Abstract
Aortic arch calcification (AoAC) is associated with cardiovascular and all-cause mortality in end-stage renal disease population. AoAC can be simply estimated with an AoAC score using plain chest radiography. The objective of this study is to evaluate the association of AoAC with brachial-ankle pulse wave velocity (baPWV) and cardiomegaly in patients who have undergoing hemodialysis (HD).
We retrospectively determined AoAC and cardiothoracic ratio (CTR) by chest x-ray in 220 HD patients who underwent the measurement of baPWV. The values of baPWV were measured by an ankle-brachial index-form device. Multiple stepwise logistic regression analysis was used to identify the factors associated with AoAC score >4.
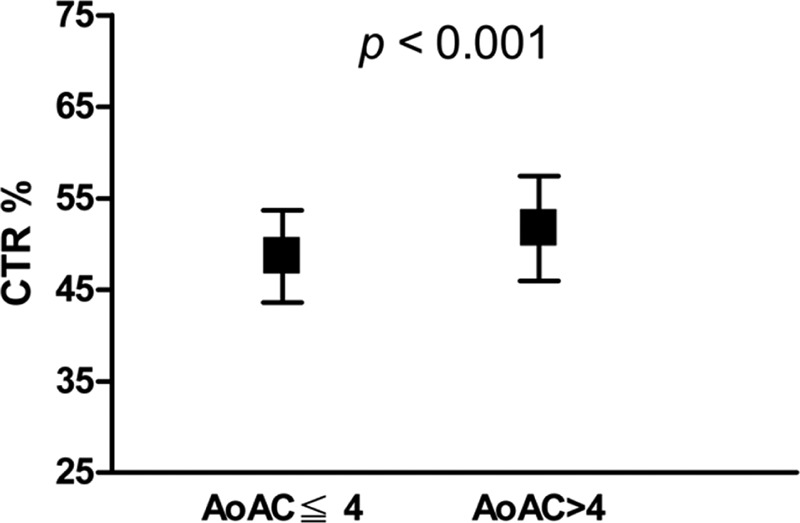
Compared patients with AoAC score ≦4, patients with AoAC score >4 had older age, higher prevalence of diabetes and cerebrovascular disease, lower diastolic blood pressure, higher baPWV, higher CTR, higher prevalence of CTR ≧50%, lower total cholesterol, and lower creatinine level. After the multivariate stepwise logistic analysis, old age, cerebrovascular disease, high baPWV (per 100 cm/s, odds ratio [OR] 1.065, 95% confidence interval [CI] 1.003–1.129, P = 0.038), CTR (per 1%, OR 1.116, 95% CI 1.046–1.191, P = 0.001), and low total cholesterol level were independently associated with AoAC score >4.
Our study demonstrated AoAC severity was associated with high baPWV and high CTR in patients with HD. Therefore, we suggest that evaluating AoAC on plain chest radiography may be a simple and inexpensive method for detecting arterial stiffness in HD patients.
INTRODUCTION
Compared with the general population, patients with end-stage renal disease have a higher prevalence of vascular calcification of up to 80% to 90%.1 Vascular calcification has also been predictive of cardiovascular morbidity and mortality among such patients.2 Increasing age is a most consistent risk factor and the need for dialysis is a known accelerator.3,4 Additional risk factors include hypertension, diabetes, dyslipidemia, inflammation, malnutrition, and oxidative stress.5–7
Vascular calcification can be assessed using several radiologic tools, including computed tomography (CT), ultrasonography, and plain x-rays.8,9 Although cardiac CT can accurately and quantitatively evaluate the extent of cardiovascular calcification, this diagnostic tool is costly and involves radiation exposure. The aortic arch calcification (AoAC) score is a semiquantitative evaluation of AoAC on chest x-rays. It is a very simple and noninvasive tool and is highly correlated with the AoAC volume as determined by multidetector CT.3 The AoAC on a chest x-ray may represent the whole AoAC and is associated with cardiovascular and all-cause mortality among patients with end-stage renal disease.10,11
In current literature, coronary artery calcium score, aorta calcium score, and AoAC volume measured on CT are independently associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction, respectively.8,12 In another study, the presence of vascular calcifications measured via B-mode ultrasonography in patients using hemodialysis (HD) is correlated with increased arterial stiffness.9 However, there is a paucity of investigations on the relationship between AoAC on plain chest x-rays and arterial stiffness and cardiomegaly. This study aimed to assess whether the presence of AoAC on plain chest radiography is associated with brachial-ankle pulse wave velocity (baPWV), a marker for arterial stiffness, and cardiothoracic ratio (CTR) in HD patients.
SUBJECTS AND METHODS
Study Patients and Design
This study enrolled all patients receiving a maintenance HD program, thrice weekly for 3 months, in a dialysis clinic of a regional hospital in Taiwan in September 2015. The exclusion criteria were as follows: patients who refused any ankle-brachial index-form device examination (n = 5), patients with atrial fibrillation (n = 4), patients with bilateral below-knee amputation (n = 2), and patients with hospitalization or antibiotic treatment in the last 4 weeks (n = 5) were excluded. Of the 220 patients included in the final analysis, 113 were males and 107 females.
All of the patients underwent routine HD 3 times a week. Each HD session was performed for 3 to 4 hours using a dialyzer with a blood flow rate of 250 to 300 mL/min and dialysate flow of 500 mL/min.
The institutional review board of Kaohsiung Medical University Hospital approved the study protocol and all of the participants provided written informed consent.
Assessment of baPWV
The baPWV was assessed 10 to 30 minutes before each HD session using an ankle-brachial index-form device, which measured the blood pressure in the arms and ankles.13,14 Briefly, occlusion and monitoring cuffs were placed tightly around the upper arm without blood access, with both sides of the lower extremities in the supine position. The baPWV values were measured as reported in previous studies.13,14 The larger bilateral baPWV value of each patient was used in the analysis.
AoAC and CTR Evaluation by Chest X-Ray
An experienced radiologist blinded to the patients’ clinical data reviewed the chest x-ray films. Aortic arch calcification was evaluated using a scale developed by Ogawa et al,3 which divided the aortic arch into 16 sections by circumference. The scale was attached to the aortic arch on chest x-rays and the number of sectors with calcification was counted. The CTR was defined as the ratio of a transverse diameter of the cardiac shadow to the transverse diameter of the chest on chest x-ray, with cardiomegaly defined as CTR ≥50%.
Collection of Demographic, Medical, and Laboratory Data
Demographic and medical data such as age, sex, body mass index (kg/m2), smoking history, and current comorbidities were obtained from medical records and by patient interviews. Laboratory data were obtained from fasting blood samples using an auto-analyzer (COBAS Integra 400; Roche Diagnostics GmbH, Mannheim, Germany). Blood samples were taken within 1 month of enrollment and the Kt/V was measured monthly as a marker of dialysis efficiency, according to the Daugirdas procedure.15
Reproducibility
Thirty patients were randomly selected for evaluation of the reproducibility of AoAC by 1 experienced radiologist and 1 trained medical doctor. Mean percent error was calculated as the absolute difference divided by the average of the 2 observations.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL). Data were expressed as percentages, mean ± standard deviation, or median (25th–75th percentile) for duration of dialysis, triglyceride, and serum intact parathyroid hormone. The differences between groups were checked using a χ2 test for categorical variables and independent t test for continuous variables. Multiple stepwise logistic regression analysis was used to identify the factors associated with AoAC score > median. Significant variables in univariate analysis were selected for multivariate analysis. A difference was considered significant at P < 0.05.
RESULTS
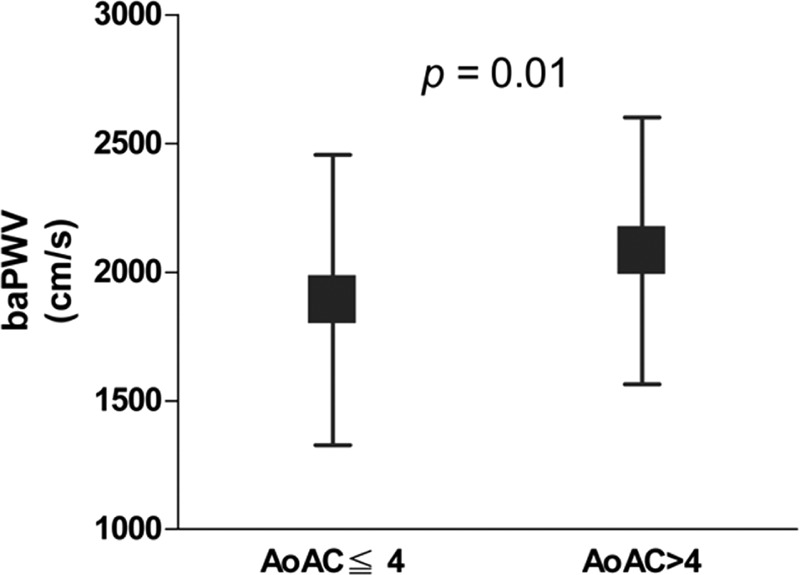
A total of 220 HD patients were included. The mean age was 60.9 ± 11.7 years with 113 men and 107 women. The median score of AoAC was 4. The mean percent error for AoAC measurements was 12.3% ± 12.3%. A comparison of the clinical characteristics between patients with AoAC score ≦ 4 and AoAC score >4 is shown in Table 1. Compared patients with AoAC score ≦4, patients with AoAC score >4 had older age, higher prevalence of diabetes and cerebrovascular disease, lower diastolic blood pressure, higher baPWV, higher CTR, higher prevalence of CTR ≧50%, lower total cholesterol and lower creatinine level. The values of baPWV in patients with AoAC score ≦4 and with AoAC score >4 were 1891.7 ± 564.0 and 2082.6 ± 518.2 cm/s, respectively (P = 0.010). Figure 1 illustrates the comparison of baPWV value in patients with AoAC score ≦4 and AoAC score >4. Patients with AoAC score >4 had higher value of baPWV than that in patients with AoAC score ≦4. The prevalence of CTR >50% in patients with AoAC score ≦4 and with AoAC score >4 were 41.6% and 58.9%, respectively (P = 0.010). Figure 2 illustrates the comparison of CTR in patients with AoAC score ≦4 and AoAC score >4. Patients with AoAC score >4 had higher value of CTR than that in patients with AoAC score ≦4 (48.7 ± 5.0% vs. 51.7 ± 5.7%, P < 0.001).
TABLE 1.
Comparison of Baseline Characteristics Between Patients With AoAC Score ≦ 4 and AoAC Score > 4
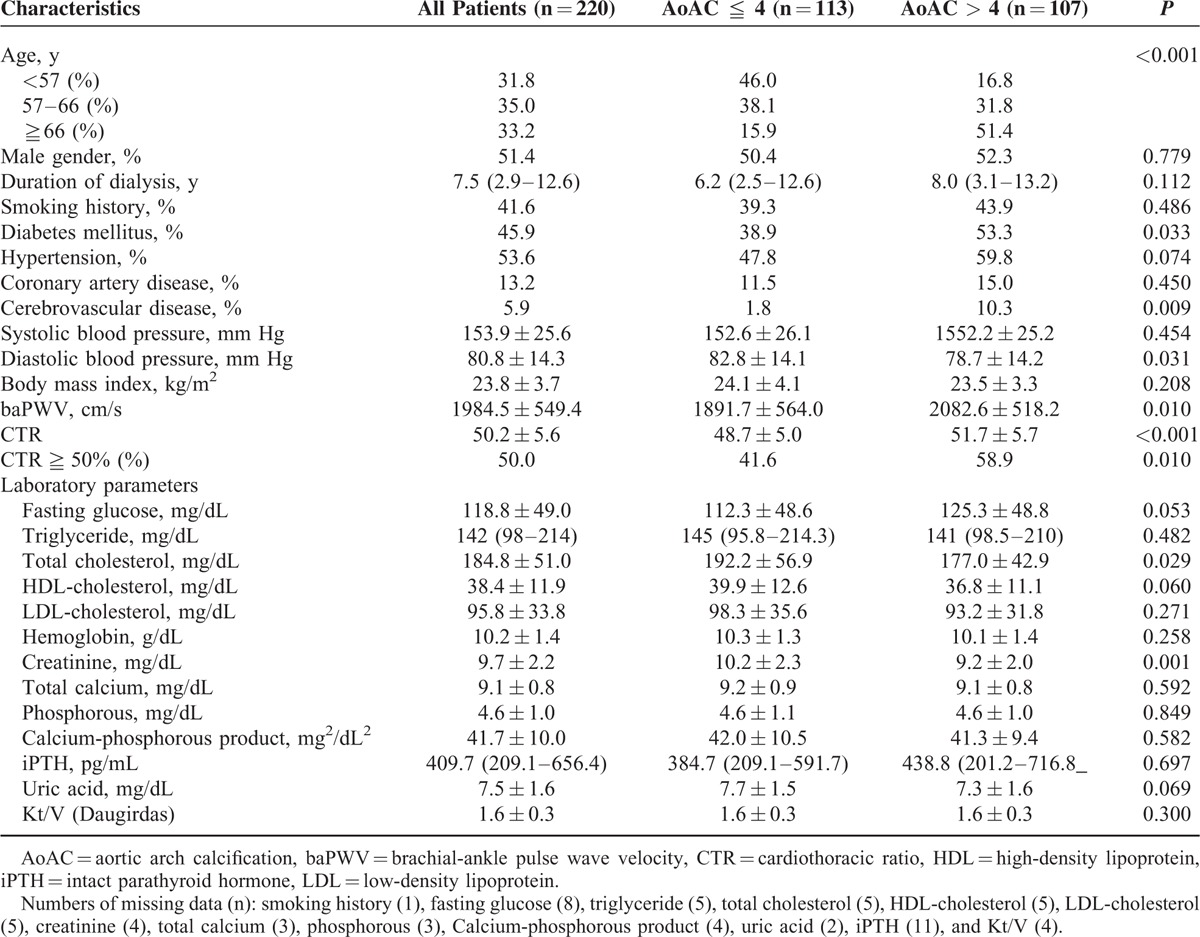
FIGURE 1.
The mean ± standard deviation value of brachial-ankle pulse wave velocity (baPWV) between patients with aortic arch calcification (AoAC) score ≦4 and AoAC score >4. Patients with AoAC score >4 had higher value of baPWV than that in patients with AoAC score ≦4 (P = 0.010).
FIGURE 2.
The mean ± standard deviation value of cardiothoracic ratio (CTR) between patients with aortic arch calcification (AoAC) score ≦4 and AoAC score >4. Patients with AoAC score >4 had higher value of CTR than that in patients with AoAC score ≦4 (P < 0.001).
Determinants of AoAC Score >4
Table 2 lists the odds ratio (OR) estimates for AoAC score >4 with adjustment for age stratification, diabetes mellitus, cerebrovascular disease, diastolic blood pressure, baPWV, CTR, total cholesterol, and creatinine. In the multivariate stepwise logistic analysis, age ≧66 years (vs <57 years, OR 5.028, 95% confidence interval [CI] 2.208–11.453, P < 0.001), cerebrovascular disease (OR, 5.343; 95% CI 1.056–27.021; P = 0.043), high baPWV (per 100 cm/s, OR 1.065, 95% CI 1.003–1.129, P = 0.038), CTR (per 1%, OR, 1.116, 95% CI 1.046–1.191, P = 0.001), and total cholesterol level (per 1 mg/dL, OR 0.992, 95% CI 0.985–0.999, P = 0.030) were independently associated with AoAC score >4.
TABLE 2.
Risk Factors for AoAC > 4 Using Binary Logistic Regression Analysis
DISCUSSION
Chest radiography is a useful and noninvasive diagnostic tool. It is also often ordered for patients on HD as it offers a quick but relatively accurate picture of cardiac dimensions, including information on aortic arch calcification. In the present study, an AoAC score >4 on plain chest x-ray is independently associated with older age, cerebrovascular disease, high baPWV, high CTR, and low total cholesterol level.
An important finding is the significant association between AoAC and baPWV. The precise mechanisms responsible for the relationship between AoAC and arterial stiffness are not completely understood. Nonetheless, the involvement of structural changes within the media and vascular calcification are hypothesized.16,17 In an animal model, there is a correlation between smooth muscle cell differentiation into osteoblast-like cells, with the associated media remodeling that includes disruption of elastic lamellas and deposition of collagen, and increased arterial stiffness and vascular calcification.18 The association between AoAC from plain x-rays and increased baPWV corroborates the clinical significance of AoAC on HD patients.
The gold standard of arterial stiffness measurement is carotid-femoral pulse wave velocity (PWV),19 which directly reflects aortic PWV.1 In contrast, baPWV reflects several arterial segments, including brachial, distal arteries, aorta and femoral arteries. Tanaka et al20 compared carotid-femoral PWV and baPWV and showed a significant correlation between them (r = 0.73, P < 0.01).20 These two parameters also exhibit similar extent of associations with cardiovascular disease risk factors and clinical events. In addition, Yamashina et al14 compared aortic PWV and baPWV and found a strong association between them (r = 0.87, P < 0.01). Therefore, it is reasonable to use baPWV as a marker of arterial stiffness. In this study, we further confirmed that increased baPWV was a risk factor for increased AoAC in HD patients.
As an easily available parameter on chest radiography, increased CTR (>50%) is a marker of cardiomegaly. In patients with essential hypertension, it is associated with increased left ventricular mass and target organ damage.21 The AoAC is correlated with cardiomegaly in the present study. Vascular calcification induces arterial wall stiffness and reduces vascular compliance, which in turn, correlates with increased left ventricular afterload and hypertrophy.21 When Li et al22 evaluated the association of AoAC and cardiomegaly with progressive loss of renal function in patients with stage 3–5 chronic kidney disease, they found that AoAC and cardiomegaly were both associated with a faster decline in estimated glomerular filtration rate. Thus, AoAC is associated with cardiomegaly and may become a useful predictive parameter for adverse renal outcomes.
Among published literature on the relationship between vascular calcification and hypercholesterolemia,23–26 a small, randomized control trial of patients with hypercholesterolemia demonstrated that stain treatment lowered aortic compliance, coronary calcification, and carotid intimal–medial thickness.27 A study by Hoff et al24 of 30,908 asymptomatic individuals revealed that hypercholesterolemia is independently associated with coronary calcification. There was an independent association between hypercholesterolemia and AoAC in the study by Iribarren et al.25 Although the relationship between cholesterol and calcification is constant, it is not consistent across vessel beds. However, in the present study, AoAC is associated with low total cholesterol level in HD patients. Such association in HD is considered “reverse epidemiology,” even pointing to the possible involvement of malnutrition, atherosclerosis, and inflammation.28 Markers of protein-energy wasting, such as hypoalbuminemia, low serum cholesterol level, and low body mass index, are associated with increased mortality in patients with chronic renal failure.29 The current findings suggest that the relationship between AoAC and total cholesterol level in HD patients is different from that in the general population.
Nonetheless, this study has some limitations. First, this study has a cross-sectional design so the causal relationship and long-term clinical outcomes are not confirmed. Prospective studies are warranted to address these issues. Second, the limited number of study patients severely reduced the power of the study. Third, plain radiography is also not sensitive for detecting early-stage vascular calcification. Although plain radiography is highly correlated with the AoAC volume as determined by multidetector CT,3 cardiac CT can accurately and quantitatively evaluate the extent of cardiovascular calcification than plain radiograph. Fourth, AoAC evaluation using the semiquantitative method is relatively crude. In addition, as no studies have documented the reliable abnormal values of AoAC, we used the median score of AoAC to classify our study patients. Finally, we did not enroll patients with peritoneal dialysis in this study; therefore, our results could not be applied in peritoneal dialysis patients.
In conclusion, this study reveals that increased AoAC score is associated with increased baPWV and CTR. Measuring AoAC on plain chest x-ray may be an easy screening test to evaluate arterial stiffness in patients undergoing hemodialysis.
Footnotes
Abbreviations: AoAC = aortic arch calcification, baPWV = brachial-ankle pulse wave velocity, CT = computed tomography, CTR = cardiothoracic ratio, HD = hemodialysis.
The authors report no conflicts of interest.
REFERENCES
- 1.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 2001; 38:938–942. [DOI] [PubMed] [Google Scholar]
- 2.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002; 39:695–701. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa T, Ishida H, Matsuda N, et al. Simple evaluation of aortic arch calcification by chest radiography in hemodialysis patients. Hemodial Int 2009; 13:301–306. [DOI] [PubMed] [Google Scholar]
- 4.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008; 118:1748–1757. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Raggi P, Chasan-Taber S, et al. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant 2004; 19:1489–1496. [DOI] [PubMed] [Google Scholar]
- 6.Demer LL, Tintut Y, Parhami F. Novel mechanisms in accelerated vascular calcification in renal disease patients. Curr Opin Nephrol Hypertens 2002; 11:437–443. [DOI] [PubMed] [Google Scholar]
- 7.Wang AY, Woo J, Lam CW, et al. Associations of serum fetuin-a with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant 2005; 20:1676–1685. [DOI] [PubMed] [Google Scholar]
- 8.Cho IJ, Chang HJ, Park HB, et al. Aortic calcification is associated with arterial stiffening, left ventricular hypertrophy, and diastolic dysfunction in elderly male patients with hypertension. J Hypertens 2015; 33:1633–1641. [DOI] [PubMed] [Google Scholar]
- 9.Guerin AP, London GM, Marchais SJ, et al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 2000; 15:1014–1021. [DOI] [PubMed] [Google Scholar]
- 10.Lee CT, Huang CC, Hsu CY, et al. Calcification of the aortic arch predicts cardiovascular and all-cause mortality in chronic hemodialysis patients. Cardiorenal Med 2014; 4:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MJ, Shin DH, Kim SJ, et al. Progression of aortic arch calcification over 1 year is an independent predictor of mortality in incident peritoneal dialysis patients. PloS One 2012; 7:e48793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiu A, Ogawa T, Matsuda N, et al. Aortic arch calcification and arterial stiffness are independent factors for diastolic left ventricular dysfunction in chronic hemodialysis patients. Circ J 2008; 72:1768–1772. [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 2003; 166:303–309. [DOI] [PubMed] [Google Scholar]
- 14.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25:359–364. [DOI] [PubMed] [Google Scholar]
- 15.Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther 1995; 2:295–304. [DOI] [PubMed] [Google Scholar]
- 16.Dao HH, Essalihi R, Bouvet C, et al. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res 2005; 66:307–317. [DOI] [PubMed] [Google Scholar]
- 17.McEniery CM, McDonnell BJ, So A, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension 2009; 53:524–531. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier-Bastien A, Ung RV, Lariviere R, et al. Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin Exp Hypertens 2014; 36:173–180. [DOI] [PubMed] [Google Scholar]
- 19.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension 1995; 26:485–490. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 2009; 27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 21.Rayner BL, Goodman H, Opie LH. The chest radiograph. A useful investigation in the evaluation of hypertensive patients. Am J Hypertens 2004; 17:507–510. [DOI] [PubMed] [Google Scholar]
- 22.Li LC, Lee YT, Lee YW, et al. Aortic arch calcification predicts the renal function progression in patients with stage 3 to 5 chronic kidney disease. Biomed Res Int 2015; 2015:131263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol 2004; 24:331–336. [DOI] [PubMed] [Google Scholar]
- 24.Hoff JA, Daviglus ML, Chomka EV, et al. Conventional coronary artery disease risk factors and coronary artery calcium detected by electron beam tomography in 30,908 healthy individuals. Ann Epidemiol 2003; 13:163–169. [DOI] [PubMed] [Google Scholar]
- 25.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: Risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA 2000; 283:2810–2815. [DOI] [PubMed] [Google Scholar]
- 26.Reilly MP, Wolfe ML, Localio AR, et al. Coronary artery calcification and cardiovascular risk factors: Impact of the analytic approach. Atherosclerosis 2004; 173:69–78. [DOI] [PubMed] [Google Scholar]
- 27.Forbat SM, Naoumova RP, Sidhu PS, et al. The effect of cholesterol reduction with fluvastatin on aortic compliance, coronary calcification and carotid intimal-medial thickness: a pilot study. J Cardiovasc Risk 1998; 5:1–10. [PubMed] [Google Scholar]
- 28.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial 2004; 17:229–232. [DOI] [PubMed] [Google Scholar]
- 29.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol 2009; 29:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


