Supplemental Digital Content is available in the text
Abstract
Multiple sclerosis (MS) is the most frequent nontraumatic disabling neurological disease among young adults. Previous studies have examined the association of rs703842 in CYP27B1 with MS susceptibility, with inconsistent results reported.
The objective of this study is to conduct a systematic literature search and perform meta-analyses to examine whether rs703842 is associated with MS risk.
We searched potential literature in PubMed, Cochrane Library, Embase, Google Scholar, Web of Science, and HuGE by using the following inclusion criteria: studies were on human subjects; the studies were case–control studies; studies included subjects who had MS and those who did not have MS; and the studies provided genotype data for rs703842 for subjects who had and did not have MS, or provided odds ratios (ORs) and the 95% confidence intervals (CIs) for assessing the association of rs703842 with MS, or provided sufficient data for the calculation of OR and the 95% CI. We used random-effects models to calculate the OR as a measure of association. We used I2 to assess between-study heterogeneity, and a funnel plot and Egger test to assess publication bias.
Seven studies published since 2008 met the eligibility criteria and were included in the meta-analyses. We found that the C allele was significantly associated with reduced MS susceptibility (OR = 0.88, 95% CI: 0.80–0.89; P < 0.0001). We also found significant association of rs703842 with MS risk using a dominant and a recessive model (both P < 0.0002). Our results remain unchanged if our meta-analysis was limited to studies that included only Caucasian participants (OR = 0.85, 95% CI: 0.80–0.90; P < 0.0001).
Our study has several limitations: The sample size is limited; We were unable to control for some important confounding factors as data for individual participant were not available; and Most of the included studies focus on MS risk in Caucasian. As a result, we could not perform meta-analysis for assessing the relationship in other ethnic groups.
In summary, we found that the genetic variant rs703842 in CYP27B1 is associated with MS risk in Caucasians. More studies with larger sample size that control for important confounding factors are needed to validate the findings from this study.
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disease in which local lymphocytic infiltration can damage myelin and axons, leading to formation of scar tissue, or sclerosis.1 In the United States, it is estimated that there are about 400,000 people who are diagnosed with MS, with about 200 new diagnoses each week.2 MS is the most frequent nontraumatic disabling neurological disease among young adults.3 It causes enormous economic burden, such as treatment cost or loss in income due to inability or reduced ability to work. The annual treatment cost alone was estimated to be about $24,000 per patient in 2009.4
The exact etiology of MS remained unclear, and epidemiological studies indicate that it probably involves complex interaction between genetic and environmental factors.5 A number of risk factors have been reported to be associated with MS susceptibility, such as gender,6 ethnicity,7 Epstein–Barr virus (EBV),8 smoking,9 latitude,10 and vitamin D.11 Notably, previous studies showed that MS incidence is inversely correlated to the degree of sunlight exposure.12,13 The observation of higher MS prevalence in both hemispheres but decreased prevalence in the tropical areas led to the hypothesis that the sunlight effect on MS susceptibility may be through vitamin D production in the skin, which was confirmed from accumulating evidence.14 Experimental autoimmune encephalomyelitis (EAE) is a model of MS. Previous research found that the development of EAE requires vitamin D,15 and treatment of EAE mice with 1,25(OH)2D3, the active form of vitamin D, or in combination with specific antigen myelin oligodendrocyte glycoprotein can potentially suppress or even block the development of EAE.16 The exact mechanism linking vitamin D with MS etiology or MS development is still an active research area, and studies indicated that the positive effect of vitamin D on reducing MS risk might be attributed to its antiinflammatory influence.17 Moreover, vitamin D can also promote recovery of central nervous system and can enhance neural stem cell proliferation and oligodendrocyte differentiation.18
Meanwhile, many genetic variants were found to be associated with MS risk. For example, HLA haplotypes, genetic loci at interleukin-2 receptor α (IL2RA), interleukin-7 receptor α (IL7RA), C-type lectindomain family 16 member A (CLEC16A), interferon regulatory factor 8 (IRF8), tumor-necrosis-factor receptor superfamily member 1A (TNFRSF1A), CD6, and CD58.19–22 To date, previous GWAS have identified more than 100 loci that were associated with MS with genome-wide significance.23 However, the association of most of these genetic variants is modest, with the exception of HLA-DRB1∗15:01 haplotype which shows the strongest association with MS risk with an odds ratio (OR) of around 3.10.23
Cytochrome P450 family 27 subfamily B member (CYP27B1), located in 12q14.1, encodes a member of the cytochrome P450 superfamily of enzymes. CYP27B1 plays a key role in converting vitamin D to its active form, 1,25-dihydroxyvitamin D3, and therefore, is essential in regulating the level of biologically active vitamin D and calcium homeostasis.24 Given the possible link of vitamin D with MS susceptibility, it is anticipated that genetic variants in CYP27B1 might have an influence on MS risk. Indeed, previous studies have reported the association of MS with multiple genetic variants in CYP27B1, such as rs118204009,25 rs12368653,26 and rs10876994.26 Many studies also examined the association of the single-nucleotide polymorphism (SNP) rs703842 in CYP27B1 with the risk of MS, with inconsistent results reported.27–33 In this study, we conducted a systematic literature search and performed meta-analyses to investigate the association between rs703842 and MS susceptibility.
METHODS
Eligibility Criteria
The following criteria were used for assessing study eligibility: studies were on human subjects; the studies were case–control studies; studies included subjects who had MS and those who did not have MS; and the studies provided genotype data for rs703842 for subjects who had and did not have MS, or provided ORs and the 95% confidence intervals (CIs) for assessing the association of rs703842 with MS risk, or provided sufficient data for the calculation of OR and the 95% CI. Studies were excluded if: they were unpublished; they were abstracts/comments, reviews, or meta-analyses; and there were no control group. If overlapping data were used, we chose the study with a larger sample size.
Search Strategy
Two authors (LL and JY) performed an independent and extensive literature search in PubMed, Cochrane Library, Embase, Google Scholar, Web of Science and HuGE (a navigator for human genome epidemiology) for papers published before October 13, 2015. The keywords used in the literature search can be found in the online supplementary file.
We retrieved all potentially relevant studies to evaluate their eligibility, and also hand searched the references in all included studies for possible studies that were missed in the literature search. The search was limited to studies published in English. No efforts were made to contact the authors for additional data. A group discussion was held to resolve any disagreement until a consensus was reached.
Data Extraction
Two authors (YB and JY) extracted the following data from the eligible studies: name of the first author, year of publication, mean age, distribution of gender, ethnicity of the participants, genetic models used for analysis, rs703842 genotype data for patients with and without MS, or OR and the corresponding 95% CI. The quality of the included studies were assessed independently by 2 authors (TJ and JY) using Newcastle–Ottawa scale.34
Data Analysis
We used ORs to assess the association between rs703842 and MS susceptibility. In all meta-analyses, the ORs were calculated using random-effects models. We used I2 to assess between-study heterogeneity, and a funnel plot and Egger test to assess publication bias.
If a study reported adjusted OR and the corresponding 95% CI for a specific genetic model, we used that information for the meta-analysis for that genetic model, even though crude OR or genotype data to calculate the crude OR were available.
As a systematic review and meta-analysis, ethical approval of this study is not needed. This work was reported according to the PRISMA guidelines.35 All statistical analyses were performed using Stata 11.2 (StataCorp LP, College Station, TX). A P-value <0.05 was considered statistically significant.
RESULTS
Study Selection and Characteristics
Literature search and selection of eligible studies are shown in Figure 1. In our initial search, we identified a total of 114 potential publications. Among them, 93 publications were excluded because they were irrelevant, reviews/abstracts, not about human subjects, or not published in English. We retrieved the remaining 21 papers for a more detailed evaluation and further excluded 14 studies because they were not case–control studies, there were insufficient data or they were not about rs703842, leading to 7 relevant publications to be included in our analyses.27–33
FIGURE 1.
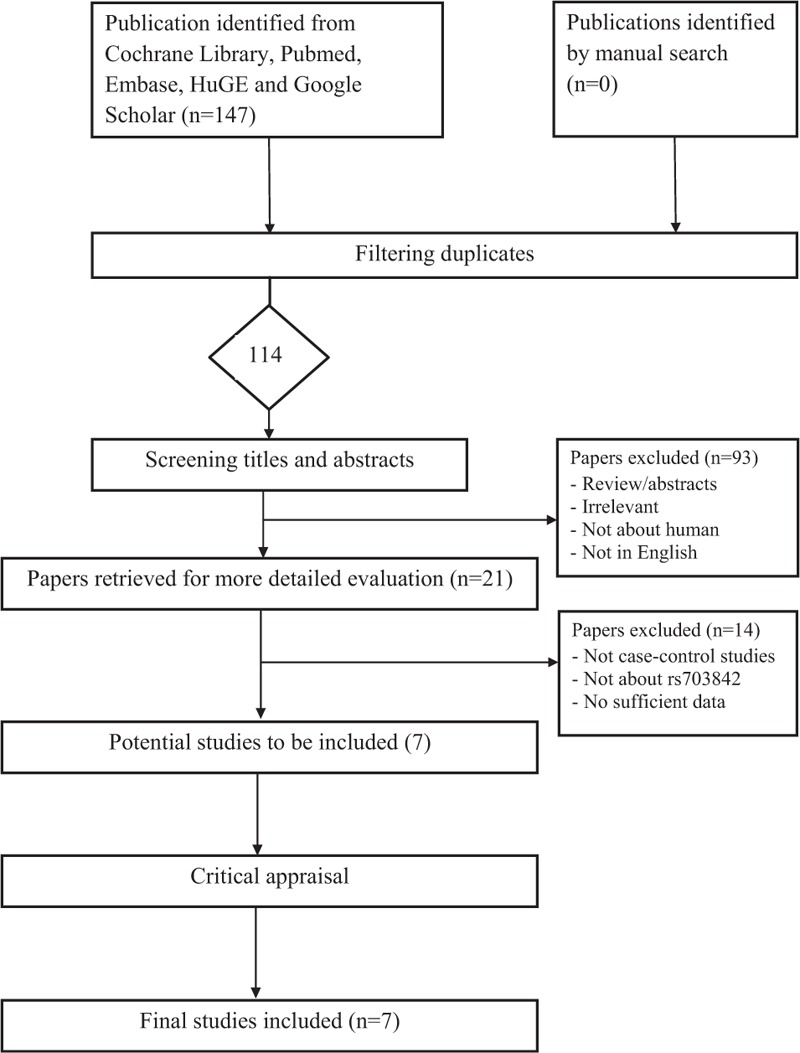
Flow diagram of the selection process of the studies included in the meta-analyses. Note: Please see the Methods section for additional details.
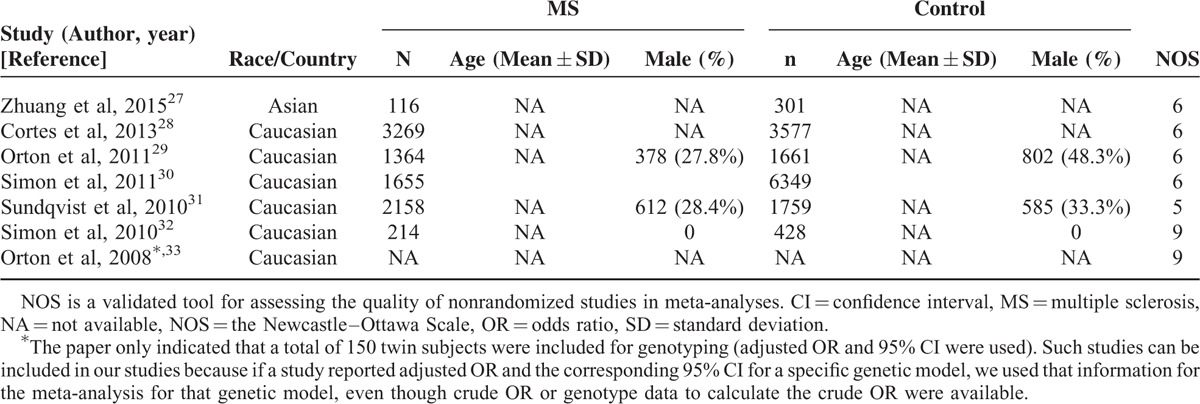
All the included studies were published since 2008. The sample size ranged from 150 to 8,004. The meta-analysis included a total of 22,851 participants. All included studies examined the association of rs703842 with MS in Caucasians except one,27 which examined the association in Chinese (Table 1).
TABLE 1.
Basic Characteristics of the Studies Included in the Meta-Analyses
Assessment of Publication Bias
We found no evidence of publication bias for the meta-analysis of rs703842 with MS using the allelic model (P = 0.49; Figure 2). Assessment of publication bias for the meta-analyses using other genetic models is not meaningful due to limited number of studies included in the corresponding analyses.
FIGURE 2.
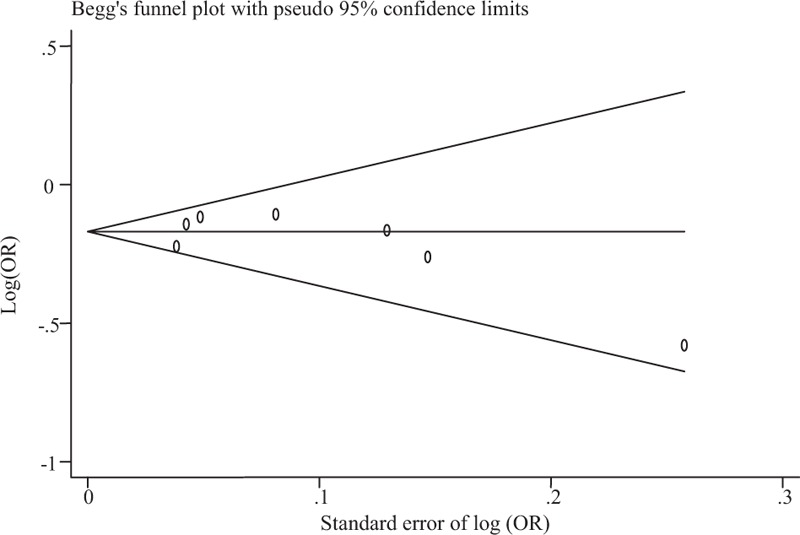
Funnel plot for meta-analysis of the association of rs703842 with multiple sclerosis. The x-axis is the standard error of the log-transformed OR (log [OR]), and the y-axis is the log-transformed OR. The horizontal line in the figure represents the overall estimated log-transformed OR. The 2 diagonal lines represent the pseudo 95% confidence limits of the effect estimate. OR = odds ratio.
Association of rs703842 With MS
Of the 7 studies included in our meta-analysis, only 3 studies provided genotype data for participants with and without MS.27,30,32 The other 4 studies provided adjusted OR and the corresponding 95% CI for the association of rs703842 with MS.28,29,31,33 Therefore, meta-analysis using allelic models utilized results from all the 7 studies, while meta-analysis using other genetic models (dominant, recessive, and additive) is only available in the 3 studies that reported genotype data.
All the studies seemed to indicate that the C allele in rs703842 was associated with decreased risk of MS, although the association was statistically significant in only 3 studies (Figure 3).28,30,31 Our meta-analysis showed that the C allele in rs703842 was significantly associated with reduced MS susceptibility (OR = 0.85, 95% CI: 0.80–0.89; P < 0.0001). There was no significant heterogeneity between studies (I2 = 14.9%, P = 0.316).
FIGURE 3.
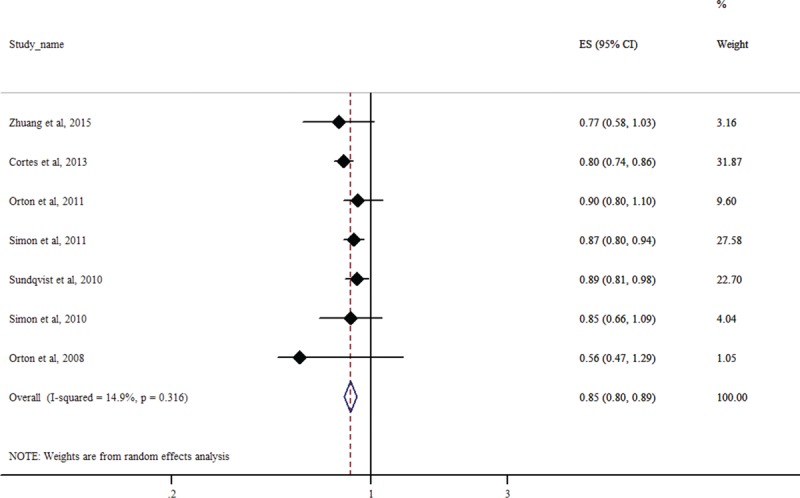
Forest plot for meta-analysis of the association of rs703842 with multiple sclerosis using the allelic model (C vs T). Each study is represented by a square whose area is proportional to the weight of the study. The overall effect from meta-analysis is represented by a diamond whose width represents the 95% CI for the estimated OR. CI = confidence interval, OR = odds ratio.
We found a significant association between rs7013842 and MS risk using a dominant model (OR = 0.75, 95% CI: 0.63–0.89: P = 0.001) and a recessive model (OR = 0.85, 95% CI: 0.77–0.94; P = 0.002), but no significant association was found using an additive model (OR = 0.87, 95% CI: 0.71–1.06; P = 0.164). However, this result should be interpreted with caution because only 3 studies were included in the meta-analysis.27,30,32
Sensitivity Analysis
We repeated our analysis by excluding studies that did not meet Hardy–Weinberg equilibrium (HWE) or those for which HWE information could not be obtained.28,30,33 The C allele in rs703842 remained to be associated with MS risk (OR = 0.88, 95% CI: 0.82–0.95; P = 0.001). There was no significant heterogeneity between studies (I2 = 0%, P = 0.795).
The observed association between the C allele in rs703842 and MS risk remained if our meta-analysis was limited to studies that included only Caucasian participants (OR = 0.85, 95%CI: 0.80–0.90; P < 0.0001). There was no significant heterogeneity between studies (I2 = 24.8%, P = 0.248). The observed association remained unchanged if we excluded the study that showed the most significant association28 (OR = 0.87, 95% CI: 0.82–0.92; P < 0.0001). There was no significant heterogeneity between studies (I2 = 0%, P = 0.545). Excluding studies of low quality31 did not change our findings (OR = 0.83, 95% CI: 0.79–0.88; P < 0.0001). There was no significant heterogeneity between studies (I2 = 9.5%, P = 0.355). Finally, to minimize the influence of a possible, albeit very unlikely, overlapping of data, we reran the analysis by excluding 1 earlier study,33 and our results remain unchanged (OR = 0.85, 95% CI: 0.81–0.89; P < 0.0001). There was no significant heterogeneity between the studies (I2 = 0%, P = 0.481).
DISCUSSION
In this study, we conducted a systematic literature search and performed meta-analyses to assess the association of rs703842 in CYP27B1 with MS. We found that the C allele showed significant association with reduced MS risk in Caucasians. The association of rs703842 with MS susceptibility did not change under different genetic models, except the additive model, probably because of reduced power due to a limited number of the included studies. The association remained when we excluded studies that violate HWE. To the best of our knowledge, this is the 1st meta-analysis on the association of rs703842 with MS susceptibility.
Previous studies have identified multiple genetic, epigenetic, and environmental risk factors that are associated with MS susceptibility.36–38 Notably, high MS frequency occurs in areas with low sunlight exposure, a major inducer of previtamin D synthesis in the skin.12 A battery of epidemiologic, experimental, and clinical evidence also suggests a link between hypovitaminosis D and increased MS susceptibility and relapses.11,17,39,40CYP27B1 encodes the enzyme 25-hydroxyvitamin D-1 alpha hydroxylase, which hydroxylates 25-hydroxyvitamin D into the bioactive form 1,25(OH)2 vitamin D. This active metabolite is a potent immuno-modulator important for immune function and development, including innate and adaptive immunity, immune tolerance and B-cell homeostasis, and type 2 antiinflammatory T helper cell generation.41–44 Recent studies have examined the association of MS with multiple genetic variants in vitamin D metabolizing genes, such as p.R389H mutation, rs12368653, rs10876994, rs118204009 and rs703842 in CYP27B1, and rs2248359 in CYP24A1.25,28,45 A previous meta-analysis found no association of 2 common SNPs (rs2228570 and rs731236) in the vitamin D3 receptor gene (VDR) with risk for MS.46 Another more recent meta-analysis of 4 polymorphisms in VDR (rs2228570, rs731236, rs1544410, and rs7975232) confirmed no association of rs731236 with MS risk, but found a significant association of rs2228570 using a dominant and codominant model.47 The conflicting results from these studies are probably due to different study designs, different genetic models used, or the heterogeneity of the ethnic background of the study participants.25,48,49 Further studies are required to clarify the relationship between these genetic variants and MS risk.
The SNP rs703842 lies 1.76 kb upstream of CYP27B1 and in the 3′-untranslated region of the neighbor gene methyltransferase-like protein 1 (METTL1). Whether and how this genetic variant regulates CYP27B1 expression and vitamin D metabolism remains unclear. A previous twin study identified 2 SNPs in CYP27B1 (rs703842 and rs4646536) as significant predictors of 25(OH)D concentrations.33 This finding, however, could not be replicated in other studies.50,51 Another study found that rs703842 was associated with altered expression level of a proximal gene Ts translation elongation factor, mitochondrial, and another strong MS candidate gene.52 Interestingly, this chromosomal region harboring METTL1-CYP27B1-CDK4 genes was also found to be associated with some other autoimmune diseases, such as type 1 diabetes, coeliac disease, and rheumatoid arthritis.53–57 More studies are needed to clarify the functional role of rs703842.58
Since previous studies suggested that lower level of blood vitamin D concentration is an important risk factor that can influence MS susceptibility, in clinical practice, it might be valuable to assess the level of blood vitamin D as well as to genotype variants associated with MS risk, such as rs703842.59 Previous studies reported beneficial effects of vitamin D supplementation in MS patients.60,61 Therefore, oral supplementation could be an alternative way to improve vitamin D level other than sunlight exposure for the prevention and treatment of MS. Preclinical studies and RCTs focusing on the safety and efficacy of vitamin D supplementation are undergoing.62
Our study has some limitations: The sample size is still limited despite our efforts to perform a literature search as systematic as possible; Because only published data were used, we were unable to control for some important confounding factors such as age, gender, and smoking as data for individual participant were not available; and Most of the included studies focus on MS risk in Caucasian. As a result, we could not perform meta-analysis for assessing the relationship in other ethnic groups, and our results might not be generalized to other ethnicities.
In summary, in this study, we conducted meta-analyses to evaluate the association between rs703842 in CYP27B1 and MS susceptibility. We found that the C allele was associated with lowered MS risk in Caucasians. Whether the association holds for other ethnic groups needs further investigation. More studies with larger sample size that control for important confounding factors are also needed to validate the findings from this study.
Supplementary Material
Acknowledgements
The authors thank the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (NO. 2013-1792), Liaoning Provincial Natural Science Foundation (2015020460 and 2013021083), the Shanghai Committee of Science and Technology (15410723200), a grant from A Joint Research Program for Management of Key Diseases by Shanghai Public Health System (2014ZYJB0007 to Xiaofeng Tao and Dexuan Ma), and Special Foundation for Science and Technology Innovation of Shenyang-Special Program for Science and Technology Development of Population and Health (F13-220-9-53), and National Natural Science Foundation (Grant NO. 81302190) for the support. The authors also thank NIH/NIA grant R01AG036042 and the Illinois Department of Public Health for the support to Dr Jingyun Yang's research.
Footnotes
Abbreviations: CI = confidence interval, CYP27B1 = cytochrome P450 family 27 subfamily B member, EAE = experimental autoimmune encephalomyelitis, HWE = Hardy–Weinberg equilibrium, MS = multiple sclerosis, OR = odds ratio, SNP = single-nucleotide polymorphism.
This study was supported by Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (NO. 2013-1792), Liaoning Provincial Natural Science Foundation (2015020460 and 2013021083), the Shanghai Committee of Science and Technology (15410723200), a grant from A Joint Research Program for Management of Key Diseases by Shanghai Public Health System (2014ZYJB0007 to Xiaofeng Tao and Dexuan Ma), and Special Foundation for Science and Technology Innovation of Shenyang-Special Program for Science and Technology Development of Population and Health (F13-220-9-53), and National Natural Science Foundation (Grant NO. 81302190) for the support; and NIH/NIA grant R01AG036042 and the Illinois Department of Public Health.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care 2013; 19:S15–S20. [PubMed] [Google Scholar]
- 3.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343:938–952. [DOI] [PubMed] [Google Scholar]
- 4.Owens GM, Olvey EL, Skrepnek GH, et al. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J Manag Care Pharm 2013; 19:S41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannoni G, Ebers G. Multiple sclerosis: the environment and causation. Curr Opin Neurol 2007; 20:261–268. [DOI] [PubMed] [Google Scholar]
- 6.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 2006; 5:932–936. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzke JF, Beebe GW, Norman JE., Jr Epidemiology of multiple sclerosis in U.S. veterans: 1. Race, sex, and geographic distribution. Neurology 1979; 29:1228–1235. [DOI] [PubMed] [Google Scholar]
- 8.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol 2006; 59:499–503. [DOI] [PubMed] [Google Scholar]
- 9.Hawkes CH. Smoking is a risk factor for multiple sclerosis: a metanalysis. Mult Scler 2007; 13:610–615. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008; 71:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010; 9:599–612. [DOI] [PubMed] [Google Scholar]
- 12.Bjornevik K, Riise T, Casetta I, et al. Sun exposure and multiple sclerosis risk in Norway and Italy: the EnvIMS study. Mult Scler 2014; 20:1042–1049. [DOI] [PubMed] [Google Scholar]
- 13.Islam T, Gauderman WJ, Cozen W, et al. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007; 69:381–388. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF, Cook S, Suarez G, et al. Vitamin D deficiency and possible role in multiple sclerosis. Eur Neurol Rev 2015; 10:131–138. [Google Scholar]
- 15.Wang Y, Marling SJ, Zhu JG, et al. Development of experimental autoimmune encephalomyelitis (EAE) in mice requires vitamin D and the vitamin D receptor. Proc Natl Acad Sci U S A 2012; 109:8501–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiuso-Minicucci F, Ishikawa LL, Mimura LA, et al. Treatment with vitamin D/MOG association suppresses experimental autoimmune encephalomyelitis. PLoS One 2015; 10:e0125836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolders J, Damoiseaux J, Menheere P, et al. Vitamin D as an immune modulator in multiple sclerosis, a review. J Neuroimmunol 2008; 194:7–17. [DOI] [PubMed] [Google Scholar]
- 18.Shirazi HA, Rasouli J, Ciric B, et al. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol 2015; 98:240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcellos LF, Oksenberg JR, Begovich AB, et al. HLA-DR2 dose effect on susceptibility to multiple sclerosis and influence on disease course. Am J Hum Genet 2003; 72:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barcellos LF, Sawcer S, Ramsay PP, et al. Heterogeneity at the HLA-DRB1 locus and risk for multiple sclerosis. Hum Mol Genet 2006; 15:2813–2824. [DOI] [PubMed] [Google Scholar]
- 21.Dyment DA, Herrera BM, Cader MZ, et al. Complex interactions among MHC haplotypes in multiple sclerosis: susceptibility and resistance. Hum Mol Genet 2005; 14:2019–2026. [DOI] [PubMed] [Google Scholar]
- 22.De Jager PL, Jia X, Wang J, et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet 2009; 41:776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawcer S, Franklin RJ, Ban M. Multiple sclerosis genetics. Lancet Neurol 2014; 13:700–709. [DOI] [PubMed] [Google Scholar]
- 24.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 2014; 55:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramagopalan SV, Dyment DA, Cader MZ, et al. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol 2011; 70:881–886. [DOI] [PubMed] [Google Scholar]
- 26.Australia New Zealand Multiple Sclerosis Genetics C. Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 2009; 41:824–828. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang JC, Huang ZY, Zhao GX, et al. Variants of CYP27B1 are associated with both multiple sclerosis and neuromyelitis optica patients in Han Chinese population. Gene 2015; 557:236–239. [DOI] [PubMed] [Google Scholar]
- 28.Cortes A, Field J, Glazov EA, et al. Resequencing and fine-mapping of the chromosome 12q13-14 locus associated with multiple sclerosis refines the number of implicated genes. Hum Mol Genet 2013; 22:2283–2292. [DOI] [PubMed] [Google Scholar]
- 29.Orton SM, Ramagopalan SV, Para AE, et al. Vitamin D metabolic pathway genes and risk of multiple sclerosis in Canadians. J Neurol Sci 2011; 305:116–120. [DOI] [PubMed] [Google Scholar]
- 30.Simon KC, Munger KL, Kraft P, et al. Genetic predictors of 25-hydroxyvitamin D levels and risk of multiple sclerosis. J Neurol 2011; 258:1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundqvist E, Baarnhielm M, Alfredsson L, et al. Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Genet 2010; 18:1349–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon KC, Munger KL, Xing Y, et al. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler 2010; 16:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orton SM, Morris AP, Herrera BM, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 2008; 88:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 1999; http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [Accessed October 01, 2015]. [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis – a quiet revolution. Nat Rev Neurol 2015; 11:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bashinskaya VV, Kulakova OG, Boyko AN, et al. A review of genome-wide association studies for multiple sclerosis: classical and hypothesis-driven approaches. Hum Genet 2015; 134:1143–1162. [DOI] [PubMed] [Google Scholar]
- 38.Correale J, Gaitan MI. Multiple sclerosis and environmental factors: the role of vitamin D, parasites, and Epstein–Barr virus infection. Acta Neurol Scand 2015; 132:46–55. [DOI] [PubMed] [Google Scholar]
- 39.Smolders J, Menheere P, Kessels A, et al. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 2008; 14:1220–1224. [DOI] [PubMed] [Google Scholar]
- 40.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009; 132:1146–1160. [DOI] [PubMed] [Google Scholar]
- 41.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 2008; 4:404–412. [DOI] [PubMed] [Google Scholar]
- 42.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007; 179:1634–1647. [DOI] [PubMed] [Google Scholar]
- 43.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004; 229:1136–1142. [DOI] [PubMed] [Google Scholar]
- 44.Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4 (+) T cells to enhance the development of Th2 cells. J Immunol 2001; 167:4974–4980. [DOI] [PubMed] [Google Scholar]
- 45.Ross JP, Bernales CQ, Lee JD, et al. Analysis of CYP27B1 in multiple sclerosis. J Neuroimmunol 2014; 266:64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Martin E, Agundez JA, Martinez C, et al. Vitamin D3 receptor (VDR) gene rs2228570 (Fok1) and rs731236 (Taq1) variants are not associated with the risk for multiple sclerosis: results of a new study and a meta-analysis. PLoS One 2013; 8:e65487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tizaoui K, Kaabachi W, Hamzaoui A, et al. Association between vitamin D receptor polymorphisms and multiple sclerosis: systematic review and meta-analysis of case-control studies. Cell Mol Immunol 2015; 12:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcina A, Fedetz M, Fernandez O, et al. Identification of a functional variant in the KIF5A-CYP27B1-METTL1-FAM119B locus associated with multiple sclerosis. J Med Genet 2013; 50:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ban M, Caillier S, Mero IL, et al. No evidence of association between mutant alleles of the CYP27B1 gene and multiple sclerosis. Ann Neurol 2013; 73:430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn J, Albanes D, Berndt SI, et al. Vitamin D-related genes, serum vitamin D concentrations and prostate cancer risk. Carcinogenesis 2009; 30:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wjst M, Altmuller J, Faus-Kessler T, et al. Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res 2006; 7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Handel AE, Handunnetthi L, Berlanga AJ, et al. The effect of single nucleotide polymorphisms from genome wide association studies in multiple sclerosis on gene expression. PLoS One 2010; 5:e10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fung EY, Smyth DJ, Howson JM, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun 2009; 10:188–191. [DOI] [PubMed] [Google Scholar]
- 54.Bailey R, Cooper JD, Zeitels L, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 2007; 56:2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhernakova A, Stahl EA, Trynka G, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet 2011; 7:e1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barton A, Thomson W, Ke X, et al. Re-evaluation of putative rheumatoid arthritis susceptibility genes in the post-genome wide association study era and hypothesis of a key pathway underlying susceptibility. Hum Mol Genet 2008; 17:2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raychaudhuri S, Remmers EF, Lee AT, et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet 2008; 40:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki A, Kochi Y, Okada Y, et al. Insight from genome-wide association studies in rheumatoid arthritis and multiple sclerosis. FEBS Lett 2011; 585:3627–3632. [DOI] [PubMed] [Google Scholar]
- 59.Runia TF, Hop WC, de Rijke YB, et al. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology 2012; 79:261–266. [DOI] [PubMed] [Google Scholar]
- 60.Stewart N, Simpson S, Jr, van der Mei I, et al. Interferon-beta and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurology 2012; 79:254–260. [DOI] [PubMed] [Google Scholar]
- 61.Holmoy T, Torkildsen O, Myhr KM, et al. Vitamin D supplementation and monitoring in multiple sclerosis: who, when and wherefore. Acta Neurol Scand Suppl 2012; 63–69. [DOI] [PubMed] [Google Scholar]
- 62.Vitamin D. 2015; http://www.nationalmssociety.org/Research/Research-News-Progress/Vitamin-D [Accessed December 31, 2015]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


