Abstract
Despite high-level evidence, the benefit of postmastectomy RT in these patients in recent years has not been fully elucidated. We investigated postmastectomy radiotherapy (RT) use and evaluated clinicopathologic and treatment factors influencing RT use in Korean women with pT1-2N1 breast cancer.
We identified women diagnosed with pT1-2N1 breast cancer between 1994 and 2009 using the Korean Breast Cancer Registry. Factors associated with RT use were evaluated using logistic regression analysis. The median follow-up was 95 months.
Of the 6196 women, 11.9% underwent postmastectomy RT. RT was applied more frequently in women with 3 positive lymph nodes (adjusted odds ratio [OR], 2.69) and larger tumors (OR per centimeter, 1.10). RT use was not significantly associated with well-established risk factors (e.g., tumor grade, hormone receptor status, and lymphovascular space invasion). Although RT utilization increased gradually during the study period (OR per year, 1.07), factors associated with RT were similar over time. The estimated 5-year overall survival increased significantly from 84.1% in 1994 to 2000 to 94.6% in 2005 to 2009.
This population-based analysis revealed that the indications for postmastectomy RT in pT1-2N1 breast cancer in Korea are based solely on conventional anatomical factors, although their survival has increased significantly in the modern treatment era. There is a significant unmet need for better risk stratification in these patients and for tailored RT with the incorporation of tumor biology-associated factors.
INTRODUCTION
In patients with breast cancer, nodal status is a significant factor for the prognostication and selection of adjuvant radiotherapy (RT).1 Randomized trials have demonstrated survival benefits for patients with node-positive breast cancer who underwent mastectomy and adjuvant RT, compared with those who underwent mastectomy alone.2–4 Despite high-level evidence, the use of postmastectomy RT is still controversial in women with the involvement of 1 to 3 axillary lymph nodes (pN1). With contemporary multidisciplinary management, overall survival (OS) has been increasing in patients treated since 2000 compared with that in those treated in earlier trials,5–7 and the locoregional recurrence (LRR) risk is likely to be correspondingly lower in these patients.5–7 In this respect, the present absolute benefits of postmastectomy RT for pT1-2N1 breast cancer are likely to be small.
Given the excellent recent treatment outcomes, there is no consensus on the definite indication for postmastectomy RT in patients with pN1 breast cancer. A number of risk factors for LRR following mastectomy alone, including large tumor size, unfavorable tumor biology, or young age, have been reported.7–9 This knowledge can guide careful patient selection for postmastectomy RT to avoid unnecessary local therapy because RT may have detrimental impacts in terms of breast cosmetics, late toxicity, and associated costs.10 A better understanding of RT use is important to optimize patient care.
We hypothesized that because OS has increased, patients may have been selected more appropriately for postmastectomy RT in Korea. Therefore, the objective of this study was to determine the survival in patients with pT1-2N1 breast cancer and describe the trend of RT use in Korea using the Korean Breast Cancer Registry nationwide database. In addition, we evaluated the impact of clinicopathologic and treatment factors on RT use.
METHODS
Data Source and Collection
The Korean Breast Cancer Society has assembled information on breast cancer since 1996. The details of the Korean Breast Cancer Registry (KBCR) have been described previously.11–13 In brief, nationwide, breast surgeons in 110 teaching hospitals have voluntarily participated in the KBCR program. These surgeons prospectively collect data on sex, age, surgical method used, and cancer stage as essential items, and operative and pathologic findings, laboratory and imaging findings, biologic markers, and adjuvant treatment as optional items. This registry comprised about 92.0% of all newly diagnosed breast cancer patients in Korea in 2011. Survival data, including dates and causes of death, were obtained from the Korean Central Cancer Registry, Ministry of Health and Welfare, Korea. The KBCR is linked to the Korean National Statistical Office with complete death statistics using unique identification numbers assigned to all Korean residents, and was recently updated in 2013. The KBCR does not include information regarding tumor recurrence.
Description of the Study Cohort
Patients were eligible for analysis if they were diagnosed with invasive ductal carcinoma of the breast and underwent mastectomy between January 1, 1994 and December 31, 2009. Exclusion criteria are shown in Figure 1. We excluded patients without information on the receipt of postmastectomy RT. The final study cohort comprised 6196 women with complete data. The median follow-up period was 95 months (range, 37–229 months) and 5-year follow-up data were available in 84% of patients. The review board of the Korean Breast Cancer Society approved this study. The institutional review board of Severance Hospital concluded that no informed consents were needed for this observational and retrospective study.
FIGURE 1.
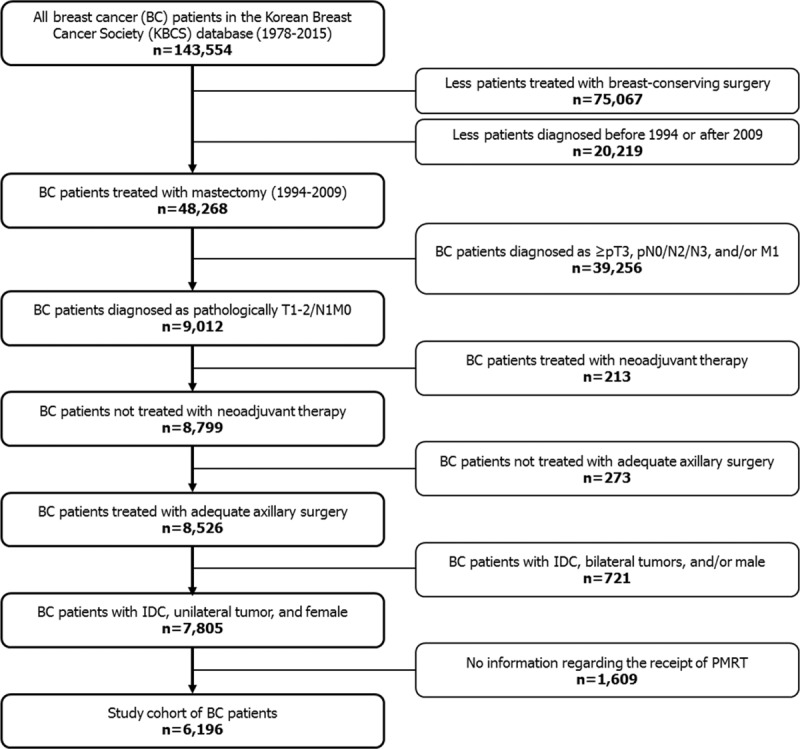
Selection of the study cohort. PMRT = postmastectomy radiation therapy.
Statistical Analyses
The proportion of women who underwent postmastectomy RT over specific periods was the primary endpoint. Univariate and multivariate logistic regression analyses were conducted to assess whether there was an association between the year of mastectomy, age, tumor size, number of lymph nodes involved, tumor grade, hormone-receptor status, lymphovascular space invasion (LVI), method of axillary clearance, number of retrieved nodes, use of adjuvant chemotherapy, breast reconstructive surgery, and receipt of postmastectomy RT. To further investigate whether patterns changed over time, the year of mastectomy was categorized into 3 periods (1994–2000, 2001–2004, and 2005–2009), and the same analyses were conducted. Secondary endpoints included OS (from mastectomy to any cause of death) and disease-specific survival (DSS, from mastectomy to death from breast cancer, women who died of other causes were censored at the time of death). Univariate and multivariate Cox proportional hazards survival analyses were performed to model the association of variables with OS or DSS. Conditional landmark analysis was used to eliminate guarantee-time bias introduced by misclassification of patients without adjuvant treatments who had died before reaching last follow-up.14 The level of statistical significance was set at 5%. All statistical analyses were performed using SPSS version 20.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).
RESULTS
Patient Clinicopathologic and Treatment Characteristics
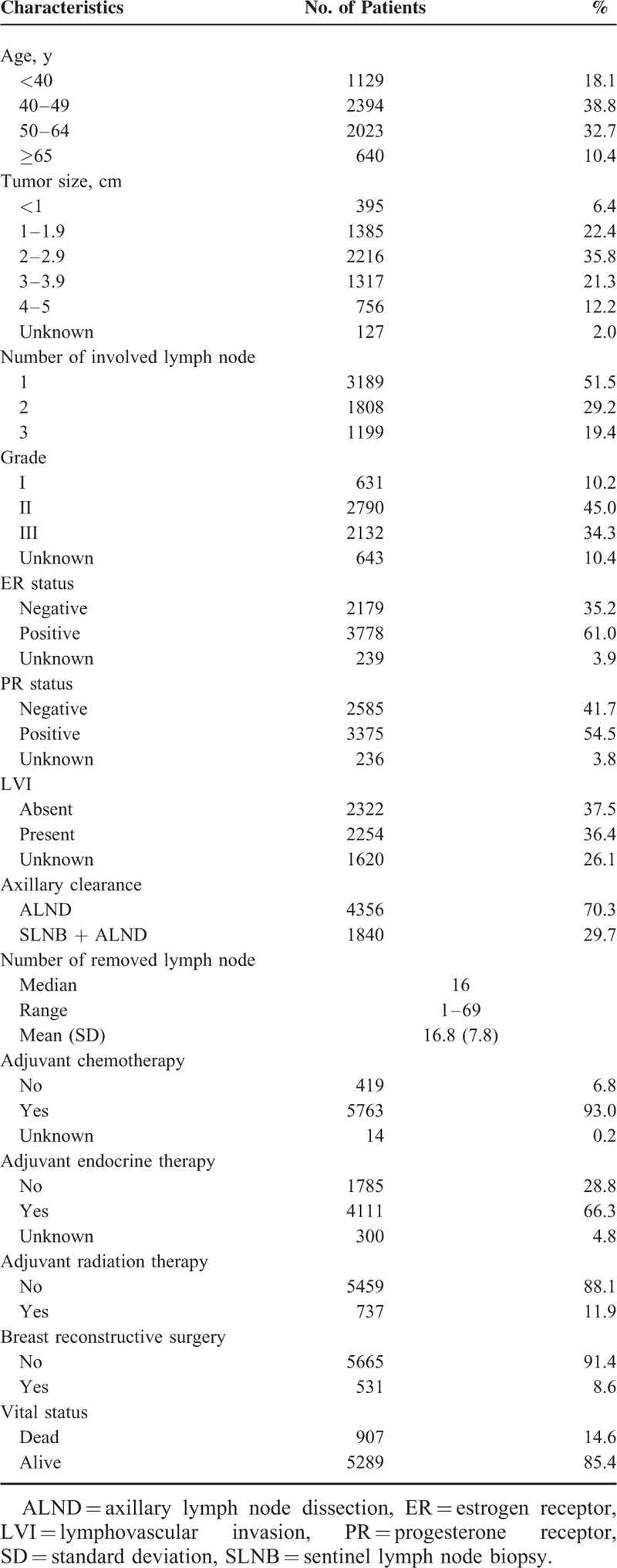
The baseline clinicopathologic and treatment characteristics of this study cohort are described in Table 1. The median patient age at diagnosis was 48 years (range, 24–94 years). Among patients with available pathology (n = 4576), 49.3% of patients had LVI. No patients underwent sentinel lymph node biopsy (SLNB) alone for pT1-2N1 after mastectomy within the study period. Among patients who underwent chemotherapy (n = 5763), 35.8%, 23.5%, and 33.6% of patients received paclitaxel-containing regimens (T-based), doxorubicin and cyclophosphamide (AC), and others (e.g., cyclophosphamide/methotrexate/fluorouracil), respectively.
TABLE 1.
Clinicopathologic and Treatment Characteristics, pT1-2N1 Breast Cancer, 1994–2009, Korean Breast Cancer Society
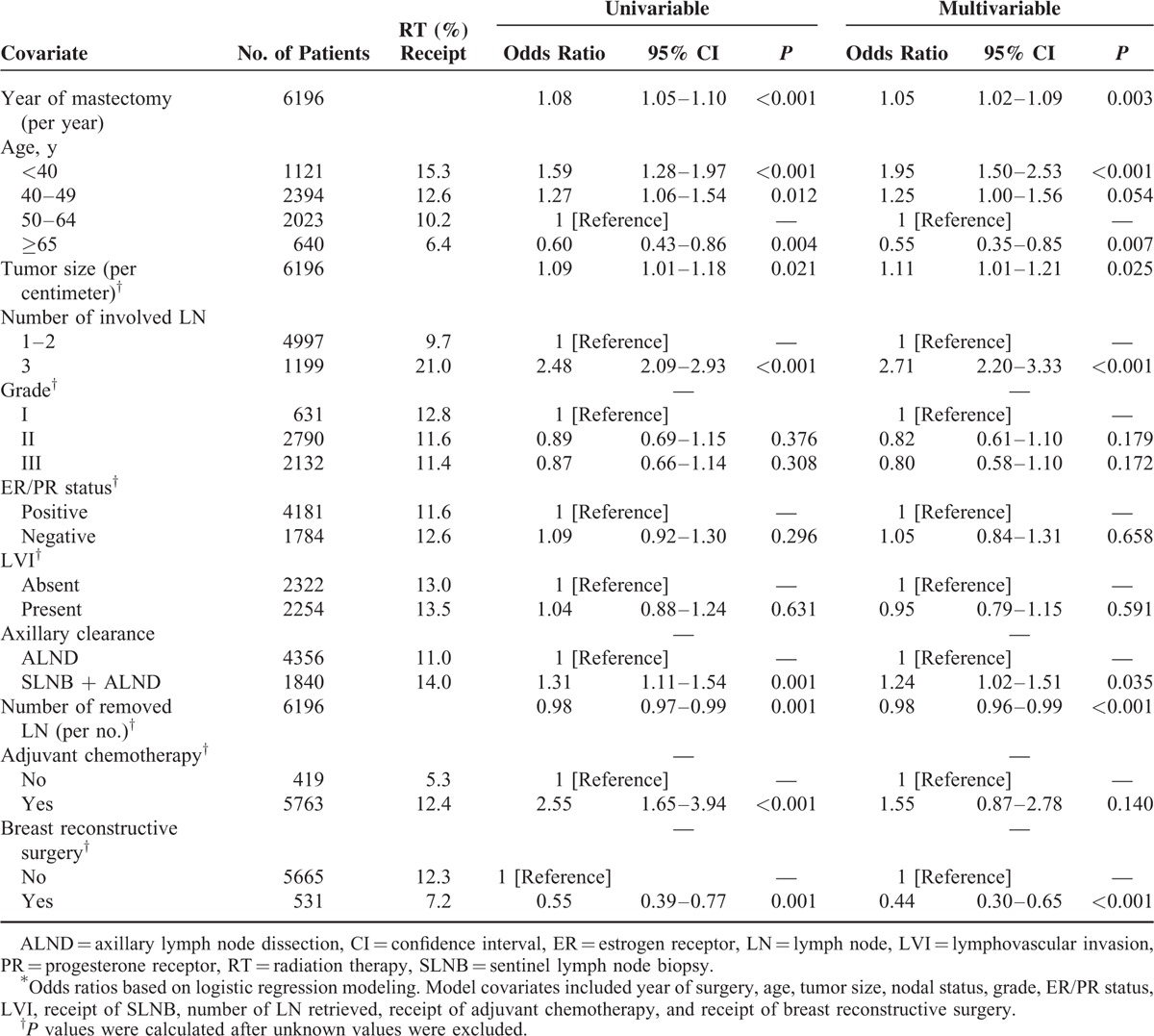
Factors Associated With Postmastectomy RT
Factors associated with having undergone postmastectomy RT were as follows: age <40 years (vs. age 50–64 years; adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.50–2.53; P <0.001); age 40–49 (vs. age 50–64 years; aOR, 1.25; 95% CI, 1.00–1.56; P = 0.054); larger tumor size (per centimeter; aOR, 1.11; 95% CI, 1.01–1.21; P = 0.025); and 3 positive lymph nodes (aOR, 2.71; 95% CI, 2.20–3.33; P <0.001). Factors inversely associated with having undergone postmastectomy RT were as follows: age ≥65 years (vs. age 50–64 years; aOR, 0.55; 95% CI, 0.35–0.85; P = 0.007); a greater number of retrieved lymph nodes (aOR, 0.98; 95% CI, 0.96–0.99; P <0.001); and breast reconstructive surgery (aOR, 0.44; 95% CI, 0.30–0.65; P <0.001) (Table 2). Multivariate analyses revealed a significant independent association between the proportion of patients undergoing postmastectomy RT and time (increasing each year) (aOR, 1.05; 95% CI, 1.02–1.09; P = 0.003).
TABLE 2.
Unadjusted and Adjusted Odds Ratios for Association With Receipt of Adjuvant Radiation Therapy for pT1-2N1 Breast Cancer for Each Clinicopathologic and Treatment Characteristic∗
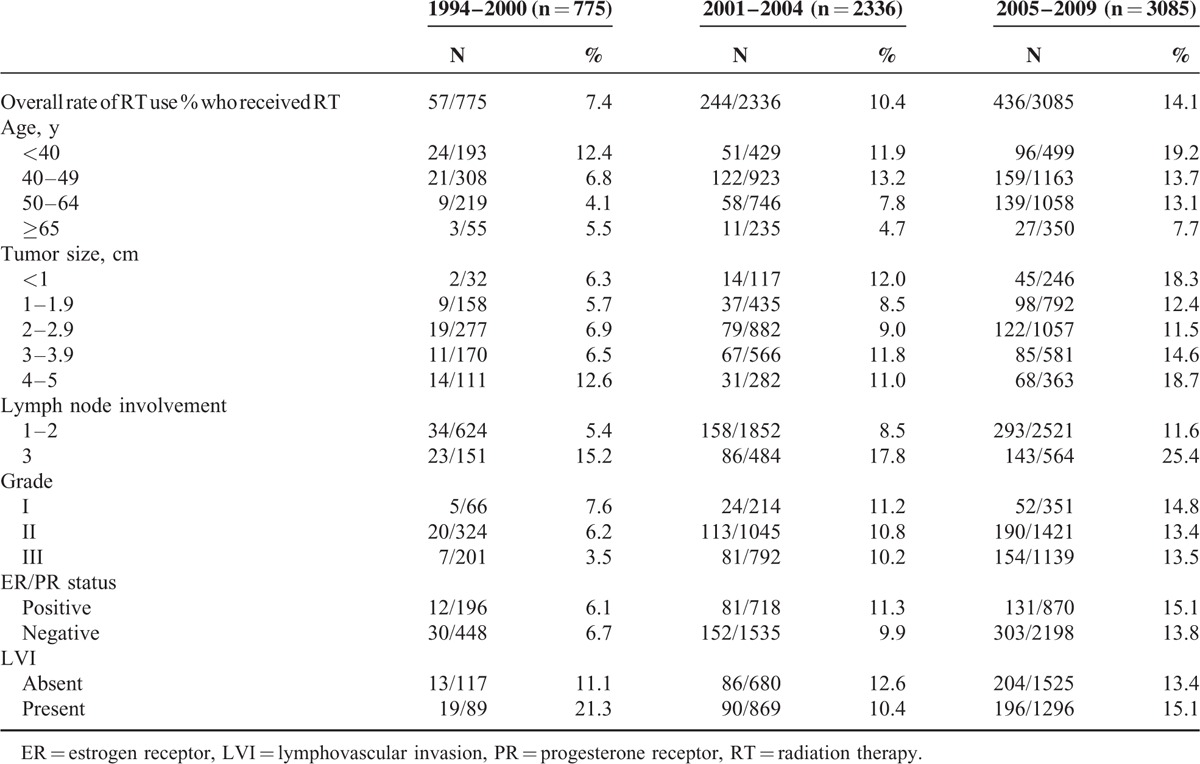
Subgroup Analysis of RT Use by Treatment Period
In the subgroup analysis, the postmastectomy RT utilization rate significantly increased as the treatment period advanced, with higher proportions of patients undergoing RT in the modern treatment period eras (X2 trend test, P <0.001, Table 3). The differences between RT use trends and patterns in adverse features during 1994 to 1999, 2000 to 2004 vs 2005 to 2009 are listed in Table 3. Age and the number of positive lymph nodes correlated with RT receipt regardless of the period, as would be expected. However, unexpectedly, the proportion of patients with small tumors (<2 cm) who underwent postmastectomy RT significantly increased between 1994 and 2000 and between 2005 and 2009 (5.8% vs. 13.8%, respectively). Postmastectomy RT use was not likely to be affected by tumor grade, hormone receptor status, or LVI across any study period.
TABLE 3.
Use of Adjuvant Radiation Therapy for pT1-2N1 Breast Cancer in Adverse Features During 1994–2000 Versus 2001–2004 Versus 2005–2009
Survival Analyses
Figure 2 and Table 4 shows the estimated survival among all patients according to period. The estimated 5-year OS and DSS significantly increased from 84.1% and 85.4% for 1994 to 2000 to 94.6% and 95.6% for 2005 to 2009, respectively (adjusted hazard ratio per year, 0.86; 95% CI, 0.83–0.88).
FIGURE 2.
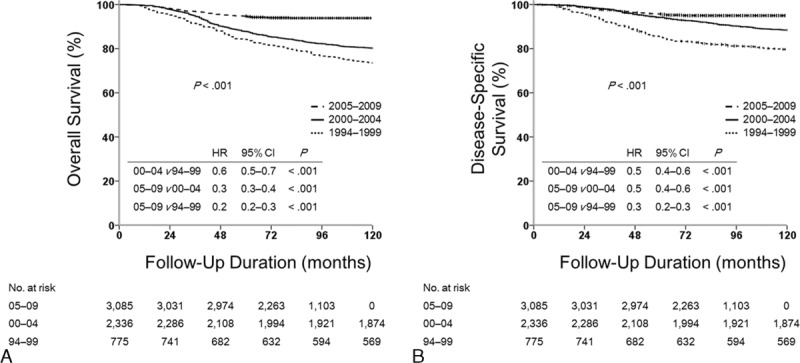
Survival outcomes for patients with pT1-2N1 breast cancer. Kaplan–Meier curves for (A) overall survival and (B) disease-specific survival. HR = hazard ratio.
TABLE 4.
Five-Year Overall and Disease-Specific Survivals for Patients With pT1-2N1 Breast Cancer According to Time Period, 1994–2009, Korean Breast Cancer Society
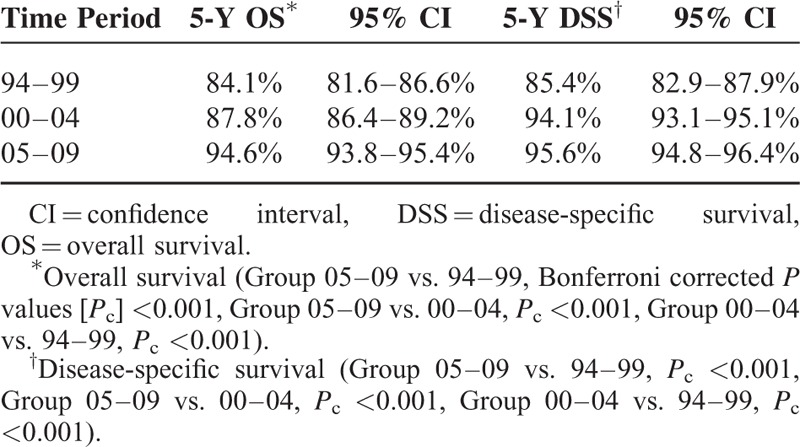
Table 5 presents the multivariate Cox proportional hazards survival analyses at the 16-month landmark point including all variables listed in Table 1. Postmastectomy RT was not associated with any differences in mortality rates. Factors associated with superior OS included later year of mastectomy, age (<50 years), SLNB followed by axillary lymph node dissection, breast reconstructive surgery, and greater numbers of retrieved lymph nodes. Factors associated with inferior OS included age (≥65 years), larger tumor size, higher grade tumor, negative hormone receptor status, and positive LVI. Findings were similar for DSS. Table 6 shows an additional subset analysis for patients who received adjuvant chemotherapy to determine whether the chemotherapy regimen affected the survival outcome. AC or T-based regimens were significantly associated with superior OS and DSS compared with other regimens.
TABLE 5.
Multivariable Cox Proportional Hazards Analyses of Overall Survival and Disease-Specific Survival at the 16-Month Landmark Point, pT1-2N1 Breast Cancer, 1994–2009, Korean Breast Cancer Society
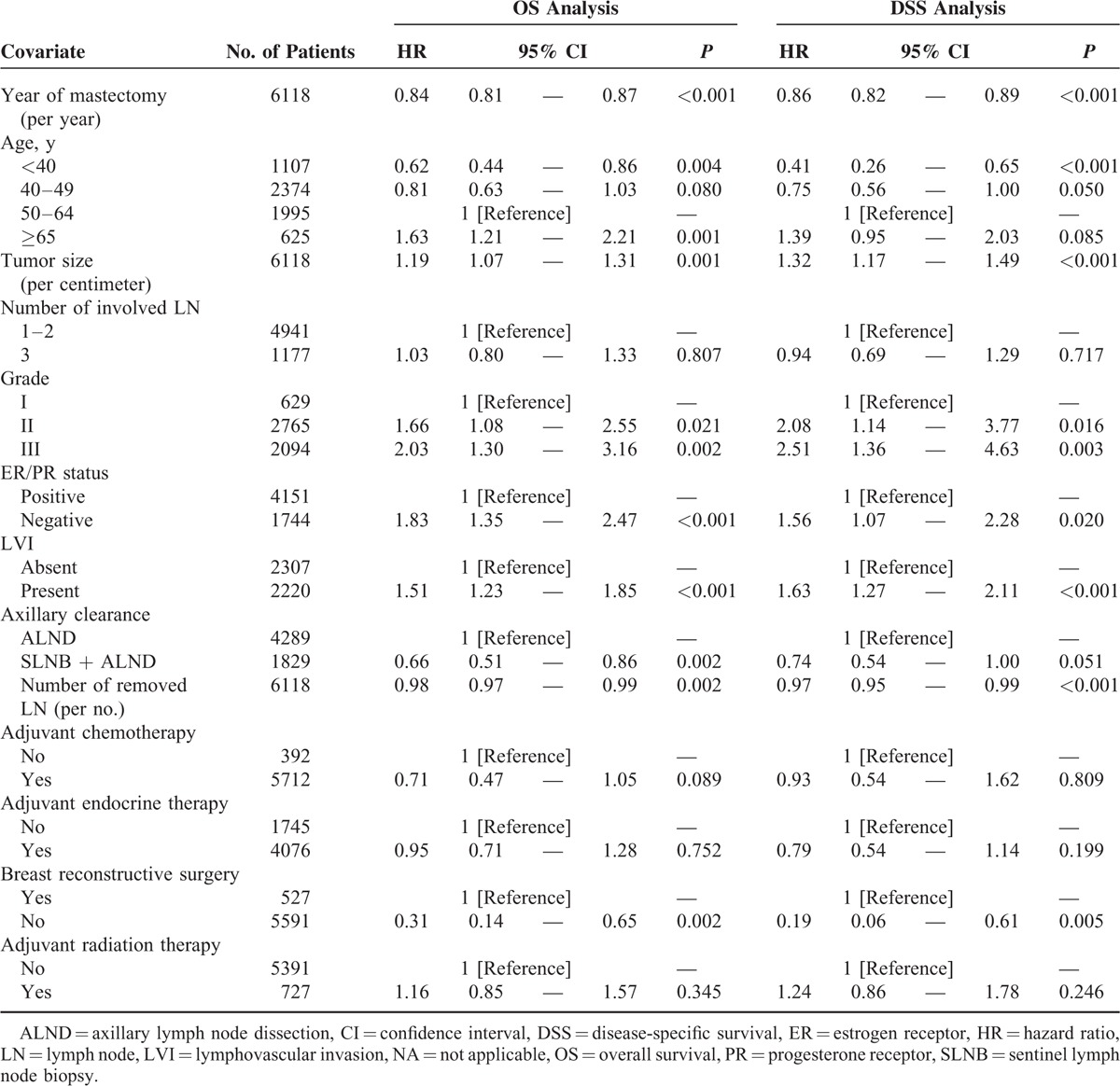
TABLE 6.
Univariable Cox Proportional Hazards Survival Analysis According to Chemotherapy Regimen Among pT1-2N1 Breast Cancer Patients Who Received Chemotherapy and Had Available Data on Regimen, 2000–2009, Korean Breast Cancer Society
DISCUSSION
The present study highlights national practice patterns in RT utilization following mastectomy in patients with pT1-2N1 breast cancer in Korea, and the factors associated with RT use, which can both be useful in guiding current clinical practice in the right direction. Surprisingly, we found that only 11.9% of patients received RT after mastectomy, but that the numbers of patients receiving RT increased gradually per year from 1994 to 2009. During this period, women with larger tumors and more positive lymph nodes were likely to receive RT, but we did not observe significant differences in relation to other well-established risk factors including LVI, high-grade tumor, and estrogen receptor-negative status. For women treated in the 2000s, who had a significantly better OS and DSS than those treated in the 1990s, this concerning trend of patient selection for RT may be against “choosing wisely.”
A number of possibilities could underlie the improvements in the survival outcome over time. Prior to 2010, the KBCR database did not record pathologic information on small volume axillary disease. However, given our findings that SLNB was independently associated with a higher OS and that the number of patients undergoing SLNB increased over time, an increase in the detection of small volume nodal metastasis, including micrometastases (pN1mi), by extensive pathologic evaluation following SLNB could be the most plausible reason for improved survival. In an analysis of >8000 patients from 2 large cohorts, Mittendorf et al15 reported that patients with pN1mi and pN0 disease have similar survival outcomes. Parallel findings were reported in 2 other recent analyses from the American College of Surgeons Oncology Group (ACOSOG) Z0010 and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B32 trials.16,17 An ongoing RxPonder trial evaluating the utility of Oncotype Dx in patients with 1 to 3 positive lymph nodes and hormone receptor-positive tumors recently amended the protocol to exclude patients with pN1mi. Taken together, we feel that the benefit of RT would be diminished in these patients and that postmastectomy RT should not be determined primarily by the presence of pN1mi disease.18
Our findings showed that patients treated in later years were more likely to undergo a modern chemotherapy (AC ± T) regimen, and that modern chemotherapy was significantly associated with improved OS. This suggests that advances in adjuvant systemic treatments might also contribute to improved survival outcomes.19 With growing evidence supporting modern chemotherapy and targeted agents, it seems essential to determine the relative contribution of each component of adjuvant treatment.20 Earlier trials that showed the 10-year LRR rate in those who did not undergo RT was 17.7%, although most of those patients also underwent a CMF chemotherapy regimen.5 On the other hand, trials from the NSABP and the Eastern Cooperative Oncology Group reported a 10-year LRR rate of <10% in those who did not undergo RT, and most of them received modern chemotherapy.6,7 This further supports the hypothesis that there is less room for improvement by RT in those undergoing modern chemotherapy.
These findings raise the question of whether RT could be omitted in T1-2N1 patients who undergo modern systemic therapy postmastectomy, especially in the era of SLNB and in those expected to have a very low risk for LRR. However, there are data emphasizing the importance of RT in these patients. A recent study by Chang et al18 showed a significant improvement in disease-free survival by postmastectomy RT in recently treated patients who were at extremely low risk of LRR, indicating recent treatment advances might not mitigate the benefit of RT in these N1 subsets of patients. Interestingly, recent data from both the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) MA.20 and the European Organization for Research and Treatment of Cancer 22922 trials indicated that the addition of comprehensive regional nodal irradiation in the treatment of breast-conserved patients provided a small but statistically significant benefit in disease-free survival.21,22 N1 patients constituted 90% of the NCIC-CTG MA.20 cohort and 43% of the European Organization for Research and Treatment of Cancer 22922 cohort. While data from the SUPREMO trial are awaited, which specifically investigates whether postmastectomy RT improves survival in patients with an intermediate risk of local recurrence, including N1 patients, physicians should pay careful attention to the selection of candidates for postmastectomy RT.23
In this study, we found that RT was underutilized in Korean patients with pT1-2N1 breast cancer patients, and that its use was considerably lower than that in North American patients (11.9% vs. 19–25%, respectively).24,25 This stands in contrast to current international guidelines, including NCCN in recent years inclusive of 2016, even after allowing for improved survivals of these subsets of patients recently.26 Our findings with respect to gradual increases in RT use also suggest conservative responsiveness of the breast cancer community to emerging evidence. While 1 overriding cause is difficult to determine, substantial underutilization of RT in N1 mastectomy patients can largely be explained by the results of a study by Jagsi et al.27 That large, population-based study found high rates of RT use in breast conserved patients, but lower rates among mastectomy patients. For most mastectomy patients with strong indications for RT who failed to undergo PMRT, patient self-report indicated that physicians either did not discuss RT or said that it was unnecessary. Concerns about the adverse effects, inconveniences, and associated costs of RT may also contribute to underutilization of adjuvant postmastectomy RT in Korean patients. However, the potential for toxicity from breast RT has decreased with the introduction of more advanced RT technologies including RT planning with computed tomography-based RT simulation and three-dimensional conformal RT delivery. Especially, CT-based RT simulation planning assists radiation oncologists in delineating the organs at risk more precisely, in estimating the complication probabilities, and in minimizing the irradiated volume. Intensity-modulated radiation therapy, which has the advantage of improving dose homogeneity and sparing normal tissue, was covered by the Korean National Health Insurance for postmastectomy RT in breast cancer in July 2015, which may alleviate concerns regarding toxicity to some degree.28 Although large randomized trials that established the role of hypofractionated RT in breast cancer excluded postmastectomy patients, there are retrospective studies supporting the use of hypofractionated RT in these patients, which compliment an ongoing phase III randomized trial (NCT00793962).29 With respect to patients’ convenience, hypofractionated RT might be possible treatment approach to reduce both cost and burden.
In a time of growing concern about local therapy under or over treatment, continued efforts are needed to investigate clinical, metabolic, and molecular markers for better risk stratification and appropriate patient selection. In our study, the number of positive lymph nodes, number of lymph nodes resected, tumor size, and age were found to be predictive clinical factors associated with RT use, which was similar to the findings of a systematic review of the NSABP trials.7 The lymph node ratio, which combined number of positive lymph nodes with the number of lymph nodes removed, could identify patients who might benefit most from RT.30,31 In our study, RT use was more common among younger than older women, reflecting the concern that younger age is in itself a risk factor for local recurrence following mastectomy.32 However, peculiarly enough, young age has not clearly been shown to be a predictor of survival from our cohort that included only early stage women. Given the fact that younger women usually present with larger tumors and higher percentage of positive lymph nodes, much care is needed to consider age in postmastectomy RT decision making in women with early-stage disease.33 Recently, many investigators have identified other risk factors for LRR in institutional cohorts who did not undergo RT, such as high-grade tumor, estrogen receptor negativity, and LVI.34–36 The present study also found that these well-established risk factors were associated with poor survival outcome, but that they did not affect RT utilization. Previous study have shown that biologic factors including tumor grade and estrogen receptor status could be used as prognostic markers and even more so in those with small-volume nodal disease.15 These results indicate that estrogen receptor status, tumor grade, and LVI should be considered alongside T and N stage in multidisciplinary discussions to decide whether to implement RT.
Recent studies reported the utility of pretreatment positron-emission tomography (PET) for predicting high-risk patients who could be candidates for RT among patients with T1-2N1 disease.37,38 In this series, hypermetabolic features in baseline PET represented a high-risk group having larger tumors, more positive lymph nodes, a higher LNR, high-grade tumors, hormone-receptor negativity, or triple-negative status, and these factors were associated with an increased LRR risk as well as poor disease-free survival. Several genetic signatures have been reported to predict risk of distant metastases, expanding the application of genetic analysis for assessing LRR risk.39–41 Mamounas et al40 retrospectively analyzed >1500 specimens from patients with N0 and estrogen-positive disease from the NSABP B-14 and B-20 trials, and found that the LRR risk was significantly associated with recurrence score risk groups that were quantified using Oncotype DX genetic analysis. Similar findings were reproduced in patients with ductal carcinoma in situ.42 Although these new findings regarding PET imaging and gene signatures need to be validated in the prospective setting or in node-positive patients, their implementation in clinical practice would prevent both RT under- and overtreatment with truly personalized treatment protocols.
There are some limitations to the present study. Given its retrospective observational study design, there were unmeasured patient factors related to prognosis, which might have influenced RT use. The KBCR database does not contain details of RT dose, fractionation, field, and technique. However, our previous study reporting RT patterns of care suggested that it is likely that a large proportion of our patients received RT in a relatively homogenous manner.43 Other limitations of this study included the lack of KBCR data on details of performance status, socioeconomic and demographic characteristics, patient preferences, physician interaction, and comorbidities. The impact of HER2 status and trastuzumab could not be analyzed in this study. As with all national large databases, miscoding of variables is possible. The KBCR database represents the majority of breast cancer patients in Korea, while the SEER database represents only approximately 26% of cancer patients in the United States.44
In summary, among women diagnosed with pT1-2N1 breast cancer in Korea, the percentage undergoing postmastectomy RT over a 15-year period was relatively low, but gradually increased during the study period. Although an international consensus recommends postmastectomy RT for N1 patients if there are additional adverse features, our study found that several important well-established risk factors have been de-emphasized in actual clinical practice in Korea. Our concern is that, with advances in surgical techniques and modern chemotherapy regimens, patients are now expected to have far superior survival outcomes with a low LRR risk when compared with those treated in previous years; therefore, more careful patient selection is needed. There is a significant unmet need to educate surgeons and medical and radiation oncologists for better risk stratification of such patients with the incorporation of tumor biologic factors, well-established risk factors, and conventional anatomic factors. As mentioned by Jagsi et al,27 surgeon participation in the RT decision has a strong impact on RT use, especially among mastectomy patients. Further effort is needed to investigate effective predictive markers for RT as well as to optimize the postmastectomy RT technique, dose, and volume.
Acknowledgments
This article was supported by the Korean Breast Cancer Society. We thank Hye Sun Lee, MS, Department of Biostatistics, Severance Hospital, for statistical consultation and analysis of the data.
Footnotes
Abbreviations: AC = doxorubicin and cyclophosphamide, ACOSOG = American College of Surgeons Oncology Group, DSS = disease-specific survival, ECOG = Eastern Cooperative Oncology Group, EORTC = European Organization for Research and Treatment of Cancer, KBCR = Korean Breast Cancer Registry, LRR = locoregional recurrence, LVI = lymphovascular space invasion, NCIC-CTG = National Cancer Institute of Canada Clinical Trials Group, NSABP = National Surgical Adjuvant Breast and Bowel Project, OS = overall survival, PET = positron-emission tomography, pN1mi = micrometastases, RT = radiotherapy, SLNB = sentinel lymph node biopsy, T-based = paclitaxel-containing regimens.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Giuliano AE, Jones RC, Brennan M, et al. Sentinel lymphadenectomy in breast cancer. J Clin Oncol 1997; 15:2345–2350. [DOI] [PubMed] [Google Scholar]
- 2.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 2005; 97:116–126. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen HM, Overgaard M, Grau C, et al. Danish Breast Cancer Cooperative G. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol 2006; 24:2268–2275. [DOI] [PubMed] [Google Scholar]
- 5.Group EEBCTC. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol 1999; 17:1689–1700. [DOI] [PubMed] [Google Scholar]
- 7.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol 2004; 22:4247–4254. [DOI] [PubMed] [Google Scholar]
- 8.Katz A, Strom EA, Buchholz TA, et al. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys 2001; 50:735–742. [DOI] [PubMed] [Google Scholar]
- 9.Untch M, Gerber B, Harbeck N, et al. 13th st. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus—opinion of a German team of experts (Zurich 2013). Breast Care (Basel) 2013; 8:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998. [DOI] [PubMed] [Google Scholar]
- 11.Moon HG, Han W, Noh DY. Underweight and breast cancer recurrence and death: a report from the Korean Breast Cancer Society. J Clin Oncol 2009; 27:5899–5905. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea—a report from the Korean Breast Cancer Society. J Clin Oncol 2007; 25:2360–2368. [DOI] [PubMed] [Google Scholar]
- 13.Ko SS. Korean Breast Cancer S. Chronological changing patterns of clinical characteristics of Korean breast cancer patients during 10 years ∗(1996-2006) using nationwide breast cancer registration on-line program: biannual update. J Surg Oncol 2008; 98:318–323. [DOI] [PubMed] [Google Scholar]
- 14.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013; 31:2963–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittendorf EA, Ballman KV, McCall LM, et al. Evaluation of the stage IB designation of the American Joint Committee on Cancer staging system in breast cancer. J Clin Oncol 2015; 33:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med 2011; 364:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011; 305:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JS, Lee J, Kim KH, et al. Do recent advances in diagnostic and therapeutic procedures negate the benefit of postmastectomy radiotherapy in N1 patients with a low risk of locoregional recurrence? Medicine (Baltimore) 2015; 94:e1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003; 21:976–983. [DOI] [PubMed] [Google Scholar]
- 20.Turner N, Biganzoli L, Di Leo A. Continued value of adjuvant anthracyclines as treatment for early breast cancer. Lancet Oncol 2015; 16:e362–e369. [DOI] [PubMed] [Google Scholar]
- 21.Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015; 373:317–327. [DOI] [PubMed] [Google Scholar]
- 22.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015; 373:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkler IH, Canney P, van Tienhoven G, et al. Group MESTM. Elucidating the role of chest wall irradiation in ’intermediate-risk’ breast cancer: the MRC/EORTC SUPREMO trial. Clin Oncol (R Coll Radiol) 2008; 20:31–34. [DOI] [PubMed] [Google Scholar]
- 24.Dragun AE, Huang B, Gupta S, et al. One decade later: trends and disparities in the application of post-mastectomy radiotherapy since the release of the American Society of Clinical Oncology clinical practice guidelines. Int J Radiat Oncol Biol Phys 2012; 83:e591–596. [DOI] [PubMed] [Google Scholar]
- 25.McBride A, Allen P, Woodward W, et al. Locoregional recurrence risk for patients with T1,2 breast cancer with 1-3 positive lymph nodes treated with mastectomy and systemic treatment. Int J Radiat Oncol Biol Phys 2014; 89:392–398. [DOI] [PubMed] [Google Scholar]
- 26.Network. NCC. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer, Version 1. 2016; Fort Washington, PA: National Comprehensive Cancer Network, 15. [Google Scholar]
- 27.Jagsi R, Abrahamse P, Morrow M, et al. Patterns and correlates of adjuvant radiotherapy receipt after lumpectomy and after mastectomy for breast cancer. J Clin Oncol 2010; 28:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho KH. The challenges faced by the Korean Society for Radiation Oncology in the national healthcare system in Korea. Int J Radiat Oncol Biol Phys 2014; 90:725–728. [DOI] [PubMed] [Google Scholar]
- 29.Montero A, Sanz X, Hernanz R, et al. Accelerated hypofractionated breast radiotherapy: FAQs (frequently asked questions) and facts. Breast 2014; 23:299–309. [DOI] [PubMed] [Google Scholar]
- 30.Kim SI, Cho SH, Lee JS, et al. Clinical relevance of lymph node ratio in breast cancer patients with one to three positive lymph nodes. Br J Cancer 2013; 109:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truong PT, Jones SO, Kader HA, et al. Patients with t1 to t2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat Oncol Biol Phys 2009; 73:357–364. [DOI] [PubMed] [Google Scholar]
- 32.Smith IE, Fribbens C. Management of breast cancer in older and frail patients. Breast 2015; 24 Suppl 2:S159–S162. [DOI] [PubMed] [Google Scholar]
- 33.Zhou P, Gautam S, Recht A. Factors affecting outcome for young women with early stage invasive breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat 2007; 101:51–57. [DOI] [PubMed] [Google Scholar]
- 34.Truong PT, Olivotto IA, Kader HA, et al. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys 2005; 61:1337–1347. [DOI] [PubMed] [Google Scholar]
- 35.Yang PS, Chen CM, Liu MC, et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys 2010; 77:516–522. [DOI] [PubMed] [Google Scholar]
- 36.Su YL, Li SH, Chen YY, et al. Post-mastectomy radiotherapy benefits subgroups of breast cancer patients with T1-2 tumor and 1-3 axillary lymph node(s) metastasis. Radiol Oncol 2014; 48:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima N, Kataoka M, Sugawara Y, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve disease recurrence prediction in postmastectomy breast cancer patients with 1 to 3 positive axillary lymph nodes. Int J Radiat Oncol Biol Phys 2013; 87:738–746. [DOI] [PubMed] [Google Scholar]
- 38.Chang JS, Lee J, Kim HJ, et al. (18)F-FDG/PET may help to identify a subgroup of patients with T1-T2 breast cancer and 1-3 positive lymph nodes who are at a high risk of recurrence after mastectomy. Cancer Res Treat 2016; 48:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 40.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 2010; 28:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzal F, Filipits M, Rudas M, et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial. Br J Cancer 2015; 112:1405–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarado M, Carter DL, Guenther JM, et al. The impact of genomic testing on the recommendation for radiation therapy in patients with ductal carcinoma in situ: a prospective clinical utility assessment of the 12-gene DCIS score result. J Surg Oncol 2015; 111:935–940. [DOI] [PubMed] [Google Scholar]
- 43.Keum K, Shim S, Lee I, et al. The 1998, 1999 patterns of care study for breast irradiation after mastectomy in Korea. J Korean Soc Ther Radiol Oncol 2007; 25:7–15. [Google Scholar]
- 44.Institute NC. Surveillance, Epidemiology, and End Results (SEER) Program SEER∗Stat Database: Incidence—SEER 18 Regs Public Use, Nov 2012 Sub (2000–2010)—Linked to County Attributes—Total U.S., 1969–2011 Counties. Bethesda: National Cancer Institute, Divi sion of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch. 2013. [Google Scholar]


