Abstract
Data on infiltrative hepatocellular carcinoma (iHCC) receiving hepatectomy are unclear. Our study assessed the outcomes, effects of anatomical resection, and prognostic factors in a cohort of Chinese patients with iHCC undergoing hepatectomy.
Data from 47 patients with iHCC undergoing hepatectomy were analyzed in a retrospective study. Independent prognostic factors of overall survival (OS) and recurrence-free survival (RFS) were identified using univariate and multivariate analyses. Correlations between microvascular invasion (MVI) and clinicopathological features were assessed using the χ2 test, Student t test, or the Mann–Whitney U test. Survival outcomes were estimated using the Kaplan-Meier method.
The median OS was 27.37 months and the 1-year RFS rate were 61.7%. Alpha-fetoprotein (AFP) level was not a specific parameter in iHCC patients undergoing hepatectomy. Anatomic resection was significantly associated with increased RFS (P = 0.007). Patients showing MVI were observed with decreased RFS (P < 0.001). A high lactate dehydrogenase (LDH) level was significantly associated with decreased OS and RFS (P = 0.003 and P = 0.020, respectively). MVI was shown correlated with the levels of aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), and LDH. Subgroup analysis indicated that in mild MVI group, survival outcome was significantly more favorable in patients with high LDH level (P = 0.019).
iHCC patients are related with higher MVI rate and patients may still derive survival benefit from anatomic resection at early and intermediate stages. MVI classification could be used to identify iHCC patients with a poorer survival, especially those with a high preoperative LDH level.
INTRODUCTION
Worldwide, hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths.1 Heterogeneity of HCC is not only due to the variables determining the stage (tumor burden and liver function) but also the gross morphologic features of the tumor, which is not included in the staging systems.2,3 HCC can present with different morphological subtypes, including single nodular type, single nodular type with extranodular growth, confluent multinodular type, and infiltrative type.4
In Chinese patients with solitary HCC, the infiltrative type accounted for a much higher proportion than other regions of the world. Infiltrative HCC (iHCC) had higher serum alpha-fetoprotein (AFP) level, hepatitis B virus (HBV) infection, and microvascular invasion (MVI) rates with poorer prognosis than other 3 types.5 Radiographically, iHCC has indistinct borders, a lack of typical enhancement pattern seen in solitary HCC.6,7 Previous studies focused on iHCC were from a cohort of patients at advanced and late stages treated with transarterial chemoembolization (TACE), sorafenib, or supportive care.8–10 Treatment options for patients with iHCC are more limited and remain poorly defined against patients at early and intermediate stages. On the basis of the Hong Kong Liver Cancer (HKLC) staging system, which was more suitable for predicting prognosis in a cohort of Chinese HCC patients, resection is a treatment option for patients at early and intermediate stages.11 Data on the presentation and outcome of patients with iHCC are not well characterized. In addition, the style of resection may also play a pivotal role in the prognosis of iHCC patients.
The primary aim of the current study was to assess the outcomes, effects of anatomical resection, and prognostic factors in a cohort of Chinese patients with iHCC undergoing hepatectomy. We also sought to deeply evaluate the demographic and clinical characteristics of iHCC.
METHODS
Ethics Statement
This study has been performed in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the 1975 Declaration of Helsinki, as revised in 1983. For this type of study, formal consent is not required.
Patients
This is a retrospective study of a cohort of 47 patients with iHCC undergoing hepatectomy between January 2003 and December 2012 in the Department of Hepatopancreatobiliary Surgery of Drum Tower Hospital. Cases of iHCC were identified by a pathological review (Figure 1). Color photographs of the resected liver specimens were reviewed according to the largest cross-section of the tumor. iHCC type was determined by 3 reviewers (1 pathologist, 1 radiologist, and 1 surgeon) who were blind to the clinical and pathological data. Disagreement in diagnosing iHCC was resolved by consensus review. Clear agreement on the identification of iHCC was established in 58 patients. Eleven patients who were lost to follow-up were then excluded. The final cohort consisted of 47 patients.
FIGURE 1.

Anatomic liver S5 resection. Infiltrative small HCC lesion.
Data Collection
The variables collected for analysis included patient demographics (age, gender), serum laboratory data [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and so on], tumor characteristics (tumor numbers, tumor size, vascular invasion, and so on), portal hypertension (gastroesophageal varices, splenomegaly with a platelet count of less than 100 000/mL, ascites), surgical data (surgical procedure, type of resection, surgical margin, and so on), and pathological data (histological grade, MVI, T category). MELD score, Eastern Cooperative Oncology Group (ECOG) performance status, and ICG retention rate in 15 minutes (R15) were also recorded.
The overall survival (OS) time was defined as the time from the date surgery started to the date of death or last contact for surviving patients. The recurrence-free survival (RFS) was defined as the duration from the date of surgery to the date of recurrence, or to the date of the last follow-up.
Statistical Analysis
Median values were used to describe continuous data, with categorical variables displayed as frequencies and percentages. OS and RFS were calculated by the Kaplan–Meier method, and curves were compared by the log-rank test. Prognostic factors associated with OS and RFS were identified by the Cox proportional hazard model. The correlation between clinicopathologic factors and the degree of MVI was analyzed by the χ2 test, Student t test, or the Mann–Whitney U test, where appropriate. Statistical analyses were carried out using SPSS software, version 19.0 (SPSS, IBM).
RESULTS
Patient Characteristics
The clinicopathologic features and tumor characteristics are summarized in Table 1 . Of the 47 patients, 38 (80.9%) were men; the median age was 51 years (range, 22–73 yrs). HBV was the most common etiology (95.7%). The median MELD score was 7.0 (range, 6.0–12.0) with most patients presenting with an ECOG of 0 (85.1%). All the patients in this study were classified as Child's class A. The median AFP level was 315.2 ng/mL (range, 0.7–311,000.0 ng/mL) at presentation; 27.7% of patients had an AFP greater than 1000 ng/mL, and 19.1% had an AFP less than 20 ng/mL. The median ICG retention rate in 15 minutes (R15), which reflects liver function, was 4.8% (range, 0.9%–13.2%). For enrolled patients, the median tumor size was 6.0 cm (range, 1.5–14.0 cm), of whom 35 (74.5%) had a single lesion. There were 11 patients (23.4%) having vascular invasion, 4 patients (8.5%) with extrahepatic spread, and 42 (89.4%) patients with cirrhosis. Ascites was not shown in the majority of patients (76.6%), and the same was presented in splenomegaly (76.6%) and gastroesophageal varices (91.5%).
TABLE 1.
Baseline Characteristics of iHCC Patients Undergoing Hepatectomy (n = 47)
Surgical Characteristics
Among these iHCC patients, 22 patients received anatomic resection and others underwent nonanatomic resection. Hepatic portal occlusion was given to 33 patients with a median occlusion time of 30 minutes (range, 0–150 min). The overall median operation time was 260 minutes (range, 90–510 min) and the surgical margin was gauged with a median result of 0.5 cm (range, 0–3.0 cm). Median bleeding volume and transfusion volume were 400 mL (range, 0–2500 mL) and 0 mL (range, 0–1925 mL), respectively.
Pathological Characteristics
Using the modified Edmondson classification,12 4 (8.5%) patients were characterized as poorly differentiated, 39 (83.0%) were moderately differentiated, and 4 (8.5%) were well differentiated. In addition, patients with iHCC more commonly had MVI (61.7%). According to T stage of AJCC, 13 were classified as stage T1, 18 were stage T2, 14 were stage T3, and 2 were stage T4.
Survival and Recurrence Analysis
As of June 2015, 30 of the 47 iHCC patients had died (63.8%). The median OS was 27.37 months (95% confidence interval, 4.52–50.22 mo). The 1-, 3-, and 5-year OS rates were 72.3%, 46.3%, and 32.8%, respectively. The 1-, 3-, and 5-year RFS rates were 61.7%, 26.1%, and 16.6%, respectively. Stratified by HKLC stage, median survival was 52.57 months for stage I + IIb (n = 31), and 8.47 months for stage IIIb + IVa (n = 16).
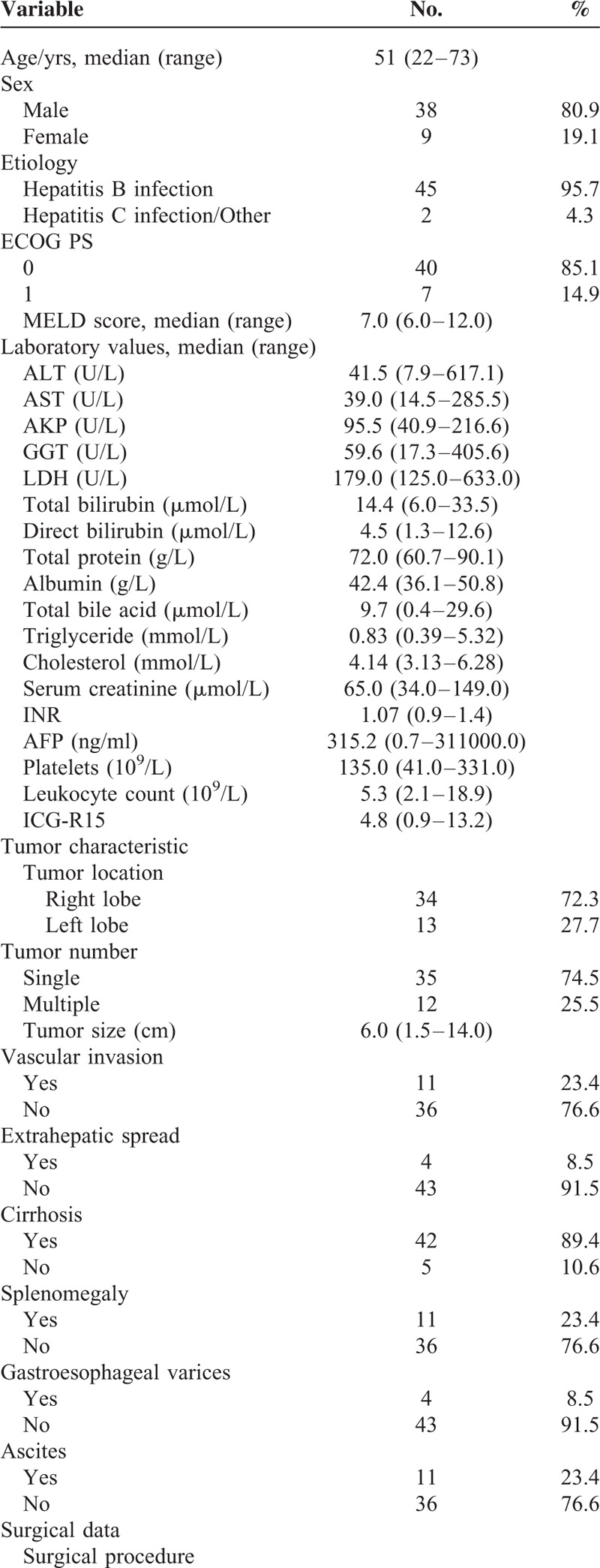
Predictors of Death and Recurrence
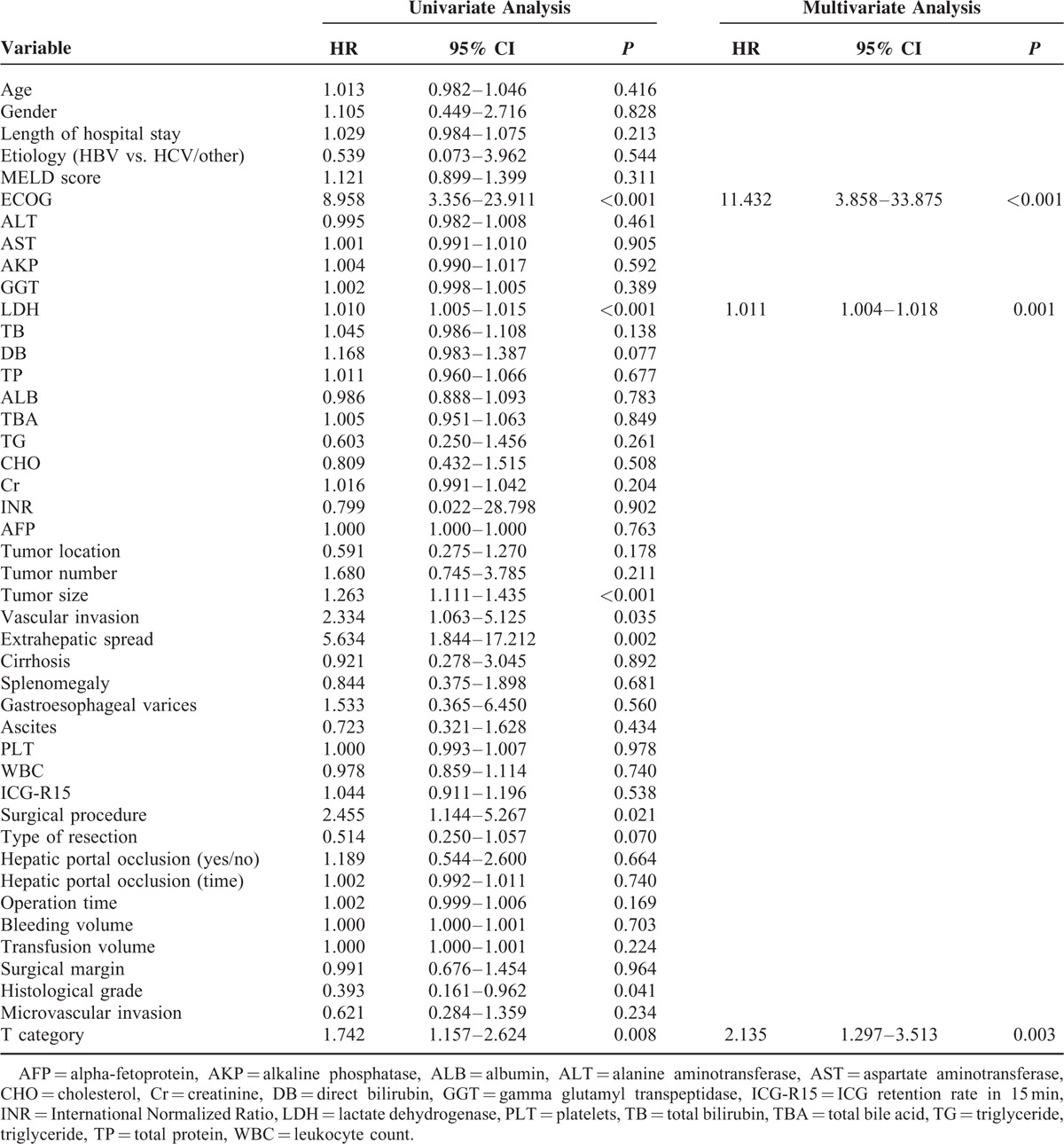
Independent predictors for OS and RFS identified through univariate and multivariate analysis are illustrated in Tables 2 and 3. On univariate analysis, the following covariates were predictive of death: ECOG, lactate dehydrogenase (LDH), tumor size, vascular invasion, extrahepatic spread, surgical procedure, histological grade, and T category. In the multivariate analysis, independent predictors of death were ECOG (P < 0.001), LDH level (P = 0.001), and T category (P = 0.003). With respect to RFS, univariate analysis identified 10 prognostic factors, including ECOG, LDH, DB, tumor size, vascular invasion, extrahepatic spread, surgical procedure, type of resection, MVI, and T category. Among them, ECOG (P = 0.018), LDH level (P = 0.001), vascular invasion (P = 0.036), surgical procedure (P = 0.004), and MVI (P = 0.027) were observed as independent risk factors of recurrence.
TABLE 1 (Continued).
Baseline Characteristics of iHCC Patients Undergoing Hepatectomy (n = 47)

TABLE 2.
Univariate and Multivariate Analysis of Prognostic Factors of OS
Correlations Between Classification of MVI and Clinicopathological Factors
On the basis of the previous classification of MVI,13,14 all patients with MVI were divided into either a mild MVI group or a severe MVI group. MVI classification was defined as follows: mild MVI group: number of vessels invaded ≤5 and furthest distance on invasion from the tumor capsule ≤1 cm; severe MVI group: number of vessels invaded >5 or furthest distance on invasion from the tumor capsule >1 cm. The relationship between the degree of MVI and clinicopathological characteristics is summarized in Table 4. The levels of AST, GGT, and LDH in the severe MVI group were significantly higher than those in the mild MVI group (P = 0.006, 0.024, and 0.043, respectively).
TABLE 3.
Univariate and Multivariate Analysis of Prognostic Factors of RFS
TABLE 4.
Relationship Between the Degree of MVI and Clinicopathological Findings (n = 29, Mean ± SD)

Comparisons of OS and RFS Rates According to LDH level, Surgical Procedure, and MVI
Overall, Kaplan–Meier curve analysis revealed that anatomic resection was significantly associated with increased RFS (P = 0.007). A high LDH level was significantly associated with decreased OS and RFS (P = 0.003 and P = 0.020, respectively). Patients showing MVI were observed with decreased RFS especially in the severe MVI group (P < 0.001) (Figure 2A–D). Subgroup analysis was performed according to LDH level (cutoff value = 174 determined by ROC curve for RFS). In patients with a high LDH level, severe MVI was associated with inferior RFS (P = 0.019) (Figure 3). However, no significant difference was observed between mild and severe MVI for RFS in patients with low LDH level (P = 0.899).
FIGURE 2.
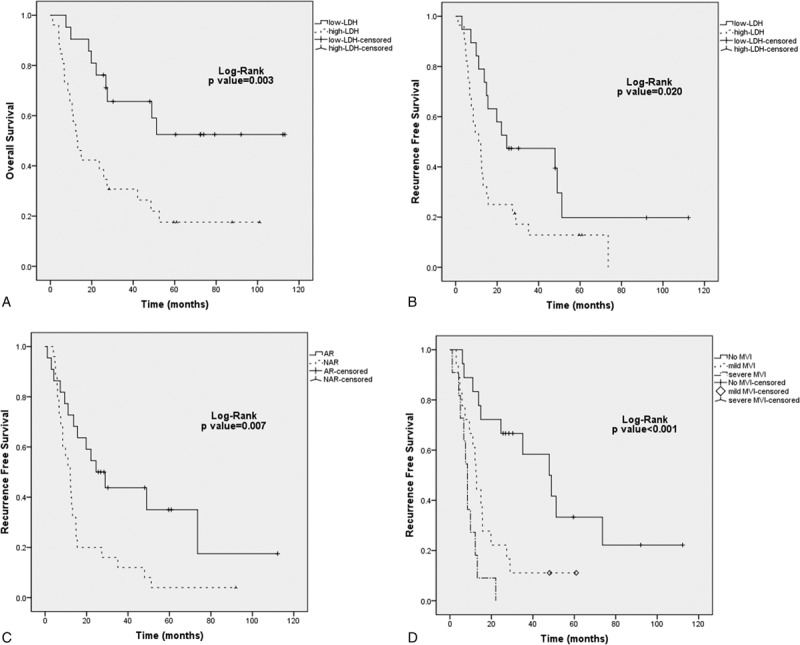
Kaplan–Meier survival analysis of LDH level, surgical procedure, and MVI in patients with iHCC undergoing curative resection. A, Overall survival according to LDH level; B, Recurrence-free survival according to LDH level; C, Recurrence-free survival according to surgical procedure; D, Recurrence-free survival according to MVI classification.
FIGURE 3.
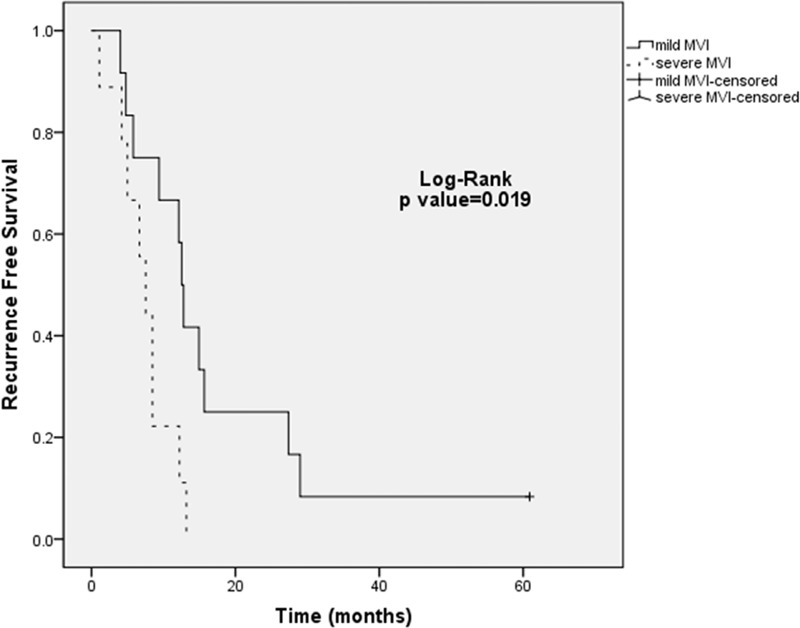
Kaplan–Meier estimated recurrence-free survival curves of the degree of MVI in iHCC patients with a high LDH level undergoing curative resection.
DISCUSSION
Recent studies referred to iHCC mainly focus on patients at advanced and late stages treated with TACE, sorafenib, or supportive care. However, almost no surgical series have been reported in iHCC patients at a relatively early stage. In this study, a cohort of Chinese patients with iHCC were selected to investigate the clinical, surgical, and pathological features, and assess the outcomes, effects of anatomical resection, and prognostic factors associated with OS and RFS.
With regard to the clinical characteristics, iHCC is reported to be commonly associated with HBV infection and markedly elevated AFP values (>10,000 ng/mL).15 Our data consisted of only 2 patients whose etiologic factor was not HBV infection. Similar to the study by Benvegnu et al16 and He et al,5 we may deduce that iHCC is characterized by a much higher HBV infection rate. Although iHCC patients may present with a significant high AFP level, it is reported that AFP serum level measurement has poor accuracy for diagnosis of HCC.17 Our cohort showed that 57.4% of iHCC patients had mildly elevated AFP (<400 ng/mL) levels and only 10.6% presented with an AFP of >10,000 ng/mL. As serum AFP concentration did not remain predictors of OS and RFS in our study, this factor may not be strongly related to prognosis of iHCC patients and seems not to be considered as 1 trait of iHCC. As iHCC patients have a poorer treatment prognosis than radiographically measureable HCC with available therapies,5,18 staging before treatment may be more emphasized to decide optimal treatment strategies. Proper therapies should be managed according to staging systems especially for the HKLC classification for HBV-related iHCC. Because most studies were enrolling iHCC patients who had advanced or late stages (e.g., BCLC stage C or D) with diffuse disease and associated major vascular invasion,6,8,19 resection for these patients was sure to be limited. It is questionable whether patients with iHCC at early or intermediate stages can survive after resection. In this study, our cohort included 66.0% of iHCC patients at stages (HKLC stage I and IIb) in which resection is recommended as the optimal treatment. Prognosis following surgical resection was 32.8% for the 5-year OS rate, which is superior to other reports.9,20,21 This is mainly because of the patient selection criteria in our study and also the surgical procedure used. By Cox univariate analysis for OS, surgical procedure was shown to be significant prognostic factors, with a P value of 0.021. Univariate analysis revealed that patients with anatomic resection had significantly better OS and RFS than those with nonanatomic resection. On multivariate analysis, surgical procedure remained an independent prognostic marker for RFS throughout the cohort.
Anatomic resection was defined as resection of the tumor together with the related portal vein branches and the corresponding hepatic territory.22 It was able to ensure the negative surgical margin and decrease the intrahepatic spread of the tumor after reforming our operation skill (Figure 1). Previous study also revealed that precise hemihepatectomy guided by middle hepatic vein resulted in fewer incidences of postoperative complications and had the potential to achieve more adequate tumor-free resection margin, which may result in higher tumor-free survival rate.23 In patients with HCC nodules equal to or less than 3 cm and with the nonboundary type, anatomic resection should be employed to the extent that liver function allows, because this procedure would be more favorable than nonanatomic resection in eradicating micrometastases that have extended away from the tumor's margin.24 That is to say, resection, especially the anatomic resection, may be more applicable for patients with iHCC at early and intermediate stages. Another treatment option that is recommended in the HKLC staging system for patients at stage I and IIa was liver transplantation. However, a high rate of MVI and propensity for early recurrence may prevent this therapy giving superior results to surgical resection in iHCC patients. As there are no relevant reports about prognosis of liver transplantation in iHCC patients, comparison of anatomic resection and liver transplantation will be accomplished in our future studies.
In addition to anatomic resection, MVI was also found to be a significantly independent predictor of RFS. Several studies have demonstrated that the majority of patients with iHCC have extensive tumor burden as well as vascular invasion.6,8 Frequent presence of macrovascular invasion is most likely secondary to advanced tumor stage in iHCC. However, the importance of MVI in iHCC has not been fully elucidated. Our previous study demonstrated the higher MVI rate of infiltrative type than the other gross types of HCC.5 Multivariate analyses indicated that the presence of MVI was a significantly independent predictor of inferior RFS (P = 0.027). With the high MVI rate in iHCC patients, the relationship between MVI and prognosis of iHCC patients should be drawn more attention. As previously stated, the presence of MVI was shown to lead to a high frequency of recurrence in iHCC after liver resection. Moreover, prevention of early recurrence of iHCC patients with MVI is the most important strategy for improving long-term survival after curative resection; unfortunately, no adjuvant therapy has been reported to show a beneficial survival. As the only standard systemic treatment capable of improving patient survival, sorafenib may act as an adjuvant treatment option to the iHCC patients with MVI. Although the recommendation of the use of sorafenib as adjuvant therapy in HCC patients after resection was seen as a disappointing consequence,25 the utility of sorafenib in iHCC patients is unclear. The time point for the use of sorafenib in iHCC patients at early or intermediate stages after resection is still controversial. With respect to iHCC patients undergoing hepatectomy, it needs to clarify whether sorafenib is a proper adjuvant therapy to reduce early recurrence and experience a better survival.26,27 Future randomized controlled trials (RCTs) should be performed to demonstrate the effectiveness of this combination therapy.
Of note, ECOG and LDH were the only 2 preoperative predictors of both OS and RFS. Interestingly, high LDH level was identified associated with decreased OS and RFS in our cohort of iHCC patients. Up to now, the biological link between LDH, hypoxia, and the tumor-driven angiogenesis pathway through the abnormal activation of the hypoxia-inducible factor 1 (HIF-1α) is well established.28 Furthermore, LDHA plays an important role in metastasis as well as in HCC tumor growth.29 Therefore, elevated serum LDH level may not only represent tumor hypoxia and/or angiogenesis but also be present along with abnormal activation of the oncogenic pathways. An increased LDH reflects an oncogenic status that favors tumor progression and impairs host immune surveillance, both of which are associated with poor oncologic outcome.30 Preoperative high LDH level was also significantly correlated with the severe degree of MVI and the poor outcome in iHCC patient. Therefore, LDH level together with MVI were shown to influence the RFS. As the role of serum LDH levels in predicting global outcome in HCC patients treated with sorafenib has been revealed,31 the application of sorafenib as adjuvant therapy following hepatectomy in iHCC patients with high LDH level and severe MVI will be possible.
The current study has several limitations. This was a single-center study and only able to identify 47 patients with iHCC undergoing hepatectomy. Because of the relatively small number of patients with iHCC selected for this study, there were limitations with regard to statistical modeling and power. Besides, not all the iHCC patients in our study were at stages (HKLC stage I to IIb) in which resection is recommended as optimal treatment. In our future prospective and multicenter study, more iHCC patients at early and intermediate stages undergoing hepatectomy will be enrolled to reduce the potential bias.
In summary, iHCC patients are related with a higher MVI rate and patients at early and intermediate stages (HKLC stage I to IIb) may still derive survival benefit from anatomic resection that could eradicate MVI as much as possible. MVI classification could be used to identify iHCC patients with a poorer survival, especially those with a high preoperative LDH level, which may guide postoperative adjuvant therapy.
Acknowledgment
The authors would like to thank the whole multiple disciplinary team (MDT) for their help in this study.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, AJCC = American Joint Committee on Cancer, AKP = alkaline phosphatase, ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinic Liver Cancer, CHO = cholesterol, Cr = creatinine, DB = direct bilirubin, ECOG = Eastern Cooperative Oncology Group, GGT = gamma glutamyl transpeptidase, HBV = hepatitis B virus, HKLC = Hong Kong Liver Cancer, ICG-R15 = ICG retention rate in 15 minutes, iHCC = infiltrative hepatocellular carcinoma, INR = International Normalized Ratio, LDH = lactate dehydrogenase, MVI = microvascular invasion, OS = overall survival, PLT = platelets, RFS = recurrence-free survival, TACE = transarterial chemoembolization, TB = total bilirubin, TBA = total bile acid, TG = triglyceride, TP = total protein, WBC = leukocyte count.
This work was supported by the National Natural Science Foundation of China (Grant No. 81470866).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64:252–271. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014; 63:844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomoto S, Hishida M, Inokawa Y, et al. Management of hepatocellular carcinoma should consider both tumor factors and background liver factors. Hepatobiliary Surg Nutr 2014; 3:82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 1987; 60:810–819. [DOI] [PubMed] [Google Scholar]
- 5.He J, Shi J, Fu X, et al. The clinicopathologic and prognostic significance of gross classification on solitary hepatocellular carcinoma after hepatectomy. Medicine (Baltimore) 2015; 94:e1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta N, Fidelman N, Sarkar M, et al. Factors associated with outcomes and response to therapy in patients with infiltrative hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013; 11:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu X, Mao L, Tang M, et al. Gross classification of solitary small hepatocellular carcinoma on preoperative computed tomography: prognostic significance after radiofrequency ablation. Hepatol Res 2016; 46:298–305. [DOI] [PubMed] [Google Scholar]
- 8.Kneuertz PJ, Demirjian A, Firoozmand A, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol 2012; 19:2897–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang ES, Yoon JH, Chung JW, et al. Survival of infiltrative hepatocellular carcinoma patients with preserved hepatic function after treatment with transarterial chemoembolization. J Cancer Res Clin Oncol 2013; 139:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han K, Kim JH, Yoon HM, et al. Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival. Korean J Radiol 2014; 15:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan X, Fu X, Cai C, et al. Validation of models in patients with hepatocellular carcinoma: comparison of Hong Kong Liver Cancer with Barcelona Clinic Liver Cancer staging system in a Chinese cohort. Eur J Gastroenterol Hepatol 2015; 27:1180–1186. [DOI] [PubMed] [Google Scholar]
- 12.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954; 7:462–503. [DOI] [PubMed] [Google Scholar]
- 13.Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009; 137:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014; 21:1002–1009. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds AR, Furlan A, Fetzer DT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics 2015; 35:371–386. [DOI] [PubMed] [Google Scholar]
- 16.Benvegnu L, Noventa F, Bernardinello E, et al. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut 2001; 48:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532. [DOI] [PubMed] [Google Scholar]
- 18.Yopp AC, Mokdad A, Zhu H, et al. Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol 2015; 22 Suppl 3:1075–1082. [DOI] [PubMed] [Google Scholar]
- 19.Demirjian A, Peng P, Geschwind JF, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg 2011; 15:2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochiai T, Sonoyama T, Ichikawa D, et al. Poor prognostic factors of hepatectomy in patients with resectable small hepatocellular carcinoma and cirrhosis. J Cancer Res Clin Oncol 2004; 130:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez RR, Jr, Pan SH, Hoffman AL, et al. Comparison of transarterial chemoembolization in patients with unresectable, diffuse vs focal hepatocellular carcinoma. Arch Surg 2002; 137:653–657. [DOI] [PubMed] [Google Scholar]
- 22.Makuuchi M. Surgical treatment for HCC – special reference to anatomical resection. Int J Surg 2013; 11 Suppl 1:S47–S49. [DOI] [PubMed] [Google Scholar]
- 23.Qiu Y, Zhu X, Zhu R, et al. The clinical study of precise hemihepatectomy guided by middle hepatic vein. World J Surg 2012; 36:2428–2435. [DOI] [PubMed] [Google Scholar]
- 24.Ueno S, Kubo F, Sakoda M, et al. Efficacy of anatomic resection vs nonanatomic resection for small nodular hepatocellular carcinoma based on gross classification. J Hepatobiliary Pancreat Surg 2008; 15:493–500. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015; 16:1344–1354. [DOI] [PubMed] [Google Scholar]
- 26.Stein LL, Sellers MT. Outcomes of additional treatment for patients with infiltrative hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013; 11:1673. [DOI] [PubMed] [Google Scholar]
- 27.He VJ. Professor Pierce Chow: neo-adjuvant and adjuvant therapy for hepatocellular carcinoma-current evidence. Hepatobiliary Surg Nutr 2013; 2:239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JP, Wang HB, Lin YH, et al. Lactate dehydrogenase is an important prognostic indicator for hepatocellular carcinoma after partial hepatectomy. Transl Oncol 2015; 8:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng SL, Liu JJ, Dai YH, et al. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J 2012; 279:3898–3910. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Ye P, Xu Q, et al. Elevation of serum GGT and LDH levels, together with higher BCLC staging are associated with poor overall survival from hepatocellular carcinoma: a retrospective analysis. Discov Med 2015; 19:409–418. [PubMed] [Google Scholar]
- 31.Faloppi L, Scartozzi M, Bianconi M, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer 2014; 14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]


