Abstract
Although the head-up tilt (HUT) test and Valsalva maneuver (VM) have been widely used to identify sympathetic adrenergic impairment, the detailed relationship between the degree of orthostatic hypotension (OH) during the HUT test and the pattern of blood pressure (BP) change during the VM remains unknown. This study was performed to investigate the relationship between the degree of OH during the HUT test and the pattern of BP change during the VM. During a 4-year period, a total of 132 consecutive patients with neurogenic OH and 60 healthy controls were enrolled. The degree of OH was defined as mild (associated with a fall in systolic BP [SBP] ≥ 20 < 30 on tilting, n = 49), moderate (associated with a fall in SBP ≥ 30 < 40 on tilting, n = 43), and severe (associated with a fall in SBP ≥ 40 on tilting, n = 40). A standardized battery of autonomic tests, including the HUT test and VM using Finometer devices for recording beat-to-beat BP and heart rate response, and a quantitative sudomotor axon reflex test, was performed. Sympathetic indexes (SIs 1–6) were calculated from the VM. A composite autonomic severity score (CASS) was also obtained to evaluate the severity and distribution of autonomic dysfunction. The degree of OH was compared with the BP decline and recovery during the VM. All indexes exhibited overall significant differences among tested groups (P < 0.001). Only SI 3 differentiated all subject groups. Compared with other SIs, SI 3 was best correlated with the amount of decrease in the mean SBP (R2 = 0.473, P < 0.001) on tilting. The decrease in mean SBP on tilting was best correlated with CASS adrenergic subscore. SI 3 can differentiate between groups with different degrees of OH. The SI 3 obtained during VM can improve the diagnostic accuracy of autonomic dysfunction in patients with different degree of OH.
INTRODUCTION
Sympathetic adrenergic failure (SAF) is typically presented with symptoms suggestive of orthostatic intolerance, such as orthostatic dizziness, and is typically diagnosed by the presence of orthostatic hypotension (OH), which is commonly accepted as a proxy for SAF.1 SAF is typically ascribed to an impairment of sympathetic nervous system innervation of the blood vessels and heart, which control the blood pressure (BP) response during orthostatic challenge.
Neurogenic cause of OH, a proxy for SAF is a less common and chronic condition than non-neurogenic cause of OH, which is due to a variety of medical conditions, such as dehydration, volume depletion, severe anemia, use of medication such as antihypertensive therapies, cardiac arrhythmia and heart failure, systemic infection, prolonged immobilization, and physical deconditioning.2 Non-neurogenic cause of OH usually resolves after the underlying cause is treated.2 Plasma norepinephrine levels (in neurogenic OH they will not go up, while in non-neurogenic OH they will go up significantly) or the Valsalva maneuver (VM) (the phase IV overshoot is blunted and the recovery time is prolonged in neurogenic OH, while it is preserved in non-neurogenic OH) are required to differentiate neurogenic OH from non-neurogenic OH. VM-induced BP changes indeed reflects sympathetic functions providing that there are no non-neurogenic causes of OH.3
Although the head-up tilt (HUT) test has been widely used to identify SAF, it can only detect the presence of severe generalized adrenergic failure due to its limited sensitivity.4,5 In contrast, the VM offers the advantage of detecting milder forms of SAF.5–7 Therefore, both techniques should be used in combination to conduct a detailed evaluation of sympathetic adrenergic function. In most laboratories that perform autonomic function tests, the HUT test and VM have been widely used to identify the presence and evaluate the degree of severity of SAF; however, the detailed relationship between the degree of OH during the HUT test and the pattern of BP changes during the VM remains unknown.
In the present study, we only included patients with neurogenic OH during the tilt and compared the various types of sympathetic indexes (SIs) during the VM to the degree of OH in patients with graded SAF. The primary purpose was to identify which SI is optimal to differentiate groups with graded SAF according to the different degrees of OH.
PATIENTS AND METHODS
Case Definition and Data Collection
From January 2011 to January 2014, we retrospectively identified 795 consecutive patients with OH from the autonomic laboratory at the Keimyung University Dongsan Medical Center who had OH during the HUT test. All patients referred to our autonomic laboratory and main reason for referral was symptoms of orthostatic intolerance such as orthostatic dizziness, lightheadedness, fatigue, and syncope. The inclusion criteria for this study were as follows: patient had classic OH that was defined as a decrease in systolic BP (SBP) ≥ 20 mm Hg or a decrease in diastolic BP ≥ 10 mm Hg within 3 min of tilting, OH continuously appeared during the entire time of tilting, patient had a definite neurological disorder responsible for OH, such as a neurodegenerative disease or diabetic neuropathy (i.e., neurogenic OH), and all hemodynamic BP responses shown as SIs (i.e., SIs 1–6) were successfully obtained from the VM. The specific disease(s) responsible for neurogenic cause of OH such as multiple system atrophy, Parkinson disease, and diabetic neuropathy was diagnosed using internationally accepted criteria for diagnosis of each disease, respectively.
Autonomic Testing
A standardized battery of autonomic tests, including the HUT test and VM using Finometer devices (FMS, Amsterdam, The Netherlands) for recording beat-to-beat BP and heart rate response, and a quantitative sudomotor axon reflex test (QSART) were performed in all patients according to a previously validated method for the diagnosis of autonomic dysfunction.8 The tilt protocol included 10 min in the supine position and 20 min of a tilt at 70°. In addition to BP monitoring using Finometer, the BP was obtained at the baseline and every minute during the tilt using a manual sphygmomanometer (Tycos, Skaneateles Falls, NY). For the VM, the patients were instructed to take a deep breath and blow into a syringe through a mouthpiece attached to a manometer for 15 s until the expiratory pressure was reached to 40 mm Hg. After a practice run, the patients performed a series of maneuvers until 2 reproducible arterial SBP responses were obtained. The results were excluded if the patient was unable to maintain a pressure of at least 30 mm Hg for at least 10 s. The BP magnitude was determined during 4 phases; that is, phase I, early and late phase II, phase III, and phase IV as previously described.7,8 The SIs, including the reduction of early phase II (SI 1), magnitude of late phase II (SI 2), difference in BP between the baseline and at the end of phase II (SI 3), magnitude of phase IV (SI 4), pressure recovery time (PRT, SI 5), and baroreflex sensitivity adrenergic (BRSa) (SI 6) that have been linked to SAF, were calculated from the VM results.4,9 SIs 1 to 4 were calculated from the mean BP, and PRT10 and BRSa11 were calculated from the SBP. PRT was defined as time interval from minimal SBP at phase III until it reaches baseline and BRSa was defined as the SBP decrement associated with phase III divided by the PRT. The dynamic range in each SI was defined as a difference between maximal value and minimal value. The postganglionic sympathetic sudomotor functions were analyzed by a quantitative QSART at the forearm, proximal leg, and distal leg using a Q-Sweat machine (WR Medical Electronics, Stillwater, MN).8,9
The pattern and severity of autonomic dysfunctions in patients with neurogenic OH were determined based on the composite autonomic severity score (CASS) that consisted of each subscore that evaluated the sympathetic adrenergic and cholinergic, and parasympathetic cardiovagal autonomic functions.7
All autonomic function testing was performed under constant environmental conditions (i.e., quiet, ambient temperature of 73–76°F, and humidity kept at approximately 50%). No coffee, food, or nicotine was permitted for 6 h before the study.
Exclusion of Non-Neurogenic Cause of OH
In the Keimyung University Autonomic Laboratory protocol, all patients with OH during the autonomic testing are routinely evaluated for identification of non-neurogenic causes. For example, hypovolemia (dehydration) was evaluated by observing absence of the jugular venous distention, dry skin and mouth, tachycardia, or tachypnea and by serologic test with high level of blood urea nitrogen. In evaluation of heart condition, cardiac arrhythmia are evaluated by performing electrocardiogram and heart failure are suspected by observing shortness of breath, distal edema, a square variant of VM, and reviewing medical history on dyspnea on exertion. The serologic test such as hemoglobin and hematocrit was routinely performed for identification of anemia and medication-induced OH was evaluated by reviewing current and recently used medication. Medication that affects autonomic testing or causing OH was stopped for 5 half-lives if this was considered to be safe.
Standard Protocol Approvals, Registrations, and Patient Consents
All experiments complied with the tenets of the Declaration of the Helsinki and the study was approved by the Institutional Review Board of the School of Medicine at Keimyung University.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to test the overall differences between subject groups. The post hoc Tukey HSD test was used for pairwise comparison if ANOVA showed overall significance. Pearson correlation coefficient was used to examine the relationship between the SBP fall during the tilt and SIs (SIs 1–6). Spearman correlation coefficient was also used to examine the relationship between the total and each item subscore of CASS and SIs during the VM. The mean value ± standard deviation is displayed. All of the analyses were performed by using SPSS 19.0 for Windows (SPSS, Inc, Chicago, IL). A probability of P < 0.05 was considered statistically significant.
RESULTS
According to above inclusion criteria, the following patients were excluded: patients with other types of OH including delayed, transient, or early types (n = 221), patients with non-neurogenic causes of OH, such as medication, hypovolemia/dehydration, anemia, systemic infection, or cardiac dysfunction such as arrhythmia or heart failure (n = 200), patients with OH who had an inadequate evaluation such as incomplete, or partial SIs from the VM (n = 172), patients with OH who showed a flat top response during the VM that was defined as an increase in the BP response of at least 20 mm Hg above baseline for at least 10 s (n = 55), or patients with OH who were unable to follow instructions due to cognitive impairment (n = 15).
After excluding a total of 663 patients with OH, 132 patients (93 men, age range 15–83 years, mean age 60.4 ± 14.2 years) were finally enrolled for this study. Group 1, 60 healthy subjects (age 59.5 ± 2.7 years; 26 men), had a normal autonomic function, defined as having SBP drop <20 mm Hg during the tilt and a normal test result from other autonomic battery (i.e., CASS = 0). The patients were divided into groups according to different degree of OH. Group 2 (n = 49, age 55.9 ± 16.0 years; 20 men) included subjects with mild OH defined by the presence of OH with fall in SBP ≥ 20 < 30 during the tilt test. Group 3 (n = 43, age 62.4 ± 12.6 years; 24 men) included those with moderate OH defined by the presence of OH with fall in SBP ≥ 30 < 40 during the tilt test. Group 4 (n = 40, age 63.9 ± 12.1 years; 23 men) included patients with severe SAF defined by the presence of OH with fall in SBP ≥ 40 during the tilt test.
There were no differences in gender among all groups, but ANOVA showed overall difference in age across all groups. Most patients had symptoms suggestive of orthostatic intolerance, such as orthostatic dizziness. The 3 most common diagnoses were diabetic peripheral neuropathy (50%), Parkinson disease (31%), and multiple system atrophy (15%).
The mean fall in SBP during the tilt showed significant differences among tested group. During the VM, all indexes showed overall differences among tested groups (P < 0.001). All indexes except for SI 1 were able to distinguish between the healthy controls and patients with different degrees of OH. SIs 2 and 4 were unable to differentiate between the moderate and severe OH groups. Additionally, SIs 5 and 6 were unable to differentiate between the mild and moderate OH groups. Only SI 3 separated all SAF groups with different degree of OH from each other. SI 3 also showed the highest dynamic range (28.1) from healthy controls (8.9 ± 5.9) to the SAF group with severe OH (−19.2 ± 12.0). The order of dynamic range of each SI decreased in the order of SI 3 > SI 4 > SI 5 > SI 2 > SI 6 > SI 1 (Table 1).
TABLE 1.
Sympathetic Indexes During Valsalva Maneuver and Blood Pressure Response to Tilt in Normal Controls and Patients With Different Degree of Orthostatic Hypotension
Illustrative cases with different patterns of SAF during the VM, according to the degree of OH, are shown in Figure 1. Among SIs, SI 3 showed the best correlation with the amount of fall in mean SBP during the tilt (Figure 2). Among CASS total and each subscore, only the CASS adrenergic subscore differentiated all tested groups. The CASS total was unable to differentiate between the moderate and severe OH groups and the CASS cardiovagal was unable to differentiate between the mid and moderate OH groups. Although the CASS sudomotor was able to differentiate the healthy controls from patients with OH, this subscore showed no significant differences among patients groups with different degree of OH (Table 2). Regarding the degree of correlation between the decrease in mean SBP during the tilt and the total CASS and each of the subscore, the strongest correlation was observed between the CASS adrenergic subscore (Figure 3).
FIGURE 1.
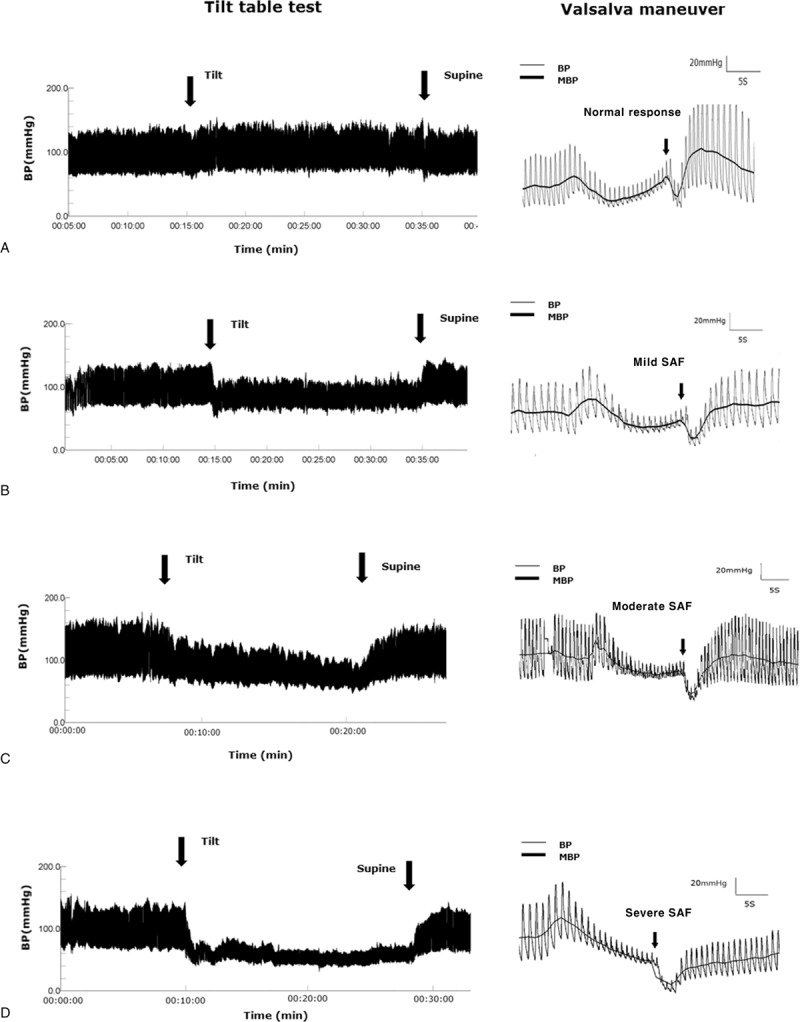
Orthostatic hypotension and Valsalva dysfunction in patients with graded sympathetic adrenergic failure. The recordings are examples that are representing typical blood pressure curve during the tilt. (A) Normal pattern. During the tilt, there was no significant decrease in the SBP or DBP. During the Valsalva maneuver, both the late phase II and phase IV exceeded the baseline. (B) Mild degree of SAF. During the tilt, there was a significant decrease in the SBP (mean, 25 mm Hg) compared with the supine position. During the Valsalva maneuver, there was a small MBP increase during the late phase II; however, the end of phase II failed to reach the baseline level, suggesting a mild degree of SI 3 impairment. (C) Moderate degree of SAF. During the tilt, there was a significant decrease in the SBP (mean, 33 mm Hg) compared with the supine position. During the Valsalva maneuver, the presence of the late phase II was ambiguous, and there was an increased difference in the MBP between the baseline and the end of phase II compared with the dysfunction shown in (B), suggesting a moderate degree of SI 3 impairment. (D) Severe degree of SAF. During the tilt, there was a significant decrease in the SBP (mean, 43 mm Hg) compared with the supine position. During the Valsalva maneuver, the recovery of the MBP at the end of phase II was completely absent, suggesting a severe degree of SI 3 impairment. DBP = diastolic blood pressure, MBP = mean blood pressure, SAF = sympathetic adrenergic failure, SBP = systolic blood pressure, SI = sympathetic index.
FIGURE 2.
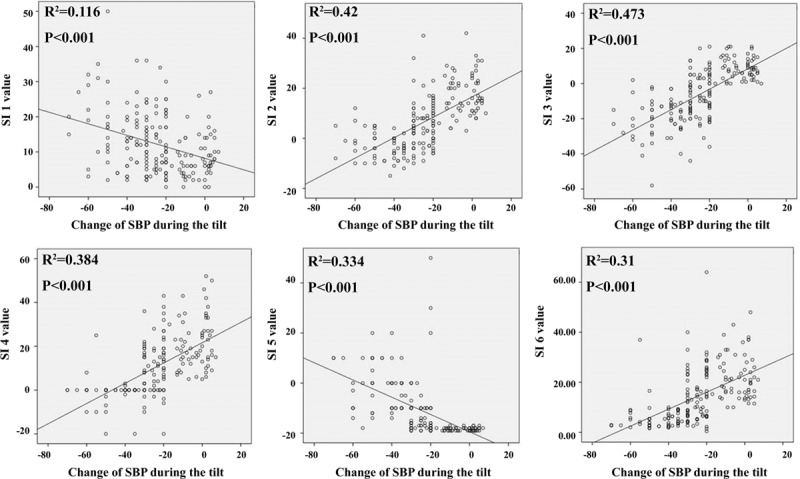
Relationship between each SI and SBP drop in patients with OH. The degree of correlation with fall in SBP decreased in the order of SI 3 > SI 2 > SI 4 > SI 5 > SI 6 > SI 1. Change of SBP means that the mean SBP values obtained while lying in the supine position for 10 min minus the mean SBP values obtained during upright posture for 20 min. OH = orthostatic hypotension, SBP = systolic blood pressure, SI = sympathetic index.
TABLE 2.
Composite Autonomic Severity Score in Normal Controls and Patients With Different Degree of Orthostatic Hypotension
FIGURE 3.
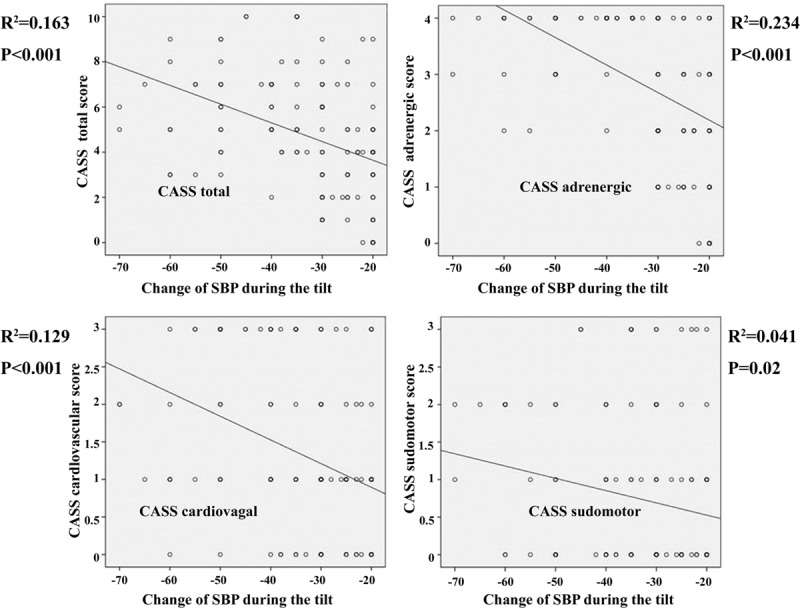
Relationship between drop in mean SBP in patients with OH and total and each scores of CASS. The degree of correlation with fall in mean SBP decreased in the order of CASS adrenergic > CASS total > CASS cardiovagal > CASS sudomotor. Change of SBP means that the mean SBP values obtained while lying in the supine position for 10 min minus the mean SBP values obtained during upright posture for 20 min. CASS = composite autonomic severity score, OH = orthostatic hypotension, SBP = systolic blood pressure.
DISCUSSION
To the best of our knowledge, this study is the first to determine the parameter of BP decline and recovery during the VM that can be used to differentiate SAF groups with different degrees of OH. In this study, only SI 3 could be used to differentiate all SAF groups from each other. Moreover, SI 3 exhibited the highest correlation with the amount of decrease in the mean SBP during the tilt.
Our result is in agreement with a previous study,4 which demonstrated that SI 3 could easily differentiate patients with SAF from healthy controls, tracked the full spectrum of SAF with different degrees of sympathetic dysfunction, and correlated with the presence of OH. However, in this study,4 certain patients with SAF did not have OH. For example, all patients with mild SAF had sympathetic cholinergic dysfunction with sudomotor impairment and showed a fall in SBP < 10 mm Hg during the tilt, which does not meet the widely accepted definition of OH (i.e., fall in SBP ≥ 20 during the tilt).12 Likewise, because the moderate degree of SAF was defined as a fall in SBP ≥ 10 < 30, a portion of the patients with moderate SAF theoretically may have exhibited a decline in SBP that was too small to meet the criteria of OH. Thus, the results from this previous study4 did not purely reflect the relationship between the degree of OH during the tilt and the pattern of BP change during the VM. By comparison, our study only included patients with OH that was defined as a decrease in the mean SBP of at least 20 mm Hg during the tilt.
All SIs, except SI 1, can differentiate healthy controls from patients with different degrees of OH. SI 1 only differentiated the healthy controls from moderate or severe OH group. The limited sensitivity of SI 1 to differentiate tested groups may partially be explained by its greater dependency on venous return.13,14 SI 2 theoretically reflects the pure vasoconstrictor response and may likely be the most sensitive indicator of total peripheral adrenergic activation. SI 2 was not sufficiently sensitive to distinguish between the moderate and severe SF groups. This finding may be explained by the fact that SI 2 had a modest dynamic range from the healthy controls to patients with severe SAF in the present study. SI 4 was also not sufficiently sensitive to distinguish between the moderate and severe SF groups, although it differentiated the healthy controls and mild OH groups from the remaining groups. In a supine position, in which the VM is routinely performed, SI 4 primarily reflects cardiac adrenergic sympathetic activity rather than peripheral vasomotor adrenergic function.6 SI 5 (PRT) and SI 6 (BRSa) were unable to differentiate between the mild OH group and the moderate OH group, although these parameters could be used to differentiate the healthy controls or severe groups from the remaining groups.
Although SIs 2, 3, and 5 represent peripheral vasomotor adrenergic sympathetic activity, our results showed that only SI 3 differentiated between all subject groups with different degrees of OH. The suboptimal performance of SI 5 may also be due to the small dynamic range from the healthy controls to severe OH group. The poor performance of SI 5 in our study is not consistent with a previous study,10 which demonstrated that SI 5 (PRT) is a valuable index of SAF and exhibits an excellent correlation with the widely used indices of late phases II and IV. However, in this study,10 the mild SAF group had sympathetic cholinergic dysfunction with sudomotor impairment rather than adrenergic impairment with OH. Additionally, because the moderate SAF group exhibited a decrease in the SBP of ≥ 10 but < 30 mm Hg, a portion of the patients with moderate SAF theoretically may have shown a decline in SBP that was not sufficiently large to meet the criteria of OH. Furthermore, the PRT was unable to differentiate the mild SAF group and healthy controls. Thus, the results from this previous study10 did not purely reflect the relationship between the degree of OH during the tilt and the pattern of BP change during the VM. SI 3, which measures the difference between the baseline BP and the BP at the end of phase II, is the only index that clearly differentiated between all SAF groups. The optimal performance of SI 3 may be explained by several factors. First, SI 3 represents the sympathetic vasoconstrictor responses due to the preceding decrease in BP. Second, SI 3 had the highest dynamic range from the healthy controls to severe OH group.
SIs correlations (rho = 0.116, 0.42, 0.473, 0.384, 0.334, and 0.31 for SIs 1–6, respectively) with the SBP change during the HUT test were also informative and lend confidence to the utilization of SI 3 as a clinical index to grade the SAF. The highest correlations occurred for SIs 3 and 2, which are both purely adrenergic. The low rho value of 0.166 for SI 1 was not surprising, as this index depends on the status of the venous capacitance bed, including the splanchnic mesenteric bed.13–16
Only the CASS adrenergic subscores can differentiate between all groups with different degree of OH. Since OH theoretically indicates sympathetic adrenergic impairment, the strong correlation of the CASS adrenergic subscores was reasonably expected. Among 3 CASS subscores, CASS adrenergic subscores showed the strongest correlation with the decrease in the mean SBP during the tilt. However, considering the fact that there was a significant correlation between the decrease in mean SBP during the tilt and CASS cardiovagal and sudomotor subscores, the extensive evaluation for clarification of autonomic function related to the sympathetic aderenergic, cardiovagal, and sudomotor should be considered as test options for patients with OH.
Our study has some limitations. First, the mean age was significantly different across all tested groups. Because loss of late phase II can occur even in normal men beyond the age of 60 years,17 the age difference in tested groups may have influenced the major result with regard to the optimal performance of SI 3. Theoretically, SIs 2 and 3 are equally obtained from phase II; however, only SI 3 could differentiate between the groups with different degrees of OH. Thus, we believe that age differences could not explain the optimal performance of SI 3 in differentiating between all SAF groups with different degrees of OH. Second, we arbitrarily classified the patients with SAF into mild, moderate, and severe groups according to the degree of OH. However, there are no established criteria for the classification of SAF according to the degree of OH. A future study to resolve this issue is necessary. Third, a direct measurement of the sympathetic function using icroneurography18 would be more accurate than the VM. However, in view of the absence of the non-neurogenic causes of OH, we believe that the VM-induced BP changes indeed reflect sympathetic functions. Finally, because our study focused on the relationship between the degree of OH and the pattern of Valsalva dysfunction, further studies are needed to investigate the frequency and pattern of orthostatic symptom associated with OH during the tilt using a patient-reported validated scale, and to determine the amount of the fall in BP enough to cause orthostatic symptom by brain hypoperfusion. In this regard, it would have also been interesting to determine which VM-related parameter is a preclinical marker of the development of the symptoms of OH.
CONCLUSION
Among many SIs, SI 3 separated all SAF groups with different degree of OH from each other and showed the best correlation with the amount of fall in mean SBP during the tilt. The SI 3 obtained during the Valsalva maneuver using the Finometer may improve the diagnostic accuracy of autonomic dysfunction in patients with different degree of OH. Our study may be a promising work in that it generates hypotheses regarding a potential gold standard for the detection of SAF. This will need further exploration in a prospective trial.
Footnotes
Abbreviations: ANOVA = 1-way analysis of variance, BP = blood pressure, BRSa = baroreflex sensitivity adrenergic, CASS = composite autonomic severity score, DBP = diastolic blood pressure, HR = heart rate, HUT = head-up tilt, MBP = mean blood pressure, OH = orthostatic hypotension, PRT = pressure recovery time, QSART = quantitative sudomotor axon reflex test, SAF = sympathetic adrenergic failure, SBP = systolic blood pressure, SI = sympathetic index, VM = Valsalva maneuver.
H-AK, J-HH, and H-AY report no disclosures.
HL serves on the editorial boards of Frontiers in Neuro-otology, Research in Vestibular Science, and Current Medical Imaging Review.
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (No. 2014R1A5A2010008).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kim HA, Yi HA, Lee H. Spectrum of autonomic dysfunction in orthostatic dizziness. Clin Neurophysiol 2014; 125:1248–1254. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Droxidopa in neurogenic orthostatic hypotension. Expert Rev Cardiovasc Ther 2015; 13:875–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res 2000; 10:285–291. [DOI] [PubMed] [Google Scholar]
- 4.Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology 2011; 76:2010–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HA, Lee H, Park KJ, et al. Autonomic dysfunction in patients with orthostatic dizziness: validation of orthostatic grading scale and comparison of Valsalva maneuver and head-up tilt testing results. J Neurol Sci 2013; 325:61–66. [DOI] [PubMed] [Google Scholar]
- 6.Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J Appl Physiol 1991; 71:1563–1567. [DOI] [PubMed] [Google Scholar]
- 7.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993; 68:748–572. [DOI] [PubMed] [Google Scholar]
- 8.Low PA, Benarroch EE. Clinical Autonomic Disorders. 3rd edPhiladelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 9.Novak P. Quantitative autonomic testing. J Vis Exp 2011; 53:e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 2005; 65:1533–1537. [DOI] [PubMed] [Google Scholar]
- 11.Schrezenmaier C, Singer W, Swift NM, et al. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol 2007; 64:381–386. [DOI] [PubMed] [Google Scholar]
- 12.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology 1996; 46:1470. [DOI] [PubMed] [Google Scholar]
- 13.Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev 1976; 56:100–177. [DOI] [PubMed] [Google Scholar]
- 14.Korner PI, Tonkin AM, Uther JB. Reflex and mechanical circulatory effects of graded Valsalva maneuvers in normal man. J Appl Physiol 1976; 40:434–440. [DOI] [PubMed] [Google Scholar]
- 15.Kooner JS, Raimbach S, Watson L, et al. Relationship between splanchnic vasodilation and postprandial hypotension in patients with primary autonomic failure. J Hypertens Suppl 1989; 7:S40–S41. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura J, Camilleri M, Low PA, et al. Effect of perturbations and a meal on superior mesenteric artery flow in patients with orthostatic hypotension. J Auton Nerv Syst 1997; 67:15–23. [DOI] [PubMed] [Google Scholar]
- 17.Denq JC, O’Brien PC, Low PA. Normative data on phases of the Valsalva maneuver. J Clin Neurophysiol 1998; 15:535–540. [DOI] [PubMed] [Google Scholar]
- 18.Krämer HH, Ament SJ, Breimhorst M, et al. Central correlation of muscle sympathetic nerve activation during baroreflex unloading—a microneurography-positron emission tomography study. Eur J Neurosci 2014; 39:623–629. [DOI] [PubMed] [Google Scholar]


