrointestinal (GI) disorders and has a prevalence of up to 30%1; however, its pathogenesis remains unclear. The Rome III classification categorized FD into 2 subtypes: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS).2–5
Transient receptor potential (TRP) channel subunits are expressed in neurons and cells in the alimentary tract.6 They play important roles in taste, mechanosensation, pain, GI motility, and secretion.6 Among the 20 TRP channels characterized so far, TRP cation channel subfamily V member 1 (TRPV1) is believed to be an important integrator of the transmission and modulation of pain (nociception).7 TRPV1 is a chemoreceptor that is stimulated by various spice-derived phytochemicals, including capsaicin (red pepper), piperine (black pepper), gingerol (ginger), and allyl isothiocyanate (mustard/wasabi).8 It is also activated by low pH (<6), heat, and inflammatory mediators such as serotonin, bradykinin, and Adenosine triphosphate.9 TRPV1 is predominantly expressed in neurons, but has also been identified in the mucosa of several GI tract regions, such as the esophagus,10,11 small intestine,12 and stomach.13–16
Since patients with dyspepsia frequently manifest a low pain threshold to chemical challenge,17,18 any part of the GI tract that detects and transmits pain might be augmented in FD. Hence, the upregulation of TRPV1 has been proposed to be associated with visceral hypersensitivity in FD.
Evidence has accumulated indicating that activation of TRPV1 causes pain and hyperalgesia in the human alimentary tract. Oral ingestion or administration of capsaicin into the human proximal jejunum has been reported to evoke epigastric pain, heartburn, and a sensation of warmth.19,20 Infusion of a capsaicin-containing chili sauce into the gastric fundus was found to enhance the mechano-sensitivity of the proximal stomach.21 In addition, esophageal instillation of capsaicin induced symptoms of epigastric burning in a dose-dependent fashion.22 Concordantly with these observations, upregulation of TRPV1 has been reported in patients with rectal hypersensitivity and non-erosive reflux disease (NERD).23,24
Gastric sensations are transmitted to the central nervous system by unmyelinated C fibers or thin myelinated Aδ fibers. These polymodal C fibers are regulated by several neurotrophic factors, such as nerve growth factor (NGF) or glial cell line-derived neurotrophic factor (GDNF).25 Neurotrophic factors are important for supporting the survival of neurons in both the central and peripheral nervous system and are also involved in pain signaling. In particular, NGF is upregulated during inflammation,26 and plays a proalgesic role.27 GDNF belongs to the transforming growth factor-β superfamily and its overexpression has also been reported in irritable bowel syndrome (IBS) patients.28
Studies evaluating the potential relationship between TRPV1 expression in the stomach and developing FD have been very limited to date. Furthermore, there have been very few reports evaluating the relationship between TRPV1 and HP infection, which is a major cause of gastric mucosal inflammation. Against this background, we hypothesized that the expression of TRPV1 is elevated in the gastric mucosa of FD patients together with that of GDNF and/or NGF, and that such changes are associated with certain dyspeptic symptoms. In addition to testing this hypothesis, we also aimed to evaluate the effects of HP infection and eradication on the expression of nociceptor-related genes.
METHODS
Study Population
From February 2011 to June 2013, subjects with dyspeptic symptoms and healthy controls recruited from a cancer-screening program were enrolled at the Seoul National University Bundang Hospital. All participants underwent upper GI endoscopy. Under the supervision of well-trained interviewers and after giving informed written consent, all participants completed a questionnaire that was modified from Talley's bowel disease questionnaire and validated in Korea.29,30 The questionnaire met the Rome III criteria. Severities of dyspeptic symptoms were scored using a 5-point Likert scale as follows. 0: none, 1: mild, 2: moderate, 3: severe, and 4: very severe. Patients were divided into 3 FD subtypes based on their symptoms: PDS, EPS, and mixed.31
Patients with peptic ulcer, reflux esophagitis, or typical IBS; concomitant systemic diseases such as diabetes mellitus or any malignancy; major depression, psychosis, or eating disorders; and daily users of NSAID or anticoagulants were excluded. Symptomatic subjects whose symptoms did not fulfill the Rome III criteria were also excluded.
A total of 55 control subjects and 117 patients with dyspeptic symptoms (PDS: n = 46, EPS: n = 33, mixed: n = 38) were analyzed. To evaluate whether changes in the expression of nociceptor-related genes correlate with variations in dyspeptic symptoms, we investigated the mRNA expression of TRPV1, GDNF, and NGF in gastric biopsy specimens at baseline and 1 year after the patients were enrolled. In addition, the expression levels of these nociceptor-related genes before and after HP eradication were compared. The study protocol was approved by the ethics committee at Seoul National University Bundang Hospital (B-1101–119–010).
Helicobacter pylori Strains and Cell Culture
Helicobacter pylori (HP) ATCC 43504 (O4 strain; cagA+, vacA+) was purchased from American Type Culture Collection (ATCC; Manassas, VA) and G27 strain (cagA+, vacA+ [s1, m1]) was obtained from Professor S. Falkow (Stanford University, Stanford, CA). Bacteria were cultured under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) at 37 °C. The effect of HP infection on the expression of TRPV1, GDNF, and NGF in human gastric cell lines was investigated. Human gastric adenocarcinoma (AGS) cells (ATCC CRL 1739) were purchased from ATCC and grown in RPMI 1640 (Gibco, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin solution at 37 °C in a 5% CO2 humidified atmosphere. Cells were seeded at a density of 2 × 105 cells/well in 6-well plates and 2 × 106 cells/well in 10 cm dishes. After the cells reached 70% to 80% confluence, H pylori were re-suspended in RPMI 1640 and co-cultured with the cells at a multiplicity of infection of 200 after overnight serum starvation. Het-1A (ATCC CRL-2692),11 MKN45 (KCLB 80103), and Colo205 (KCLB 10222) cells were purchased from ATCC and Korean Cell Line Bank (KCLB; Seoul, South Korea).
Gastric Mucosa Specimens and H pylori Evaluation
During endoscopy, 2 biopsy specimens were taken for histology and determining of HP infection status.32 One biopsy was taken from the gastric antrum and the other from the gastric body. Two additional biopsies were obtained from the greater curvature of the high body and were used for measuring the expression of TRPV1, GDNF, and NGF and preparing paraffin blocks.
The baseline HP infection status was determined based on modified Giemsa staining, culture, and rapid urease test (CLO test; Delta West, Bentley, Australia). If one of these invasive HP tests was positive, the patient was diagnosed with HP infection. As for the histologic features, the degree of inflammatory cell infiltration, atrophy, and intestinal metaplasia was recorded using the updated Sydney scoring system (0: none, 1: slight, 2: moderate, and 3: marked).33
Measurement of TRPV1, GDNF, and NGF mRNA Expression in Cells and Tissues
After 1, 6, 12, and 24 hours of cell co-culture, the medium was removed and cells were washed with phosphate-buffered saline. Total RNA was isolated from cells and tissue biopsy specimens using an RNeasy Plus Mini Kit (Qiagen, Valencia, CA). Reverse transcription was performed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time quantitative polymerase chain reaction (qPCR) for mRNA expression was performed using SYBR Green probes and an ABI 7500 thermal cycler (Applied Biosystems). The following primers were used, based on a previous study. TRPV1 forward, 5′-GAGTTTCAGGCAGACACTGGAA-3′; TRPV1 reverse, 5′-CTATCTCGAGCACTTGCCTCTCT-3′; GDNF forward, 5′-CTTGGGTCTGGGCTATGAAAC-3′; GDNF reverse, 5′-CAAAGGCGATGGGTCTGC-3′; NGF forward, 5′-AGCAAGCGGTCATCATCC-3′; and NGF reverse, 5′-GTGGCGGTGGTCTTATCC-3′. GAPDH was used as an endogenous reference. The amplification protocol consisted of an initial denaturation step at 95 °C for 10 seconds, followed by 40 cycles of denaturation for 5 seconds at 95 °C and annealing/extension for 33 seconds at 60 °C. Relative expression levels were normalized by dividing the target gene Ct values by the endogenous GAPDH Ct values.
Western Blot Analysis
After 24 hours of co-culture, cells were washed twice with phosphate-buffered saline and then lysed with radioimmunoprecipitation assay buffer (Cell Signaling Technology, Beverly, MA), and bicinchoninic acid protein assay was performed to determine the protein concentration of the lysates (Pierce, Rockford, IL). Cell extracts (30 μg protein) were subjected to 10% (v/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis and the separated proteins were transferred to polyvinylidene difluoride membranes. After blocking the non-specific binding sites with non-fat dry milk, the membranes were incubated with anti-TRPV1 antibody (1:2000; H00007442-M01; Abnova, Taiwan) at 4 °C overnight, then blots were incubated with secondary antibody (goat-anti mouse antibody, 1:4000; Santa Cruz Biotechnology, Dallas, TX). Detection was achieved using enhanced chemiluminescence reagent (Amersham Biosciences, Buckinghamshire, UK).
Immunohistochemistry
Immunostaining of TRPV1 was performed using core tissue biopsies (2 mm in diameter) obtained from paraffin-embedded gastric mucosa. Test procedures included a human control slide for immunohistochemistry analysis (SuperBioChips Laboratories, Seoul, Korea). A primary antibody against TRPV1 (1:100 dilution; ab63083; Abcam, Cambridge, UK) was used. For each tissue section, the intensity of immunohistochemical staining was scored as 0, no staining; 1+, faint/barely perceptible partial staining; 2+, weak to moderate staining; or 3+, strong staining. A composite measure of staining area and intensity was then calculated by multiplying the intensity by the positively stained area (%) in the epithelial glands. The resulting score ranged from 0 to 300. Each sample was scored by a blinded reviewer.
Medication for Dyspepsia
HP eradication was recommended for HP-positive patients. Some HP-positive control subjects with a family history of gastric cancer also underwent HP eradication. As the first-line anti-HP therapy, proton-pump inhibitor (PPI)-based triple therapy for 7 days was prescribed. At least 4 weeks after the completion of the therapy,13 C-urea breath test (UBiTkit; Otsuka Pharmaceutical, Tokushima, Japan) was performed. If the first line therapy failed, bismuth-based quadruple therapy was given for 14 days. HP-negative FD patients or HP-positive patients who refused the anti-HP therapy received symptomatic management with PPI, H2-antagonists, or prokinetics.
Evaluation of Symptom Response and Follow-Up
Every 2 to 3 months after enrollment, the severity of the dyspeptic symptoms of participants was assessed using a global patient assessment (GPA) score.35 The GPA score was used to assess whether symptoms remained the same, improved, or deteriorated over time compared with the measurements made at baseline. Patients were asked: “How were your overall dyspeptic symptoms during the past 2–3 months, compared with your symptoms at enrollment in the study?” Responses were scored on a 7-point Likert scale (1: extremely improved, 2: improved, 3: slightly improved, 4: not changed, 5: slightly aggravated, 6: aggravated, and 7: extremely aggravated).35 Participants received a second upper GI endoscopy assessment at 1 year after enrollment. Those who had agreed to mucosal biopsy were included in the follow-up mRNA analysis. At the 1-year follow-up evaluation, participants who consistently answered “1” or “2” were defined as responders, while patients who answered “4”, “5”, “6”, or “7” were assigned to the non-responders group. Participants who answered “3” were assigned to a separate, unclear response group. The schematic diagram for follow-up was summarized in Supplementary Digital Content 2.
Statistical Analysis
Categorical variables were analyzed by chi-square test and parametric variables were analyzed by Student t-test or 1-way analysis of variance (ANOVA) followed by Scheffe test. Spearman correlation test was used to evaluate potential correlations between nociceptor gene expression and dyspeptic symptoms. Changes in values over time were compared with paired t-test or Wilcoxon rank sum test. SPSS Statistics version 20.0 (IBM, Armonk, NY) was used. All statistical tests were 2-sided, and a value of P < 0.05 was considered to be statistically significant.
RESULTS
Identification of TRPV1 Expression in Gastric Cell Lines
To confirm the presence of TRPV1 mRNA in gastric cells, quantitative reverse transcription polymerase chain reaction was performed (Supplementary Digital Content 1). AGS and MKN45 cells showed 1.1-fold and 1.3-fold higher expression of TRPV1 mRNA than HET-1A, respectively.11 A 37.18-kDa band was detected using a TRPV1 monoclonal antibody, confirming the presence of TRPV1 receptors in these gastric epithelial cell lines (Supplementary Digital Content 1).
Upregulation of NGF, GDNF, and TRPV1 in Gastric Cell Lines after H pylori Infection
We next examined whether HP infection upregulates the expression of NGF, GDNF, and TRPV1 mRNAs. As shown in Figure 1, AGS cells were cultured with the 43504 (O4) and G27 strains of HP for 1, 6, 12, and 24 hours. NGF expression quickly increased and was maintained during the infections. For example, 12 hours after infection by HP O4 or G27, the AGS cells still showed almost the same level of NGF expression as they did at 1 and 6 hours. AGS cells incubated with HP G27 did not show a significant change in NGF expression at 12 hours (Figure 1A). GDNF expression started to increase at 6 and 12 hours, but it was significantly increased only at 6 hours after incubation with HP O4 (Figure 1B). TRPV1 expression was significantly elevated 12 hours after infection with HP O4 or G27 (Figure 1C). Western blot analysis demonstrated a significant increase in the abundance of TRPV1 in AGS cells that were co-incubated with HP O4 or G27 (Figure 1D).
FIGURE 1.
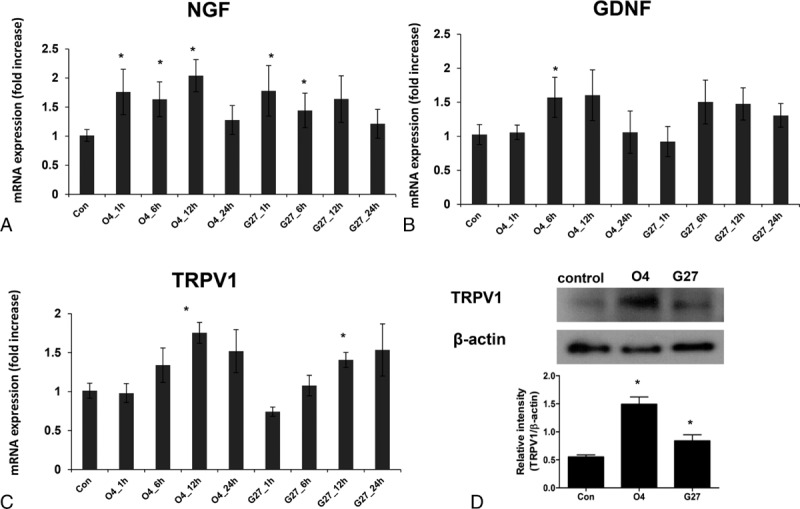
Expression of NGF, GDNF, and TRPV1 in AGS cell lines co-cultured with H pylori 43504 and G27 strains. (A–C) mRNA expression of (A) NGF, (B) GNF, and (C) TRPV1. (D) Western blot data of TRPV1 protein abundance after 24 hours of co-culture. Results are expressed as the mean ± standard error of 3 experiments. ∗P < 0.05, significantly different from the control non-HP-infected group at 1 hour after the infection. GDNF = glial cell-line derived neurotrophic factor, NGF = nerve growth factor, TRPV1 = transient receptor potential vanilloid receptor 1.
General Characteristics of Subjects
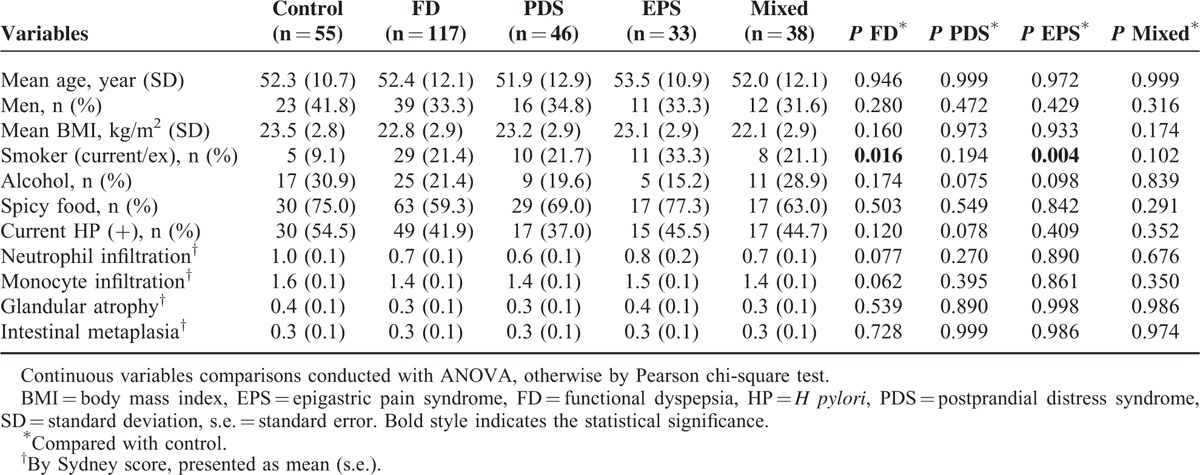
Based on our in vitro results, we addressed the possibility that TRPV1 may be associated with FD in human. Baseline characteristics of 55 control subjects and 117 FD patients are shown in Table 1. When comparing the controls and FD patients, never-smokers were more common in controls (P = 0.016) Otherwise, age, body mass index, alcohol consumption, regular spicy food ingestion, current HP infection status, degree of neutrophil/monocyte infiltration, atrophic gastritis, and intestinal metaplasia were not significantly different between the controls and FD patients. When these variables were compared with controls and the 3 FD subtype groups, there were no significant differences in the aforementioned variables, except for smoking; that is, a higher proportion of patients with EPS smoked, compared with the control subjects.
TABLE 1.
Baseline Characteristics in 55 Healthy Controls and 117 FD Patients
Comparison of NGF, GDNF, and TRPV1 Expression in the Functional Dyspepsia and Control Groups
Next, we evaluated the expression of NGF, GDNF, and TRPV1 mRNAs as possible etiological factors for FD. When control subjects and the overall FD group were compared, the FD group showed significantly higher expression of NGF, GDNF, and TRPV1, compared with that of the control group (Table 2). After subgroup analyses, the PDS, EPS, and mixed groups showed a higher expression of NGF (Figure 2A), GDNF (Figure 2B), and TRPV1 (Figure 2C) mRNAs than that of the control group. However, the expression of NGF mRNA in the PDS group did not reach statistical significance (Table 2 and Figure 2A).
TABLE 2.
Relative mRNA Expression of NGF, GDNF, and TRPV1 and TRPV1 Immunohistochemistry
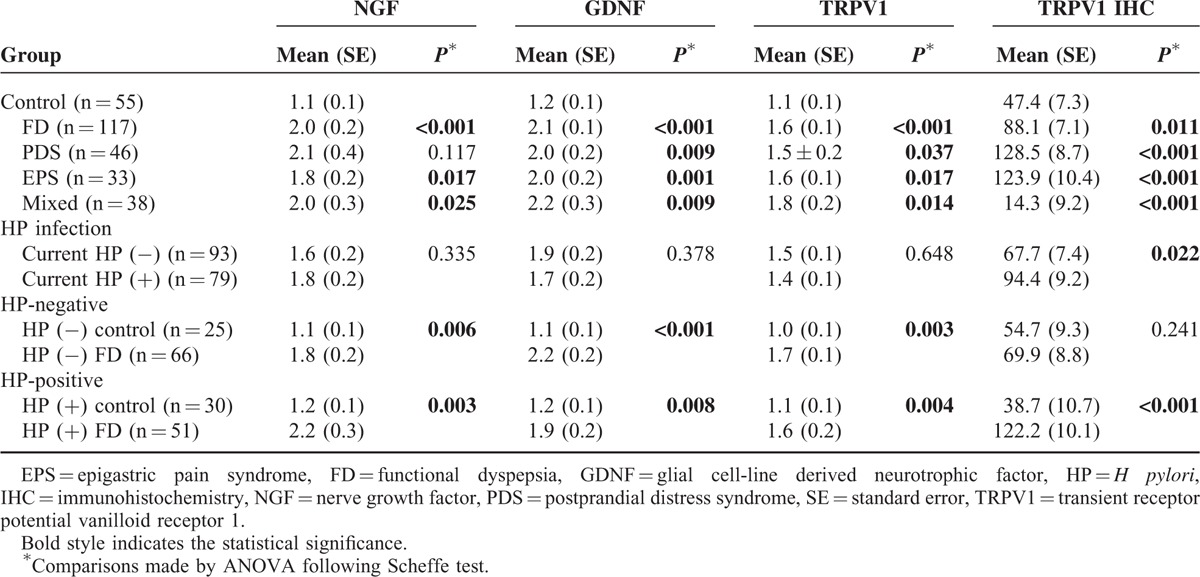
FIGURE 2.
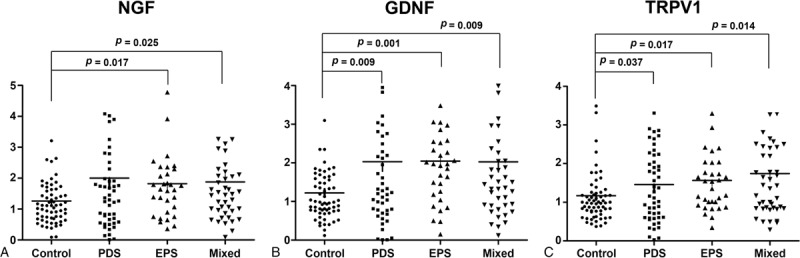
Comparison of gastric NGF, GDNF, and TRPV1 expression in control and FD subgroups. (A) NGF expression in the EPS and mixed groups was higher than that in control (P = 0.017, P = 0.025). (B) GDNF expression in the PDS, EPS, and mixed groups was higher than in control (P = 0.009, P = 0.001, and P = 0.009, respectively). (C) TRPV1 expression was higher in the PDS, EPS, and mixed groups than in the control group (P = 0.037, P = 0.017, and P = 0.014, respectively). Comparisons were made by analysis of variance followed by Scheffe test. EPS = epigastric pain syndrome, FD = functional dyspepsia, GDNF = glial cell-line derived neurotrophic factor, NGF = nerve gsrowth factor, PDS = postprandial distress syndrome, TRPV1 = transient receptor potential vanilloid receptor 1.
We observed TRPV1 immunoreactivity in the base or neck region of gastric mucosa (Figure 3). Immunoreactivity of TRPV1 in the gastric mucosa was compared between patients with FD and control subjects. The mucosa from FD patients showed significantly stronger staining than that of control subjects (Table 2). The specimens from control subjects showed weaker TRPV1 immunoreactivity compared with that of specimens from the PDS, EPS, and mixed groups (Table 2).
FIGURE 3.
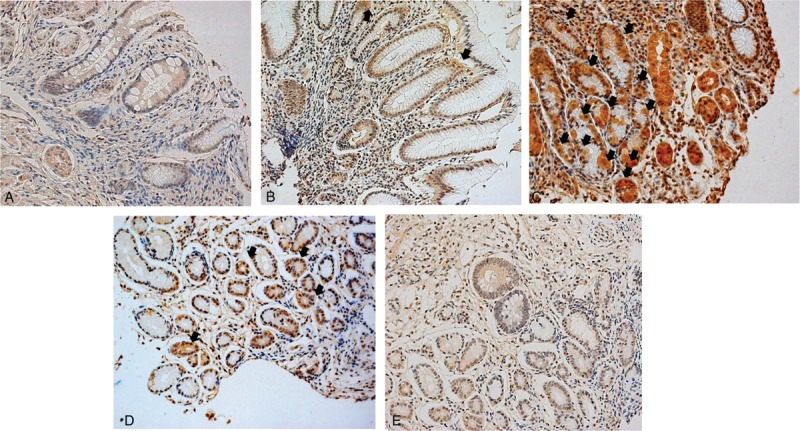
Representative images of TRPV1 immunostaining of human gastric mucosa. TRPV1 staining of (A) H pylori-negative healthy control, (B) H pylori-positive healthy control, (C) H pylori-positive epigastric pain syndrome patient, and (D) H pylori-positive postprandial distress syndrome patient. (E) The same patient shown in (D), 1-year after H pylori eradication. Cytoplasms and cell junctions were stained (arrow) (×20 magnification). TRPV1 = transient receptor potential vanilloid receptor 1.
Association Between Expression of Nociceptor-Related Genes and Dyspeptic Symptoms
Spearman rank correlation tests were performed to evaluate whether the expression of nociceptor-related genes was associated with specific dyspeptic symptoms including early satiety, postprandial fullness, or epigastric pain/burning. For the epigastric pain/burning and postprandial fullness scores, the expression of the 3 nociceptor-related genes showed weak but significant correlations with the symptom score (epigastric pain/burning: NGF: r = 0.291; GDNF: r = 0.316; and TRPV1: r = 0.288, all P < 0.001) (postprandial fullness scores: NGF: r = 0.156, P = 0.042; GDNF: r = 0.185, P = 0.016; and TRPV1: r = 0.160, P = 0.037). However, the early satiety score was not significantly associated with the expression of any of the 3 genes (NGF: r = 0.115, P = 0.136; GDNF: r = 0.111, P = 0.149; and TRPV1: r = 0.104, P = 0.176).
Expression of TRPV1 was strongly correlated with that of NGF and GDNF (r = 0.769, P < 0.001 and r = 0.768, P < 0.001, respectively). Furthermore, the expression levels of NGF and GDNF were also positively correlated with each other (r = 0.664, P < 0.001).
Expression of NGF, GDNF, and TRPV1 in Relation to H pylori Infection Status in Human Gastric Mucosa
In order to evaluate the possible effects of HP infection on the human gastric expression of NGF, GDNF, and TRPV1, we analyzed the expression of these genes in gastric mucosa according to HP infection status. There were no significant differences in nociceptor-related gene expression between HP-positive and HP-negative subjects (Table 2). Higher expression of NGF, GDNF, and TRPV1 was observed in FD compared with controls in both the HP-negative and HP-positive groups (Table 2). In terms of TRPV1 immunohistochemistry, HP-positive subjects showed stronger immunostaining intensity than HP-negative subjects did (Table 2) (Figure 3A and B). While there was no significant difference in TRPV1 immunostaining between FD patients and control subjects in the HP-negative group, the FD group showed a significantly increased immunostaining intensity compared with that of control subjects in the HP-positive group (Table 2) (Figure 3B and C).
Changes in NGF, GDNF, and TRPV1 Expression According to the Alteration of Dyspeptic Symptoms at the 1-Year Follow-Up Evaluation
We performed re-biopsy and repeated symptom assessments 1 year after the first assessment. The expression of NGF, GDNF, and TRPV1 was evaluated in 44 FD patients and 17 healthy control subjects (Table 3). The mean duration of follow-up was 12.1 months. The FD patients consisted of 5 PDS, 16 EPS, and 23 mixed patients. Of the 44 followed-up FD patients, only 23 patients answered that their symptoms were either “extremely improved” or “improved” upon assessment. The 3 symptom response groups (control, symptom improved, and non-improved groups) were compared (Table 3). There were no differences in age, sex, distribution of FD subtypes, current HP positivity, or follow-up duration. Baseline epigastric burning/pain score was higher in the symptom improved group than in the other groups (P = 0.018), but postprandial fullness and early satiation scores were not significantly different among groups (Table 3). Among the 11, 16, and 9 HP-positive subjects in each symptom response group, HP eradication was achieved in 10, 14, and 9 patients, respectively. Among the 23 HP-eradicated FD patients, 14 (60.9%) reported that their symptoms had improved. Ten (71.4%) of these patients received only anti-HP therapy, while all of the patients in the non-improved group maintained empirical treatment after anti-HP therapy (P < 0.001).
TABLE 3.
Dyspeptic Symptoms and Expression of Nociceptor-Related Genes at the Baseline and 1-Year Follow-Up
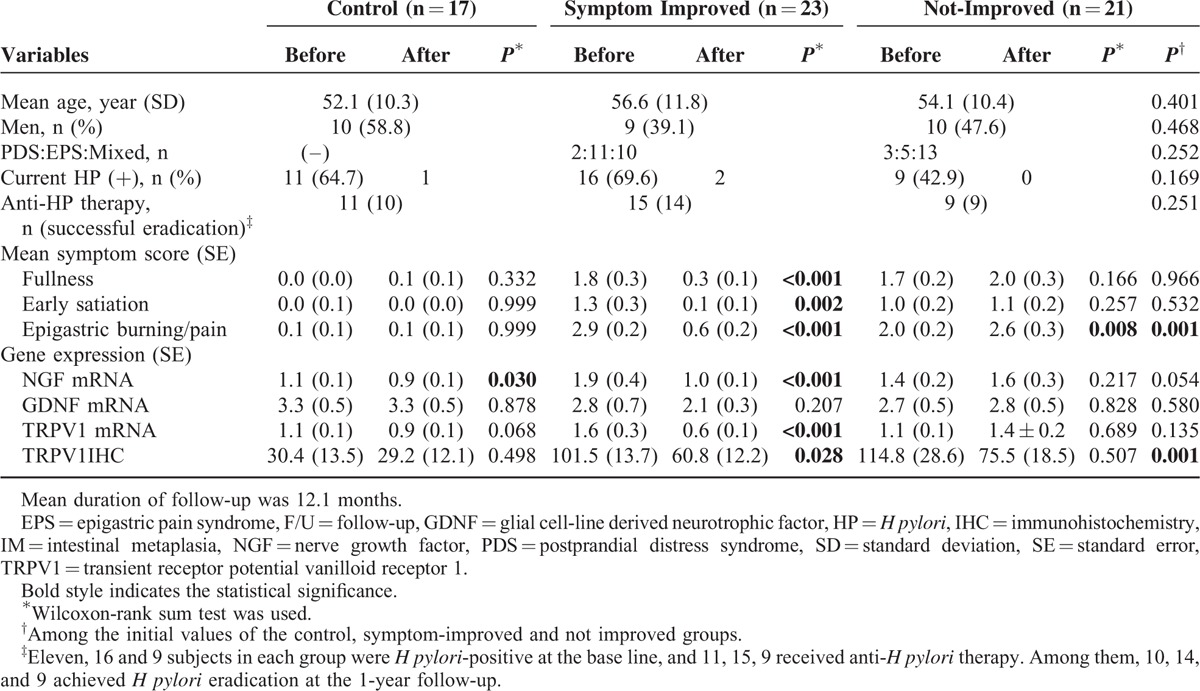
Approximately 1 year after enrollment, changes in 3 representative dyspeptic symptoms were analyzed (Table 3). While the patients who reported symptomatic improvements showed a significant reduction in all 3 symptoms, the group without symptomatic improvement displayed an increase in epigastric burning/pain symptoms (Table 3). HP was eradicated in 10 of 11 controls. None of the healthy controls presented dyspeptic symptoms during the follow-up period.
The expression of NGF and TRPV1 significantly decreased in the control and symptom-improved groups, while their expression in the non-improved FD group did not change significantly (Table 3). GDNF expression did not change significantly during the follow-up period in any of the groups.
At the 1-year follow-up evaluation, the symptom-improved group showed a reduced intensity of TRPV1 immunostaining compared with that at baseline, while the healthy controls and non-improved group did not present significant changes (Table 3).
Expression of NGF, GDNF, and TRPV1 after HP Eradication at the 1-Year Follow-Up Evaluation
To evaluate the effects of HP eradication on the expression of nociceptor-related genes and the reported severity of symptoms, the relative expression levels of NGF, GDNF, and TRPV1 were compared based on changes in HP status and FD symptoms (Table 4). There was a significant improvement of gastritis in all of the HP-eradicated groups.
TABLE 4.
Change of Nociceptor-Related Gene Expressions in Different Symptom Groups According to the Existence of Successful H pylori Eradication
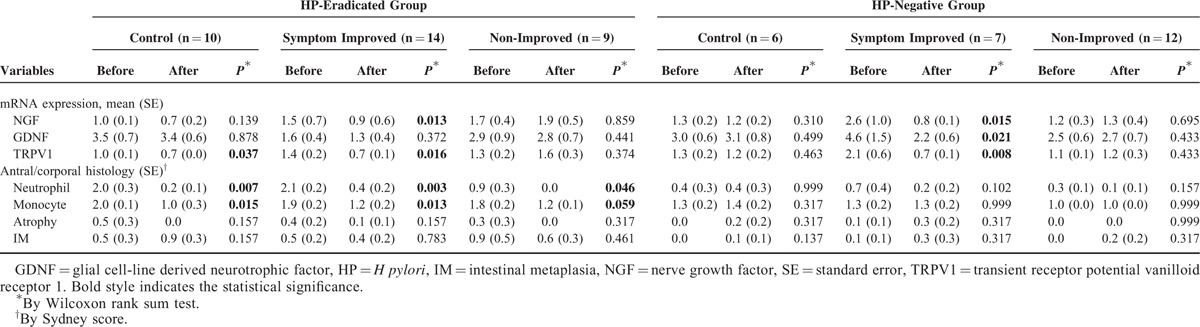
The HP-eradicated healthy control group showed a reduction in the expression of NGF and TRPV1, but the change in the expression of the former gene did not reach statistical significance. However, the HP-negative control group did not present any changes in the expression of the 3 nociceptor-related genes.
In the symptom-improved groups, the expression of NGF, GDNF, and TRPV1 was significantly decreased in both the HP-eradicated and HP-negative groups, although the change in GDNF expression in symptom-improved patients who underwent HP eradication did not reach statistical significance. Unlike the symptom-improved group, there was no significant change in the expression of the 3 nociceptor-related genes or the gastritis score.
The intensity of TRPV1 immunostaining was compared before and after HP eradication. The HP-eradicated group showed a decreased immunostaining score, compared with that at baseline (Figure 3D and E).
Responses to Treatment by the Patients With Functional Dyspepsia
The responses of the FD patients to different treatments are illustrated in Figure 4. Among the 49 HP-positive FD patients, 37 received anti-HP therapy. At the 1-year follow-up evaluation, HP eradication had been achieved for 27 patients, but 8 patients failed to achieve HP eradication due to low compliance, adverse effects, or resistance. The remaining 2 patients were lost during follow-up. As a result, 18 (66.7%) of 27 patients with successful HP eradication showed improved dyspeptic symptoms at a higher rate compared with the patients who failed or refused HP eradication (18/27 vs. 2/19, P < 0.001). The median duration for the improvement of symptoms after the completion of anti-HP therapy was 6.3 months (1.4–14.2 months). Among the 18 symptom-improved patients in whom HP was eradicated, 13 (72.2%) maintained their improved status without any supportive drugs for a median of 15.1 months (4.5–38.1 months). The other 5 symptom-improved patients maintained the improvements for around 6 to 9 months after the eradication, but they needed supplementary prokinetics and PPI thereafter and answered “extremely improved or improved since enrollment” at the 1-year follow-up evaluation. On the other hand, the 68 HP-negative FD patients received supportive care with PPI, H2-antagonists, or prokinetics. Excluding 13 patients who could not be followed up, 25 (45.5%) of 55 patients reported an improvement of their symptoms at a median of 12.1 months after enrollment.
FIGURE 4.
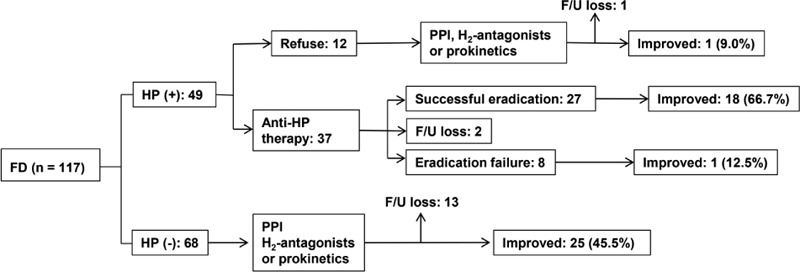
Summary of the responses to different treatments by 117 patients with functional dyspepsia based on the global patient assessment score. FD = functional dyspepsia, F/U = follow-up, HP = H pylori, PPI = proton-pump inhibitor.
DISCUSSION
Our study showed that the expression of NGF, GDNF, and TRPV1 in FD patients was higher than in the control group. The severities of epigastric pain/burning and postprandial fullness significantly correlated with the expression of these 3 nociceptor-related genes. These results imply that TRPV1 and neurotrophic factors play an important role in the pathogenesis of FD, epigastric pain/burning, and fullness after meals. Moreover, since the in vitro data showed that infection with HP induced NGF, GDNF, and TRPV1 in human gastric cell lines, the present study provides a clue as to the possible mechanism of HP-related dyspepsia.
Similar to the results reported here, previous studies have also indicated that TRPV1 was upregulated in other functional GI diseases including IBS,36 NERD, and erosive reflux disease.24,37
We also demonstrated that the gastric epithelium contains TRPV1 receptors, as has previously been shown in gastric cell lines and human gastric tissue. This confirms that TRPV1 receptors are present not only in the neuronal structures of the submucosa,23,27 but also in the epithelial cells, in agreement with previous data.11,13,15,16 TRPV1 immunoreactivity was previously demonstrated in parietal cells15 and gastrin cells16 of the human gastric antrum and body, and in small nerves in the mucosa.
The precise role of HP infection in FD is still under debate,38,39 although there are several studies supporting the therapeutic effect of HP eradication on FD.40,41 Patients with active inflammatory bowel disease42 or erosive reflux disease34 showed enhanced TRPV1 expression in the inflamed tissues, suggesting that inflammation can trigger overexpression of TRPV1. Moreover, since inflammation induces NGF expression and NGF protein directly activates the TRPV1 receptor,43 HP, which is a leading cause of gastric inflammation, could be involved in promoting FD development via TRPV1 upregulation. In vitro experiments with AGS cells in the present study demonstrated that HP induced the overexpression of NGF, GDNF, and TRPV1, supporting the abovementioned hypothesis. However, the association between HP and FD in human is not straightforward. There were no significant differences in gastric TRPV1, GDNF, or NGF expression among the FD groups according to their HP infection status, although the TRPV1 immunostaining score was increased in the HP-positive subjects, compared with the HP-negative group. The proportion of HP-positive cases did not differ between the control and FD groups. Together, these data indicate that multiple factors are involved in FD development.
Nonetheless, at the 1-year follow-up evaluation, not only symptom-improved patients but also healthy controls showed reductions in NGF and TRPV1 expression, suggesting that HP eradication could resolve some of the inflammation-induced changes in nociceptor-related gene expression. Although it has not so far been possible to predict which FD patients will show reductions in their dyspeptic symptoms after anti-HP therapy, the findings of this study support the usefulness of anti-HP therapy for some subsets of HP-positive dyspepsia patients.44
Although IBS could be associated with inflammation-induced TRPV1 upregulation,36 inflammation does not explain all instances of TRPV1 overexpression or the generation of functional GI disorder symptoms. When comparing these genes even in the HP-negative subjects, TRPV1 and neurotrophic factors were upregulated in the FD group compared with the control group (Table 2). In contrast, at the 1-year follow-up evaluation of the empirically treated category, which was HP-negative, the 3 nociceptor-related genes were reduced in the symptom-improved patients, but not in the non-improved patients. This implies that TRPV1 expression can be closely associated with dyspeptic symptoms, even when HP-associated inflammation is absent. In line with our results, some previous studies have reported TRPV1 overexpression in GI diseases without overt inflammation, such as in NERD24 and idiopathic rectal or fecal urgency.23 The cause of the increased TRPV1 expression in HP-negative FD patients might be attributed to central and peripheral factors leading to increased NGF levels. Interestingly, NGF levels have been shown to alter with psychosocial influences45 and to increase with stress.46 Furthermore, chronic stress induces hypersensitivity in the absence of inflammation,47 while acid secretion may also drive TRPV1 overexpression.48 Consequently, HP infection could play a role in the overexpression of inflammation-induced neurotrophic factors and TRPV1, but cannot be considered an exclusive factor of FD.
Unlike NGF, details about the role of GDNF in the GI system are very limited, although some studies have suggested its importance in GI sensory transduction together with TRPV1.34 The present study showed that the expression of GDNF, along with that of NGF and TRPV1, was higher in FD patients than in control subjects. However, its reduction was not as prominent as that of NGF or TRPV1 in subjects whose dyspepsia symptoms were improved or in HP-eradicated subjects. There were no significant changes in GDNF expression at the 1-year follow-up evaluation across all symptom groups in HP-eradicated subjects, in comparison with baseline levels. This result is not consistent with the assumption that the expression pattern of neurotrophic factors would be similar to that of TRPV1. The reason for this discordance is unclear, but NGF expression appeared to be more related to the early phase of inflammation, while GDNF showed a delayed response compared with NGF.49 In the present study, there were differences among the 3 nociceptor-related genes in the timing of their elevated expression. NGF expression was elevated first, followed by that of GDNF and TRPV1. The elevation of GDNF mRNA was only sustained for a relatively short time. Further study of these 2 neurotrophic factors is needed.
There are several limitations to the present study. Since this is not a clinical trial, but rather an observational study, a few patients for whom HP eradication was achieved took prokinetics. This makes it difficult to isolate the effect of HP eradication on the dyspeptic symptoms. The sample size for the re-biopsy of patients was not large, since a majority of the patients rejected it. Whether increased TRPV1 expression is the cause of dyspeptic symptoms and the significance of the TRPV1-stained mucosa remain be clarified.
Nonetheless, the present study is the first to have demonstrated upregulation of TRPV1 and the expression of NGF genes in FD. The association of these molecular changes with FD suggests that their further study is warranted, since they may represent etiological factors. The present study also provided in vitro HP infection data and human results after HP eradication. These findings suggested that HP-induced inflammation and the accompanying elevation of TRPV1 mRNA and protein may be involved in the pathogenesis of HP-related dyspepsia, and indicated that anti-HP therapy could be useful for this disease category.
Supplementary Material
Acknowledgments
The authors are indebted to J. Patrick Barron, Professor Emeritus, Tokyo Medical University and Adjunct Professor, Seoul National University Bundang Hospital for his pro bono editing of this manuscript. In addition, the authors thank the Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
Footnotes
Abbreviations: EPS = epigastric pain syndrome, FD = functional dyspepsia, GDNF = glial cell-line derived neurotrophic factor, GI = gastrointestinal, GPA = global patient assessment, HP = Helicobacter pylori, IBS = irritable bowel syndrome, NERD = non-erosive reflux disease, NGF = nerve growth factor, PDS = postprandial distress syndrome, PPI = proton-pump inhibitor, qPCR = quantitative polymerase chain reaction, TRP = transient receptor potential, TRPV1 = transient receptor potential cation channel subfamily V member 1.
This work was supported by a National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korean government (MSIP) (No. 2011–0030001).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
The authors declare that they have no conflict of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol 2006; 12:2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130:1466–1479. [DOI] [PubMed] [Google Scholar]
- 3.Tack J, Piessevaux H, Coulie B, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998; 115:1346–1352. [DOI] [PubMed] [Google Scholar]
- 4.Perri F, Clemente R, Festa V, et al. Patterns of symptoms in functional dyspepsia: role of Helicobacter pylori infection and delayed gastric emptying. Am J Gastroenterol 1998; 93:2082–2088. [DOI] [PubMed] [Google Scholar]
- 5.Tack J, Caenepeel P, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology 2001; 121:526–535. [DOI] [PubMed] [Google Scholar]
- 6.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Aliment Pharmacol Ther 2011; 131:142–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui M, Honore P, Zhong C, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci 2006; 26:9385–9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everaerts W, Gees M, Alpizar YA, et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 2011; 21:316–321. [DOI] [PubMed] [Google Scholar]
- 9.Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 2003; 300:1284–1288. [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, de la Monte S, Ma J, et al. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 2009; 297:G135–G143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Harnett KM, Behar J, et al. Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2010; 298:G233–G240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Jones S, Brody K, et al. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 2004; 286:G983–G991. [DOI] [PubMed] [Google Scholar]
- 13.Nozawa Y, Nishihara K, Yamamoto A, et al. Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci Lett 2001; 309:33–36. [DOI] [PubMed] [Google Scholar]
- 14.Kato S, Aihara E, Nakamura A, et al. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol 2003; 66:1115–1121. [DOI] [PubMed] [Google Scholar]
- 15.Faussone-Pellegrini MS, Taddei A, Bizzoco E, et al. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem Cell Biol 2005; 124:61–68. [DOI] [PubMed] [Google Scholar]
- 16.Kechagias S, Botella S, Petersson F, et al. Expression of vanilloid receptor-1 in epithelial cells of human antral gastric mucosa. Scand J Gastroenterol 2005; 40:775–782. [DOI] [PubMed] [Google Scholar]
- 17.Hammer J, Führer M, Pipal L, et al. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol Motil 2008; 20:125–133. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Cao Y, Wong R, et al. Visceral and somatic sensory function in functional dyspepsia. Neurogastroenterol Motil 2013; 25:246–253. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt B, Hammer J, Holzer P, et al. Chemical nociception in the jejunum induced by capsaicin. Gut 2004; 53:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer J, Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol Motil 2007; 19:279–287. [DOI] [PubMed] [Google Scholar]
- 21.Lee K-J, Vos R, Janssens J, et al. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am J Physiol Gastrointest Liver Physiol 2004; 286:G278–G284. [DOI] [PubMed] [Google Scholar]
- 22.Kindt S, Vos R, Blondeau K, et al. Influence of intra-oesophageal capsaicin instillation on heartburn induction and oesophageal sensitivity in man. Neurogastroenterol Motil 2009; 21:1032–1038. [DOI] [PubMed] [Google Scholar]
- 23.Chan C, Facer P, Davis J, et al. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003; 361:385–391. [DOI] [PubMed] [Google Scholar]
- 24.Guarino M, Cheng L, Ma J, et al. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol Motil 2010; 22:746–751. [DOI] [PubMed] [Google Scholar]
- 25.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2001; 2:83–91. [DOI] [PubMed] [Google Scholar]
- 26.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 1999; 51:159–212. [PubMed] [Google Scholar]
- 27.McKelvey L, Shorten GD, O’Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem 2013; 124:276–289. [DOI] [PubMed] [Google Scholar]
- 28.Steinkamp M, Geerling I, Seufferlein T, et al. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 2003; 124:1748–1757. [DOI] [PubMed] [Google Scholar]
- 29.Noh YW, Jung H-K, Kim S-E, et al. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J Neurogastroenterol Motil 2010; 16:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song KH, Jung H-K, Min B-H, et al. Development and validation of the Korean Rome III questionnaire for diagnosis of functional gastrointestinal disorders. J Neurogastroenterol Motil 2013; 19:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vakil N, Halling K, Ohlsson L, et al. Symptom overlap between postprandial distress and epigastric pain syndromes of the Rome III dyspepsia classification. Am J Gastroenterol 2013; 108:767–774. [DOI] [PubMed] [Google Scholar]
- 32.Kim SE, Park YS, Kim N, et al. Effect of Helicobacter pylori eradication on functional dyspepsia. J Neurogastroenterol Motil 2013; 19:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon MF, Genta RM, Yardley JH, et al. the participants in the International Workshop on the Histopathology of Gastritis, Houston 1994. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 1996; 20:1161–1181. [DOI] [PubMed] [Google Scholar]
- 34.Shieh K, Yi C, Liu T, et al. Evidence for neurotrophic factors associating with TRPV1 gene expression in the inflamed human esophagus. Neurogastroenterol Motil 2010; 22:971–977. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Matsuzaki J, Fukushima Y, et al. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia—a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil 2014; 26:950–961. [DOI] [PubMed] [Google Scholar]
- 36.Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008; 57:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews PJ, Aziz Q, Facer P, et al. Increased capsaicin receptor TRPV1 nerve fibers in the inflamed human oesophagus. Eur J Gastroenterol Hepatol 2004; 16:897–902. [DOI] [PubMed] [Google Scholar]
- 38.Chey WD. Current consensus and remaining questions regarding the diagnosis and treatment of Helicobacter pylori. Gastroenterol Hepatol 2012; 8:623–625. [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JC. Asian consensus report on functional dyspepsia: necessary and ready? J Gastroenterol Hepatol 2012; 27:624–625. [DOI] [PubMed] [Google Scholar]
- 40.Jin X, Li Ym. Systematic review and meta-analysis from Chinese Literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter 2007; 12:541–546. [DOI] [PubMed] [Google Scholar]
- 41.Gwee K-A, Teng L, Wong R-K, et al. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol 2009; 21:417–424. [DOI] [PubMed] [Google Scholar]
- 42.Yiangou Y, Facer P, Dyer N, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 2001; 357:1338–1339. [DOI] [PubMed] [Google Scholar]
- 43.Chuang H-h, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns (4, 5) P2-mediated inhibition. Nature 2001; 411:957–962. [DOI] [PubMed] [Google Scholar]
- 44.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelucci F, Ricci E, Padua L, et al. Music exposure differentially alters the levels of brain-derived neurotrophic factor and nerve growth factor in the mouse hypothalamus. Neurosci Lett 2007; 429:152–155. [DOI] [PubMed] [Google Scholar]
- 46.Wijngaard RM, Klooker TK, Welting O, et al. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in marternally seperated rats. Neurogastroenterol Motil 2009; 21:1107–1117. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Winston J, Sarna S. Neurological and cellular regulation of visceral hypersensitivity induced by chronic stress and colonic inflammation in rats. Neuroscience 2013; 248:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, Altomare A, Guarino M, et al. HCl-induced and ATP-dependent up-regulation of TRPV1 receptor expression and cytokine production by human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2012; 303:G635–G645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malin SA, Molliver DC, Koerber HR, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 2006; 26:8588–8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


