Supplemental Digital Content is available in the text
Abstract
To assess the effects of radial extracorporeal shock wave therapy (rESWT) on plantar flexor muscle spasticity and gross motor function in very young patients with cerebral palsy (CP).
The design was case-control study (level of evidence 3).
The setting was the Department of Pediatric Neurology and Neurorehabilitation, First Hospital of Jilin University, Changchun, China.
Those with a diagnosis of CP and spastic plantar flexor muscles were recruited between April 2014 and April 2015.
According to the parents’ decision, patients received 1 ESWT session per week for 3 months, with 1500 radial shock waves per ESWT session and leg with positive energy flux density of 0.03 mJ/mm2, combined with traditional conservative therapy (rESWT group) or traditional conservative therapy alone (control group).
The Modified Ashworth Scale (MAS) (primary outcome measure) and passive range of motion (pROM) measurements were collected at baseline (BL), 1 month (M1), and 3 months (M3) after BL. The Gross Motor Function Measure (GMFM)-88 was collected at BL and M3.
Sixty-six patients completed the final review at 3 months and were included in the study. Subjects ranged in age from 12 to 60 months (mean age 27.0 ± 13.6 months; median age 22.0 months; 33.3% female). For the rESWT group (n = 34), mean MAS grades at BL, M1, and M3 were 2.6, 1.9, and 1.5 on the left side and 1.9, 1.7, and 1.2 on the right side. For the control group (n = 32), mean MAS grades at BL, M1, and M3 were 2.5, 2.4, and 2.1 on the left side and 1.8, 1.8, and 1.5 on the right side. The within-subject effects time × side and time × treatment were statistically significant (P < 0.01). Similar results were found for the improvement of mean pROM. GMFM-88 improved from BL to M3, but showed no statistically significant difference between the groups. There were no significant complications.
This study demonstrates that the combination of rESWT and traditional conservative therapy is more effective than traditional conservative therapy alone in the treatment of spasticity in very young patients with CP.
INTRODUCTION
Cerebral palsy (CP) is a clinical syndrome characterized by a persistent disorder of posture or movement caused by a nonprogressive disorder of the immature brain.1 The prevalence of CP has been reported to be between 1.86 cases per 1000 population in the United Kingdom,1 and 3.6 cases per 1000 in 8-year-old children in the United States,2 with little variation among Western nations.3 In a very recent systematic review analyzing a total of 49 studies, the pooled overall prevalence of CP was 2.11 cases per 1000 live births.4 Rates of CP in population-based settings in India and China gave figures of 2 to 2.8 cases per 1000 births.5 A systematic literature review for a period spanning between 1965 and 2004 found CP more prevalent in more deprived socioeconomic populations.6 The same study identified low birth weight, intrauterine infections, and multiple gestation as the most important risk factors for CP.6
Most children with CP suffer from spasticity as the main motor disorder.1,7 Spasticity is a major challenge for rehabilitation of children with CP. This is because spasticity can cause pain, prevent or hamper function, and may disturb sleep.1,7 Spasticity of plantar flexor muscles is a particular problem in CP because it causes toe walking. This can result in major functional implications such as disturbances in balance and walking, and interfere with gross motor function.8
The management of spasticity in CP is complex and is a major challenge to the treatment team.7,9 The ultimate goal of any therapy program must be to achieve the child's maximum potential in motor skills.9 Unfortunately, the scientific evidence for various physical therapy treatment options for children with CP is limited.10,11 Botulinum neurotoxin (BoNT) is a widely used and effective pharmacological treatment for focal muscle overactivity.2,12–14 An alternative to BoNT treatment is focal intramuscular treatment with phenol and alcohol, with the aim to improve activity limitations and other outcomes in children and adults with spasticity.15–17 However, focal intramuscular injection of BoNT, phenol, and alcohol is not without problems: BoNT is expensive and not available in many countries; a significant risk of focal intramuscular injection of alcohol and phenol is persisting pain15; and all these procedures are invasive and, thus, not without risk when applied under difficult hygienic conditions. With regard to poststroke spasticity, a recent Cochrane review18 concluded that, at best, there was ’low-level’ evidence for the effectiveness of outpatient multidisciplinary rehabilitation in improving active function and impairments after BoNT treatment for upper limb spasticity in adults with chronic stroke.
Orthopedic surgery is considered as a last resort in managing spasticity in children with CP, but is not an option for managing spasticity per se. Instead, it is used to help correct the secondary problems that occur with growth alongside spastic muscles and poor motion control. Those problems include muscle shortening, joint contractures, and bony deformities.19
Recently, extracorporeal shock wave therapy (ESWT) has become an alternative in the treatment of spasticity (summarized in Supplemental Table 1).20–37 A by product of extracorporeal shock wave lithotripsy, ESWT has emerged as a noninvasive management option for tendon and other pathologies of the musculoskeletal system,38 with only a few unwanted side effects such as temporary skin redness and pain during treatment. Prior studies on tendinopathy showed that ESWT can be as or more effective than other forms of treatment such as eccentric exercise, traditional physiotherapy, steroid injections, and surgery.38 There are 2 different types of extracorporeal shock waves—focused (fESWT) and radial (rESWT)—and several modes of operation of focused and radial extracorporeal shock wave generators.38
Among the studies on fESWT and rESWT for spasticity performed so far, 6 out of 18 (33%) were pilot studies without control group, 7 (39%) were pseudo-controlled studies (ie, each patient served as her/his own control, with 1 placebo treatment followed by 1 ESWT treatment 1 or 2 weeks later), and 5 (28%) were randomized controlled trials (RCTs) (Supplemental Table 1).
It is of note that none of the studies on fESWT and rESWT for spasticity summarized in Supplemental Table 1 was performed on patients younger than an average of 4.8 years of age. However, it has been argued that the management of spasticity in children with CP should be started as early as possible,7 and there is evidence that early intervention (ie, before the age of 36 months) can minimize secondary complications of CP.9
Acknowledging the particular problem of spastic plantar flexor muscles in CP,8 the limited scientific evidence for various physical therapy treatment options for children with CP,10,11 the risks and limitations associated with BoNT and focal intramuscular treatment with phenol and alcohol,15,18 and the proven effectiveness of rESWT in the treatment of spasticity in patients with CP aged between 10 and 46 years of age,24 the aim of the present study was to determine whether rESWT combined with traditional conservative therapy (consisting of physical therapy, Chinese massage, meridian mediation, and muscle stimulation) is safe and more effective than traditional conservative therapy alone for the management of spastic plantar flexor muscles in patients with CP younger than averaged 3 years of age. Although in all studies listed in Supplemental Table 1, a positive effect of fESWT or rESWT on spasticity was reported, no preliminary data were available for patients with CP younger than an average of 3 years of age. Therefore, the null hypothesis of the present study was that rESWT combined with traditional conservative therapy is as safe as, but not more effective in, the treatment of spastic plantar flexor muscles in very young patients with CP than traditional conservative therapy alone.
METHODS
Ethics
The present study was approved by the Ethics Committee of the First Hospital of Jilin University, Changchun, China, before starting the study, and was carried out in accordance with the World Medical Association Declaration of Helsinki.39 It has been registered at the Chinese Clinical Trial Registry (Identifier ChiCTR-OCC-15006095) and with ClinicalTrials.gov (Identifier NCT02719483). Parents of the patients were allowed to withdraw the free and informed consent term to participate in the present study at any time.
Participants
A total of n = 86 young children with CP were assessed for eligibility to be enrolled in the present prospective case control trial between April 2014 and April 2015. All patients were from the community of Changchun or Jilin province, China, and referred to the Department of Pediatric Neurology and Neurorehabilitation, First Hospital of Jilin University. Accordingly, the patients assessed for eligibility to be enrolled in the present study were representative of the citizens of the city of Changchun and Jilin Province, China. Patients were diagnosed based on their history and physical examination at the Department of Pediatric Neurology and Neurorehabilitation, First Hospital of Jilin University. Patients were considered for participation in the present study according to the inclusion and exclusion criteria summarized in Table 1.
TABLE 1.
Inclusion and Exclusion Criteria of Children With Spastic Plantar Flexor Muscles Due to Cerebral Palsy Enrolled in the Present Study

Allocation of Patients to One of the Treatment Groups, Dropouts, and Loss to Follow-up
The flow of patients in the present study according to the Consolidated Standards of Reporting Trials (CONSORT)40 is shown in Figure 1. After the diagnosis was confirmed, 20 out of the 86 patients assessed for eligibility were excluded because they did not meet the inclusion criteria and/or met 1 or more of the exclusion criteria, or the parents of the patients declined to participate in the study. The age distribution of the remaining 66 patients is shown in Supplemental Figure 1.
FIGURE 1.
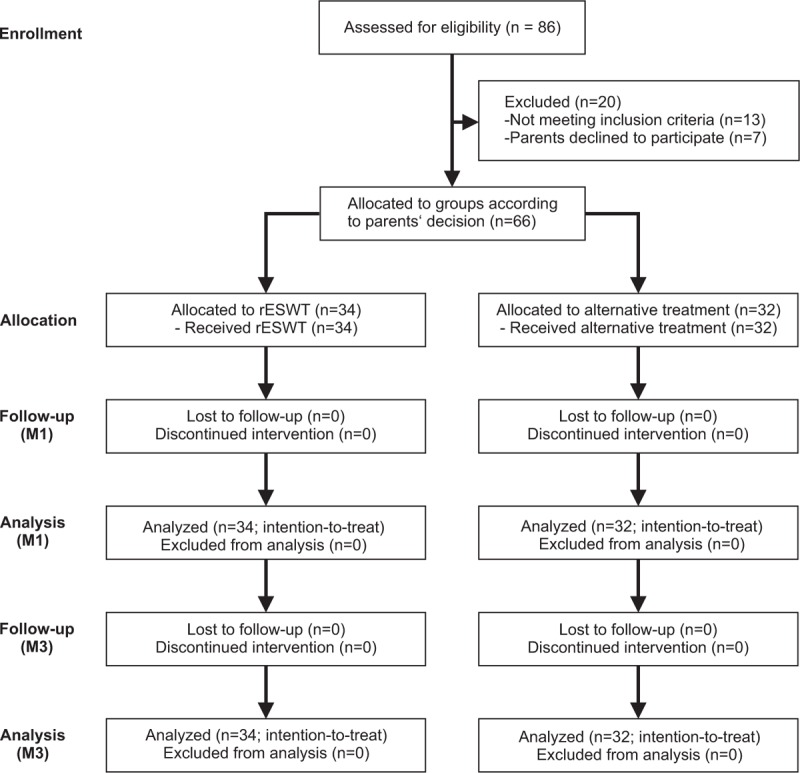
Flow of patients in the present study according to the Consolidated Standards of Reporting Trials (CONSORT).40 M1 = 1 month after baseline, M3 = 3 months after baseline, rESWT = radial extracorporeal shock wave therapy.
For the parents of these 66 patients, a thorough explanation of the various options, and also the potential risks, benefits, and outcomes associated with the various options, took place. Parents of all patients who met the inclusion criteria, did not meet the exclusion criteria, and agreed to participate in the present study were offered traditional conservative therapy consisting of physical therapy, Chinese massage, meridian mediation, and muscle stimulation for 3 months; or rESWT plus traditional conservative therapy for 3 months. After making an informed decision, those patients whose parents chose to treat the condition of their child with rESWT were assigned to the rESWT group (n = 34). Those patients whose parents chose to treat the condition of their child only with traditional conservative therapy (ie, without rESWT) were considered for inclusion in the control group (n = 32).
All patients received treatment as allocated. None of the patients were lost to follow-up at 1 month (M1) and 3 months (M3) after the first treatment, resulting in full analysis of all patients at M1 and M3 that were allocated to one of the treatment groups.
Treatment
The design of the present study is shown in Supplemental Figure 2. The patients in the rESWT group were treated with the radial shock wave device, Swiss DolorClast (EMS Electro Medical Systems, Nyon, Switzerland) using the “radial” (blue) handpiece with the 15-mm applicator (the same device was used in the aforementioned study by Vidal et al24). Each patient received 1 rESWT session per week for 3 months, with 1500 radial shock waves per session and leg, that is, a total of 3000 radial shock waves per session or a total of 36,000 radial shock waves within 12 weeks. The application of radial shock waves to the plantar flexor muscles is shown in Figure 2. Radial shock waves were applied using coupling gel, and evenly distributed over the gastrocnemius and soleus muscles. The air pressure of the device was set at 0.6 bar, resulting in a positive energy flux density (EFD+) of 0.03 mJ/mm2. Radial extracorporeal shock waves were applied at a frequency of 8 Hz. Accordingly, a single rESWT session (1 leg) took approximately 3 minutes and 8 seconds. Local or general anesthesia was not applied.
FIGURE 2.
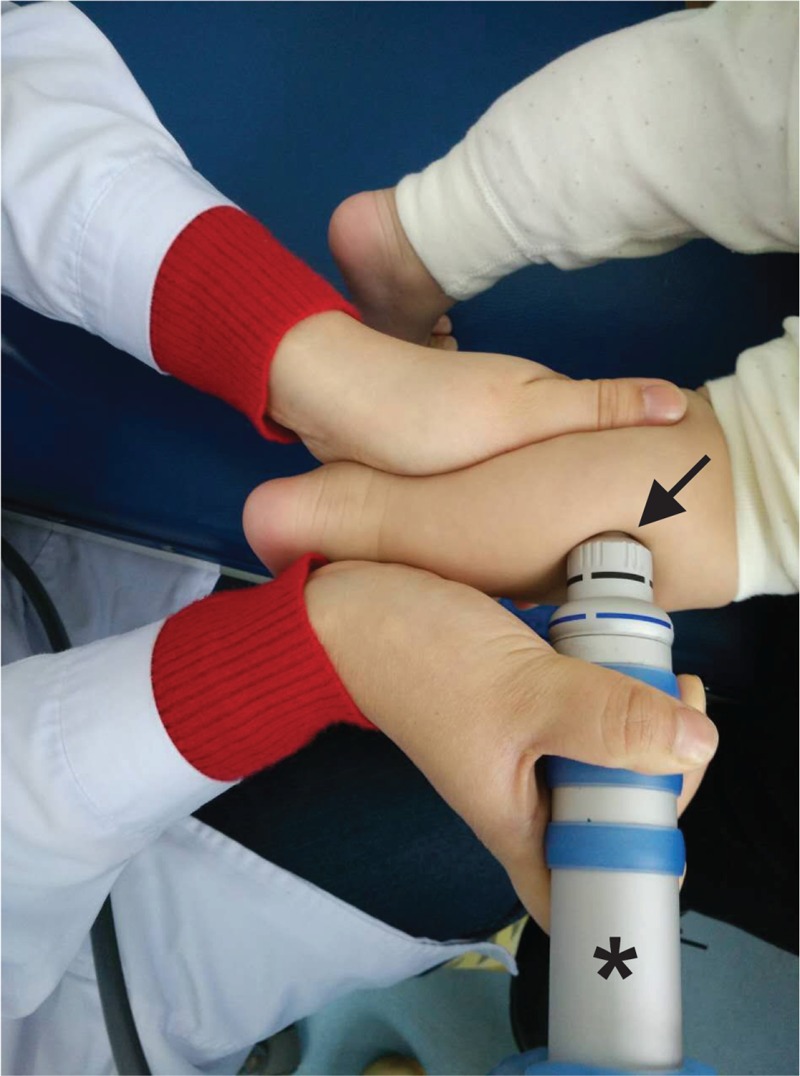
Application of radial extracorporeal shock waves (rESWs) on the gastrocnemius muscle (lateral head) of a 12-month-old patient suffering from cerebral palsy (note that rESWs were evenly distributed over the gastrocnemius and soleus muscles). The applicator of the handpiece (asterisk) of the rESW device is coupled with ultrasound gel to the skin directly over the spastic muscle (arrow).
Patients in both groups received traditional conservative therapy consisting of physical therapy, Chinese massage, meridian mediation, and muscle stimulation for 3 months (6 days per week, 30 minutes per type of therapy).
Treatments were performed at the Department of Pediatric Neurology and Neurorehabilitation, First Hospital of Jilin University. Accordingly, the intervention was undertaken in a specialist center unrepresentative of hospitals and clinics most of the source population would attend. However, all treatments were performed on an outpatient basis and, thus, representative of that in use in the source population.
Outcome Measures
Outcome measures included the Modified Ashworth Scale (MAS) grade of the plantar flexor muscles (primary outcome measure), the passive range of motion (pROM) of the foot, and the Gross Motor Function Measure (GMFM)-88 (secondary outcome measures).
The MAS41 grade was collected on each side at baseline (BL), M1, and M3 after BL. For the patients in the rESWT group, the MAS grade was collected before rESWT at BL and after rESWT at M1 and M3 (Supplemental Figure 2). MAS grades were, respectively, 0 (no increase in muscle tone), 1 (slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) moved in flexion or extension), 1+ (slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM), 2 (more marked increase in muscle tone through most of the range of motion, but affected part(s) easily moved), 3 (considerable increase in muscle tone, passive movement difficult), or 4 (affected part(s) rigid in flexion or extension).41
Treatment success was defined as individual improvement of the primary outcome measure (MAS grade) by more than 1 grade at M3 compared with BL.
The pROM was measured at BL, M1, and M3 as the dorsiflexion of the ankle joint. Measurements were performed using a goniometer in supine position with the knee extended. For the patients in the rESWT group, pROM measurements were performed before rESWT at BL and after rESWT at M1 and M3 (Supplemental Figure 2). These data were used to calculate differences between the rESWT group and the control group at BL, and also treatment-related differences. Besides this, on the left side, pROM measurements were also performed after rESWT at BL and before rESWT at M1 and M3 (Supplemental Figure 2). These additional data were used to calculate differences in pROM immediately before and after rESWT (ΔpRom).
The GMFM-8842,43 was collected at BL and M3. For the patients in the rESWT group, the GMFM-88 was collected before rESWT at BL, and after rESWT at M3 (Supplemental Figure 2). The GMFM-88 is a standardized criterion referenced measurement tool designed to measure gross motor function over time for children with disabilities between 5 months and 6 years of age.44 It considers 5 dimensions of gross motor function: lying and rolling (17 items), sitting (20 items), crawling and kneeling (14 items), standing (13 items), and walking, running, and jumping (24 items). A recent literature review considered the GMFM-88 useful as an outcome measure to detect changes in gross motor function in children with CP undergoing interventions.44
Complications, adverse effects, and complaints during treatment were documented.
Blinding
The design of the present study prevented blinding of the patients, their parents, and the therapists who applied the treatments. On the other hand, the assessors who measured treatment outcome did not have access to the patients’ treatment records, including patient allocation, until all patients had completed the 3-month follow-up examination.
Power Analysis
Based on the outcome of the studies listed in Supplemental Table 1, previous pilot experience with rESWT, and extensive experience with traditional conservative therapy, we expected treatment success (ie, individual reduction of the MAS grade by more than 1 grade at M3 compared with BL) in 50% of the patients in the rESWT group and in 10% of the patients in the control group. Considering a 2-sided significance level of 95%, power of 0.9, and equal samples, the power analysis determined a minimum number of n = 28 (according to Kelsey et al45) or n = 31 (according to Fleiss et al46) per group to be enrolled in the present study. Power analysis was performed with the online tool, Open Source Epidemiologic Statistics for Public Health.47
Statistical Analysis
Statistical analysis was performed on an intention-to-treat basis using the “Last Observation Carried Forward” approach.48 However, because all patients received treatment as allocated and none of the patients were lost to follow-up at M1 and M3, it was not necessary to separately perform statistical analysis for the intention-to-treat population and the per-protocol completers.
Mean and standard deviation (SD) were calculated for all investigated variables. The D’Agostino and Pearson omnibus normality test was used to determine whether the distribution of the investigated variables of the patients in the rESWT group and the patients in the control group were consistent with a Gaussian distribution.
Differences at BL between the patients in the rESWT group and the patients in the control group were tested with nonparametric Mann–Whitney U test for the mean age of the patients and the mean GMFM-88 score; Fisher exact test for the relative numbers of female and male patients; and 2-way repeated-measures analysis of variance (ANOVA) with side (left, right) as within-subject factor for the mean MAS grades and the mean pROM values.
Treatment-related differences in mean MAS grades and mean pROM values between the patients in the rESWT group and the patients in the control group were tested with 2-way repeated-measures ANOVA, with the different times (BL, M1, M3) as within-subject factor, group (rEWST, control) and treated side (left, right) as between-subject factors, and age and sex of the patients as covariances.
Treatment success (ie, number of patients with reduction of the MAS grade by more than 1 grade at M3 compared with BL) was tested with Fisher exact test. This was separately done for the left and the right side.
For the patients in the rESWT group, the change in pROM on the left side immediately after rESWT compared with the situation immediately before rESWT (henceforth referred to as ΔpROM) was calculated at BL, M1, and M3. Differences in mean ΔpROM between BL, M1, and M3 were tested using repeated-measures ANOVA with time (BL, M1, M3) as within-subject factor, followed by Bonferroni multiple comparison test comparing all pairs of groups.
Treatment-related differences in mean GMFM-88 scores between the patients in the rESWT group and the patients in the control group were tested with 2-way repeated-measures ANOVA, with the different times (BL, M3) as within-subject factor, group (rEWST, control) as between-subject factor, and age and sex of the patients as covariances.
Dependency of the individual MAS grades on the patients’ age was tested with nonparametric Spearman rank-order correlation, whereas the variables pROM, ΔpROM, and GMFM-88 were tested with linear regression analysis.
In all analyses, an effect was considered statistically significant if its associated P value was smaller than 0.05. Calculations were performed using SPSS (Version 23 for Windows; IBM, Armonk, NY) and GraphPad Prism (Version 5; Graph Pad Software, San Diego, CA).
Data Deposition
All data of the present study were deposited at ClinicalTrials.gov (Identifier NCT02719483).
RESULTS
Patients
All BL data of the patients in the rESWT group and the patients in the control group are summarized in Table 2, and also the P values of the corresponding statistical analyses.
TABLE 2.
Characteristics of Included Patients at Baseline
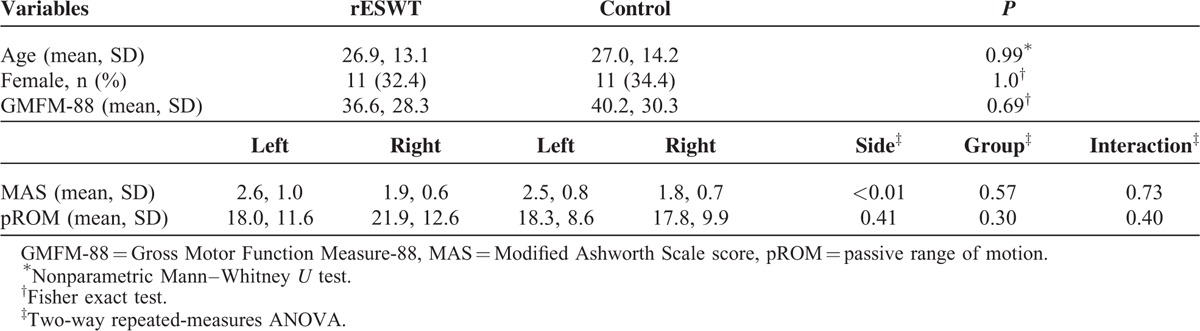
The median age of the patients in the rESWT group was 22.3 months (range 12–57.5), and of the patients in the control group, 21.5 months (range 13–60). The D’Agostino and Pearson omnibus normality test showed that the age distribution of the patients in the control group was not consistent with a Gaussian distribution (PrESWT = 0.13; Pcontrol = 0.03). The mean age of the patients in the rESWT group was not significantly different statistically from the mean age of the patients in the control group (Table 2).
There were 11 female and 23 male patients in the rESWT group, and 11 female and 21 male patients in the control group, with no statistically significant difference between the groups (Table 2).
At BL, there were no statistically significant differences between the patients in the rESWT group and the patients in the control group with regard to the mean MAS grade, the mean pROM, and the mean GMFM-88 score (Table 2). However, patients in both the rESWT group and the control group showed higher mean MAS grades on the left side than on the right side (Table 2).
Modified Ashworth Scale (MAS; Primary Outcome Measure)
Radial ESWT combined with traditional conservative therapy reduced the mean MAS grade on the left side from 2.6 ± 1.0 (mean ± SD) at BL to 1.5 ± 1.0 at M3 (-42%), and on the right side from 1.9 ± 0.6 at BL to 1.2 ± 0.7 at M3 (−37%). In contrast, traditional conservative therapy alone reduced the mean MAS grade on the left side from 2.5 ± 0.8 to only 2.1 ± 0.5 at M3 (−16%), and on the right side from 1.8 ± 0.7 degrees to only 1.5 ± 0.7 at M3 (−17%). Two-way repeated-measures ANOVA showed that the within-subject effects time × side and time × treatment were statistically significant (P < 0.01), and also the between-subject effect side (P < 0.01) (Table 3). Individual MAS grades did not depend on the patients’ age (Figure 3).
TABLE 3.
Results of the Present Study (pROM, MAS, and GMFM-88)
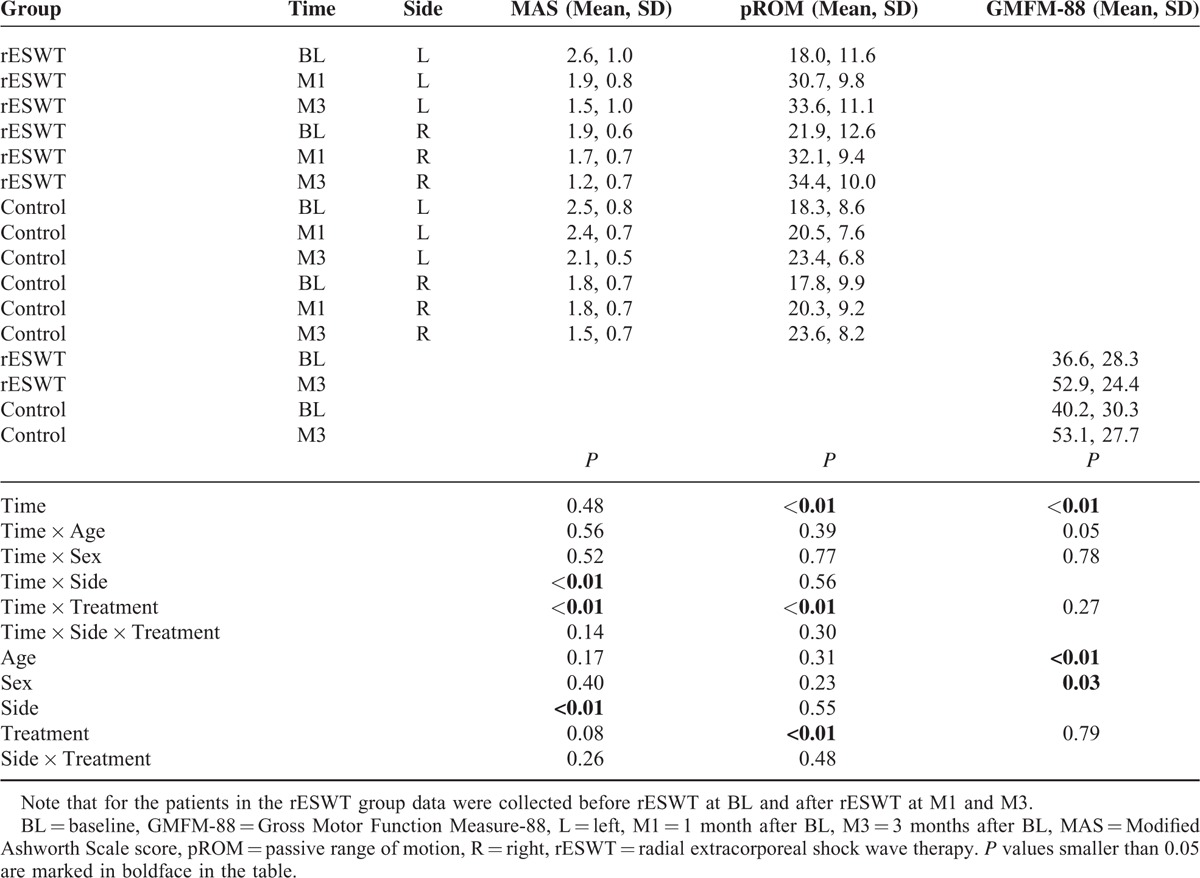
FIGURE 3.
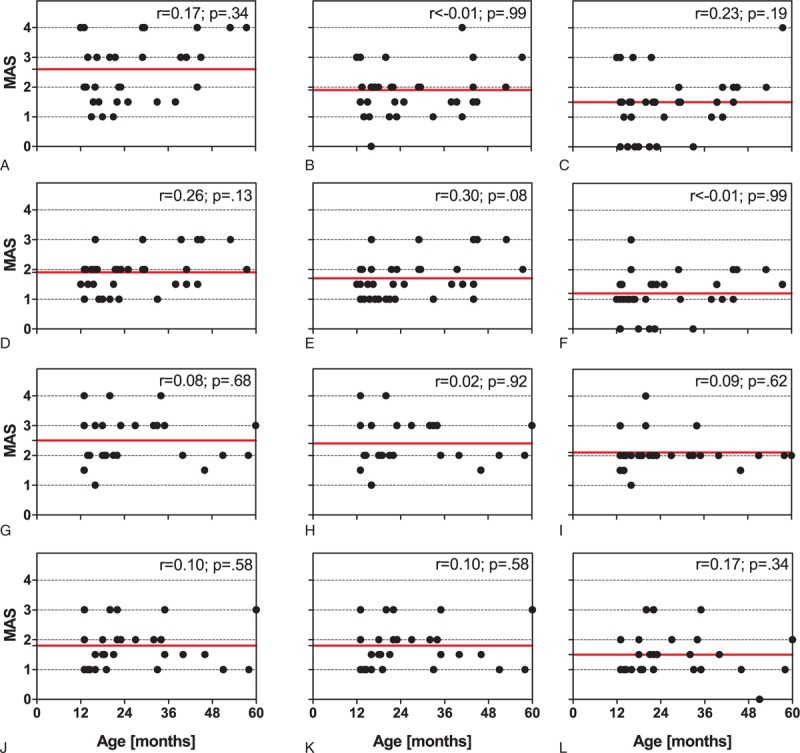
Modified Ashworth Scale grades as a function of the patient's age of the patients in the rESWT group on the left (top row of panels; A–C) and the right side (second row of panels; D–F), and also of the patients in the control group on the left (third row of panels; G–I) and the right side (bottom row of panels; J–L) at baseline (left column of panels: A, D, G, and J), 1 month after baseline (middle column of panels: B, E, H, and K), and 3 months after baseline (right columns of panels: C, F, I, and L). Each panel shows individual data (dots) and the corresponding mean value (red solid lines). Results of nonparametric Spearman rank-order correlation are provided in the upper right corner of each panel. r = Spearman r, rESWT = radial extracorporeal shock wave therapy.
Treatment Success
Thirteen patients (13/34 = 38.2%) in the rESWT group, but no patients in the control group, showed individual improvement of the MAS grade on the left side by more than 1 grade at M3. For the right side, the corresponding data were 2 patients (2/34 = 5.9%) in the rESWT group and no patients in the control group. Differences between groups were statistically significant on the left side (P < 0.01), but not on the right side (P = 0.49). Accordingly, the null hypothesis was rejected for the left side, but not for the right side.
Passive Range of Motion
Radial ESWT combined with traditional conservative therapy increased the mean pROM on the left side from 18.0 ± 11.6 degrees (mean ± SD) at BL to 33.6 ± 11.1 degrees at M3 (+87%), and on the right side from 21.9 ± 12.6 degrees at BL to 34.4 ± 10.0 degrees at M3 (+57%). In contrast, traditional conservative therapy alone increased the mean pROM on the left side from 18.3 ± 8.6 degrees at BL to only 23.4 ± 6.8 degrees at M3 (+28%), and on the right side from 17.8 ± 9.9 degrees at BL to only 23.6 ± 8.2 degrees at M3 (+33%). Two-way repeated-measures ANOVA showed that the within-subject effects time and time × treatment were statistically significant (P < 0.01), and also the between-subject effect treatment (P < 0.01) (Table 3). Individual pROM values did not depend on the patients’ age (Figure 4).
FIGURE 4.
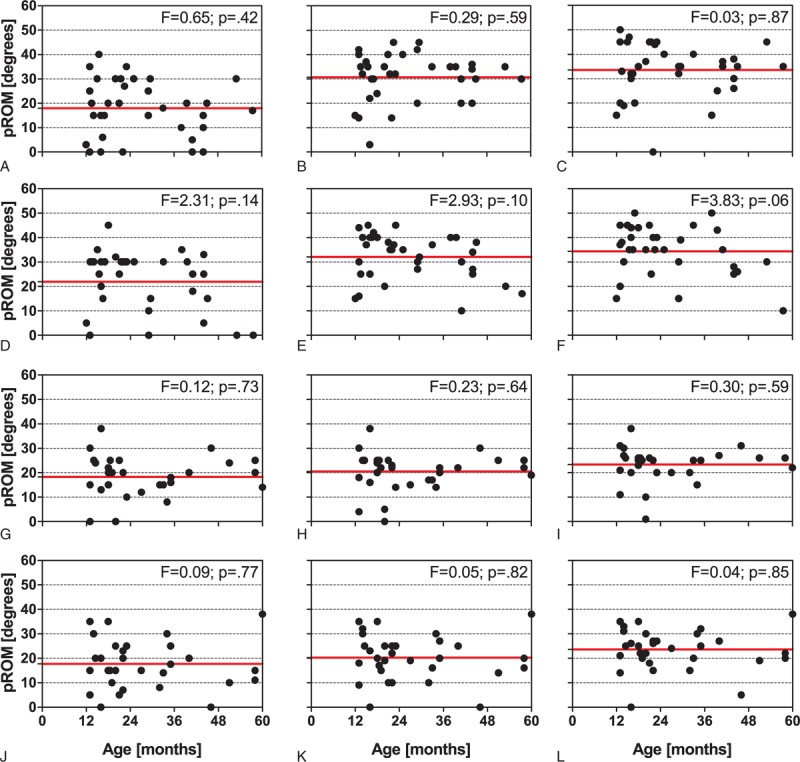
Passive range of motion as a function of the patient's age of the patients in the rESWT group on the left (top row of panels: A–C) and the right side (second row of panels: D–F), and also of the patients in the control group on the left (third row of panels: G–I) and the right side (bottom row of panels: J–L) at baseline (left column of panels: A, D, G, and J), 1 month after baseline (middle column of panels: B, E, H, and K), and 3 months after baseline (right columns of panels: C, F, I, and L). Each panel shows individual data (dots) and the corresponding mean value (red solid lines). Results of linear regression analysis are provided in the upper right corner of each panel. rESWT = radial extracorporeal shock wave therapy.
Immediately after rESWT, most of the patients showed a greater individual pROM than immediately before rESWT (ΔpROM) (tested only on the left side of the patients in the rESWT group) (Figure 5). The mean ΔpROM decreased from 6.9 ± 4.7 degrees (mean ± SD) at BL to 5.4 ± 3.9 degrees at M1 and 4.2 ± 2.6 degrees at M3. Repeated-measures ANOVA showed statistically significant differences in mean ΔpROM between the times (P = 0.02), and Bonferroni multiple comparison test revealed a statistically significant difference in mean ΔpROM between BL and M3 (P < 0.05), but not between BL and M1 and between M1 and M3 (P < 0.05 each). Individual ΔpROM values did not depend on the patient's age (Figure 5).
FIGURE 5.
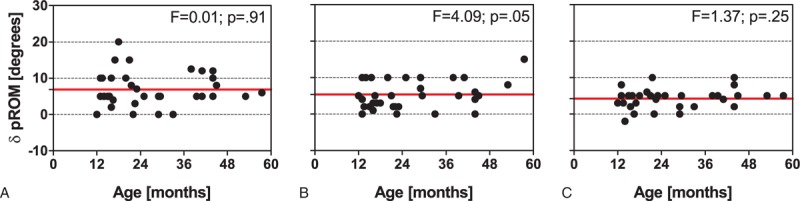
Change in passive range of motion immediately after rESWT compared with the situation immediately before rESWT (ΔpROM) on the left side of the patients in the rESWT group at baseline (A), 1 month after baseline (B), and 3 months after baseline (C). Each panel shows individual data (dots) and the corresponding mean value (red solid lines). Results of linear regression analysis are provided in the upper right corner of each panel. rESWT = radial extracorporeal shock wave therapy.
Gross Motor Function Measure-88
Radial ESWT combined with traditional conservative therapy increased the mean GMFM-88 score from 36.6 ± 28.3 (mean ± SD) at BL to 52.9 ± 24.4 at M3 (+45%). For traditional conservative therapy alone, the corresponding values were 40.2 ± 30.3 at BL and 53.1 ± 27.7 at M3 (+32%). Two-way repeated-measures ANOVA showed that the within-subject effect time was statistically significant (P < 0.01), and also the between-subject effects age (P < 0.01) and sex (P = 0.03) (Table 3). For the patients in the rESWT group, but not for the patients in the control group, the individual GMFM-88 scores were depending on the patients’ age (Figure 6).
FIGURE 6.
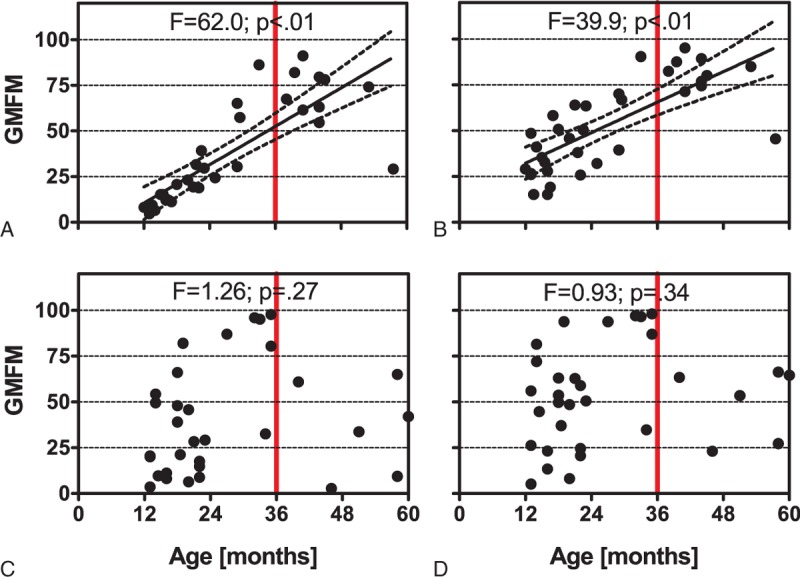
Gross Motor Function Measure (GMFM)-88 as a function of the patient's age of the patients in the rESWT group (A, B) and the patients in the control group (C, D) at baseline (A, C) and 3 months after baseline (B, D). Each panel shows individual data (dots). Results of linear regression analysis are provided at the top of each panel. Regression lines with a slope significantly nonzero statistically are indicated (black solid lines) together with their 95% confidence intervals (black dotted lines). The reason for the red vertical lines at X = 36 months is explained in the “Discussion” section.
Complications, Adverse Effects, and Complaints During Treatment
No complications or adverse effects were observed. No complaints directly related to rESWT (such as pain during treatment) were reported.
DISCUSSION
The findings of the present study can be summarized as follows: radial ESWT (rESWT), as applied in the present study (1 ESWT session per week for 3 months, with 1500 radial shock waves per ESWT session and leg with EFD+ of 0.03 mJ/mm2 applied at a frequency of 8 Hz, combined with traditional conservative therapy consisting of physical therapy, Chinese massage, meridian mediation, and muscle stimulation), resulted in reduced mean MAS grades of spastic plantar flexor muscles and increased mean pROM of the feet of very young patients with CP (median age below 24 months; mean age below 36 months) compared with traditional conservative therapy alone. On the other hand, rESWT, as applied in the present study, was not superior to traditional conservative therapy alone in improving the mean GMFM-88 score of very young patients with CP.
With regard to the latter finding, it is of note that the patients in the rESWT group showed a different age distribution of individual GMFM-88 scores than the patients in the control group (Figure 6). This was most likely due to the fact that patients were not randomly allocated to the groups, but rather according to the decision of the patients’ parents after explanation of the various options, and also the potential risks, benefits, and outcomes associated with the various options. The latter strategy was chosen in agreement with the Ethics Committee of the First Hospital of Jilin University, Changchun, China, considering the very young age of the patients and the novelty of rESWT to the citizens of Jilin province, China. Specifically, parents of patients older than 36 months with GMFM-88 scores lower than 50 tended to choose traditional conservative therapy alone (4/5 = 80%; Figure 6), whereas parents of patients older than 36 months with GMFM-88 scores higher than 50 tended to choose rESWT (9/11 = 81.8%; Figure 6). In other words, parents of patients older than 36 months with better motor skills were apparently more open for a new, innovative treatment modality than parents of patients older than 36 months with worse motor skills. One cannot rule out that this observation was linked to sociological and/or psychological factors particular to the population of the citizens of Jilin Province, China. However, this observation should be considered in future studies on novel interventions on very young patients with CP.
To answer the question whether the aforementioned observation had impact on the results of the present study, we repeated the statistical analysis of the GMFM-88 data after exclusion of all patients older than 36 months of age. In this case, the groups were not significantly different statistically at BL (Fisher exact test; P = 0.05); patients in the rESWT group showed an increase of the mean GMFM-88 score by 79% at M3 compared with BL, whereas patients in the control group showed an increase of the mean GMFM-88 score by only 30% at M3 compared with BL; but this difference was not statistically significant (Supplemental Table 2). It therefore remains to be shown in future studies that rESWT as applied in the present study can improve the mean GMFM-88 score of very young patients with CP.
We could reject our null hypothesis on the left side of the patients (with mean MAS grades at BL of 2.6 ± 1.0 [mean ± SD] of the patients in the rESWT group and 2.5 ± 0.8 of the patients in the control group), but not on the right side of the patients (with mean MAS grades at BL of 1.9 ± 0.6 of the patients in the rESWT group and 1.8 ± 0.7 of the patients in the control group). This may indicate that rESWT is more effective the more increased the tone of the affected muscle is. The reason of the higher mean MAS grades on the right side than on the left side in our sample of very young patients with CP is unknown.
The present study is not the first one on ESWT for spasticity, but the first one on ESWT for spasticity in very young children with CP with median age below 24 months and mean age below 36 months (Supplemental Table 1). Thus, the present study is the first one on ESWT acknowledging the recommendation in the literature that the management of spasticity in children with CP should be started as early as possible,7 and the evidence in the literature indicating that early intervention (ie, before the age of 36 months) can minimize secondary complications of CP.9 However, it remains to be shown in future studies with longer follow-up that rESWT as applied in the present study can minimize secondary complications of CP.
The Taskforce on Childhood Motor Disorders of the US National Institutes of Health (NIH) defined spasticity in 2001 as hypertonia in which 1 or both of the following signs are present: resistance to externally imposed movement that increases with increasing speed of stretch and varies with the direction of joint movement; and resistance to externally imposed movement that rapidly rises above a threshold speed of joint angle.49 The Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society pointed out in 2010 (Delgado et al2) that, according to a study by Nielsen and Sinkjaer,50 the MAS41 measures a broader set of neural and musculoskeletal factors of nonvelocity-dependent hypertonia in addition to spasticity itself. According to Delgado et al,2 the Tardieu scale (TS)51 is a tool that is more consistent with the proposed definition of spasticity provided by the NIH Taskforce on Childhood Motor Disorders.49 The TS accounts for the joint angle measure of the spastic phenomenon at different velocities of joint movement.51 For the following reasons, the MAS, but not the TS, was used in the present study: it was recently shown that the MAS can have a higher intraobserver reliability (based on repeated measurements on the same child at different times) than the TS in the assessment of spasticity in children with CP52; in most of the studies summarized in Supplemental Table 1, the MAS was applied, but in none of these studies, the TS was applied; and our primary goal was not to assess rESWT-induced changes in spasticity according to the proposed definition of spasticity provided by the NIH Taskforce on Childhood Motor Disorders,49 but to compare our results to data reported in other studies on ESWT for spasticity.
The results of the present study (particularly the fact that we could not reject our null hypothesis on the right side of the patients with the lower mean MAS grades than on the left side) raised the question as to whether our treatment protocol can be further optimized. To answer this question, Supplemental Table 3 summarizes the treatment protocols of all studies on ESWT for spasticity reported so far, and Supplemental Table 4 provides an overview on the MAS grades reported in these studies.
We found that a major difference between the present study and the studies listed in Supplemental Table 1, 3, and 4 was that neither a hypothesis nor a definition of treatment success was provided in any of the latter studies, and (with the exception of a study by Santamato et al29), no power analysis was performed (as is required by CONSORT40). Besides this, we found substantial variation in the different treatment protocols, with numbers of ESWT sessions varying between 1 and 12, intervals between ESWT sessions varying between 1 day and 1 week, number of shock waves per ESWT session varying between 500 and 3000, and energy flux densities varying between 0.03 and 0.15 mJ/mm2 (in most studies it was not specified whether the reported energy flux density was the positive or the total energy flux density). Furthermore, the treatment protocols of the studies with control groups (ie, RCTs and case-control studies) did not reflect the treatment protocols of the studies without control groups (ie, pilot studies and pseudo-placebo controlled studies). On the other hand, with a few exceptions, the treatment protocols of the studies with control groups shared an interval between ESWT sessions of 1 week, a number of 1500 shock waves per ESWT session, and an energy flux density of 0.03 mJ/mm2. In fact, our treatment protocol (1 ESWT session per week for 3 months; 1500 shock waves per ESWT session, and leg with EFD+ of 0.03 mJ/mm2) was very similar to the treatment protocol used by El-Shamy et al.32
As shown in Supplemental Table 4, the mean MAS grade on the right side in the present study was lower than any other mean MAS grade at BL in studies on ESWT for spasticity reported so far. Thus, one cannot currently answer the question as to whether patients with spasticity and MAS grade of less than 2 at BL are good candidates for ESWT or not (as suggested by the present study). On the other hand, the data summarized in Supplemental Table 4 collectively show that for patients with spasticity and MAS grade of more than 2, ESWT can improve the MAS grade by 1 grade on average. To our knowledge, this is more than what is known for any other conservative, nonpharmacological treatment of spasticity in children with CP.10,11
Moreover, the data summarized in Supplemental Table 4 do not indicate a striking advantage of higher energy flux densities over lower energy flux densities in ESWT for spasticity. This does not rule out the possibility that a treatment protocol similar to the one used in the present study, but with higher EFD than 0.03 mJ/mm2, may be even more effective in the treatment of spasticity of very young children with CP. On the other hand, higher EFDs can increase pain associated with ESWT and thus limit its usability on very young children. Besides this, it must be ruled out in future studies that ESWT for spasticity with higher EFD, particularly in very young children, does not result in myolysis as it was repeatedly reported in early studies on ESWL and ESWT with high EFD.53–55 It is of note that this question was not addressed in any of the studies summarized in Supplemental Tables 1, 3, and 4.
The data summarized in Supplemental Table 4 also show that a single or only a few ESWT sessions may not be effective in treating spasticity in children with CP. Rather, continuous treatment with ESWT (once per week) seems useful. In this regard, the data of the present study on MAS grades (Figure 3), pROM (Figure 4), and particularly ΔpROM (Figure 5) (ie, change in pROM immediately after rESWT compared with the situation immediately before rESWT) indicate that repeated rESWT did not let the treated muscles merely “fluctuate” between higher and lower muscle tone, but actually caused lasting reduction in muscle tone. Unfortunately, the molecular and cellular mechanisms of ESWT on spastic muscles causing this lasting reduction in muscle tone are largely unknown. Taking biopsy samples of spastic muscles after repeated ESWT has not yet been performed and seems very problematic from an ethical point of view in very young children.
Kenmoku et al56 exposed the gastrocnemius muscle of Sprague–Dawley rats to radial extracorporeal shock waves (rESWs) using the same rESWT device as in the present study (2000 radial shock waves; EFD+ = 0.18 mJ/mm2). Using a rhodamine-α-bungarotoxin binding method, the authors found that rESWs induced degeneration of acetylcholine receptors. Compared with untreated control muscles, the compound muscle action potential (CMAP) amplitude of the treated muscles was significantly decreased immediately after rESWT, and this reduction in CMAP amplitude lasted for 8 weeks without delaying latency. Kenmoku et al56 concluded that these results suggest a transient dysfunction of nerve conduction at the neuromuscular junction.
Recently, we exposed Caenorhabditis elegans worms (C elegans) to rESWs generated with the same rESWT device as used in the present study, and found that increased exposure to rESWs resulted in decreased mean speed of movement of the worms (analyzed under the microscope similarly to gait analysis of patients) while increasing the proportion of worms rendered paralyzed.57 Recovery of these 2 behavioral symptoms was observed during increasing postexposure waiting periods.57 At first glance, one may challenge the significance of data obtained by exposing C elegans to rESWs to the application of rESWT on humans. On the other hand, C elegans express many of the neurotransmitters and associated receptors that are found in higher eukaryotes, including humans. These include dopamine, acetylcholine, γ-aminobutyric acid (GABA), glutamate, and serotonin (reviewed in58). In addition, more than 100 neuropeptide genes encoding over 250 distinct neuropeptides were identified in C elegans so far, among them 40 genes encoding insulin-like peptides.59 Many of the neurons that express specific neurotransmitters were identified.60–62 In other words, the nervous system of C elegans provides a unique opportunity to understand how behavior emerges from activity in the nervous system of an organism. We may therefore expect novel major insights into the molecular and cellular mechanisms of ESWT on spastic muscles causing lasting reduction in muscle tone by exposing C elegans to rESWs (and also to focused extracorporeal shock waves) in future studies.
Based on reports in the literature that both rESWT and fESWT can induce neovascularization,63,64 thereby increasing the blood supply to the tissue and modulating the activation of growth factors,63–66 some authors have hypothesized that these mechanisms may also play a role in reducing muscle tone in ESWT for spasticity.21,27,37 One cannot rule out that these mechanisms may indeed play a role after repeated exposure of spastic muscles to extracorporeal shock waves. However, it is of note that these mechanisms cannot explain reduction of muscle tone and improvement of the pROM of the corresponding joints immediately after ESWT.
The present study is an audit of prospectively collected data, and has therefore inherent limitations. First, there was no randomization in the present study. Second, the small number of patients could potentially confound the clinical results. Third, no muscle biopsies were taken, and, thus, the molecular and cellular mechanisms of reducing muscle tone by rESWs could not be investigated. Fourth, muscle tone was assessed with the MAS,41 but not with the TS51. However, the symptoms and physical findings used to define spasticity in children with CP in the present study are generally accepted and considered appropriate for this condition.
CONCLUSIONS
The results of the present study suggest that the use of rESWT for spasticity in very young patients with CP is safe and effective, leading to a significant reduction in MAS grade and improvement of joint function without adverse effects. For this reason, clinicians should consider rESWT before invasive intervention in the management of spasticity in very young patients with CP.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: BL = baseline, BoNT = botulinum neurotoxin, CP = cerebral palsy, ESWT = extracorporeal shock wave therapy, fESWT = focused extracorporeal shock wave therapy, GMFM-88 = Gross Motor Function Measure-88, M1 = 1 month after baseline, M3 = 3 months after baseline, MAS = Modified Ashworth Scale, pROM = passive range of motion, RCT = randomized controlled trial, rESWs = radial extracorporeal shock waves, rESWT = radial extracorporeal shock wave therapy.
Supplemental Digital Content is available for this article.
The authors have not received external funding for this study. Dr Christoph Schmitz serves as paid consultant for and receives benefits from Electro Medical Systems, the manufacturer and distributor of the radial extracorporeal shock wave device, Swiss DolorClast. However, Electro Medical Systems had no any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No other potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.National Collaborating Centre for Women's and Children's Health (UK). Spasticity in children and young people with non-progressive brain disorders: management of spasticity and co-existing motor disorders and their early musculoskeletal complications. London, England: RCOG Press; 2012. [PubMed] [Google Scholar]
- 2.Delgado MR, Hirtz D, Aisen M, et al. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2010; 74:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol 2006; 33:251–267. [DOI] [PubMed] [Google Scholar]
- 4.Oskoui M, Coutinho F, Dykeman J, et al. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol 2013; 55:509–519. [DOI] [PubMed] [Google Scholar]
- 5.Gladstone M. A review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settings. Ann Trop Paediatr 2010; 30:181–196. [DOI] [PubMed] [Google Scholar]
- 6.Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil 2006; 28:183–191. [DOI] [PubMed] [Google Scholar]
- 7.Shamsoddini A, Amirsalari S, Hollisaz MT, et al. Management of spasticity in children with cerebral palsy. Iran J Pediatr 2014; 24:345–351. [PMC free article] [PubMed] [Google Scholar]
- 8.Krigger KW. Cerebral palsy: an overview. Am Fam Physician 2006; 73:91–100. [PubMed] [Google Scholar]
- 9.Oleszek J, Davidson L. Braddom RL. Cerebral Palsy. Physical Medicine and Rehabilitation. Philadelphia, PA: Elsevier; 2011. 1253–1273. [Google Scholar]
- 10.Turnbull JD. Early intervention for children with or at risk of cerebral palsy. Am J Dis Child 1993; 147:54–59. [PubMed] [Google Scholar]
- 11.Damiano DL. Rehabilitative therapies in cerebral palsy: the good, the not as good, and the possible. J Child Neurol 2009; 24:1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker JA, Pereira G. The efficacy of Botulinum Toxin A for limb spasticity on improving activity restriction and quality of life: a systematic review and meta-analysis using the GRADE approach. Clin Rehabil 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Strobl W, Theologis T, Brunner R, et al. Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins 2015; 7:1629–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker HW, Lee MY, Bahroo LB, et al. Botulinum toxin injection techniques for the management of adult spasticity. PMR 2015; 7:417–427. [DOI] [PubMed] [Google Scholar]
- 15.Tilton AH. Injectable neuromuscular blockade in the treatment of spasticity and movement disorders. J Child Neurol 2003; 18 Suppl 1:S50–S66. [DOI] [PubMed] [Google Scholar]
- 16.Patel DR, Soyode O. Pharmacologic interventions for reducing spasticity in cerebral palsy. Indian J Pediatr 2005; 72:869–872. [DOI] [PubMed] [Google Scholar]
- 17.Chung CY, Chen CL, Wong AM. Pharmacotherapy of spasticity in children with cerebral palsy. J Formos Med Assoc 2011; 110:215–222. [DOI] [PubMed] [Google Scholar]
- 18.Demetrios M, Khan F, Turner-Stokes L, et al. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst Rev 2013; 6:CD009689. [DOI] [PubMed] [Google Scholar]
- 19.Lynn AK, Turner M, Chambers HG. Surgical management of spasticity in persons with cerebral palsy. PMR 2009; 1:834–838. [DOI] [PubMed] [Google Scholar]
- 20.Lohse-Busch H, Kraemer M, Reime U. Pilotuntersuchung zur Wirkung von niedrigenergetischen, extrakorporalen Stosswellen auf Muskelfunktionsstorungen bei spastischen Bewegungsstorungen von Kindern [article in German]. Schmerz 1997; 11:108–112. [DOI] [PubMed] [Google Scholar]
- 21.Manganotti P, Amelio E. Long-term effect of shock wave therapy on upper limb hypertonia in patients affected by stroke. Stroke 2005; 36:1967–1971. [DOI] [PubMed] [Google Scholar]
- 22.Trompetto C, Avanzino L, Bove M, et al. External shock waves therapy in dystonia: preliminary results. Eur J Neurol 2009; 16:517–521. [DOI] [PubMed] [Google Scholar]
- 23.Amelio E, Manganotti P. Effect of shock wave stimulation on hypertonic plantar flexor muscles in patients with cerebral palsy: a placebo-controlled study. J Rehabil Med 2010; 42:339–343. [DOI] [PubMed] [Google Scholar]
- 24.Vidal X, Morral A, Costa L, et al. Radial extracorporeal shock wave therapy (rESWT) in the treatment of spasticity in cerebral palsy: a randomized, placebo-controlled clinical trial. NeuroRehabilitation 2011; 29:413–419. [DOI] [PubMed] [Google Scholar]
- 25.Sohn MK, Cho KH, Kim YJ, et al. Spasticity and electrophysiologic changes after extracorporeal shock wave therapy on gastrocnemius. Ann Rehabil Med 2011; 35:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manganotti P, Amelio E, Guerra C. Shock wave over hand muscles: a neurophysiological study on peripheral conduction nerves in normal subjects. Muscles Ligaments Tendons J 2012; 2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 27.Gonkova MI, Ilieva EM, Ferriero G, et al. Effect of radial shock wave therapy on muscle spasticity in children with cerebral palsy. Int J Rehabil Res 2013; 36:284–290. [DOI] [PubMed] [Google Scholar]
- 28.Troncati F, Paci M, Myftari T, et al. Extracorporeal shock wave therapy reduces upper limb spasticity and improves motricity in patients with chronic hemiplegia: a case series. NeuroRehabilitation 2013; 33:399–405. [DOI] [PubMed] [Google Scholar]
- 29.Santamato A, Notarnicola A, Panza F, et al. SBOTE study: extracorporeal shock wave therapy versus electrical stimulation after botulinum toxin type a injection for post-stroke spasticity-a prospective randomized trial. Ultrasound Med Biol 2013; 39:283–291. [DOI] [PubMed] [Google Scholar]
- 30.Moon SW, Kim JH, Jung MJ, et al. The effect of extracorporeal shock wave therapy on lower limb spasticity in subacute stroke patients. Ann Rehabil Med 2013; 37:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YW, Shin JC, Yoon JG, et al. Usefulness of radial extracorporeal shock wave therapy for the spasticity of the subscapularis in patients with stroke: a pilot study. Chin Med J 2013; 126:4638–4643. [PubMed] [Google Scholar]
- 32.El-Shamy SM, Eid MA, El-Banna MF. Effect of extracorporeal shock wave therapy on gait pattern in hemiplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil 2014; 93:1065–1072. [DOI] [PubMed] [Google Scholar]
- 33.Santamato A, Micello MF, Panza F, et al. Extracorporeal shock wave therapy for the treatment of poststroke plantar-flexor muscles spasticity: a prospective open-label study. Top Stroke Rehabil 2014; 21:S17–24. [DOI] [PubMed] [Google Scholar]
- 34.Mirea A, Onose G, Padure L, et al. Extracorporeal shockwave therapy (ESWT) benefits in spastic children with cerebral palsy (CP). J Med Life 2014; 7:127–132. [PMC free article] [PubMed] [Google Scholar]
- 35.Daliri SS, Forogh B, Emami Razavi SZ, et al. A single blind, clinical trial to investigate the effects of a single session extracorporeal shock wave therapy on wrist flexor spasticity after stroke. NeuroRehabilitation 2015; 36:67–72. [DOI] [PubMed] [Google Scholar]
- 36.Marinelli L, Mori L, Solaro C, et al. Effect of radial shock wave therapy on pain and muscle hypertonia: a double-blind study in patients with multiple sclerosis. Mult Scler 2015; 21:622–629. [DOI] [PubMed] [Google Scholar]
- 37.Park DS, Kwon DR, Park GY, et al. Therapeutic effect of extracorporeal shock wave therapy according to treatment session on gastrocnemius muscle spasticity in children with spastic cerebral palsy: a pilot study. Ann Rehabil Med 2015; 39:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz C, Császár NBM, Milz S, et al. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br Med Bull 2015; 116:115–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards SD, McNamee MJ. Ethical concerns regarding guidelines for the conduct of clinical research on children. J Med Ethics 2005; 31:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010; 7:e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67:206–207. [DOI] [PubMed] [Google Scholar]
- 42.Russell DJ, Rosenbaum PL, Cadman DT, et al. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol 1989; 31:341–352. [DOI] [PubMed] [Google Scholar]
- 43.Russell DJ, Rosenbaum PL, Avery L, et al. Gross Motor Function Measure (GMFM-66 and GMFM-88) User's Manual: Clinics in Developmental Medicine. London, England: Mac Keith Press; 2002. [Google Scholar]
- 44.Alotaibi M, Long T, Kennedy E, et al. The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): a literature review. Disabil Rehabil 2014; 36:617–627. [DOI] [PubMed] [Google Scholar]
- 45.Kelsey JL, Whittemore AS, Evans AS, et al. Methods in Observational Epidemiology. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 46.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd edNew Jersey, NY: Wiley; 2003. [Google Scholar]
- 47.OpenEpi, 2003. Open Source Epidemiologic Statistics for Public Health Web Site. Available at: http://www.openepi.com. Updated May 4, 2015 (Version 3.03a) Accessed February 1, 2016. [Google Scholar]
- 48.European Medicine Agency. Guideline on missing data in confirmatory clinical trials. European Medicine Agency Web Site. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC5000967 93.pdf. Updated July 2, 2010 Accessed February 1, 2016. [Google Scholar]
- 49.Sanger TD, Delgado MR, Gaebler-Spira D, et al. Task Force on Childhood Motor Disorders. Classification and definition of disorders causing hypertonia in childhood. Pediatrics 2003; 111:e89–e97. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen JF, Sinkjaer T. A comparison of clinical and laboratory measures of spasticity. Mult Scler 1996; 1:296–301. [DOI] [PubMed] [Google Scholar]
- 51.Haugh AB, Pandyan AD, Johnson GR. A systematic review of the Tardieu scale for the measurement of spasticity. Disabil Rehabil 2006; 28:899–907. [DOI] [PubMed] [Google Scholar]
- 52.Numanoğlu A, Günel MK. Intraobserver reliability of modified Ashworth scale and modified Tardieu scale in the assessment of spasticity in children with cerebral palsy. Acta Orthop Traumatol Turc 2012; 46:196–200. [DOI] [PubMed] [Google Scholar]
- 53.Kishimoto T, Yamamoto K, Sugimoto T, et al. Side effects of extracorporeal shock-wave exposure in patients treated by extracorporeal shock-wave lithotripsy for upper urinary tract stone. Eur Urol 1986; 12:308–313. [DOI] [PubMed] [Google Scholar]
- 54.Parr KL, Lingeman JE, Jordan M, et al. Creatinine kinase concentrations and electrocardiographic changes in extracorporeal shock-wave lithotripsy. Urology 1988; 32:21–23. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda K, Tomita K, Takayama K. Application of extracorporeal shock wave on bone: preliminary report. J Trauma 1999; 47:946–950. [DOI] [PubMed] [Google Scholar]
- 56.Kenmoku T, Ochiai N, Ohtori S, et al. Degeneration and recovery of the neuromuscular junction after application of extracorporeal shock wave therapy. J Orthop Res 2012; 30:1660–1665. [DOI] [PubMed] [Google Scholar]
- 57.Angstman NB, Kiessling MC, Frank HG, et al. High interindividual variability in dose-dependent reduction in speed of movement after exposing C. elegans to shock waves. Front Behav Neurosci 2015; 9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyd WA, Smith MV, Kissling GE, et al. Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol Teratol 2010; 32:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Kim K. Neuropeptides. In: The C. elegans Research Community, ed. WormBook. doi/10.1895/wormbook.1.142.1; www.wormbook.org. [Google Scholar]
- 60.Brownlee DJ, Fairweather I. Exploring the neurotransmitter labyrinth in nematodes. Trends Neurosci 1999; 22:16–24. [DOI] [PubMed] [Google Scholar]
- 61.McDonald PW, Jessen T, Field JR, et al. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol 2006; 26:593–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuske K, Beg AA, Jorgensen EM. The GABA nervous system in C. elegans. Trends Neurosci 2004; 27:407–414. [DOI] [PubMed] [Google Scholar]
- 63.Contaldo C, Högger DC, Khorrami Borozadi M, et al. Radial pressure waves mediate apoptosis and functional angiogenesis during wound repair in ApoE deficient mice. Microvasc Res 2012; 84:24–33. [DOI] [PubMed] [Google Scholar]
- 64.Wang CJ, Wang FS, Yang KD, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res 2003; 21:984–989. [DOI] [PubMed] [Google Scholar]
- 65.Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J 2003; 26:220–232. [PubMed] [Google Scholar]
- 66.Mariotto S, Cavalieri E, Amelio E, et al. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide 2005; 12:89–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


