Supplemental Digital Content is available in the text
Abstract
Limited data exist on the burden of serious adverse drug reactions (ADRs) in sub-Saharan Africa, which has high HIV and tuberculosis prevalence. We determined the proportion of adult admissions attributable to ADRs at 4 hospitals in South Africa. We characterized drugs implicated in, risk factors for, and the preventability of ADR-related admissions.
We prospectively followed patients admitted to 4 hospitals’ medical wards over sequential 30-day periods in 2013 and identified suspected ADRs with the aid of a trigger tool. A multidisciplinary team performed causality, preventability, and severity assessment using published criteria. We categorized an admission as ADR-related if the ADR was the primary reason for admission.
There were 1951 admissions involving 1904 patients: median age was 50 years (interquartile range 34–65), 1057 of 1904 (56%) were female, 559 of 1904 (29%) were HIV-infected, and 183 of 1904 (10%) were on antituberculosis therapy (ATT). There were 164 of 1951 (8.4%) ADR-related admissions. After adjustment for age and ATT, ADR-related admission was independently associated (P ≤ 0.02) with female sex (adjusted odds ratio [aOR] 1.51, 95% confidence interval [95% CI] 1.06–2.14), increasing drug count (aOR 1.14 per additional drug, 95% CI 1.09–1.20), increasing comorbidity score (aOR 1.23 per additional point, 95% CI 1.07–1.41), and use of antiretroviral therapy (ART) if HIV-infected (aOR 1.92 compared with HIV-negative/unknown, 95% CI 1.17–3.14). The most common ADRs were renal impairment, hypoglycemia, liver injury, and hemorrhage. Tenofovir disoproxil fumarate, insulin, rifampicin, and warfarin were most commonly implicated, respectively, in these 4 ADRs. ART, ATT, and/or co-trimoxazole were implicated in 56 of 164 (34%) ADR-related admissions. Seventy-three of 164 (45%) ADRs were assessed as preventable.
In our survey, approximately 1 in 12 admissions was because of an ADR. The range of ADRs and implicated drugs reflect South Africa's high HIV and tuberculosis burden. Identification and management of these ADRs should be considered in HIV and tuberculosis care and treatment programs and should be emphasized in health care worker training programmes.
INTRODUCTION
Data on the burden of serious adverse drug reactions (ADRs) in sub-Saharan Africa are limited. In this region, colliding epidemics of infectious and noncommunicable diseases,1–5 prevalent pharmacogenetic variants associated with increased risk of ADRs,6–9 widespread concomitant use of traditional remedies,8,10,11 and overburdened health care systems4,8,12–14 potentially contribute to the burden of drug-related harm.
In South Africa, an estimated 6.8 million people were HIV-infected by 2014, with approximately 2.9 million receiving antiretroviral therapy (ART).15 Moreover, in 2013, the country's tuberculosis incidence was estimated at 860 new cases per 100,000 population, and 62% of tuberculosis patients were HIV-co-infected.16 A 2005 South African hospital survey found that HIV infection was a risk factor for serious ADRs, especially in patients receiving ART.17 Subsequently, as with other sub-Saharan African countries, there has been massive scale-up of the ART programme with earlier initiation and use of less-toxic first-line ART regimens.18,19
We prospectively assessed medical admissions at 4 South African hospitals to determine the burden of ADRs resulting in hospital admission, to identify common serious ADRs, to identify the drugs implicated in these ADRs, to evaluate whether these ADRs were preventable, and to determine the influence of HIV infection on ADR burden and pattern.
METHODS
Setting and Study Design
We conducted a cross-sectional survey during 2013 in the adult medical wards of 4 hospitals. We prospectively reviewed all admissions over sequential 30-day periods at the sites. The survey sites represent 3 different provinces of South Africa and are a mixture of tertiary and regional hospitals. (See Supplementary Methods, Supplemental Digital Content 1, for a detailed description of the 4 hospitals).
We conducted this survey in parallel with a survey of ADR-related deaths, which has previously been published.20 The sample size was calculated to ensure adequate power for the mortality survey20: we calculated that a sample size of 2000 would be needed to detect a mortality rate because of ADR of 0.3%, with a 95% confidence interval of 0.1% to 0.7% (using exact confidence interval method of Clopper-Pearson). (See figure, Supplemental Digital Content 2, which demonstrates the extent of overlap between the 2 surveys’ participants).
Processes and Study Team
One medical doctor and 2 pharmacists collected the data. For every admission, we recorded demographic information, drug exposure history, and diagnoses. From the admission records and investigation results, we identified the reason for the admission and flagged this where it was potentially related to an ADR, with the aid of a trigger tool (see Table, Supplemental Digital Content 3, for the trigger tool used), adapted from Rozich.21 For flagged cases, we collected more detailed data, including laboratory results, and a more detailed drug history, which included start and stop dates, dosage regimens, and indications. We performed an exit clinical record review at patients’ discharge, death, or approximately 1 week after the end of the survey period for those still in hospital at survey end, to verify and augment data and to add information on the management and outcome of potential ADRs. Clinicians and nursing staff were aware of the survey, but did not actively report potential ADRs to the study team. When the data collection team identified information of direct potential benefit to the patient, this was communicated to the hospital clinical team.
Admissions identified as potentially ADR-related were assessed by a multidisciplinary case review panel for ADR causality, preventability, and severity. The panel reached its assessment through consensus discussion. (See Supplementary Methods, Supplemental Digital Content 1, which describes details of the panel composition, case identification, and case assessment procedures).
Record Linkage to Identify All-Cause Mortality
We identified in-hospital deaths from hospital administrative records. We performed linkage to the national population register, using South African personal identity numbers, to identify deaths occurring within 30 days of discharge from hospital.
Definitions and Taxonomies Used
We recorded drug exposure for 30 days before admission. We identified patients documented to have zero drug exposure, and distinguished those from patients for whom no drug history was documented in the medical records. We coded drugs according to the Anatomic Therapeutic Chemical (ATC) Classification System of the World Health Organization Collaborating Center for Drug Statistics Methodology,22 with a few exceptions and assumptions (see Supplementary Methods, Supplemental Digital Content 1, for an exposition of the exceptions and assumptions used in drug coding). We defined the drug count as the number of drugs with unique ATC codes over 28 days before admission, excluding topical preparations, vitamins, and mineral supplements. We defined first-line ART as 2 nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) in combination with a non-nucleoside reverse transcriptase inhibitor, and second-line ART as 2 NRTIs in combination with a ritonavir-boosted protease inhibitor, as per South African adult ART guidelines.23
We recorded patients’ diagnoses or clinical problems according to the International Statistical Classification of Disease and Related Health Problems, 10th Revision (ICD-10) codes. We calculated a comorbidity score for each admission, modified from the Charlson comorbidity score.24 Our modification did not allocate points for a diagnosis of HIV/AIDS, as the HIV score assigned by the original Charlson method is probably inappropriately high in the era of ART,25 and as we wanted to explore the independent effect of HIV infection on ADR-related admissions.
We performed causality assessment of suspected ADRs using the World Health Organization-Uppsala Monitoring Center (WHO-UMC) system for standardized case causality assessment.26 We defined an ADR according to the definition of Aronson and Ferner.27 We did not include intentional drug overdose, therapeutic failure, or cases of ART-associated immune reconstitution syndrome as ADRs. We coded ADRs to “preferred terms” contained in version 17.1 of the Medical Dictionary for Regulatory Activities (MedDRA, MedDRA Maintenance and Support Services Organization, McLean, VA, USA).
We considered a case to be an ADR-related admission if the multidisciplinary case review panel assessed the ADR as “possible,” “probable,” or “certain” according to WHO-UMC criteria, and the ADR was the main reason for the admission.
We considered ADRs to be preventable if the case review panel held that one or more of the Schumock and Thornton criteria28 was present. We assessed ADR severity according to guidance from Temple et al29 as causing temporary harm, permanent harm, near-death (including anaphylaxis and cardiorespiratory arrest), or death. We classified ADRs as type A (predictable from the pharmacological action of the drug) or type B (idiosyncratic) according to the Rawlins and Thompson classification30 and used this classification to inform our assessment of causality and preventability in the event of >1 drug suspected in the ADR. For type B reactions with >1 drug suspect, we assessed causality and preventability for each drug suspect separately. However, for type A reactions, we assessed causality and preventability attributable to the combined action of all drug suspects. When a combination of type A and type B mechanisms was considered to have caused the ADR, we classified the ADR as type B.
Data Entry and Statistical Analysis
We entered data into an Access 2010 database (Microsoft Corporation, Redmond, WA), and analyzed data using Stata 13.1 (Stata Corporation, College Station, TX). We used cross-tabulation and χ2 statistics to explore associations between binary and categorical variables. We summarized continuous variables using medians and interquartile ranges, owing to their non-normal distribution, and used the Wilcoxon rank-sum test for between-group comparisons of continuous variables. A P value of <0.05 was taken to indicate significant difference.
We constructed a generalized estimation equation model, with admissions clustered on the patient level for those with readmissions, to explore independent associations between ADR-related admission and age, sex, HIV infection, ART, ATT, drug count, and modified comorbidity score. Variables were selected for inclusion in the model a priori, at the time of study design. To categorize HIV infection and ART exposure, we constructed a 3-category variable: HIV-negative/unknown; HIV-infected and not on ART; or HIV-infected and on ART. In the main model, we excluded admissions wherein patients were documented as not exposed to any drug before the admission, but included those in whom no drug history was recorded. In sensitivity analyses, we constructed 2 additional models, the first including all admissions, and the second excluding patients with missing drug histories as well as patients with documented zero drug exposure.
Ethics
The Human Research Ethics Committee of the Faculty of Health Sciences at the University of Cape Town approved this study (reference no 576/2011). We received permission to conduct the research by the respective hospitals’ management and/or provincial Departments of Health.
RESULTS
Description of the Cohort: All Admissions
During the survey period, 1904 patients were admitted on 1951 occasions (1858 patients were each admitted once, 45 patients were each admitted twice, and 1 patient was admitted 3 times). In 242 of 1951 (12%) admissions, drug histories were not recorded in the medical records. Linkage with the population register was possible for only 866 of 1904 (45%) patients, because of missing personal identity numbers.
Patient characteristics at the time of their first admission are given in Table 1. HIV-infected patients were younger than HIV-negative/unknown patients (median age 36 years [interquartile range, IQR: 30–44 years] vs median age 58 years [IQR: 43–70 years], P < 0.001). Of the HIV-infected patients, 251 of 559 (45%) were not on ART, 253 of 559 (45%) were on first-line ART, 18 of 559 (3.2%) on second-line ART, 3 of 559 (0.54%) on other ART combinations, 33 of 559 (5.9%) on unspecified ART, and 1 of 559 (0.18%) on zidovudine monotherapy for prevention of mother-to-child-transmission. The drugs most commonly included in ART regimens were efavirenz (240), lamivudine (213), and TDF (207). The median CD4 count was 142.5 cells/mm3 (IQR: 42–328) in the 494 HIV-infected patients with CD4 cell counts available. A total of 128 of 559 (23%) HIV-infected patients were on treatment for tuberculosis at the time of first admission, versus 54 of 1345 (4.0%) HIV-negative/unknown patients (P < 0.001).
TABLE 1.
Characteristics of Patients at Their First Admission to the Medical Wards of 4 Hospitals in South Africa, 2013 (n = 1904)
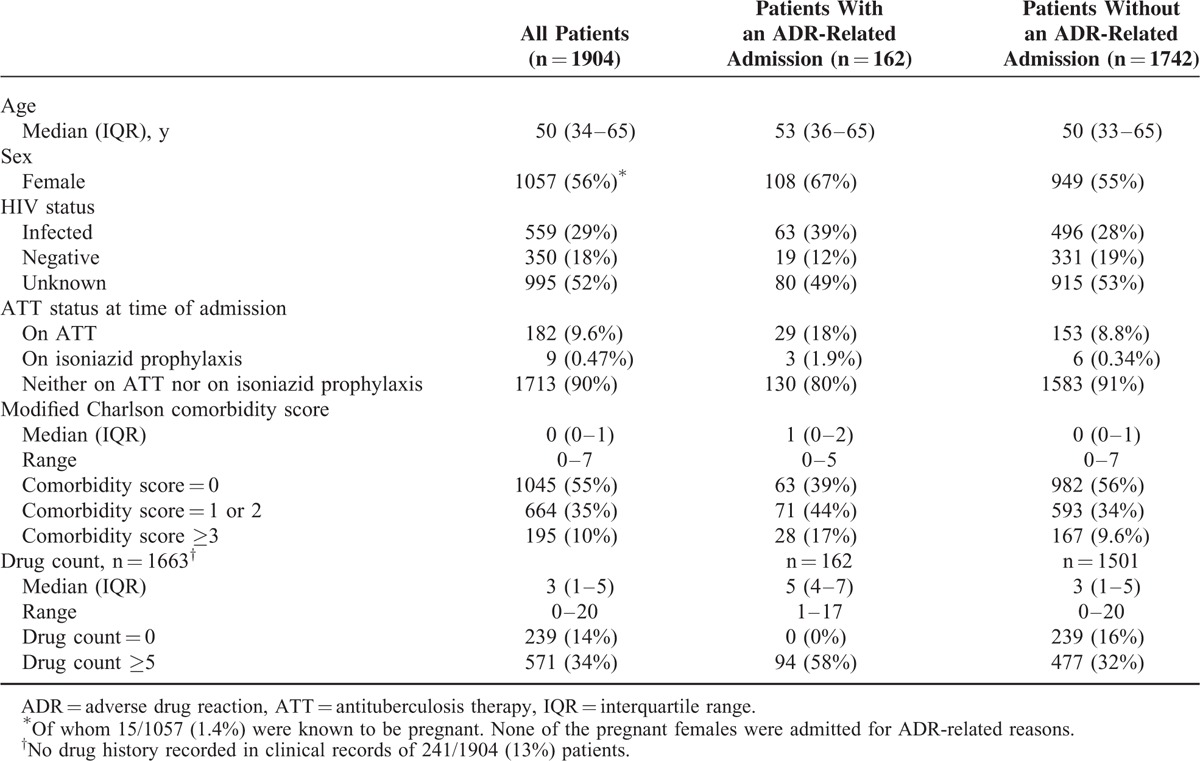
Other common comorbidities included cardiovascular disease in 44% of patients, endocrine/metabolic disease in 31%, renal failure in 17%, and chronic lower respiratory disease in 8.8%. Forty percent of patients were exposed to cardiac agents before their first admission (including 29% to diuretics, 20% to angiotensin-converting enzyme [ACE] inhibitors/angiotensin receptor blockers, 13% to calcium channel blockers, 12% to “statins”), 16% to antithrombotic agents (including 13% to aspirin), and 17% to blood glucose-lowering agents (including 6.8% to insulin). A total of 239 of 1904 (13%) patients were documented to have had zero drug exposure before their first admission.
The most common reasons for admission were cardiovascular disease (496/1951 [25%] admissions), respiratory disease (313/1951 [16%]), and infectious/parasitic disease (285/1951 [15%]) (See tables, Supplemental Digital Content 4, Supplemental Digital Content 5, and Supplemental Digital Content 6, which describe the prevalence of comorbidities, patients’ exposure to drugs before admission, and the cause of admission, respectively).
ADR-Related Admissions: Presentations and Implicated Drugs
We identified 164 ADR-related admissions, which represented 8.4% of all admissions (95% confidence interval [CI] 7.2%–9.7%). These 164 ADRs occurred in 162 patients, 2 of whom were each admitted twice for ADR-related reasons during the survey period.
Drugs used in the management of HIV and tuberculosis (ART, ATT, and co-trimoxazole) were implicated in 56 of 164 (34%) ADR-related admissions, and 63 of 164 (38%) ADR-related admissions occurred in HIV-infected patients.
The 5 most common ADRs resulting in admission were renal impairment (24 cases), hypoglycemia (22 cases), drug-induced liver injury (DILI) (20 cases), hemorrhage (19 cases), and blood dyscrasias (14 cases). These cases are summarized in Table 2 (Also see tables, Supplemental Digital Content 7, Supplemental Digital Content 8, Supplemental Digital Content 9, Supplemental Digital Content 10, and Supplemental Digital Content 11, for patient-level detail regarding each of the five most common presentations). Renal impairment, DILI, and blood dyscrasias predominantly occurred in HIV-infected and younger patients, whereas hypoglycemia and hemorrhage predominantly occurred in HIV-negative/unknown and older patients.
TABLE 2.
Summary of Characteristics of the 5 Most Common Adverse Drug Reactions That Caused Admission to the Medical Wards of 4 Hospitals in South Africa, 2013
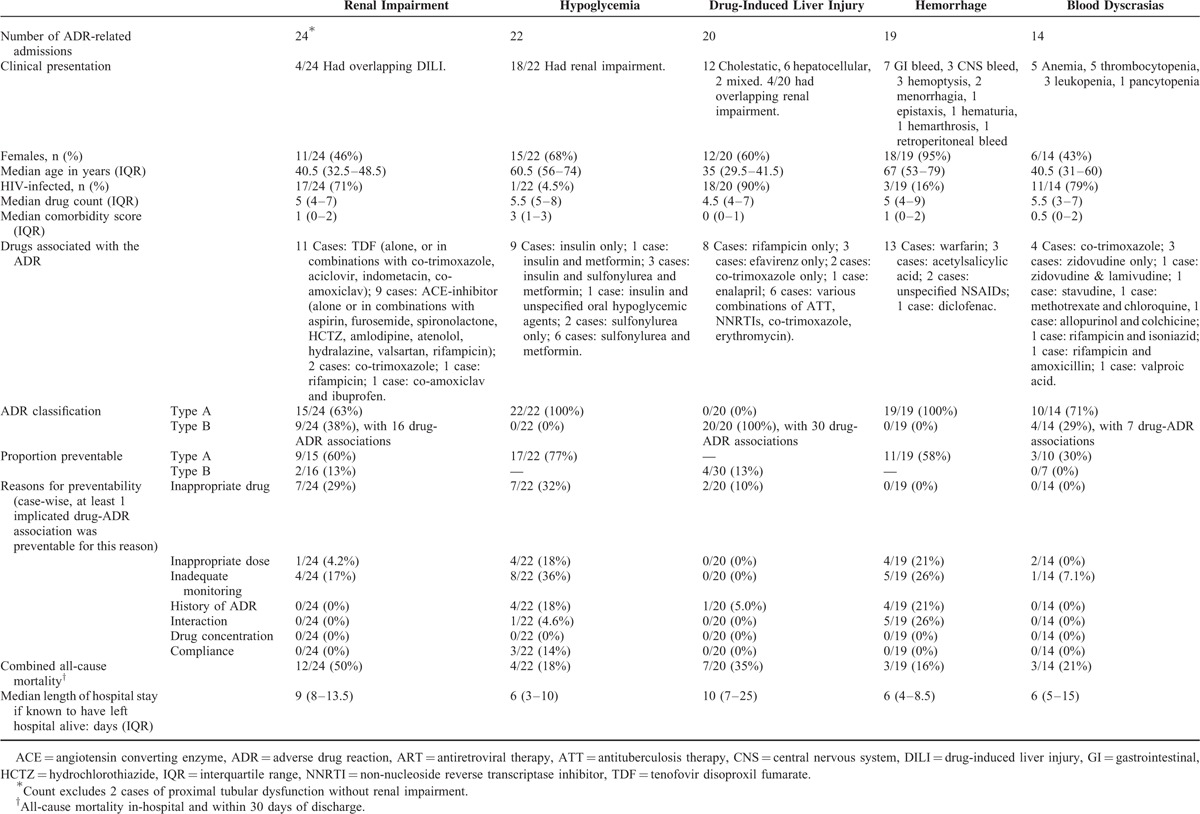
Other common ADRs resulting in admission included cardiac failure (9 cases), confusion (8 cases), electrolyte disturbances (7 cases), antineoplastic-, corticosteroid-, and immunosuppressant-associated pneumonia (4 cases), hypotension (4 cases), drug reaction with eosinophilia and systemic symptoms (DRESS) (4 cases), and ataxia (3 cases) (See tables, Supplemental Digital Content 12 and Supplemental Digital Content 13, for patient-level details of these ADR-related admissions. Also see table, Supplemental Digital Content 14, which maps the 164 ADRs to the multi-level MedDRA taxonomy).
Drugs and drug classes most commonly implicated in ADRs were NRTIs (23 times in 21 cases, with TDF implicated in 14, zidovudine in 4, stavudine in 3, and lamivudine in 2), rifampicin in 17, ACE-inhibitors in 15, insulin in 14, and warfarin in 13 cases (See table, Supplemental Digital Content 15, for a full list of drugs implicated in ADR-related admissions). Figure 1 plots the proportion of ADRs in which the drug was implicated against the drug's frequency of use, for the 19 drugs implicated in ≥4 ADR-related admissions. Warfarin, phenytoin, and co-trimoxazole were the 3 drugs most frequently implicated in ADR-related admissions, relative to their frequency of use.
FIGURE 1.
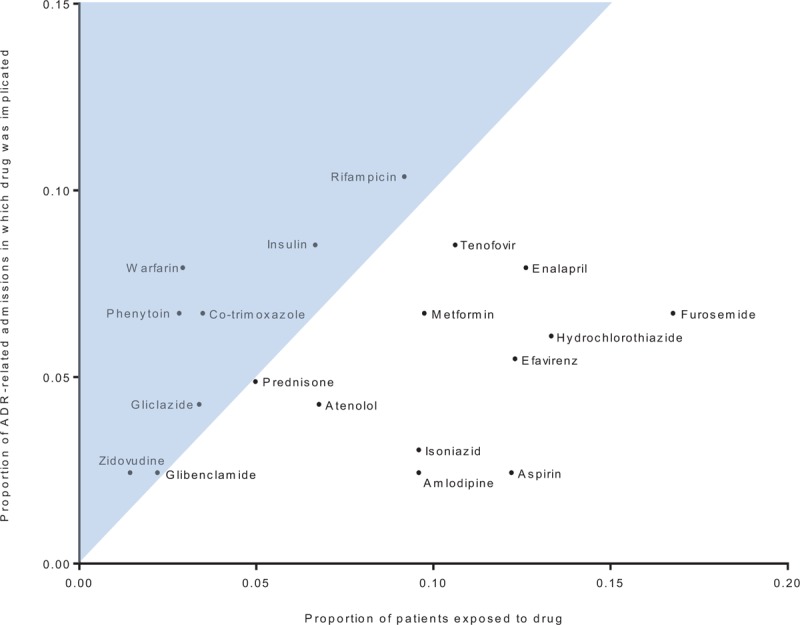
Scatterplot of 19 drugs implicated in at least 4 adverse drug reaction (ADR)-related admissions, showing proportion of patients exposed to the drug before their first admission versus the proportion of ADR-related admissions in which the drug was implicated. The shaded area represents those cases where the proportion of ADRs in which the drug was implicated was higher than the proportion of patients exposed to the drug.
ADR-Related Admissions: Associations
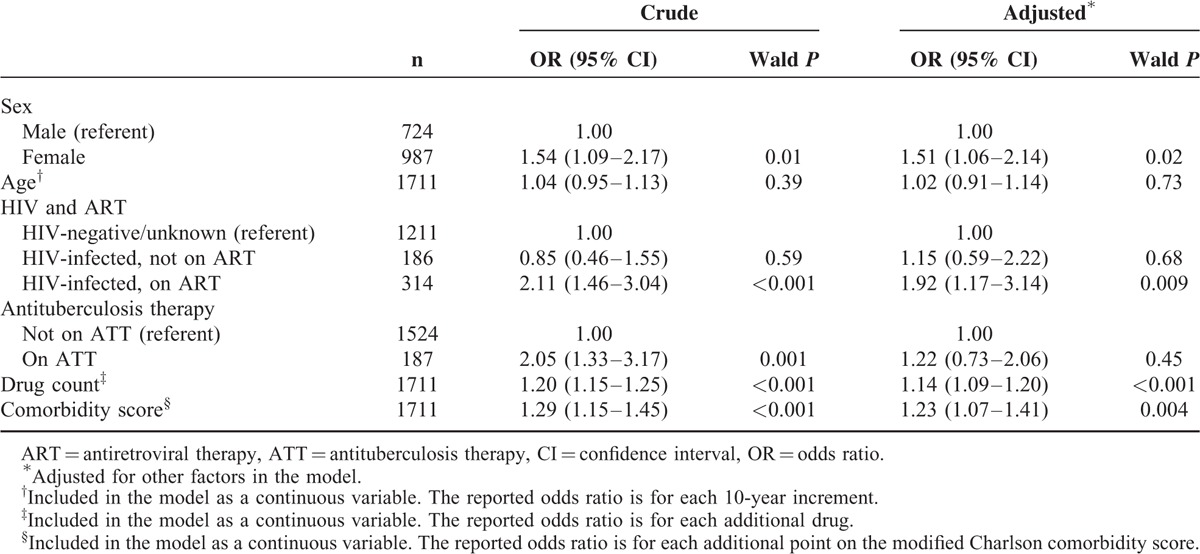
Results of the generalized equation estimation model (excluding patients who were not exposed to drugs over the 30 days before admission) are presented in Table 3. ADR-related admission was independently associated with female sex, higher drug count, higher comorbidity score, and HIV-infection with ART. We did not find an association between ADR-related admission and age. Associations were similar in the sensitivity analyses (See table, Supplemental Digital Content 16, for an alternative model including patients documented to have had zero drug exposure, and table, Supplemental Digital Content 17, for an alternative model excluding patients in whom no drug history was recorded in the medical records).
TABLE 3.
Generalized Estimation Equation Model of Associations With Adverse Drug Reaction-Related Admission (n = 1711 Admissions in 1669 Patients), Excluding Patients Documented to Have Had Zero Drug Exposure Before Admission
ADR-Related Admissions: ADR Classification, Causality, Preventability, and Severity
A total of 126 of 164 (77%) ADRs were classified as type A, and 38 of 164 (23%) as type B. Thirty-six of 63 (57%) ADRs in HIV-infected patients were type A and 27 of 63 (43%) type B. This differed significantly from HIV negative/unknown patients in whom 90 of 101 (89%) ADRs were type A and 11 of 101 (11%) type B (P < 0.001). The causality assessment of ADRs, stratified by ADR type and HIV status, is described in Table 4.
TABLE 4.
Causality Assessment of Adverse Drug Reactions, Stratified by Type of Adverse Drug Reaction and by Human Immunodeficiency Virus Infection Status
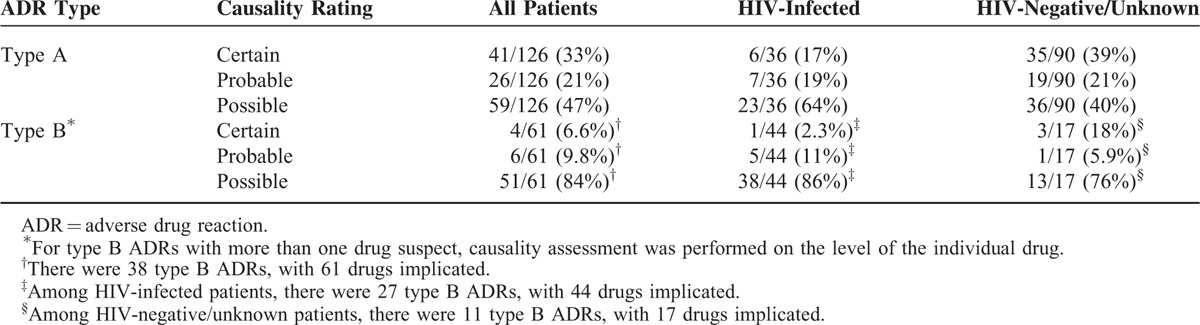
Seventy-three of 164 (45%) ADRs resulting in admission were assessed as preventable. The drugs most commonly implicated in preventable ADRs were warfarin (11), insulin (10), phenytoin (9), furosemide (8) and metformin (8). Sixty-nine of 126 (55%) type A ADRs were assessed as preventable versus 4 of 38 (11%) type B ADRs (P < 0.001). In 24 of 126 (19%) type A ADRs, the drug was inappropriate; in 14 of 126 (11%), the dose, route, or frequency was inappropriate; in 25 of 126 (20%), monitoring was insufficient; in 11 of 126 (8.7%), the patient previously had an ADR to the drug; in 13 of 126 (10%), a drug interaction played a role; in 9 of 126 (7.1%), a supratherapeutic drug concentration was found; and in 4 of 126 (3.2%), adherence played a role. In 3 of 38 (7.9%) type B ADRs, at least 1 implicated drug was inappropriate for the patient, and in 1 of 38 (2.6%), the patient had a previous ADR to an implicated drug.
Only 20 of 63 (32%) ADRs in HIV-infected patients were preventable versus 53 of 101 (52%) in patients who were HIV-negative/unknown (P = 0.009). In 10 of these 20 cases, the ADR was assessed as preventable because the implicated drug was considered inappropriate.
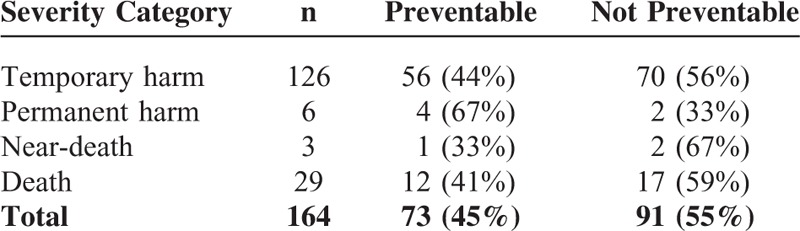
We assessed 126 of 164 (77%) ADRs to have resulted in temporary harm, 6 of 126 (3.7%) to have resulted in permanent harm, 3 of 126 (1.8%) to have resulted in near-death, and 29 of 164 (18%) to have resulted in death. Table 5 shows that there was no association between the severity of ADRs and their preventability (P = 0.71).
TABLE 5.
Severity and Preventability of Adverse Drug Reactions Resulting in Admission
ADR-Related Admissions: Patient Outcomes
The median length of stay was 7 days (IQR: 4–11 days) in the 1528 admissions wherein the patient exited the hospital alive, and did not differ between admissions for ADRs (median 7 days, IQR: 4–10 days) and admissions for other reasons (median 7 days, IQR: 4–11 days, P = .87).
Combined all-cause mortality (in-hospital death or death within 30 days of discharge) among patients with an ADR-related admission was in 38 of 162 (23%) patients. This was no different from combined all-cause mortality in patients admitted for other reasons (362/1742 [21%], P = 0.42). However, combined all-cause mortality was significantly worse in HIV-infected patients with an ADR-related admission (24/63 [38%]) versus HIV-infected patients admitted for other reasons (117/496 [24%], P = 0.01).
DISCUSSION
We found that 8.4% of admissions to adult medical wards of 4 hospitals in 3 provinces of South Africa were directly attributable to ADRs, with drugs used in the management of tuberculosis and HIV implicated in one-third of these admissions. ADRs in HIV-infected patients were more likely to be severe type B ADRs, were less likely to be preventable, and were associated with higher all-cause mortality. Nearly half of ADRs resulting in admission were preventable.
Six systematic reviews, of studies with a design comparable to ours, have estimated the proportion of adult medical admissions attributable to ADRs to range between 3.1% and 6.3%31–36 (See table, Supplemental Digital Content 18, for a summary of these six reviews). Studies from low- and middle-income countries (LMICs) are notably underrepresented in these reviews. Previous LMIC hospital-based surveys found the proportion of adult medical admissions attributable to ADRs to be 19% in Argentina,37 7.0% in Lebanon,38 and 6.8% in India.39 ADR burden data from sub-Saharan Africa, a setting of high HIV and tuberculosis prevalence, are scarce. In a previous South African survey, 6.3% of adult medical admissions were found to have been ADR-related.17 Although these differences may be because of variations in study design, they may also, when combined with our figure of 8.4%, suggest that ADRs may result in proportionally more medical admissions in LMICs than is the case in high-income countries.
The different burden and pattern of ADR-related admissions we observed may be explained as an effect of the colliding epidemics of infectious and noncommunicable diseases in South Africa. In our study, a group of mostly elderly patients with multimorbidity presented with type A ADRs such as hypoglycemia, hemorrhages, and hypotension, which is not unlike typical ADR presentations seen in studies from high-income settings. A second group of patients in our survey were younger, with a burden of chronic infectious diseases (which is not allocated a score on the original or modified Charlson comorbidity score), and presented with more type B ADRs including DILI and blood dyscrasias. HIV-infected patients are known to be at increased risk for drug hypersensitivity reactions, particularly to co-trimoxazole40,41 and ATT,40–42 but the pathophysiology is not fully understood and likely to be multifactorial.40,41
A meta-analysis of 6 observational studies found a decline in creatinine clearance attributable to TDF43 that was approximately 10 times the rate of normal age-related decline44, which was significantly greater than that seen in randomized controlled trials43,44 and probably reflects the “real-life” situation.43–45 TDF-associated renal impairment is not uncommon in sub-Saharan Africa,46,47 and our survey identified 11 cases with high mortality. In all of our cases of TDF-associated renal impairment, causality was assessed as “possible,” because of the presence of concomitant diseases and/or nephrotoxic drugs. TDF use in acutely ill patients with renal dysfunction in this region is of concern, particularly when TDF is co-prescribed with other potentially nephrotoxic drugs, as there is limited facilities for renal replacement therapy in sub-Saharan Africa.
All of the patients admitted because of DILI in our survey were HIV-infected and/or taking tuberculosis treatment. A very high incidence (15%) of DILI was reported in Ethiopians on ART, ATT, and cotrimoxazole.48 Most of our patients with DILI showed a cholestatic liver enzyme pattern, which is similar to the Ethiopian study, and which often implicated rifampicin rather than isoniazid or pyrazinamide as the causative agent. Combined all-cause mortality among our DILI patients was 35%, which is considerably worse than the mortality rates of 5.2% to 11.5% reported in 4 observational studies from high-income settings.49
We identified a large number of ADR admissions for hypoglycemia. In the majority of our cases, insulin was implicated and many patients had concomitant renal impairment. Most of the admissions for hypoglycemia were assessed as preventable, mostly because of insufficient blood glucose monitoring. Insufficient blood glucose monitoring was identified as a major problem in a rural setting in South Africa, where 48% of patients with diabetes mellitus had no blood glucose value recorded in their clinic records during the previous year.50
Our finding that 45% of ADRs resulting in admission were preventable is in keeping with a 2012 meta-analysis, which estimated that 52% (95% CI: 42%–62%) of ADR-related admissions are because of preventable ADRs.51
Our study has a number of limitations. First, we relied on clinical data recorded in hospital clinical records and we did not have access to patients’ primary care medical records. Despite educating clinicians about our study, 1 in 8 folders had no drug history recorded, and the documented drug exposure before admission suggests there may have been poor documentation of contraceptives, over-the-counter agents, topical agents, and herbal remedies, which may have resulted in under-ascertainment of ADR-related admissions. Second, linkage with the South African population register was not possible for the majority of the patients included in our survey, as accurate personal identity numbers were not routinely documented at all sites, and thus we have under-ascertained post-discharge death. Third, assessment of ADR causality, severity, and preventability is subjective and assessment by expert judgment has previously been criticized.52–54 We used a 2-step process, applying a sensitive screening instrument followed by a multidisciplinary panel discussion of screened cases, to help mitigate the subjectivity associated with expert assessments.
Our study findings have limited generalizability outside secondary and tertiary settings in South Africa. Rather, it emphasizes how the local disease burden and drug use pattern influences the ADRs occurring in our setting. Public health programmes should factor in anticipated risks, including ADRs, and should incorporate risk-reduction measures. Our study demonstrates the feasibility of generating locally relevant pharmacoepidemiological data in a resource-limited setting, and the methodology we used is suited to be periodically repeated, scaled up, and/or transferred elsewhere. The key risk drivers that an approach such as this identifies can be turned into opportunities to improve quality of care.
Supplementary Material
Acknowledgments
We thank Andrew Boulle and Nicky Maxwell from the Centre for Infectious Disease Epidemiology and Research (CIDER) at the University of Cape Town for data linkage with the South African population registry. We also thank the clinical, nursing, administrative, and managerial staff at the four hospitals for their assistance and support in this study.
Footnotes
Abbreviations: ACE = angiotensin converting enzyme, ADR(s) = adverse drug reaction(s), AIDS = acquired immunodeficiency syndrome, aOR = adjusted odds ratio, ART = antiretroviral therapy, ATC = anatomic therapeutic chemical, ATT = antituberculosis therapy, CI = confidence interval, DILI = drug-induced liver injury, DRESS = drug reaction with eosinophilia and systemic symptoms, HIV = human immunodeficiency virus, ICD-10 = International Statistical Classification of Disease and Related Health Problems, 10th Revision, IQR = interquartile range, LMIC(s) = low- and middle-income country (countries), MedDRA = Medical Dictionary for Regulatory Activities, NRTI(s) = nucleoside/nucleotide reverse transcriptase inhibitor(s), TDF = tenofovir disoproxil fumarate, WHO-UMC = World Health Organization-Uppsala Monitoring Centre.
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under terms of Cooperative Agreement Number GGH000371. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
MedDRA, the Medical Dictionary for Regulatory Activities terminology, is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA trademark is owned by IFPMA on behalf of ICH.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Coovadia H, Jewkes R, Barron P, et al. The health and health system of South Africa: historical roots of current public health challenges. Lancet 2009; 374:817–834.doi:10.1016/S0140-6736(09)60951-X. [DOI] [PubMed] [Google Scholar]
- 2.Karim SSA, Churchyard GJ, Karim QA, et al. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009; 374:921–933.doi:10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayosi BM, Flisher AJ, Lalloo UG, et al. The burden of non-communicable diseases in South Africa. Lancet 2009; 374:934–947.doi:10.1016/S0140-6736(09)61087-4. [DOI] [PubMed] [Google Scholar]
- 4.Mayosi BM, Lawn JE, Van Niekerk A, et al. Health in South Africa: Changes and challenges since 2009. Lancet 2012; 380:2029–2043.doi:10.1016/S0140-6736(12)61814-5. [DOI] [PubMed] [Google Scholar]
- 5.Oni T, Youngblood E, Boulle A, et al. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis 2015; 15:1–8.doi:10.1186/s12879-015-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warnich L, Drögemöller BI, Pepper MS, et al. Pharmacogenomic Research in South Africa: Lessons Learned and Future Opportunities in the Rainbow Nation. Curr Pharmacogenomics Person Med 2011; 9:191–207.doi:10. 2174/187569211796957575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aminkeng F, Ross CJD, Rassekh SR, et al. Higher frequency of genetic variants conferring increased risk for ADRs for commonly used drugs treating cancer, AIDS and tuberculosis in persons of African descent. Pharmacogenomics J 2014; 14:160–170.doi:10.1038/tpj.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isah AO, Pal SN, Olsson S, et al. Specific features of medicines safety and pharmacovigilance in Africa. Ther Adv Drug Saf 2011; 3:25–34.doi:10.1177/2042098611425695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subbaraman R, Chaguturu SK, Mayer KH, et al. Adverse effects of highly active antiretroviral therapy in developing countries. Clin Infect Dis 2007; 45:1093–1101.doi:10.1086/521150. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Pharmacovigilance for Antiretrovirals in Resource-Poor Countries. Geneva: World Health Organization; 2007. Available at: http://www.who.int/entity/medicines/areas/quality_safety/safety_efficacy/PhV_for_antiretrovirals.pdf?ua=1 Accessed on March 16, 2016. [Google Scholar]
- 11.Mudzviti T, Maponga CC, Khoza S, et al. The impact of herbal drug use on adverse drug reaction profiles of patients on antiretroviral therapy in Zimbabwe. AIDS Res Treat 2012; 434171.doi:10.1155/2012/434171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopra M, Lawn JE, Sanders D, et al. Achieving the health Millennium Development Goals for South Africa: challenges and priorities. Lancet 2009; 374:1023–1031.doi:10.1016/S0140-6736(09)61122-3. [DOI] [PubMed] [Google Scholar]
- 13.Olsson S, Pal SN, Stergachis A, et al. Pharmacovigilance activities in 55 low- and middle-income countries: a questionnaire-based analysis. Drug Saf 2010; 33:689–703.doi:10.2165/11536390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Health Systems Trust. The National Health Care Facilities Baseline Audit. National Summary Report 2012, Revised February 2013. Westville; 2013. Available at: http://www.health-e.org.za/wp-content/uploads/2013/09/National-Health-Facilities-Audit.pdf Accessed on March 16, 2016. [Google Scholar]
- 15.UNAIDS. How AIDS Changed Everything: MDG6: 15 Years, 15 Lessons of Hope from the AIDS Response. Geneva; 2015. Available at: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf Accessed on March 16, 2016. [Google Scholar]
- 16.World Health Organization. Global Tuberculosis Report 2014. Geneva: World Health Organization; 2014. Available at: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf Accessed on March 16, 2016. [Google Scholar]
- 17.Mehta U, Durrheim DN, Blockman M, et al. Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol 2008; 65:396–406.doi:10.1111/j.1365-2125.2007.03034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2010. Available at: http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf Accessed on March 16, 2016. [PubMed] [Google Scholar]
- 19.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf Accessed on March 16, 2016. [Google Scholar]
- 20.Mouton JP, Mehta U, Parrish AG, et al. Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: A cross-sectional survey. Br J Clin Pharmacol 2015; 80:818–826.doi:10.1111/bcp.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rozich JD, Haraden CR, Resar RK. Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care 2003; 12:194–200.doi:10.1136/qhc.12.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2013. 16th ed. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2012. Available at: http://www.whocc.no/filearchive/publications/1_2013guidelines.pdf Accessed on March 16, 2016. [Google Scholar]
- 23.Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria: Department of Health; 2013. Available at: http://www.sahivsoc.org/upload/documents/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf Accessed on March 16, 2016. [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139.doi:10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 25.Zavascki AP, Fuchs SC. The need for reappraisal of AIDS score weight of Charlson comorbidity index. J Clin Epidemiol 2007; 60:867–868.doi:10.1016/j.jclinepi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Uppsala Monitoring Centre. The use of the WHO-UMC system for standardized case causality assessment. Available at: http://who-umc.org/Graphics/24734.pdf Accessed on March 16, 2016. [Google Scholar]
- 27.Aronson JK, Ferner RE. Clarification of terminology in drug safety. Drug Saf 2005; 28:851–870.doi:10.2165/00002018-200528100-00003. [DOI] [PubMed] [Google Scholar]
- 28.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992; 27:538. [PubMed] [Google Scholar]
- 29.Temple ME, Robinson RF, Miller JC, et al. Frequency and preventability of adverse drug reactions in paediatric patients. Drug Saf 2004; 27:819–829.doi:10.2165/00002018-200427110-00005. [DOI] [PubMed] [Google Scholar]
- 30.Rawlins MD. Clinical pharmacology. Adverse reactions to drugs. Br Med J 1981; 282:974–976.doi:10.1136/bmj.282.6268.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Einarson TR. Drug-related hospital admissions. Ann Pharmacother 1993; 27:832–840.doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- 32.Muehlberger N, Schneeweiss S, Hasford J. Adverse drug reaction monitoring--cost and benefit considerations. Part I: frequency of adverse drug reactions causing hospital admissions. Pharmacoepidemiol Drug Saf 1997; 6 Suppl 3:S71–S77.doi:10.1002/(SICI)1099-1557(199710)6:3+<S71::AID-PDS282>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.doi:10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 34.Wiffen P, Gill M, Edwards JJ, Moore A. Adverse drug reactions in hospital patients: A systematic review of the prospective and retrospective studies. Zanger U, ed. Bandolier Extra. 2002;(June):1-15. http://www.medicine.ox.ac.uk/bandolier/Extraforbando/ADRPM.pdf Accessed March 16, 2016. [Google Scholar]
- 35.Beijer HJM, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 2002; 24:46–54.doi:10.1023/A:1015570104121. [DOI] [PubMed] [Google Scholar]
- 36.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42:1017–1025.doi:10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez PA, Bril F, Castro V, et al. Adverse drug reactions as a reason for admission to an internal medicine ward in Argentina. Int J Risk Saf Med 2013; 25:185–192.doi:10.3233/JRS-130596. [DOI] [PubMed] [Google Scholar]
- 38.Major S, Badr S, Bahlawan L, et al. Drug-related hospitalization at a tertiary teaching center in Lebanon: incidence, associations, and relation to self-medicating behavior. Clin Pharmacol Ther 1998; 64:450–461.doi:10.1016/S0009-9236(98)90076-5. [DOI] [PubMed] [Google Scholar]
- 39.Patel KJ, Kedia MS, Bajpai D, et al. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: a prospective study. BMC Clin Pharmacol 2007; 7:8.doi:10.1186/1472-6904-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips E, Mallal S. Drug hypersensitivity in HIV. Curr Opin Allergy Clin Immunol 2007; 7:324–330.doi:10.1097/ACI.0b013e32825ea68a. [DOI] [PubMed] [Google Scholar]
- 41.Lin D, Tucker MJ, Rieder MJ. Increased adverse drug reactions to antimicrobials and anticonvulsants in patients with HIV infection. Ann Pharmacother 2006; 40:1594–1601.doi:10.1345/aph.1G525. [DOI] [PubMed] [Google Scholar]
- 42.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003; 167:1472–1477.doi:10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 43.Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010; 51:496–505.doi:10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 44.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol 2013; 24:1519–1527.doi:10.1681/ASN.2012080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez-Nóvoa S, Alvarez E, Labarga P, et al. Renal toxicity associated with tenofovir use. Expert Opin Drug Saf 2010; 9:545–559.doi:10.1517/14740331003627458. [DOI] [PubMed] [Google Scholar]
- 46.Mulenga L, Musonda P, Mwango A, et al. Effect of baseline renal function on tenofovir-containing antiretroviral therapy outcomes in Zambia. Clin Infect Dis 2014; 58:1473–1480.doi:10.1093/cid/ciu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bygrave H, Kranzer K, Hilderbrand K, et al. Renal safety of a tenofovir-containing first line regimen: experience from an antiretroviral cohort in rural Lesotho. PLoS One 2011; 6:e17609.doi:10.1371/journal.pone.0017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yimer G, Gry M, Amogne W, et al. Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: a prospective four arm observational study in Ethiopian patients. PLoS One 2014; 9:e94271.doi:10.1371/journal.pone.0094271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Björnsson ES. Incidence and outcomes of DILI in Western patients. Clin Liver Dis 2014; 4:9–11.doi:10.1002/cld.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rotchford AP, Rotchford KM. Diabetes in rural South Africa--an assessment of care and complications. South African Med J 2002; 92:536–541. [PubMed] [Google Scholar]
- 51.Hakkarainen KM, Hedna K, Petzold M, et al. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions - a meta-analysis. PLoS One 2012; 7:e33236.doi:10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arimone Y, Bégaud B, Miremont-Salamé G, et al. Agreement of expert judgment in causality assessment of adverse drug reactions. Eur J Clin Pharmacol 2005; 61:169–173.doi:10.1007/s00228-004-0869-2. [DOI] [PubMed] [Google Scholar]
- 53.Arimone Y, Miremont-Salamé G, Haramburu F, et al. Inter-expert agreement of seven criteria in causality assessment of adverse drug reactions. Br J Clin Pharmacol 2007; 64:482–488.doi:10.1111/j.1365-2125.2007.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch-Weser J, Sellers EM, Zacest R. The ambiguity of adverse drug reactions. Eur J Clin Pharmacol 1977; 11:75–78.doi: 10.1007/BF00562895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


